Abstract
Vectors based on primate-derived adeno-associated virus (AAV) are being considered in the development of genetic vaccines against a number of diseases including infection with HIV-1. Preexisting immunity to the vaccine carrier as a result of natural infections could potentially compromise vaccine efficacy. This study evaluates the impact of neutralizing antibodies against AAV capsids on the ability of HIV-1 Gag-expressing vectors to elicit transgene-specific T and B cell responses. Mice were passively transferred with pooled human immunoglobulin at various doses to simulate human antivector humoral immunity. Vectors based on serotype 2, which were evaluated in the clinic, were compared with those created from the novel monkey isolates AAV7 and AAV8. Inhibition of AAV2-directed Gag responses occurred at doses of human immunoglobulin 10- to 20-fold less than was required to inhibit immunogenicity of AAV7 and AAV8 vectors. Cynomolgus macaques were screened for preexisting immunity to AAV7 and AAV8 and sera from individual animals were passively transferred into mice that were analyzed for AAV vaccine efficacy. There was a correlation between the level of preexisting capsid neutralizing titers and diminution of vaccine efficacy; sera from a number of animals with no detectable neutralizing antibodies showed partial vaccine inhibition, suggesting that the in vitro assay is less sensitive than the in vivo passive transfer assay for detecting neutralizing antibodies to AAV.
Introduction
Adeno-associated viral (AAV) vectors are routinely used to deliver transgenes for therapeutic or experimental reasons. In some experimental models, AAV-mediated expression of a non-self gene product has been shown to be extinguished by antibody or cellular-mediated immune responses (Brockstedt et al., 1999; Wang et al., 2005b). These findings suggested that AAV vectors may be useful as vaccine carriers for cancer or infectious diseases (Manning et al., 1997; Gallez-Hawkins et al., 2004). AAV as a vector for eliciting immune protection against human immunodeficiency viral (HIV) antigens is being evaluated in large-animal models and clinical trials (IAVI Report, http://www.iavireport.org/Issues/Issue9-5/VaccineBriefs.asp; Johnson et al., 2005). Most studies have focused on AAV serotype 2 as a vaccine carrier. However, vectors based on other AAV serotypes are also under development, many of which seem to elicit higher levels of transgene-specific T cells. Studies have suggested that the T cells generated by AAV vectors may have limited proliferation potential (Lin et al., 2007).
An important consideration in the use of any viral vector for in vivo gene therapy or genetic vaccines is the impact of preexisting immunity on the vector. Prior natural AAV infections can result in long-lasting production of AAV-specific neutralizing antibodies (nAbs). The AAV capsid is the sole antigen shared by both wild-type virus and vectors and is susceptible to antibody-mediated neutralization. In gene therapy settings with AAV2, in vivo transduction is indeed diminished even at low circulating antibody titer (Peden et al., 2004; Scallan et al., 2006). Here we modeled, characterized, and quantified the ability of preexisting anti-AAV nAbs to impair transgene immune responses after intramuscular immunization with AAV vectors of various serotypes.
Materials and Methods
Mice and immunization
All animal procedures were performed in accordance with protocols approved by institutional animal care and use committees (University of Pennsylvania, Philadelphia, PA). CB6F1 mice (6–8 weeks old) were purchased from Jackson Laboratories (Bar Harbor, ME). Mice were immunized with recombinant AAV vector vaccines diluted in 50 μl of phosphate-buffered saline (PBS) and administered intramuscularly.
Vaccine vectors
All AAV vectors used in this study were manufactured as described previously (Wang et al., 2005a) by PennVector at the University of Pennsylvania. In brief, the cDNA encoding a truncated and codon-optimized form of Gag of HIV-1 clade B was cloned into an AAV cis-plasmid containing the cytomegalovirus (CMV) promoter, poly(A), and AAV2 inverted terminal repeats. The recombinant vector was packaged by triple transfection of HEK293 cells with an adenoviral helper plasmid; a chimeric packaging construct containing the AAV2 rep gene and the cap gene derived from various AAV serotypes; and the vector plasmid to produce pseudotyped AAV2/7 and AAV2/8 HIV Gag vectors. AAV2 vector vaccine was purified by a single-step gravity-flow heparin column method (Auricchio et al., 2001). AAV2/7 and AAV2/8 vector vaccines were purified by three rounds of cesium chloride gradient centrifugation. The genome titer (genome copies [GC] per milliliter) of AAV vectors was determined by real-time polymerase chain reaction (PCR).
Peptides
The identified immunodominant cytotoxic T lymphocyte (CTL) epitope contained in the p24 portion of the protein is Gag 197–205 (AMQMLKETI), which has been shown to be H2-Kd restricted (Mata et al., 1998). This peptide was synthesized by Mimotopes (Clayton, Victoria, Australia) and dissolved in dimethyl sulfoxide (DMSO) at 1 mg/ml. The peptide was used at a concentration of 4 μg/ml in all experiments and DMSO concentrations were kept below 0.1% (v/v) in all final assay mixtures.
Mouse model reconstituted with human immunoglobulin or nonhuman primate sera
In this model, CB6F1 mice underwent intravenous transferral of various doses of pooled human intravenous immunoglobulin (pooled and purified from thousands of healthy donors; ZLB Bioplasma, Bern, Switzerland) or 300 μl of naive nonhuman primate (NHP)-derived serum before vaccination. Transfer was done twice, 24 and 2 hr before immunization. Recipient mouse serum was harvested immediately before immunization via retro-orbital bleed for nAb titer detection in vitro. Subsequently, serum-recipient mice were immunized with HIV Gag-expressing AAV vaccines, serotypes 2, 7, and 8, at a dose of 3 × 1010 or 1 × 1011 GC per mouse. Gag immunoreactivity was characterized by Gag tetramer staining and p24 IgG production.
Neutralizing antibody assay
Serum samples were heat inactivated at 56°C for 40 min. Recombinant AAV-CMV-lacZ (109 GC/well) was diluted in serum-free Dulbecco's modified Eagle's medium (DMEM) and incubated with 2-fold serial dilutions of heat-inactivated serum samples in DMEM for 1 hr at 37°C. Subsequently, serum–vector mixture was added to 96-well plates with seeded Huh7 cells at 1 × 105 cells per well. After 1 hr, the cells in each well were supplemented with 100 μl of 20% fetal bovine serum (FBS)–DMEM and incubated for 18–22 hr at 37°C and 5% CO2. After that, cells were washed twice in PBS and developed with a Galacto-Star kit (Applied Biosystems, Foster City, CA). The resulting luminescence was measured with a luminometer (Clarity; BioTek Instruments, Winooski, VT). The nAb titer was reported as the highest serum dilution that inhibited AAV-CMV-LacZ transduction (β-galactosidase expression) by 50% or more, compared with the naive mouse serum control.
MHC class I tetramer staining and flow analysis
Phycoerythrin (PE)-conjugated MHC class I Kd tetramer HIV Gag was obtained from Beckman Coulter (Fullerton, CA). At various time points after vector injection, tetramer staining was performed on heparinized whole blood cells, isolated splenocytes, or hepatic lymphocytes. Cells were costained for 30 min at room temperature with PE-conjugated tetramers and the fluorescein isothiocyanate (FITC)-conjugated anti-CD8a (Ly-2) antibody (BD Biosciences Pharmingen, San Diego, CA) at predetermined optimal concentrations. Red blood cells were then lysed and cells were fixed with iTAg MHC tetramer lysing solution with fix solution (Beckman Coulter) for 10 min at room temperature. The cells were then washed three times in PBS and eventually fixed again with BD CytoFix (BD Biosciences Pharmingen) for 20 min at 4°C. Data were gathered with an FC500 flow cytometer (Beckman Coulter) and were analyzed with FlowJo analysis software (Tree Star, San Carlos, CA). In the analysis, lymphocytes were selected on the basis of forward and side scatter characteristic, and CD8+ cells were selected.
Enzyme-linked immunosorbent assay
Polypropylene plates (96-well; USA Scientific, Ocala, FL) were coated with 100 μl of recombinant HIV-1 IIIB Gag p24 (0.5 μg/ml; International Immuno-Diagnostics, Foster City, CA) overnight at 4°C. The plates were blocked with PBS containing 3% fetal calf serum for 2 hr at room temperature. Serum samples were 2-fold serially diluted with PBS containing 1% fetal calf serum and added to the plates for incubation for 2 hr at room temperature. After that, the plates were washed three times with PBS containing 0.1% Tween 20, and then incubated for 1 hr with peroxidase-conjugated anti-mouse IgG (diluted 1:1000 in PBS; Sigma-Aldrich, St. Louis, MO). After three washes, the plates were incubated with TMB substrate (100 μl/well; Sigma-Aldrich) for 15 min at room temperature, and then reaction was stopped with stop reagent (100 μl/well; Sigma-Aldrich). The optical density was measured at a wavelength of 450 nm. IgG titers are reported as the highest serum dilution detectable by enzyme-linked immunosorbent assay (ELISA) as measured by the optical density at 450 nm.
Interferon-γ enzyme-linked immunospot assay
The interferon (IFN)-γ enzyme-linked immunospot (ELISPOT) assay was performed with a mouse IFN-γ ELISPOT set (BD Biosciences Pharmingen) according to the protocol provided by the manufacturer. Briefly, a 96-well ELISPOT plate was coated with anti-mouse IFN-γ capture antibody (5.0 μg/ml) overnight at 4°C. The next day, the wells were washed and blocked with complete culture medium for 2 hr at room temperature. The purified lymphocytes from an immunized mouse were added to microwells along with the Gag-dominant peptide AMQMLKETI. Cells were incubated at 37°C and 5% CO2 for 18 to 20 hours. Control cells were incubated either without peptide or with nonspecific stimulator (staphylococcal enterotoxin B [SEB], 200 ng/ml). The wells were then washed extensively with PBS containing 0.05% Tween 20 and subsequently incubated with biotinylated anti-mouse IFN-γ detection antibody (2.0 μg/ml) for 2 hr at room temperature. After washing, the wells were incubated with streptavidin–horseradish peroxidase antibody (5 μg/ml) for 1 hr at room temperature. The wells were washed again, and the final substrate was added to the wells. Color development was monitored and stopped by washing with water. After drying overnight at room temperature, the wells were counted with an ELISPOT reader.
Intrahepatic T cell isolation
Liver tissue was diced, using sterile blades, into 1-mm3 pieces in RPMI 1640 containing collagenase IV (1 mg/ml; Sigma-Aldrich) and incubated at 37°C for 1 hr. After enzymatic digestion, tissue was passed through a nylon mesh filter (pore size, 100 μm) to remove cell clumps and undissociated tissue. Cells were washed three times in PBS to remove collagenase IV, and the cell suspension was layered on a Percoll density gradient (64/56%) and centrifuged for 30 min at 2000 rpm. The lymphocyte band was then removed from the interface between 56 and 64% Percoll and further washed three times in PBS.
Statistical analysis
All analyses were carried out on Prism software (version 5.00 for Windows; GraphPad Software, San Diego, CA). p < 0.05 was considered to be significant. For comparison of the difference between two groups, a two-sided unpaired Student t test was carried out. When comparing the means of three or more unmatched groups, one-way analysis of variance (Newman–Keuls multiple comparison test) was used for analysis. Results are expressed as means ± SD.
Results
To quantify the effect of nAbs in humans on the potency of AAV vector-based HIV Gag vaccines, mice were passively transferred with pooled human immunoglobulin before vaccination. Titers of nAbs against AAV2, AAV7, and AAV8 were evaluated in a stock solution of human immunoglobulin (240 mg/ml) by performing an in vitro assay that measures inhibition of vector transduction in Huh7 cells. As described previously, the prevalence of nAbs in human sera is higher against AAV2 compared with AAV7 and AAV8 (1:2560 vs. 1:320 and 1:640, respectively) (Table 1).
Table 1.
Pooled Human Immunoglobulin-Reconstituted Mouse Model
| |
|
Percent positive micea |
|||||
|---|---|---|---|---|---|---|---|
| |
|
Circulating human Ig dose (mg) |
|||||
| Serotype | Stock nAb titer (240 mg/ml) | 0.08 | 0.24 | 0.8 | 2.4 | 8 | 24 |
| AAV2/2 | 1:2560 | 0 | 10 | 10 | 20 | 30 | — |
| AAV2/7 | 1:320 | — | — | — | 0 | 0 | 50 |
| AAV2/8 | 1:640 | — | — | — | 20 | 40 | 90 |
Abbreviations: Ig, immunoglobulin; nAb, neutralizing antibody.
n = 10 mice; mice were defined as positive with serum nAb titer ≥1:20 posttransfer.
A quantitative assessment of neutralization in vivo was done by dosing mice with various quantities of human immunoglobulin before intramuscular vaccination with AAV2, AAV7, or AAV8 Gag vectors. Human immunoglobulin doses varied by 0.5 log from 0.08 to 8 mg for AAV2 and from 2.4 to 24 mg for AAV7 and AAV8. The higher doses of human immunoglobulin were used with the novel serotypes, based on the lower levels of nAbs to the corresponding vectors.
Animals were evaluated for vector-induced transgene responses by evaluating peripheral blood mononuclear cells (PBMCs) for Gag-specific T cells, using a tetramer to the mapped dominant epitope and measuring Gag antibodies in an ELISA. Figure 1 summarizes peak T cell responses (Fig. 1A) and peak Gag antibodies (Fig. 1B) for cohorts of animals (n = 10) dosed with various quantities of human immunoglobulin and vaccinated with AAV2 (3 × 1010 or 1 × 1011 GC) or AAV7 or AAV8 (3 × 1010 GC). Peak T cell responses in the absence of human immunoglobulin were substantially higher with AAV7 and AAV8 as we described previously (Lin et al., 2007). The lowest dose of human immunoglobulin (2.4 mg) resulted in partial inhibition of AAV7- and AAV8-induced Gag-specific T cells (50 and 30% reduction, respectively) with further reductions noted at higher doses of human immunoglobulin. A virtually complete inhibition of AAV2 vaccine efficacy in terms of T cells was seen at a dose of human immunoglobulin as low as 0.08 mg. This suggests that in vivo neutralizing activity of pooled human sera is at least 30-fold more active against AAV2 than against AAV7 or AAV8. This effect is partially overcome by increasing the dose of vector as evidenced by studies with a 3-fold higher dose of AAV2 (1 × 1011 GC), which showed detectable T cells at the lowest doses of human immunoglobulin. A reduction of tetramer-positive cells was also noted in cells from liver and spleen after passive transfer of 0.24 and 2.4 mg of human immunoglobulin and vaccination with AAV2 Gag (Fig. 2C).
FIG. 1.

Pooled human immunoglobulin inhibits adoptive immune responses induced by AAV-based HIV Gag vaccines. CB6F1 mice were passively transferred with pooled human immunoglobulin at the indicated doses 24 and 2 hr before immunization. A similar regimen was used for the PBS control group. Immunization, performed intramuscularly, consisted of 3 × 1010 genome copies (GC) of AAV2/2, AAV2/7, or AAV2/8 or 1 × 1011 GC of AAV2/2 viral vector encoding HIV Gag. (A) Three weeks after vector injections, HIV Gag tetramer-specific T cells were evaluated by staining with FITC-conjugated anti-CD8 antibody and PE-conjugated Gag tetramer complex (H2-Kd-AMQMLKETI), and Gag-specific CD8+ T cells are presented as the percentage of double-positive cells among total CD8+ T cells. (B) HIV Gag-specific B cell immune responses were determined by the titers of p24-specific IgG antibodies in collected mouse sera, as indicated by ELISA at week 4. Optical density was measured at a wavelength of 450 nm. IgG titers are reported as the highest serum dilution detectable by ELISA as measured by optical density at 450 nm. p Values were obtained by comparison with PBS control groups, using one-way analysis of variance: *p < 0.05; **p < 0.001. Data are shown as means ± SD (n = 10 mice).
FIG. 2.
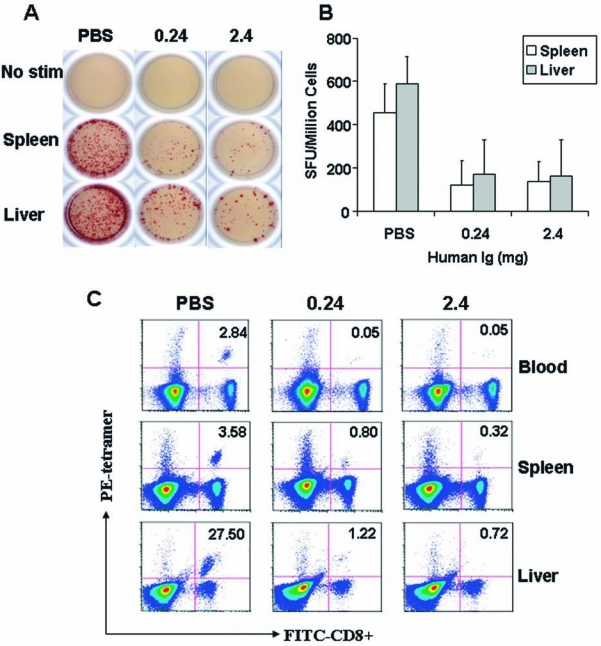
Preexisting immunity from human immunoglobulin decreased IFN-γ-releasing lymphocytes as well as Gag tetramer-positive CD8+ T cells. Human immunoglobulin-reconstituted mice that received 3 × 1010 GC of AAV2/2 Gag vaccine were sacrificed on day 40 postimmunization. (A) In an ELISPOT assay lymphocytes were isolated from livers and spleens and then seeded at 5 × 105 cells per well into plates and stimulated with Gag-dominant peptide at a final concentration of 4 μg/ml for 18 hr. IFN-γ secreted by activated cells was captured locally by the coated antibody. A biotinylated polyclonal antibody specific to IFN-γ was employed to detect the captured cytokine, which could be visualized with an avidin–HRP-precipitating substrate. Representative data from five mice in each group are shown here. (B) Numbers of IFN-γ-releasing cells among 1 × 106 lymphocytes are reported from the human immunoglobulin- or PBS-transferred mouse model that was subsequently injected intramuscularly with 3 ×1010 GC of AAV2/2 Gag. Data are shown as means ± SD. (C) Lymphocytes were isolated from blood, spleen, and liver and stained with PE-conjugated H2-Kd-AMQMLKETI tetramer complex together with FITC-conjugated anti-CD8 antibody. Numbers in the top right quadrants indicate the percentage of Gag tetramer-specific CD8+ T cells among total CD8+ T cells. Representative data for each group (n = 10) are shown.
More detailed analysis of the impact of nAbs on the production of functional T cells was measured in ELISPOT assays; mice passively transferred with PBS or 0.24 and 2.4 mg of human immunoglobulin were immunized with 3 × 1010 GC of AAV2 Gag vector. The ELISPOT assay confirmed that this translated to a reduction in peptide-induced IFN-γ expression in spleen and liver (Fig. 2A and B).
Vector-induced B cell responses as measured by ELISA were more substantially affected by human immunoglobulin passive transfer than were T cell responses (Fig. 1). The lowest dose of human immunoglobulin tested with the novel serotypes diminished Gag antibodies for AAV7 and AAV8 by 10- and 2-fold, respectively. A 3-fold reduction in AAV2-induced Gag antibody was measured with 0.08 mg of human immunoglobulin. As with the T cell responses, a 3-fold increase in the dose of AAV2 partially overcame human immunoglobulin inhibition as evidenced by full vaccine efficacy at 0.08 mg and 3-fold reduction at 0.24 mg of human immunoglobulin.
We showed in previous studies that cynomolgus macaques have a high prevalence of nAbs to both AAV7 and AAV8 partly because these viruses are natural pathogens to this species. We screened a population of cynomolgus macaques for nAbs to AAV7 and AAV8 and then passively transferred sera from individual animals into mice before vaccination with AAV7 and AAV8 Gag vectors. Peak Gag tetramer response and Gag antibodies were measured. AAV7 is a different serotype than AAV8, so there is no direct correlation in nAbs between these two viruses from individual animals. Sera were stratified into different groups based on the level of nAbs to either AAV7 (1:1280 to 1:2560 [n = 2], 1:160 to 1:640 [n = 10], 1:20 to 1:80 [n = 14], and undetectable [n = 24]) or AAV8 (1:1280 to 1:2560 [n = 2], 1:160 to 1:640 [n = 4], 1:20 to 1:80 [n = 4], and undetectable [n = 15]). Sera were harvested from the recipient mice immediately before vaccination and analyzed for nAbs by the in vitro assay. As shown in Table 2, nAbs were indeed detected in animals who received the higher titers of passively transferred sera although they were always less than the input titer presumably representing dilution.
Table 2.
Individual Nonhuman Primate Serum-Reconstituted Mouse Model
| |
AAV2/7 (n = 50) |
AAV2/8 (n =25) |
||
|---|---|---|---|---|
| NHP serum anti-AAV titer | Positive micea | Titer (ex vivo) | Positive micea | Titer (ex vivo) |
| 1:1280–1:2560 | 2/2 | 1:160, 1:320 | 2/2 | 1:40, 1:160 |
| 1:160–1:640 | 4/10 | 1:20–1:80 | 4/4 | 1:40 |
| 1:20–1:80 | 5/14 | 1:20 | 0/4 | No |
| No nAb | 0/24 | No | 0/15 | No |
Abbreviations: NHP, nonhuman primate.
Mice were defined as positive with serum nAb titer ≥1:20 posttransfer.
The data, as measured by peak T cell response and Gag antibodies, are segregated into groups based on levels of input nAbs titers as described above (Fig. 3A and B). There were diminutions in T cell responses and Gag antibody levels in all groups with a direct correlation between the titer of input nAbs and extent of suppression. Interestingly, sera in some animals with no detectable nAbs by in vitro assay substantially decreased T and B cell responses for both AAV7 and AAV8. As noted with human immunoglobulin, the individual macaque sera affected Gag antibodies more than T cell frequencies.
FIG. 3.

Nonhuman primate (NHP)-derived neutralizing antibodies (nAbs) to AAV vectors interfere with the adaptive immune response induced by AAV HIV Gag vaccines. Various titers of NHP-derived nAbs were reconstituted in CB6F1 mice as described for the human immunoglobulin mouse model. Mice were immunized with 3 × 1010 GC of AAV2/7 or AAV2/8 HIV Gag vaccine after having received corresponding NHP serum (AAV2/7, n = 50; AAV2/8, n = 25). HIV Gag-specific T cell immune responses (A) and the titers of p24-specific antibodies (B) were used to evaluate vaccine efficacy. p Values were obtained by comparison with PBS control groups, using one-way analysis of variance: *p < 0.05; **p < 0.001. Data are shown as means ± SD.
Discussion
The impact of preexisting immunity to viral vectors has been evaluated extensively in applications of gene therapy. The greatest focus has been on the impact of nAbs to the vector on gene transfer efficiency. A clinical trial of AAV2 gene transfer in subjects with hemophilia B suggested a potential role of memory T cells to the AAV2 capsid in mediating toxicity, thereby expanding the spectrum of host–vector interactions that need to be considered (Mingozzi et al., 2007). The focus of this study was to evaluate the role of nAbs to AAV on vaccine efficacy, using HIV-1 Gag as a model antigenic transgene.
Our studies do indeed demonstrate that human sera are capable of diminishing vaccine efficacy in vivo in the passive transfer model. In fact, the level of vaccine interference was remarkable, especially for AAV2, where T and B cell activation was significantly diminished with quantities of pooled human immunoglobulin that yield no detectable nAbs in the sera of passively transferred mice (data not shown). Human immunoglobulin inhibited AAV2 vaccine efficacy 20-fold more effectively than did AAV7 or AAV8, which is a much greater effect than would have been predicted by the relative nAb activity based on in vitro transduction inhibition, which showed a 4- to 8-fold difference between AAV2 and AAV7/8.
These data suggest that the in vitro nAb assay is not sufficiently sensitive to detect nAb levels capable of interfering with in vivo vector performance. To directly evaluate this we screened a large number of cynomolgus macaques for nAbs due to natural infections and correlated this with the ability of sera from these animals to interfere with AAV7 and AAV8 vaccine efficacy. There was indeed a correlation between the level of nAbs as measured by the in vitro assay and diminution of vaccine performances. However, there were a number of animals that demonstrated no in vitro nAb activity despite significant reduction in vaccine performance after passive transfer.
The most plausible explanation for our findings is simply insufficient sensitivity of the in vitro transduction inhibition assay in predicting meaningful neutralization in vitro. The relative resistance of most cells and cell lines to AAV transduction in vitro complicates the development of assays with improved sensitivity; relatively high MOIs are required to establish a baseline of transduction, which diminishes sensitivity. Alternatively, other protein components in the blood that may act cooperatively with AAV antibodies to enhance neutralization in vivo may be missing in the in vitro assays. Another explanation to explain the discrepancy between in vitro and in vivo findings is that anti-AAV antibodies may effectively diminish in vivo transduction by mechanisms other than by directly interfering with uptake of the vector. For example, antibody bound to vector particles may promote sequestration. Finally, it is plausible that non-antibody-dependent processes may contribute to diminished vector performance in vivo. However, the general correlation between diminished vector performance and AAV nAb levels does suggest a role for antigen-specific immunity. An understanding of the mechanisms responsible for diminished vaccine/vector efficacy in vivo will be critical in designing more sensitive in vitro neutralizing assays.
The limitations of the in vitro AAV nAb assay should be taken into account when assessing the potential of preexisting immunity in interfering with AAV vaccine efficacy.
Acknowledgments
The work was supported by the NIH (NIDDKP30-DK47757 and NHLBI P01-HL-059407) and GlaxoSmithKline. The Penn Vector Core at the University of Pennsylvania provided the vectors. Deirdre McMenamin and Regina Munden provided help with the animal studies.
Author Disclosure Statement
J.M.W. has a grant from GlaxoSmithKline (GSK) and is an inventor on patents licensed to several companies. Jianping Lin, Roberto Calcedo, and Joanita M. Figueredo have no competing financial interests. Luk Vandenberghe is an inventor on patents licensed to GSK.
References
- Auricchio A. O'Connor E. Hildinger M. Wilson J.M. A single-step affinity column for purification of serotype-5 based adeno-associated viral vectors. Mol. Ther. 2001;4:372–374. doi: 10.1006/mthe.2001.0462. [DOI] [PubMed] [Google Scholar]
- Brockstedt D.G. Podsakoff G.M. Fong L. Kurtzman G. Mueller-Ruchholtz W. Engleman E.G. Induction of immunity to antigens expressed by recombinant adeno-associated virus depends on the route of administration. Clin. Immunol. 1999;92:67–75. doi: 10.1006/clim.1999.4724. [DOI] [PubMed] [Google Scholar]
- Gallez-Hawkins G. Li X. Franck A.E. Thao L. Lacey S.F. Diamond D.J. Zaia J.A. DNA and low titer, helper-free, recombinant AAV prime–boost vaccination for cytomegalovirus induces an immune response to CMV-pp65 and CMV-IE1 in transgenic HLA A*0201 mice. Vaccine. 2004;23:819–826. doi: 10.1016/j.vaccine.2004.06.048. [DOI] [PubMed] [Google Scholar]
- Johnson P.R. Schnepp B.C. Connell M.J. Rohne D. Robinson S. Krivulka G.R. Lord C.I. Zinn R. Montefiori D.C. Letvin N.L. Clark K.R. Novel adeno-associated virus vector vaccine restricts replication of simian immunodeficiency virus in macaques. J. Virol. 2005;79:955–965. doi: 10.1128/JVI.79.2.955-965.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. Zhi Y. Mays L. Wilson J.M. Vaccines based on novel adeno-associated virus vectors elicit aberrant CD8+ T-cell responses in mice. J. Virol. 2007;81:11840–11849. doi: 10.1128/JVI.01253-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning W.C. Paliard X. Zhou S. Pat Bland M. Lee A.Y. Hong K. Walker C.M. Escobedo J.A. Dwarki V. Genetic immunization with adeno-associated virus vectors expressing herpes simplex virus type 2 glycoproteins B and D. J. Virol. 1997;71:7960–7962. doi: 10.1128/jvi.71.10.7960-7962.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mata M. Travers P.J. Liu Q. Frankel F.R. Paterson Y. The MHC class I-restricted immune response to HIV-gag in BALB/c mice selects a single epitope that does not have a predictable MHC-binding motif and binds to Kd through interactions between a glutamine at P3 and pocket D. J. Immunol. 1998;161:2985–2993. [PubMed] [Google Scholar]
- Mingozzi F. Maus M.V. Hui D.J. Sabatino D.E. Murphy S.L. Rasko J.E. Ragni M.V. Manno C.S. Sommer J. Jiang H. Pierce G.F. Ertl H.C. High K.A. CD8+ T-cell responses to adeno-associated virus capsid in humans. Nat. Med. 2007;13:419–422. doi: 10.1038/nm1549. [DOI] [PubMed] [Google Scholar]
- Peden C.S. Burger C. Muzyczka N. Mandel R.J. Circulating anti-wild-type adeno-associated virus type 2 (AAV2) antibodies inhibit recombinant AAV2 (rAAV2)-mediated, but not rAAV5-mediated, gene transfer in the brain. J. Virol. 2004;78:6344–6359. doi: 10.1128/JVI.78.12.6344-6359.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scallan C.D. Jiang H. Liu T. Patarroyo-White S. Sommer J.M. Zhou S. Couto L.B. Pierce G.F. Human immunoglobulin inhibits liver transduction by AAV vectors at low AAV2 neutralizing titers in SCID mice. Blood. 2006;107:1810–1817. doi: 10.1182/blood-2005-08-3229. [DOI] [PubMed] [Google Scholar]
- Wang L. Calcedo R. Nichols T.C. Bellinger D.A. Dillow A. Verma I.M. Wilson J.M. Sustained correction of disease in naive and AAV2-pretreated hemophilia B dogs: AAV2/8-mediated, liver-directed gene therapy. Blood. 2005a;105:3079–3086. doi: 10.1182/blood-2004-10-3867. [DOI] [PubMed] [Google Scholar]
- Wang L. Dobrzynski E. Schlachterman A. Cao O. Herzog R.W. Systemic protein delivery by muscle-gene transfer is limited by a local immune response. Blood. 2005b;105:4226–4234. doi: 10.1182/blood-2004-03-0848. [DOI] [PMC free article] [PubMed] [Google Scholar]


