Abstract
Malignant glioma is a lethal form of brain cancer that is very difficult to treat. The aggressive behavior of these neoplasms and their limited responsiveness to therapy has been attributed in part to the ability of these tumors to evade the immune system. Gliomas, like many other solid tumors, express components of numerous immune escape mechanisms, including immunosuppressive proteins such as TGF-β, IL-10, and FasL. Here, we show that FasL expression can support the growth of experimental intracranial glioma. We show that FasL is readily detected in human glioblastoma multiforme clinical specimens. FasL was found to be expressed by three well-characterized rat glioma cell lines (9L, F98, and C6) and glioma cell-derived FasL mediated the death of phytohemagglutinin-stimulated Jurkat T-lymphocytes when cocultured with glioma cells in vitro. We asked if inhibiting 9L-derived FasL altered the growth of experimental glioma. FasL expression knockdown using shRNA reduced the growth of subcutaneous and intracranial 9L gliomas by ∼50% in immune competent Fisher 344 rats. In contrast, FasL expression knockdown had no affect on the growth of intracranial 9L glioma in T-cell deficient athymic rats. Intracranial tumors derived from FasL knockdown 9L glioma cells contained up to 3-fold more tumor infiltrating T-cells than tumors derived from control 9L cells. These results demonstrate that down-regulating FasL expression and/or function in glial malignancies can enhance T-cell tumor infiltration and inhibit tumor growth. The findings suggest that targeting endogenous FasL in glial malignancies could enhance the efficacy of emerging immune-based treatment strategies.
Keywords: CD95L, Fas ligand, glioma, immunotherapy, immunity
Malignant glioma is the most common form of brain cancer in the central nervous system. Currently, the mean survival of patients affected by glioblastoma multiforme (GBM; the most aggressive form of malignant glioma) after conventional therapy consisting of surgical resection followed by radiotherapy and chemotherapy, remains just over 14 months.1 It is known that the immune system can provide some degree of surveillance against immunogenic solid tumors, including glioma. However, malignant glioma and most other malignancies are known for their ability to evade antitumor immunity via mechanisms that are likely to hamper the effectiveness of many emerging immunotherapeutic strategies.2 A variety of tumor immune escape mechanisms have been described including the expression of TGF-β, IL-10, and FasL (CD95L) by tumor cells.3–10
The recognition that FasL is expressed by a variety of tumors has led to the belief that the Fas/FasL system can play a prominent role in the inhibition of immune responses by neutralizing activated T and B cells.11–13 FasL is a 42 kDa protein belonging to the tumor necrosis factor family of proteins. When bound to its receptor, Fas, FasL activates an intracellular signaling cascade that results in caspase activation and the initiation of apoptosis. FasL is involved in many normal cellular processes including immune cell interactions and the maintenance of immune privilege in the eyes, ear, and testis.14–16 In these sites FasL acts to limit inflammation by inducing apoptosis of infiltrating, pro-inflammatory lymphocytes.15 FasL is expressed by many human tumors and expression levels are associated with poor prognosis in breast, liver, and ovarian cancers, supporting the immunosuppressive “counterattack” hypothesis.5,17,18
On the basis of the premise that FasL expression by glioma contributes to its ability to evade antitumor immunity, we hypothesized that down-regulating brain tumor FasL expression will enhance innate antitumor immune responses and cooperate/synergize with other T-cell activating immunotherapies. To investigate whether FasL mediates glioma immune privilege, we used siRNA to knock-down FasL expression in rat glioma cell lines and analyzed its effects on T-cell/glioma cell interactions in vitro and in vivo. We show that inhibiting FasL expression abrogates the ability of glioma cells to induce T-cell apoptosis and enhances the number of tumor-infiltrating T-cells, which in turn leads to decreased tumor growth. We conclude that this method of modulating immunotherapy may be applicable to a wide range of FasL-expressing tumor types, including CNS malignancies, and may prove useful in enhancing immune-based treatment strategies such as interleukin and vaccine therapies.
Materials and Methods
Cell Culture
The rat glioma cell lines 9L, F98, and C6, as well as the human Jurkat A3 T-cell line were purchased from American type culture collection and grown in Dulbecco's modified Eagle's medium (DMEM; Mediatech Inc.) supplemented with 10% fetal bovine serum (FBS; Gemini Bioproducts Inc.) and 1% penicillin-streptomycin (Mediatech Inc.). All cells were grown at 37°C in a humidified incubator with 5% CO2.
Antibodies
Antibodies were obtained from the following sources and used at the indicated concentrations: Santa Cruz Biotechnology, FasL N-20 (1:200); Sigma, actin (1:5000); BD Pharmingen (Franklin Lakes, New Jersey), FasL MFL4 (62.5 µg/mL), and CD3 (1:200). Secondary antibodies conjugated to IRDyeTM 680 and 800 were purchased from Li-Cor (Lincoln, Nebraska) and used at a 1:20 000 dilution.
Immunoblotting
SDS–polyacrylamide gel electrophoresis (PAGE) and immunoblotting were performed by the method of Towbin et al.19 with minor modifications.20 Aliquots of 50 µg of total protein were combined with Laemmli loading buffer containing β-mercaptoethanol and heated at 100°C for 5 minutes. The proteins were separated on precast SDS–PAGE gels (Cambrex) at 130 V for 90 minutes. Proteins were electrophoretically transferred to nitrocellulose with a semidry transfer apparatus (GE Healthcare) at 50 mA per gel stack for 60 minutes. Transfer was confirmed by ponceau (Sigma) staining for 5 minutes at room temperature. Membranes were incubated with Odyssey blocking buffer (Li-Cor) for 1 hour at room temperature and then with primary antibody for 18–24 hours at 4°C in 5% BSA in Tris Buffered Saline with Tween 20 (TBS/T). Membranes were then washed three times with TBS/T, incubated with secondary antibody for 1 hour in Odyssey blocking buffer, washed three times with TBS/T, once in TBS, and analyzed using the Odyssey Infrared Imaging System (Li-Cor). A competitive binding assay was performed using a blocking peptide (sc-834; Santa Cruz) against the FasL N-20 Ab. This assay allowed us to exclude nonspecific bands from our analysis of FasL protein expression. All immunoblots depict membrane bound FasL with a MW of ∼42 kDa.
Real-time Reverse Transcription Polymerase Chain Reaction
Cells were collected in ice-cold PBS using a cell scraper and centrifuged at 1000 × g for 5 minutes. RNA was isolated using the RNeasyTM Kit (Qiagen) according to the manufacturers' recommendations. Real-time RT–PCR was conducted using 100 ng RNA and the iSCRIPT kit (BioRad) according to the manufacturers' instructions. The following sets of primers were used; FasL sense (5′-TGCCTCCACTAAGCCCTCTA-3′), FasL antisense (5′-AGGCTGTGGTTGGTGAACTC-3′), 18s sense (5′-ACAGGATTGACAGATTGATAGCTC-3′), and 18s antisense (5′-CAAATCGCTCCACCAACTAAGAA-3′). Each sample was analyzed in triplicate and end products were subjected to melt curve analysis and agarose gel electrophoresis to ensure accurate synthesis of the correct product according to manufacturer's protocol. The critical-cycle analysis method was used to determine the ratio of FasL to 18S RNA expression in each sample according to manufacturer's protocol.
FasL siRNA and Stable Cell Line Generation
Predesigned siRNAs directed against rat FasL mRNA (GenBank: NM_012908) were purchased from Ambion (Austin, Texas; siRNA IDs 197271, 48716, and 197272). SiRNAs were transiently transfected into wild-type 9L and F98 glioma cells and screened by immunoblotting for optimal knock-down 48 hours after transfection. A scramble siRNA was used as negative control (Ambion product # AM4636). Transient transfections were performed using 100 nM siRNA combined with siPORT lipid transfection reagent (Ambion, Inc.) in optiMEM media (Gibco) according to the manufacturers' recommendations. Briefly, glioma cells were plated in 100-mm-diameter tissue culture dishes at 5 × 105 cells/plate in medium supplemented with 10% FBS. The following day medium was removed and replaced with medium containing 0.1% FBS. Approximately 1 hour later, siRNA:lipid complexes were added to the cells and allowed to incubate for 48 hours before cells were harvested as described above. For the generation of glioma cell lines that stably express anti-FasL and scrambled siRNAs (shFasL and shSCR, respectively), RNA sequences validated by transient transfection (FasL: siRNA ID 197272, Scramble: product # AM4636) were used to design 42 bp hairpin oligos that were cloned into the pSilencer4.1 plasmid (Ambion product #5775) using the BamH1 and HindIII restriction sites. The pSilencer4.1-shFasL3 and pSilencer4.1-shSCR plasmids were then transfected into glioma cells plated at low density using siPORT XP (Ambion, Inc.) according to the manufacturer's instructions. Clonal cell lines were selected in the presence of 1 mg/ml puromycin (Sigma). Immunoblotting was used to screen for FasL knock-down. For each parental glioma cell line, one shSCR and two shFasL clonal lines were chosen for subsequent experiments. Stocks of transfected cell lines were maintained in wild-type medium supplemented with 500 µg/mL puromycin. All subsequent experiments were performed in the absence of antibiotic.
In Vitro Coculture Assay
T-cell killing by glioma cells was quantified according to the method of Posmantur et al.21 with some modification. Glioma cells were distributed to 48-well tissue culture plates (1.5 × 104 cells/well) and allowed to grow for 48 hours. Jurkat A3 T-lymphocytes were stimulated with phytohemagglutinin (PHA, 5 µg/mL) for 24 hours, rinsed, re-suspended in medium, and added to wells containing glioma cells (105 cells/well) or to wells lacking glioma cells as control. Cultures were incubated for 48 hours at 37° in the absence or presence of neutralizing anti-FasL antibody MFL4 after which conditioned media were collected, centrifuged at 100 000 × g to eliminate cells and debris and assayed for lactate dehydrogenase (LDH) using the Cytotox96 nonradioactive assay kit (Promega) according to the manufacturers' recommendations.
Tumor Growth and Histology
For intracranial implantations, glioma cells (105 cells in 10 µL DMEM) were implanted to the right striatum as previously described.22 Animals were sacrificed after 2 weeks and perfusion-fixed using 4% paraformaldehyde. Cryoprotected frozen brains were sectioned 30 µm thick using a cryostat and sections were mounted on gelatin-coated slides and stained for T-cells using anti-CD3 and sodium citrate antigen retrieval. Maximum tumor cross-sectional areas were determined in hematoxylin and eosin stained sections by computer-based morphometry using the Odyssey infrared imaging system and used to calculate estimated tumor volumes using the formula: Signal to noise ratios (SNR) ([Maximum tumor intensity] − [Mean background intensity])/Standard deviation (SD) of mean background.23 For subcutaneous xenografts, glioma cells (105 cells in 100 µL DMEM) were implanted into the right flank of Fisher 344 rats. Tumor volumes were measured using calipers at the indicated time points and volumes estimated using the formula for an ellipsoid tumor = pi/6(length)(width)(height).
Statistical Analysis
Statistical analysis was performed using GraphPad Prism version 3.03. Evaluation of significance was determined using ANOVA with a Bonferonni post test for multiple simultaneous comparisons. P-values less than .05 were considered statistically significant and * is P < .05, ** is P < .01, and *** is P < .001.
Results
FasL Expression in Human GBM and Rat Glioma Cell Lines
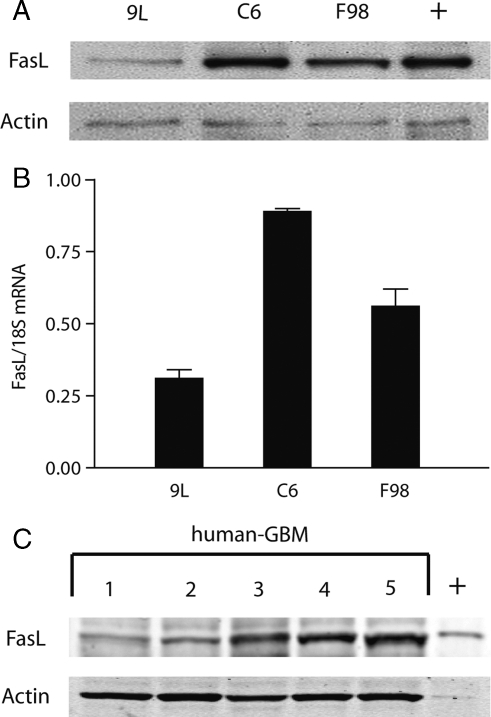
We analyzed the expression of FasL in three common rat glioma cell lines (9L, F98, and C6). Each cell line was found to express FasL protein and mRNA as determined by immunoblot and real-time polymerase chain reaction analyses, respectively (Fig. 1). The relative levels of FasL protein expressed by these three cell lines mirrored their mRNA levels (Fig. 1A and B). FasL expression was also assayed by immunoblot in 5 clinical glioblastoma specimens (hGBM) obtained from patients at the time of tumor resection (Fig. 1C). All tumors expressed FasL at variable levels. These data show that FasL is commonly expressed by experimental rat glioma cell lines and by human glioblastoma.
Fig. 1.
Rat glioma cell lines and human GBM specimens express FasL. FasL protein expression was assessed in three commonly used rat glioma cell lines by (A) immunoblotting and (B) the real-time polymerase chain reaction (RT-PCR). The results show that each cell line examined expresses FasL protein and mRNA. (C) Total tissue proteins from human GBM clinical specimens were subjected to immunoblot analysis for FasL. Every human-GBM tested was positive for FasL expression. + indicates lanes with positive control, FasL expressing HL60 leukaemia cells.
FasL Mediates In Vitro T-cell Killing
Glioma cells were cocultured with PHA-activated, FasL sensitive Jurkat A3 T-lymphocyte cells to determine if glioma cells can kill activated T-cells via a FasL-dependant mechanism. Each cell line specifically killed Jurkat cells as assessed by LDH release assay (Fig. 2). Up to 30% of Jurkat cell death induced by glioma cells was specifically inhibited by the neutralizing FasL mAb (MFL4) and thus was mediated by FasL. Of the three cell lines tested, the F98 cell line induced the highest level of Jurkat cell death. These results demonstrate that rat glioma cells can kill activated T-cells by paracrine mechanisms and that a significant percentage of T-cell death induced by the glioma cells is FasL mediated. This assay also confirms that FasL expressed by the cell lines used in this study is functional and capable of activating a lymphocytic death response.
Fig. 2.
Glioma cell lines induce FasL dependent T-cell death in vitro. LDH release was measured after FasL sensitive, PHA activated T-cells were cocultured with FasL expressing glioma cell lines in the absence (black bars) or presence (white bars) of a FasL neutralizing antibody (MFL4). The results indicate that up to 30% of T-cell death was inhibited by the FasL neutralizing antibody and thus mediated by FasL.
FasL Expression Knock-down Inhibits Glioma-induced T-cell Death
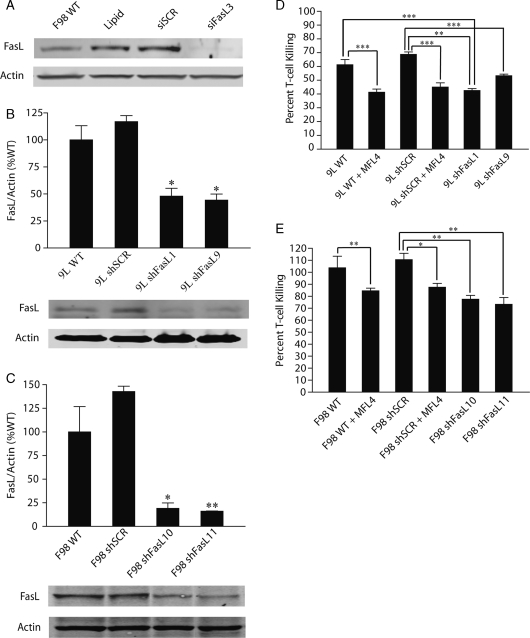
Stable 9L and F98 FasL knockdown cell lines were created to determine the potential for inhibiting FasL to improve the viability and antitumor actions of tumor-infiltrating T-cells. Three commercially available anti-FasL siRNAs and one scramble siRNA were tested using transient tranfection to determine which target sequence knocked-down FasL expression most effectively. The sequence selected (Ambion siRNA ID 197272) inhibited FasL expression (RNA and protein) by over 75% in both the 9L and F98 cell lines although the scramble siRNA sequence had negligible effects (Fig. 3A). 9L and F98 cell lines were transfected with shFasL (derived from siFasL sequence ID 197272) and shSCR plasmid expression vectors. Clonal cell lines were screened for FasL expression by immunoblot analysis. Four cell lines with >60%–80% FasL expression inhibition (9LshFasL1, 9LshFasL9, F98shFasL10, F98shFasL11) are shown in (Fig. 3B and C). The transfection and selection procedures had no effect on the in vitro growth characteristics of the cloned cell lines (data not shown).
Fig. 3.
Stable FasL knockdown decreases T-cell death induced by glioma cells in vitro. FasL siRNA sequences were tested for their ability to knock-down FasL protein expression when transiently transfected into gloma cells. (A) Immunoblot results indicate that the FasL specific siRNA sequence (designated siFasL3) knocked down FasL protein expression to less than 10% when transiently transfected into glioma cells. A similarly high level of FasL knockdown was seen in immunoblots when shFasL derived from the siFasL3 sequence (designated shFasL3) was stably expressed in the (B) 9L and (C) F98 glioma cell lines. Shown are stable clonal FasL knockdown lines 9LshFasL1 and 9LshFasL9 (B) and F98shFasL10 and F98shFasL11 (C). A control siRNA hairpin sequence (shSCR) was also stably expressed in 9L and F98 cells and immunoblotting showed that FasL protein expression was relatively unchanged when compared with wild-type cells. (D and E) 9L and F98 transfected cell lines cocultured in the absence or presence of the MFL4 antibody. The results show that stable FasL knockdown inhibits glioma cells from initiating T-cell death at levels comparable to the presence of the MFL4 antibody.
The effects of FasL knock-down on Jurkat cell death was examined in the glioma:Jurkat cell cocultures. The magnitude of Jurkat cell protection in cocultures using FasL knockdown cells was essentially identical to that seen in response to neutralizing anti-FasL mAb MFL4 (Fig. 3D and E). This further demonstrates that FasL expression by glioma cells can induce FasL-dependent T-cell death and suggested that FasL expression inhibition could enhance the survival of activated glioma-infiltrating T-cells.
Stable FasL Knock-down Decreases Tumor Growth In Vivo by a T-cell Dependent Mechanism
The finding that FasL knock-down can enhance T-cell survival in glioma:T-cell cocultures suggested that FasL knock-down might increase T-cell dependent antiglioma immune responses. Therefore, we asked if FasL knockdown alters glioma growth in immune competent hosts. In order to test this hypothesis we selected the 9L glioma model. 9L was selected for its low levels of FasL expression, which provide a theoretical advantage to achieve a biologically relevant magnitude of FasL expression inhibition in vivo.
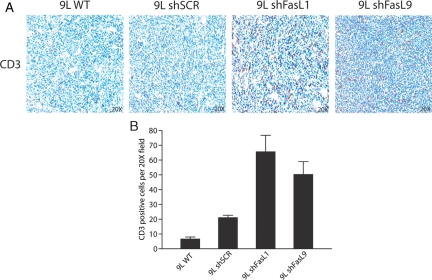
9L-WT, 9L-shFasL, and control 9L-shSCR cell lines were implanted to the flanks of syngeneic Fisher rats and tumor growth was quantified (Fig. 4A). Tumors derived from 9L-WT and 9L-shSCR grew at comparable rates. In contrast, the growth of tumors derived from 9L-siFasL cell lines was ∼50% slower than control tumors. Similar differences were observed following tumor cell implantation to brain (Fig. 4B). We examined the effect of FasL knockdown on the number and distribution of CD3-positive T-cells surrounding and infiltrating orthotopic glioma xenografts. Substantially more CD3-positive T-cells were associated with 9L-shFasL tumors compared with controls (Fig. 5A and B).
Fig. 4.

Stable FasL knockdown inhibits the ability of glioma cells to grow in vivo. (A) 9L FasL knock-down cell lines were injected subcutaneously into Fisher rats. FasL knockdown caused a 50% decrease in tumor volume after 26 days of growth when compared with wild-type and shSCR transfected cell lines. (B, top) 9L transfected cell lines were implanted in the brains of Fisher rats. The results show that after 14 days of growth a greater than 50% reduction in tumor volume was observed. (B, bottom) Immunoblotting of protein extracted from tumors after 14 days of growth confirmed the persistance of FasL knock-down in vivo. (C) When 9L transfected cell lines were implanted into the brains of athymic rats no change in tumor volume was observed after 14 days of growth. These results indicate that the presence of T-cells helps to mediate decreased growth in FasL knockdown tumors.
Fig. 5.
Stable FasL knock-down promotes T-cell infiltration in intracranial gliomas. (A) Tumor sections from 9L transfected cell lines implanted into the brains of Fisher rats were stained for the presence of CD3. (B) The number of CD3-positive cells was quantified using computer-based methodology as described in Materials and Methods. The results indicate a significantly higher number of CD3 positive infiltrating cells in sections from 9L FasL knock-down tumors than in 9L wild-type or 9L shSCR tumors.
We hypothesized that the diminished growth of 9L-siFasL gliomas resulted from T-cell dependent host-glioma interactions since FasL knockdown did not alter glioma cell growth rates in vitro and increased T-cell infiltration in vivo. This notion was supported by the finding that 9L-WT, 9L-shSCR, and 9L-shFasL tumors grew at the same rates in T-cell deficient hosts (Fig. 4C).
Discussion
The role of the Fas/FasL system in modulating the biological behavior of neoplasms and their response to therapeutic strategies has been widely studied. Cell apoptosis resulting from the activation of this system has been identified as a potentially exploitable biological mechanism to control tumor growth. In particular, previous studies24, 25 have reported that chemotherapy can sensitize glioma cells to Fas-dependent apoptosis. Wei et al.26 postulated that a Fas/FasL mediated mechanism of glioma cell death is the preponderant biological mechanism responsible for the synergistic antitumor effect of combining chemotherapy with immunotherapy in gliomas. In their study they conclude that since activated immune cells express FasL, a chemotherapy (topotecan) induced up-regulation of the Fas receptors on glioma cells could potentially enhance immune clearance of tumors.
The expression of FasL in a variety of tumor types supports the notion that it may help promote tumor immune privilege. Despite this, the role of FasL in facilitating immune privilege remains controversial. In gliomas, a high number of apoptotic tumor-infiltrating lymphocytes have been observed, suggesting that tumor-derived factors promote immune evasion via immune cell death.27 To conclusively determine if FasL can contribute to glioma immune privilege, we first analyzed the expression of FasL in rat glioma cell lines and human glioma specimens. Expression of FasL by each cell line and all human specimens tested indicates that it may contribute to glioma immune privilege in the unique context of the brain. Our findings suggest that inhibiting FasL either alone or in conjunction with other immune-based therapies could be a beneficial treatment strategy for FasL-expressing brain malignancies.
In the past, some studies have relied on associations between FasL expression and pathological analysis of tumor grade alone to validate the FasL counterattack theory in the brain. To establish a more functional and biological relationship between FasL expression and T-cell apoptosis we used an in vitro coculture assay. We were able to show that glioma cell lines were able to induce apoptosis of activated T-cells. This activity was inhibited using a FasL neutralizing Ab and by stably knocking-down FasL using siRNA in two independent rats glioma cell lines. This in vitro data shows that glioma cell lines are capable of inducing FasL-dependent apoptosis in T-cells. It also confirms that FasL expressed by glioma cells is functional and capable of activating the Fas death cascade. Although each cell line tested was still able to induce apoptosis of T-cells in the presence of a neutralizing Ab, indicating that multiple mechanisms contribute to the induction of apoptosis by glioma cells, it is clear that FasL contributes significantly to the total amount of apoptosis induced. It may be the case that the ability of glioma cells to induce T-cell apoptosis does not have to be completely inhibited to see a large increase in antitumor immunity. It has often been the case using other immunotherapy modalities that a small change in biological function ultimately leads to a large physiological response.28,29 Most importantly, this assay established a functional link between glioma cell FasL expression and T-cell viability.
To test the belief that FasL knock-down has a physiological effect on in vivo tumor growth we implanted FasL knock-down clones into immune-competent animals. The result of this experiment was a dramatic decrease in tumor volume that we associate with an increased antiglioma immune response as evidenced by an increase in tumor-associated CD3 positive T-cells. Additionally, FasL knock-down clones grew comparably to control cell lines when implanted into immune-deficient, athymic animals, further indicating that the observed change in tumor volume is immune cell dependent. In the future, it will be interesting to establish the role of specific T-cell subsets involved in the glioma response to FasL knockdown. To our knowledge, this is the first time that the FasL counterattack has been shown in the unique environment of the brain using both biological and physiological endpoints.
We propose that FasL knock-down therapy has the potential to enhance emerging immunotherapies, including interleukin delivery and tumor vaccines. For example, we have seen that IL-2 facilitates enhanced glioma rejection, as indicated by decreased volume and increased survival, partly due to an increase in the recruitment and activation of T-cells.30 It is plausible that this therapy could be even more beneficial in the presence of a treatment that further increases the viability of the activated immune cells. Additionally, there is extensive evidence that many solid tumors of the body express FasL indicating that FasL knock-down could be broadly applicable across a large spectrum of tumor types once placed in a clinical setting.
Funding
This work was supported by NIH grants CA112148 (A.O.) and NS43987 (J.L.).
Acknowledgments
The authors would like to thank Dr Bachchu Lal for his expertise in tumor implantation, processing and analysis, as well as Dr Cliona O'Driscoll for her expert technical advice.
Conflict of interest statement. None declared.
References
- 1.Stupp R, Pavlidis N, Jelic S ESMO Guidelines Task Force. ESMO Minimum Clinical Recommendations for diagnosis, treatment and follow-up of malignant glioma. Ann Oncol. 2005;16(suppl 1):i64–i65. doi: 10.1093/annonc/mdi834. [DOI] [PubMed] [Google Scholar]
- 2.Kim R, Emi M, Tanabe K, Arihiro K. Tumor-driven evolution of immunosuppressive networks during malignant progression. Cancer Res. 2006;66(11):5527–5536. doi: 10.1158/0008-5472.CAN-05-4128. [DOI] [PubMed] [Google Scholar]
- 3.Fynan TM, Reiss M. Resistance to inhibition of cell growth by transforming growth factor-beta and its role in oncogenesis. Crit Rev Oncology. 1993;4:493–540. [PubMed] [Google Scholar]
- 4.Markowitz S, Roberts A. Tumor suppressor activity of the TGF-β pathway in human cancers. Cytokine Growth Factor Rev. 1996;7:93–102. doi: 10.1016/1359-6101(96)00001-9. [DOI] [PubMed] [Google Scholar]
- 5.Grady WM, Markowitz SD. Genetic and epigenetic alterations in colon cancer (published online ahead of print April 15, 2002) Annu Rev Genomics Hum Genet. 2002;3:101–128. doi: 10.1146/annurev.genom.3.022502.103043. Review. [DOI] [PubMed] [Google Scholar]
- 6.Gottfried E, Kreutz M, Mackensen A. Tumor-induced modulation of dendritic cell function (published online ahead of print December 03, 2007) Cytokine Growth Factor Rev. 2008;19(1):65–77. doi: 10.1016/j.cytogfr.2007.10.008. Review. [DOI] [PubMed] [Google Scholar]
- 7.Larmonier N, Marron M, Zeng Y, et al. Tumor-derived CD4(+)CD25(+) regulatory T cell suppression of dendritic cell function involves TGF-beta and IL-10. Cancer Immunol Immunother. 2007;56:48–59. doi: 10.1007/s00262-006-0160-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hattori E, Okumoto K, Adachi T, et al. Possible contribution of circulating interleukin-10 (IL-10) to antitumor immunity and prognosis in patients with unresectable hepatocellular carcinoma. Hepatol Res. 2003;27:309–14. doi: 10.1016/j.hepres.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Saas P, Walker PR, Hahne M, et al. Fas ligand expression by astrocytoma in vivo: maintaining immune privilege in the brain? Clin Invest. 1997;99(6):1173–1178. doi: 10.1172/JCI119273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gratas C, Tohma Y, Van Meir EG, et al. Fas ligand expression in glioblastoma cell lines and primary astrocytic brain tumors. Brain Pathol. 1997;7(3):863–869. doi: 10.1111/j.1750-3639.1997.tb00889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Song E, Chen J, Ouyang N, et al. Soluble Fas ligand released by colon adenocarcinoma cells induces host lymphocyte apoptosis: an active mode of immune evasion in colon cancer. Br J Cancer. 2001;85(7):1047–1054. doi: 10.1054/bjoc.2001.2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashley DM, Kong FM, Bigner DD, et al. Endogenous expression of transforming growth factor beta1 inhibits growth and tumorigenicity and enhances Fas-mediated apoptosis in a murine high-grade glioma model. Cancer Res. 1998;58(2):302–309. [PubMed] [Google Scholar]
- 13.Yang BC, Lin HK, Hor WS, et al. Mediation of enhanced transcription of the IL-10 gene in T cells, upon contact with human glioma cells, by Fas signaling through a protein kinase A-independent pathway. J Immunol. 2003;171(8):3947–3954. doi: 10.4049/jimmunol.171.8.3947. [DOI] [PubMed] [Google Scholar]
- 14.Griffith S, Brunner T, Fletcher S, et al. Fas ligand-induced apoptosis as a mechanism of immune privilege. Science. 1995;270(5239):189–1192. doi: 10.1126/science.270.5239.1189. [DOI] [PubMed] [Google Scholar]
- 15.Ferguson TA, Griffith TS. The role of Fas ligand and TNF-related apoptosis-inducing ligand (TRAIL) in the ocular immune response. Chem Immunol Allergy. 2007;92:140–154. doi: 10.1159/000099265. Review. [DOI] [PubMed] [Google Scholar]
- 16.Bodmer D, Brors D, Pak K, et al. Inflammatory signals increase Fas ligand expression by inner ear cells. J Neuroimmunol. 2002;129(1–2):10–17. doi: 10.1016/s0165-5728(02)00143-1. [DOI] [PubMed] [Google Scholar]
- 17.Zhang B, Sun T, Xue L, et al. Functional polymorphisms in FAS and FASL contribute to increased apoptosis of tumor infiltration lymphocytes and risk of breast cancer (published online ahead of print December 20, 2006) Carcinogenesis. 2007;28(5):1067–1073. doi: 10.1093/carcin/bgl250. [DOI] [PubMed] [Google Scholar]
- 18.Minas V, Rolaki A, Kalantaridou SN, et al. Intratumoral CRH modulates immuno-escape of ovarian cancer cells through FasL regulation (published online ahead of print July 31, 2007) Br J Cancer. 2007;97(5):637–645. doi: 10.1038/sj.bjc.6603918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Towbin H, Ozbey O, Zingel O. An immunoblotting method for high-resolution isoelectric focusing of protein isoforms on immobilized pH gradients. Electrophoresis. 2001;22(10):1887–1893. doi: 10.1002/1522-2683(200106)22:10<1887::AID-ELPS1887>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 20.Reznik TE, Sang Y, Ma Y, et al. Transcription-dependent epidermal growth factor receptor activation by hepatocyte growth factor. Mol Cancer Res. 2008;6(1):139–150. doi: 10.1158/1541-7786.MCR-07-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Posmantur R, Wang KK, Gilbertsen RB. Caspase-3-like activity is necessary for IL-2 release in activated Jurkat T-cells. Exp Cell Res. 1998;244(1):302–309. doi: 10.1006/excr.1998.4214. [DOI] [PubMed] [Google Scholar]
- 22.Tamargo RJ, Myseros JS, Epstein JI, et al. Interstitial chemotherapy of the 9L gliosarcoma: Controlled release polymers for drug delivery in the brain. Cancer Res. 1993;53:329–333. [PubMed] [Google Scholar]
- 23.Kovar JL, Johnson MA, Volcheck WM, et al. Hyaluronidase expression induces prostate tumor metastasis in an orthotopic mouse model. Am J Pathol. 2006;169(4):1415–1426. doi: 10.2353/ajpath.2006.060324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xia S, Rosen EM, Laterra J. Sensitization of glioma cells to Fas-dependent apoptosis by chemotherapy-induced oxidative stress. Cancer Res. 2005;65(12):5248–5256. doi: 10.1158/0008-5472.CAN-04-4332. [DOI] [PubMed] [Google Scholar]
- 25.Giraud S, Bessette B, Boda C, et al. In vitro apoptotic induction of human glioblastoma cells by Fas ligand plus etoposide and in vivo antitumour activity of combined drugs in xenografted nude rats. Int J Oncolog. 2007;30:273–281. [PubMed] [Google Scholar]
- 26.Wei J, deAngulo G, Sun W, et al. Topotecan enhances immune clearance of gliomas (published online ahead of print July 02, 2008) Cancer Immunol Immunother. 2009;58(2):259–270. doi: 10.1007/s00262-008-0550-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Didenko VV, Ngo HN, Minchew C, Baskin DS. Apoptosis of T lymphocytes invading glioblastomas multiforme: a possible tumor defense mechanism. J Neurosurg. 2002;96(3):580–584. doi: 10.3171/jns.2002.96.3.0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Herzer K, Hofmann TG, Teufel A, et al. IFN-alpha-induced apoptosis in hepatocellular carcinoma involves promyelocyticleukemia protein and TRAIL independently of. Cancer Res. 2009;69((3):855–862):53. doi: 10.1158/0008-5472.CAN-08-2831. [DOI] [PubMed] [Google Scholar]
- 29.Bertoli C, Copetti T, Lam EW, et al. Calpain small-1 modulates Akt/FoxO3A signaling and apoptosis through PP2A. Oncogene. 2008;28:721–733. doi: 10.1038/onc.2008.425. [DOI] [PubMed] [Google Scholar]
- 30.Nam M, Johnston P, Lal B, Indurti R, Wilson MA, Laterra J. Endothelial cell-based cytokine gene delivery inhibits 9L glioma growth in vivo. Brain Res. 1996;731(1–2):161–170. doi: 10.1016/0006-8993(96)00471-4. [DOI] [PubMed] [Google Scholar]