Abstract
Glioblastoma is a frequent brain malignancy with a dismal prognosis. The molecular changes causing its aggressive phenotype are under investigation. We report that the cytoskeletal-related proteins neurofibromatosis type 2 (NF2) and ezrin have opposite yet interdependent activities in glioblastoma growth. We show that NF2 is absent in approximately one-third of glioblastoma cell lines and tumors, and that it suppresses growth when expressed in cells. Although ezrin overexpression was previously observed in glioblastoma, we show here that ezrin enhanced cell proliferation and anchorage-independent growth but only in cells expressing NF2. Ezrin interacted and delocalized NF2 from the cortical compartment releasing its inhibition on Rac1. By using swap NF2-ezrin molecules, we identified that the opposite effects on cell growth of NF2 and ezrin depend on their amino-terminal FERM domain. The subcellular cortical localization appeared important for NF2 suppressive activity. In contrast, the ability of ezrin to enhance growth or complex NF2 did not depend on the molecular conformation or subcellular localization. In conclusion, these studies show 2 mechanisms for NF2 inactivation in glioblastoma: (i) decreased protein expression and (ii) increasing dosages of ezrin that disable NF2 by intermolecular association and aberrant intracellular recruitment.
Keywords: ezrin, FERM domain, glioblastoma, NF2
Gliomas are tumors derived from glial cells and are the most common primary brain tumors in adults. Glioblastoma multiforme (GBM) is the most frequent and most aggressive type of glioma, with a median patient survival of only 1 year.1 The study of hereditary cancer syndromes that lead to tumors in the central nervous system is key to uncovering how brain tumors develop and progress. Among familial cancer syndromes exhibiting brain tumors is the neurofibromatosis type 2 (NF2) syndrome that is characterized by the development of schwannomas.2 In sporadic cancers, mutations of NF2 have been documented in meningiomas, schwannomas, and ependymomas, which is a type of glioma, revealing its role as a tumor suppressor.3
The NF2 gene encodes a protein called merlin or schwannomin that displays significant homology to ezrin.4,5 Ezrin, radixin, and moesin (ERM) and NF2 form the ERM protein family and function to link membrane proteins to the actin cytoskeleton.6 Unlike NF2, ezrin has been associated with tumor invasion in various types of cancer cells7 and with increasing malignancy of astrocytic tumors.8,9 NF2 and ezrin share a similar molecular structure comprised of 3 functional domains: an amino (N)-terminal four-point-one-ERM (FERM) domain, an α-helical region, and a carboxyl (C)-terminal domain.6 NF2 lacks the C-terminal F-actin binding region that is present in the other ERM proteins10 but contains a unique N-terminal 17 residue sequence that confers localization to cell–cell boundaries.11 Functional and structural studies have shown that ERM proteins fold in a head-to-tail closed conformation in which the C-terminal region and parts of the α-helical region dock onto the FERM domain and mask the FERM domain sites for association to other molecules.12,13 Three FERM domain interaction sites have been mapped so far: 2 distinct but neighboring pockets for association with Na/H exchanger regulatory factors (NHERF) and with transmembrane proteins, such as CD44, respectively,14 and one positively charged cleft for contact with phosphatidylinositol-4,5-bisphosphate (PIP2).15,16 The switch from a closed to an open conformation is triggered by phosphorylation of a C-terminal Thr in ERM proteins (Ezrin-T567, Radixin-T564, Moesin-T558)17 or Ser518 in NF2.18 This event triggers also translocation of ERM from the cytoplasm to the plasma membrane19 where these proteins bridge transmembrane receptors to F-actin, but it is not clear if it controls the intracellular localization of NF2 as well.
The mechanism by which NF2 exerts its tumor suppression in mammalian cells is not elucidated. Even less clear is the molecular difference between NF2 and ezrin that confers growth suppression to NF2 and invasiveness to ezrin. In the present study, we found that GBM cell lines show distinct patterns of NF2-ezrin expression and that overexpression of NF2 and ezrin have contrasting consequences on GBM cell proliferation and anchorage-independent growth. Interestingly, ezrin required the presence of NF2 to enhance proliferation. Ezrin appeared to suppress NF2 by interacting and displacing the tumor suppressor from the cell membrane. By generating chimeric ezrin-NF2 molecules we found that the FERM domain of each molecule is responsible for the growth response. Our findings show interplay between NF2 and ezrin for GBM cell growth and propose the observed increase of ezrin expression in tumors as a mechanism for NF2 inhibition.
Materials and Methods
Plasmids and Small Hairpin (sh)RNAs
Mouse NF2 (gift of A. McClatchey) was inserted in the pCXn retroviral vector (neomycin selection). N-terminally Myc-tagged NF2, human ezrin, and NF2-ezrin swap chimera, Ez/NF2, and NF2/Ez, were inserted in the pCXb vector (blasticidin selection). The swap chimera were generated by molecular cloning taking advantage of an unique SmaI site in ezrin cDNA situated at the boundary between the FERM domain and the α-helical region. At the equivalent position in NF2, 1 nucleotide was mutated by PCR to restore the SmaI site. Myc-tagged ezrin and EzΔ53 mutant in pCDNA vector were described.20 Ezrin shRNAs were designed and cloned in the pSIREN-RetroQ retroviral vector (Clontech) and ezrin sh11 (GTGGGATGCTCAAAGATAA) and sh8 (GGAAGGAATCCTTAGCGAT) were used for these studies. The NF2 shRNAs (Open Biosystems) sh74 (GCTCTGGATATTCTGCACAAT) in pLKO or sh375 (CAGCAAGCACAATACCATT) in pGIPZ lentiviral vectors deplete both NF2 isoform I and II.
Cells, Retroviral Infections, Proliferation, and Soft Agar Colony Assays
293T, Bosc, and the GBM cell lines LN18, LN229, LN308, U373, U251-MG, D54, and A172 were grown in Dulbecco modified Eagle's medium supplemented with 10% fetal calf serum (FCS). Transfections, retroviral infections, proliferation, and soft agar colony assays for cells stably expressing proteins were previously described.21 All proliferation experiments were performed by plating equal numbers of cells in triplicate in 5% FCS followed by counting after 5 days of growth by using a hemocytometer.
Protein Analysis
The protocols for cell lysis, Western blotting, and immunoprecipitation were previously described.21 The GST-PAK2-Rac binding domain (RBD) construct (gift of S. Tanaka) for active Rac1 pull-down was used as described.22 For subcellular fractionation, cells were homogenized in hypotonic buffer (10 mM HEPES, pH 7.5, 10 mM KCl, 3 mM MgCl2), clarified at 600 × g for 5 minutes, and centrifuged at 100 000 × g for 1 hour. The resulting supernatant represented the cytoplasmic fraction and the pellet resuspended in membrane solubilization buffer (10 mM Tris–HCl, pH 7.4, 500 mM NaCl, 1 mM EDTA, 1% Triton X-100) was centrifuged at 16 000 × g for 30 minutes. The supernatant corresponded to the Triton-X soluble fraction and the insoluble pellet, resuspended in SDS-containing buffer, to the Triton-X insoluble fraction. For sucrose-gradient fractionation, cells scraped in 1.5 mL of sucrose buffer (0.25 M, buffered with 10 mM Tris–HCl, pH 7.4) were lysed by passing the suspension 20 times through a 25-gauge needle and incubated for 30 minutes. After centrifugation at 600 × g for 10 minutes, the supernatant was added on top of a sucrose gradient (1.5 mL of 0.5 M sucrose, 2 mL of 0.8 M sucrose, 2 mL of 1.0 M sucrose, and 2 mL of 1.5 M sucrose). Equal amounts of protein loaded and separated by ultracentrifugation at 100 000 × g for 16 hours were recovered in 500-µL aliquots from the top of the gradient. All procedures were performed on ice and all buffers contained protease and phosphatase inhibitors (1 mM sodium orthovanadate, 0.1 mM sodium molybdate, 1 mM phenylmethylsulfonyl fluoride, 21 µg/mL aprotinin, and 5 µg/mL leupeptin). Antibodies were obtained as follows: Ezrin (4A5), Moesin (C15), Erk1 (C-16), Erk2 (C-14), GAPDH (sc-47724), Myc (9E10), Rac1 (C14), NF2 C-terminal (C18), NF2 N-terminal (A19) (Santa Cruz Biotechnology), NF2 (9168), phospho-ERM (3141) (Cell Signaling), N-cadherin (Zymed), NHERF1 (Calbiochem), Myc (Invitrogen), phospho-Ser518-NF2, and actin (Chemicon).
Immunofluorescence Analysis and TMA Staining
The immunofluorescence analysis of formaldehyde-fixed cells was performed as described23 with some modifications. Briefly, formaldehyde-fixed, Triton-X-permeabilized cells were treated with Image-iT FX signal enhancer (Invitrogen) for 30 minutes and blocked with 5% donkey serum diluted in PBS-gel (0.2% gelatin in PBS) for 30 minutes. The primaries antibodies c-Myc (9E10), P-ERM, and merlin IC4 (Cell Signaling) and the secondary antibodies Alexa Fluor 488 and 568 donkey antimouse and antirabbit IgG (Molecular Probes) were used. The coverslips were mounted with Prolong antifade reagent (Invitrogen). The GBM TMAs slides were obtained from the M. D. Anderson Neuro-Pathology Core and stained with NF2 C-terminal (C18) antibody according to the described immunohistochemistry (IHC) protocol.24
Results
NF2 Tumor Suppressor is Absent or Reduced in GBM Cell Lines and Tumors
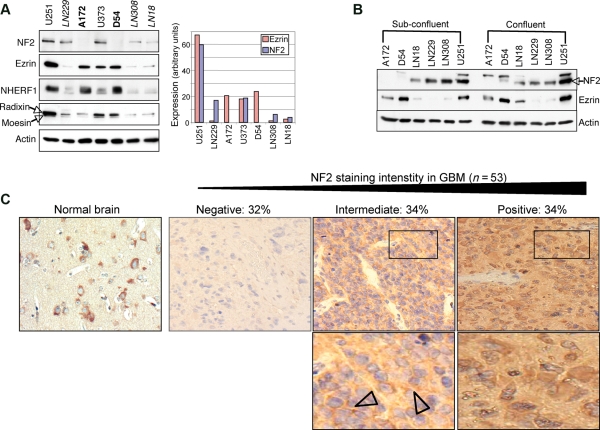
To examine the role of NF2 and ezrin in GBM cell growth, the expression level of these proteins was first analyzed in a panel of 7 GBM cell lines (Fig. 1A). Three patterns of protein expression were found: (i) lack of NF2 with high ezrin expression in A172 and D54 cells, (ii) high NF2 and ezrin expression in U251 and U373 cells, and (iii) lower NF2 and ezrin expression in LN229, LN308, and LN18 cell lines. The expression levels of NHERF1, an important ERM-interacting protein,25 paralleled ezrin levels. Radixin and moesin harbored also levels paralleling ezrin levels in all but 1 cell line (A172). The lack or reduced levels of NF2 in GBM cell lines suggested that NF2 might behave as a tumor suppressor in GBM. Interestingly, the total levels of NF2 varied depending on the cell confluency but retained the same expression pattern among the GBM cell lines (Fig. 1B). No differences were observed in the levels of ezrin due to cell confluency (Fig. 1B). To detect the levels of NF2 expression in GBM tumors, IHC with NF2 antibodies was performed in a GBM tissue microarray. NF2 localization in normal brain (Fig. 1C) was consistent with its reported expression in both neurons and glia.26 The quantification of NF2 staining intensity showed lack of NF2 in 32% of GBM tumors (Fig. 1C). The other two-thirds of the samples analyzed showed a progressive loss of NF2 staining with 34% of positive staining and 34% of intermediate staining (Fig. 1C). These results are in concordance with those from a recent study documenting the loss of NF2 expression in nearly one-third of GBM tumor samples.27
Fig. 1.
NF2 and ezrin expression patterns in GBM. (A) Western blot analysis with indicated antibodies of protein extracts from 7 GBM cell lines shows 3 ERM–NF2 expression patterns grouped and labeled in either regular, bold, or italic letters. The graph shows the quantification of NF2 and ezrin levels normalized to actin levels performed with Image J program (NIH). (B) Western blot analysis shows varying NF2 levels, in cells rated as 70% (Sub-confluent) and 100% (Confluent). Arrowhead indicates NF2. (C) Immunohistochemistry analysis (×40) with NF2 antibody of GBM tissue microarray (n = 53 samples) showing the patterns of progressive loss of NF2 staining and the corresponding % of tumors. The lower zoomed fields (rectangles) show cytoplasmic and membrane (arrowheads) NF2 localization in tumors with intermediate staining and mainly cytoplasmic localization in tumors with pronounced positive staining. NF2 intensity was quantified by ImageJ software and intensity scores were computed as x = [255–(gray scale value)]. Tumors were scored as NF2 positive (x ≥ 90), intermediate (36 < x < 90), or negative (x ≤ 36).
NF2 Suppresses Proliferation in GBM Cells and Decreases Ezrin Expression
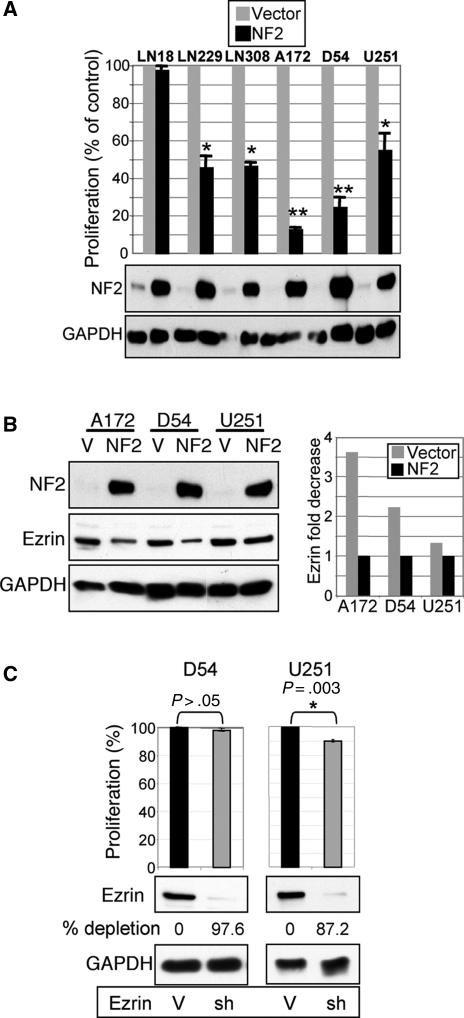
The ability to alter cell proliferation was tested in GBM cells by stable overexpression of NF2 (Fig. 2A). NF2 strongly suppressed proliferation in all but 1 GBM cell line, with more potency in the 2 NF2-negative GBM cell lines, supporting a tumor suppressor role for NF2 in GBM.
Fig. 2.
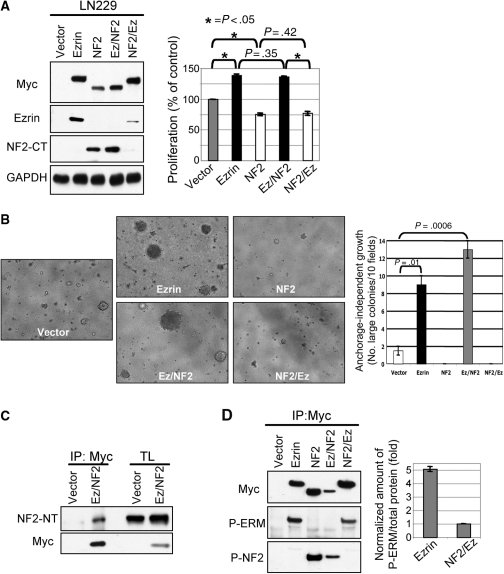
NF2 suppresses proliferation and decreases ezrin expression in GBM cell lines. (A) Proliferation analysis of indicated GBM cell lines stably expressing either NF2 or vector control. The lower panels show the corresponding NF2 and control GAPDH expression levels. (B) NF2 overexpression decreases endogenous ezrin levels in the indicated GBM cells compared with vector control cells (V). The densitometry quantification of ezrin levels normalized to GAPDH levels is shown. (C) Ezrin depletion by shRNA (sh-11) in NF2-negative D54 cells showed 97.6% expression decrease but no proliferation change compared with vector control (V). In contrast, ezrin depletion in NF2-positive U251 cells induced a small but very reproducible proliferation decrease. Values represent means ± SEM from 3 independent experiments. Statistically significant differences relative to vector control were calculated by using paired t-test and are marked with a single (P ≤ .01) or double (P ≤ .005) asterisk.
We observed that NF2 overexpression reduced the levels of endogenous ezrin in GBM cells (Fig. 2B). This effect may occur because of the interaction between overexpressed NF2 and endogenous ezrin with mislocalization of ezrin and faster degradation. Changes in the actin cytoskeleton may be also involved as well as competition between NF2 and ezrin for common binding partners with displacement and degradation of ezrin. These possibilities need further investigation.
To determine if the antiproliferative effects induced by NF2 are a consequence of ezrin downregulation, ezrin was depleted in D54 cells that express high levels of endogenous ezrin but lack NF2 (Fig. 2C). Efficient downregulation of 97% of ezrin in these cells by ezrin sh11 had no effect on proliferation, suggesting that the reduction of ezrin levels following NF2 expression is not the mechanism responsible for NF2 growth suppression. In contrast, ezrin depletion in cells expressing endogenous NF2 induced a small but very reproducible decrease in proliferation (Fig. 2C), suggesting a repressive effect of ezrin on NF2. These results were confirmed by using a second shRNA (sh8) for ezrin silencing (not shown).
Ezrin Increases Cell Proliferation by Altering the Subcellular Distribution of NF2
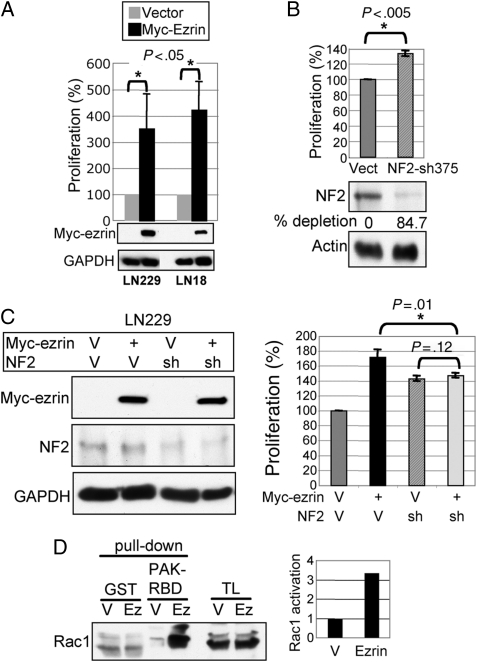
We next performed complementary experiments to assess the role of ezrin in GBM proliferation. In cell lines with low endogenous ezrin levels but expressing NF2, ezrin overexpression increased cell proliferation (Fig. 3A), indicating an oncogenic role for ezrin in GBM, as opposed to NF2. The silencing of NF2 expression by 2 distinct shRNAs increased significantly cell proliferation, again demonstrating a tumor suppressor function for NF2 in GBM (Fig. 3B and Supplementary Material, Fig. S1). However, although ezrin overexpression did not influence the levels of endogenous NF2, it caused proliferation in cells expressing NF2 rather than in cells depleted of endogenous NF2 (Fig. 3C and Supplementary Material, Fig. S2). In fact, the increase in proliferation in the LN229 cells simultaneously overexpressing ezrin and depleted of NF2 was at the level of that of cells depleted of NF2 alone, suggesting that the oncogenic effects of ezrin require the presence of NF2. Nevertheless, the possibility of a direct effect of ezrin on Rac activation cannot be excluded.28
Fig. 3.
Ezrin overexpression promotes cell growth in NF2-positive GBM cells. (A) Proliferation analysis of Myc-tagged ezrin stably expressed in the NF2-positive LN229 and LN18 cells. (B) NF2 silencing by shRNA (sh375) in LN229 cells increased cell proliferation compared with vector control cells (Vect). The Western blot and quantification analyses show the depleted NF2 levels normalized to actin levels, in comparison to vector control levels. (C) Western blot and proliferation analyses of LN229 cells with combinatorial infections of Myc-tagged ezrin, NF2 shRNA-375 (sh), and their corresponding vectors (V), showing no effect of ezrin overexpression on the cells depleted of NF2. Statistically significant differences are indicated with asterisk. (D) GST pull-down assay of lysates from LN229 cells overexpressing ezrin (Ez) or vector (V) with PAK-RBD domain that precipitates active GTP-bound Rac1. Rac 1 fold activation was quantified by normalizing activated Rac1 levels to GST-PAK-RBD input. TL, total lysate.
Previous studies showed that NF2 might exert its tumor suppressor effects by decreasing the activation of Rac1.18 As we have shown that ezrin requires the presence of NF2 for inducing oncogenic effects (Fig. 3C), a possible functional explanation is that ezrin inactivates endogenous NF2, thus counteracting its growth-controlling activity. The analysis of Rac1 activation by GST pull down assay with GST-PAK2-RBD that interacts only with active GTP-bound Rac1 showed that indeed, ezrin overexpression in LN229 cells that express endogenous NF2 increased the levels of active Rac1 (Fig. 3D). Of note is that we could not detect activation of the MAPK or Akt by ezrin overexpression in these cells (not shown). These experiments implied that ezrin overexpression might indeed exert oncogenic effects by suppressing NF2.
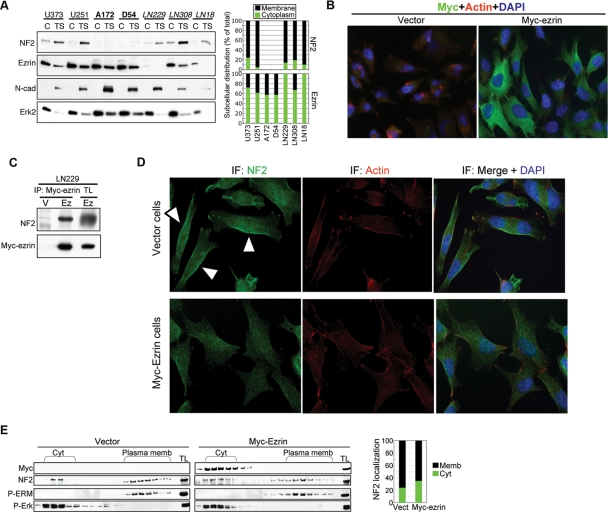
Because the ERM proteins are activated by phosphorylation and recruitment to the membrane compartment, we examined the subcellular distribution of these proteins in cytoplasmic and membrane (Triton X-soluble) fractions from GBM cells (Fig. 4A and Supplementary Material, Fig. S3). Endogenous NF2 was mainly localized in the membrane compartment whereas endogenous ezrin was mainly cytoplasmic in GBM cells (Fig. 4A), corresponding to a distribution pattern previously reported in other types of cells.6 Overexpressed ezrin exhibited the same intracellular localization as endogenous ezrin, with predominant expression in the cytoplasm (Figs 4B and 5B). Radixin and moesin were almost equally distributed between the cytoplasmic and membrane fraction, presenting overall an intracellular distribution intermediate between NF2 and ezrin (Supplementary Material, Fig. S3).
Fig. 4.
Ezrin interacts and delocalizes NF2 in GBM cells. (A) Fractionation in cytoplasmic (C) and membrane Triton X-soluble fractions (TS) shows the subcellular localization of endogenous NF2 and ezrin in the GBM cell lines grouped as in Fig. 1A. Erk2 and N-cadherin (N-cad) were used as cytoplasmic and membrane fractionation markers, respectively. The densitometric intensities of the cytoplasmic and membrane bands of NF2 or ezrin are represented graphically as percentage from the summed cytosolic and membrane distributions. (B) Immunofluorescence analysis (×40) of LN229 cells containing vector or Myc-tagged ezrin (Myc-ezrin) for ezrin (Myc), actin (Rhodamin-phalloidin), and nucleus (DAPI) detection. Images were acquired with MetaVue program using a Leica Epifluorescence Inverted Microscope. These cells were obtained by infection with retroviruses containing vector or Myc-ezrin. The efficiency of infection was almost 100% in these experiments and all cells expressed Myc-ezrin to various extents. (C) Coimmunoprecipitation of Myc-tagged ezrin with endogenous NF2 from LN229 cells overexpressing Myc-tagged ezrin (Ez) or vector control (V). TL, total lysate. (D) Immunofluorescence analysis with NF2 antibody of the cells from (B) that express either vector or Myc-ezrin, as indicated on the left. Arrowheads indicate membrane NF2 in vector-expressing cells. Note decreased membrane localization of endogenous NF2 in the cells expressing Myc-ezrin. (E) The vector and Myc-ezrin-expressing cells from (B) were subjected to sucrose gradient fractionation to separate cytoplasm (Cyt) and membrane (plasma memb) compartments. The fractions were analyzed with the antibodies indicated on the left. P-ERM and P-Erk were used as plasma membrane and cytoplasmic fractionation markers, respectively. Note extended cytoplasmic cofractionation of endogenous NF2 with Myc-tagged ezrin. TL, total cell lysate. The densitometry analysis shows the cytoplasmic and membrane distribution of NF2 in the vector- and Myc-ezrin-expressing cells. The intensities of the individual bands were measured with Image J program. These were summed separately for the cytoplasmic or the membrane fractions and computed to a percentage from the total summed distribution.
Fig. 5.
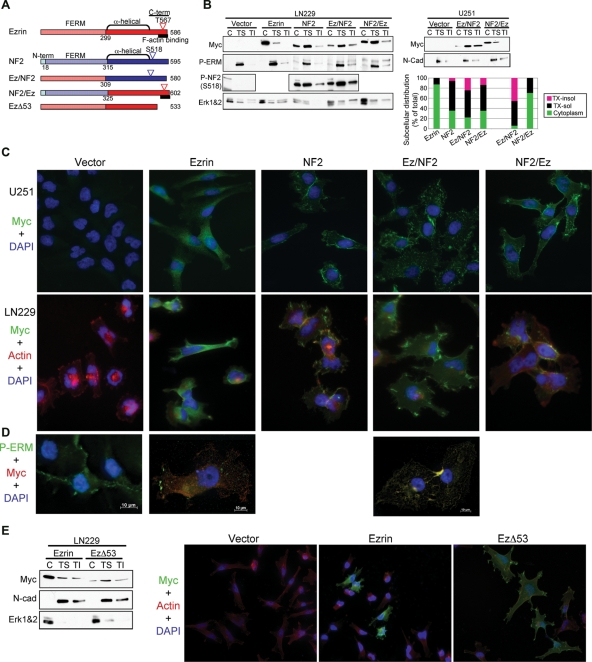
Subcellular distribution of ezrin-NF2 chimeric proteins. (A) Molecular organization of ezrin, NF2, ezrin-NF2 swap chimera, and EzΔ53 mutant. (B) LN229 and U251 cells stably expressing Myc-tagged ezrin, NF2, Ez/NF2, and NF2/Ez were processed in cytoplasmic (C), Triton-X soluble (TS), and Triton-X insoluble (TI) fractions. Erk1 and 2 (Erk1&2) and N-cadherin were used as cytoplasmic and membrane markers, respectively. The intensity of each fraction of a Myc-tagged protein is represented graphically as percent ratio from the protein's summed cytosolic, Triton-X-soluble, and Triton-X-insoluble intensities. (C) Immunofluorescence analysis (×40) of U251 and LN229 cells labeled as indicated on the left side revealing the subcellular localization of the proteins indicated on top. Note cytoplasmic expression of ezrin, contrasting with the localization at membrane spike-like structures of Ez/NF2, and membrane localization of NF2 and NF2/Ez. (D) Deconvolved images (63× , oil immersion) show colocalization of P-ERM proteins (green) and Ez/NF2 mutant stained with anti-Myc antibody (red) at the membrane spike-like structures. (E) Subcellular fractionation as in (A) and immunofluorescence analysis (×40) of LN229 cells transiently expressing Myc-tagged ezrin and EzΔ53 mutant. Image stacks were acquired with a Zeiss Axiovert 200M inverted microscope and deconvolved with the AxioVision Rel 4.5 SP1 software.
The cytoplasmic distribution of ezrin is attributed to its closed conformation, in which the FERM domain and actin-binding site are reciprocally masked by a head-to-tail intramolecular or intermolecular interaction. Ezrin has been reported to interact in a head-to-tail manner not only with itself but also with the other members of the ERM family, including NF2.6,29–31 By coimmunoprecipitation experiments, we observed that a fraction of endogenous NF2 was recruited in complex with overexpressed ezrin (Fig. 4C). This observation, coupled with that of opposite intracellular distribution observed for NF2 and ezrin, lead us to hypothesize that ezrin might inactivate NF2 by forming complexes that alter its intracellular distribution. Overexpression of ezrin in LN229 GBM cells that contain endogenous NF2 determined delocalization of NF2 from the cortical/membrane compartment, as evidenced by immunofluorescence analysis (Fig. 4D) and sucrose gradient fractionation (Fig. 4E). A similar effect on endogenous NF2 localization was observed in LN18 cells (Supplementary Material, Fig. S4). These data suggested that ezrin exerts its oncogenic effects by sequestering and altering NF2 subcellular localization in GBM cells.
Ezrin-NF2 Chimeric Proteins Localize to the Membrane Compartment in GBM Cells
To map the domains within ezrin and NF2 responsible for their different patterns of intracellular localization and their opposite phenotypes, we engineered chimeric ezrin-NF2 molecules by interchanging the N-terminal regions containing the FERM domains (Fig. 5A). Myc-tagged parental and chimeric molecules were stably expressed in LN229 and U251 GBM cells that have either low or high levels of endogenous ezrin and NF2, respectively (Fig. 1A), and their intracellular localization was initially examined by fractionation in cytoplasmic, Triton X-soluble (membrane-enriched), and Triton X-insoluble (cytoskeleton-enriched) compartments (Fig. 5B). Surprisingly, Ez/NF2 showed predominant expression in the membrane/cytoskeletal compartments in sharp contrast to ezrin, although NF2/Ez had similar distribution as NF2. The immunofluorescence analysis confirmed these findings and revealed that Ez/NF2 specifically localizes to membrane spike-like structures resembling microvilli and modifies the morphology of cells, which appeared enlarged and flattened (Fig. 5C, 4th column panels and Supplementary Material, Fig. S5). This localization was very distinct from that of the other proteins, being reminiscent of the localization of phosphorylated-ERM (P-ERM) proteins. Costaining with Myc and P-ERM (T567/T564/T558) antibodies showed that the Ez/NF2 molecule colocalized with P-ERM proteins in apical spike-like structures (Fig. 5D and Supplementary Material, Fig. S6).
Phospho-ERM proteins are open and have 2 regions that could recruit them to the membrane, the FERM domain that binds to PIP2 and transmembrane proteins, and the C-terminal F-actin binding site (Fig. 5A). Because Ez/NF2 displayed the same subcellular localization as P-ERM proteins, its molecular conformation is probably open, with the NF2 C-terminal moiety away from ezrin FERM domain. Examining the phosphorylation of Ser518 that was also implicated in opening up the closed conformation of NF2,18 we found that both NF2 and Ez/NF2 were phosphorylated on this residue (Figs 5B and 6D), a finding that could explain the open conformation of Ez/NF2. In contrast to ezrin, both NF2 and Ez/NF2 lack the C-terminal F-actin binding region of ezrin. To determine if this region is involved in the regulation of the intracellular localization, we transiently expressed in LN229 cells Myc-tagged ezrin and EzΔ53 that lacks the F-actin binding region (Fig. 5A). Because the C-terminal region is also involved in FERM domain interaction, the EzΔ53 mutant is also bound to adopt an open conformation. Strikingly, EzΔ53 had the same subcellular distribution as Ez/NF2 and induced the same modifications of cell morphology (Fig. 5E). These results indicated that (i) Ez/NF2 is indeed in an open conformation by analogy with EzΔ53, (ii) the unmasked FERM domain of ezrin is responsible for the localization in membrane/cytoskeletal spike-like structures and for the change of GBM cell shape, and (iii) the actin-binding region is possibly required for the exclusive localization in the membrane of the P-ERM proteins, as a small proportion of the EzΔ53 mutant was still present in the cytoplasmic fraction.
Fig. 6.
The FERM domain controls the opposite phenotype of ezrin and NF2. (A) Proliferation of LN229 cells expressing ezrin, NF2, and chimeric ezrin-NF2 proteins, as shown in the Western blot panels. Ezrin and NF2 antibodies detect the C-terminal (CT) region of the proteins. (B) The anchorage-independent growth was assessed by colony formation assay after 3 weeks of incubation in soft agar. Images (10×) were acquired on a Zeiss Axiovert 200 microscope using the Cool SNAP ES Photometrics Camera (Ropert Scientific) and Meta Imaging Corporation Series software (Universal Imaging). Colonies were counted in 10 different fields from duplicate plates. Large colonies presented a diameter > 2 mm and their average numbers ± SD are represented graphically. Statistically significant differences relative to vector control were calculated by using the t-test. (C) Coimmunoprecipitation of Myc-tagged Ez/NF2 chimera with endogenous NF2 in LN229 cells. The precipitated sample was probed with an NF2 antibody recognizing the N-terminal (NT) region to reveal only endogenous NF2 (upper panel) and further re-probed with anti-Myc antibody to reveal Myc-tagged Ez/NF2 (lower panel). (D) Immunoprecipitation of Myc-tagged proteins expressed in D54 cells followed by Western blotting with indicated antibodies show phosphorylation of the C-terminal residues in all proteins. To quantify the relative phosphorylation on Thr567 of ezrin and NF2/Ez, the intensities of the P-ERM bands were normalized to those of the Myc bands (total protein immunoprecipitated) and the analysis is shown in the graph as mean ± SEM from 3 separate experiments.
The FERM Domain Is the Molecular Determinant that Imparts the Opposing Effects of Ezrin and NF2 on Cell Growth
To examine the contribution of the ERM N-terminal and C-terminal domains to GBM cell growth, LN229, LN18, and D54 cells stably expressing parental and chimeric proteins were subjected to proliferation and colony formation assays (Fig. 6A and B and Supplementary Material, Fig. S2). The experiments showed that compared with vector control cells, ezrin and Ez/NF2 that contain the FERM domain of ezrin increased both cell proliferation and anchorage-independent growth. In contrast, NF2 and NF2/Ez that contain the FERM domain of NF2 had the opposite effect. These data imply that the type of FERM domain is responsible for the consequences on GBM cell growth. In the case of ezrin and Ez/NF2 molecules, this effect appeared to be independent of the intracellular distribution or of the open or closed conformation of the proteins. To ascertain that Ez/NF2 acts like ezrin, by sequestering NF2 in complex, we immunoprecipitated Myc-EZ/NF2 and probed for endogenous NF2 (Fig. 6C). Similarly to ezrin, Ez/NF2 associated with endogenous NF2.
Since NF2 and NF2/Ez exhibited similar intracellular localization, we examined the conformation status by assessing the phosphorylation in the C-terminal region. Immunoprecipitation of proteins with Myc antibody followed by immunoblotting with either P-S518-NF2 or P-ERM antibodies showed that both proteins were phosphorylated in their C-terminal region (Fig. 6D). Only a small proportion of NF2/Ez appeared phosphorylated on the threonine recognized by the P-ERM antibody in comparison to the amount of ezrin phosphorylated on T567. This may indicate that NF2/Ez is in a closed conformation similar to ezrin and that the tail of NF2/Ez is even less available than the tail of ezrin for phosphorylation. This finding reinforces the notion that the closed conformation of NF2 is the active, growth suppressive form.32 However, our data on similar levels of S518 phosphorylatation in both NF2 and Ez/NF2 (Fig. 6D), although supporting an open conformation for Ez/NF2 in concordance with the membrane localization of this chimera, would also indicate an open and therefore inactive conformation of NF2 that is not compatible with the potent effect of NF2 on growth (Table 1). One explanation for this apparent conundrum may be that a nonphosphorylated fraction of active NF2 is present in cells. Another possibility could be that, in contrast to Thr567 in ezrin, Ser518 phosphorylation may not be sufficient to open up NF2 and the open conformation of Ez/NF2 might be due to additional structural constraints present in this mutant in comparison to NF2.
Table 1.
Summary of the roles of ezrin, NF2, and chimeric ezrin-NF2 proteins in GBM cells
| Protein | Proliferation colony formation | Cell shape | Intracellular localization |
||
|---|---|---|---|---|---|
| Immunofluorescence | Coloc. P-ERM | Fractionation | |||
| Ezrin | ↑ | Elongated | Cytoplasm | No | Cyt. |
| NF2 | ↓ | Unchanged | Memb-cell junctions | n.t. | TX-sol/Cyt. |
| Ez/NF2 | ↑ | Spread | Memb-spikes | Yes | TX-sol/TX-insol |
| NF2/Ez | ↓ | Unchanged | Memb-cell junctions | n.t. | TX-sol/Cyt. |
| EzΔ53 | n.t. | Spread | Memb-spikes | n.t. | TX-sol/TX-insol |
↑, increase; ↓, decrease; n.t., not tested; Coloc, colocalization; memb, membrane; Cyt., cytoplasm, TX-sol, Triton-X soluble; TX-insol, Triton-X insoluble.
Discussion
On the basis of our observation that NF2 and ezrin reveal a pattern of expression in GBM cell lines in which either relatively lower or relatively higher expression of both proteins is present, as if ezrin expression parallels NF2 expression, we analyzed the possibility of NF2 inactivation by ezrin overexpression. NF2 is a bona fide tumor suppressor in 3 types of brain and spinal cord tumors—meningioma, schwannoma, and ependymoma, and is the tumor suppressor responsible for the inherited NF2 syndrome.3–5 NF2 belongs to the ERM family of proteins, but surprisingly, the other members of the family have been assigned rather oncogenic and pro-metastatic roles.7,33 In astrocytic tumors, of which GBM is the most malignant type, ezrin overexpression was detected by microarray, proteomic, and IHC analyses.8,9,34,35 Most importantly, ezrin expression increased with the grade of the tumor and high expression correlated with poor overall survival.8,9 We carried out a similar analysis for NF2 in GBM tissue microarrays and found that one-third of the tumors lost NF2 and another third had low expression of the tumor suppressor. From these results, an opposite ERM expression tendency became apparent in GBM, characterized by high expression of ezrin, and progressive loss of NF2. We subsequently firmly established that NF2 behaves as a tumor suppressor in GBM by performing both overexpression experiments in 6 GBM cell lines and shRNA silencing experiments. With one exception, exogenous NF2 efficiently suppressed GBM cell growth, and the depletion of the endogenous NF2 resulted in enhanced proliferation.
Analyzing the role of ezrin in GBM cells, we found that overexpression of ezrin increased GBM cell growth only in cell lines expressing NF2 (Fig. 3C and Supplementary Material, Fig. S2). Moreover, ezrin silencing in cells lacking NF2 had no effect on cell proliferation whereas it reproducibly decreased proliferation in cells expressing endogenous NF2 (Fig. 2C). These results implied a paradoxical requirement of NF2 tumor suppressor expression for ezrin oncogenic consequences that could be explained only if ezrin acts by inhibiting NF2. Ezrin and NF2 have been previously shown to interact in vitro and in vivo and colocalize in GBM U251 cells.29–31,36 We have shown that both ezrin and Ez/NF2 molecules that have oncogenic activity bind to endogenous NF2 in GBM cells. Ezrin delocalizes NF2 from the membrane compartment, where NF2 is thought to exert its growth suppressive function.32 In addition, the downstream signaling to Rac1 that is restricted by NF218 is released by ezrin expression in GBM cells. These findings suggest that ezrin blocks the activity of NF2 by association and displacement from the membrane. Coupling these data to the expression patterns of ezrin and NF2 in GBM tumors and cell lines, we conclude that the overexpression of ezrin is a mechanism of NF2 inactivation in GBM. Since the expression of ezrin appears homogenous and very high in 75% of GBM,9 this mechanism might be used in the majority of GBM tumors. A critical question would then be whether this mechanism is likely to be used in other types of cancers as well. Ependymomas are known to express high levels of ezrin8 and thus the interference with NF2 in the cases without NF2 LOH appears to be an attractive possibility. Other tumors, such as meningiomas, schwannomas, and mesotheliomas that harbor frequent NF2 LOH,3 prostate cancer where NF2 appears inactivated in some cases by phosphorylation,37 colorectal cancer with rare NF2 genetic alterations,38 or osteosarcoma that exhibits ezrin overexpression,39,40 might constitute likely targets for NF2 inactivation by ezrin heterodimerization, and await investigation.
Analyzing the molecular requirements for the opposite growth phenotypes by utilizing chimeric NF2-ezrin molecules, we found that the FERM domain specifies the phenotypic behavior whereas the region composed of the α-helical stretch and the C-terminal domain is interchangeable between molecules without consequences on cell growth (Table 1). In the case of NF2/Ez, the extreme N-terminal 17-residue sequence that localizes NF2 to cell boundaries is most likely also required beside the FERM domain to confer growth suppression, as an NF2 mutant lacking this sequence has been shown to lose suppressive ability.11 However, the same study has shown that the transfer of this sequence to ezrin induced a change in localization but not in growth properties.11 As the difference between ezrin tagged with the N-terminal sequence of NF2 and NF2/ezrin is only the FERM domain of NF2, it ensues that the combination of NF2 N-terminal membrane localization sequence and FERM domain is necessary and sufficient for growth suppression. Reciprocally, Ez/NF2 displays a growth phenotype similar to ezrin, making of the ezrin FERM domain the sole determinant for enhanced growth. The FERM domains of ERM are 86% identical whereas that of NF2 bears only 62% amino acid identity with ezrin's.6 In spite of this divergence, the NF2 FERM domain has the same fold as those of radixin or moesin.13,41 The difference may stem from the presence of surfaces with opposite electrostatic potential in the NF2 FERM domain that may account for differential interaction with binding partners. Importantly, NF2-specific residues in these areas are targeted for missense mutations in the NF2 syndrome.41 Whether these residues account for the difference in phenotype between NF2 and ezrin is currently under investigation.
That the ezrin FERM domain is responsible for ezrin's oncogenic properties would not be surprising in light of the mechanism that we propose for the NF2-dependent effects of ezrin on cell growth. The FERM domain of ezrin has been reported to interact strongly with the C-terminal tail of NF2 isoform I in intermolecular association assays29,31 and this interaction is most likely key to the association of overexpressed ezrin and Ez/NF2 with endogenous NF2. The NF2 splice isoforms I and II differ in that the very last 16 residues of isoform I are replaced by 11 new residues in isoform II. This change disrupts the interaction of the tail with the FERM domain and seems sufficient to inactivate the growth suppressive ability of isoform II, therefore leaving isoform I as the functional NF2 form.42 It appears that the FERM domain of ezrin has to be in the context of the full molecule to be oncogenic. The isolated ezrin FERM domain has been previously shown to act as a dominant negative molecule and inhibit the growth of GBM cells that express both NF2 and ezrin, probably by interfering with endogenous ezrin.43
Interestingly, it was shown that NF2 has stronger tendency to homodimerize than to heterodimerize with ezrin.29,30 It would be thus tempting to speculate that only high levels of ezrin, as those observed in GBM tumors,8,9 would be effective to bind and de-localize NF2, resulting in the cytoplasmic NF2 localization that we observed in NF2 positive GBM tumors (Fig. 1C). Therefore, beside the loss of NF2, a more extensive NF2 inactivation due to ezrin overexpression occurs in GBM. In conclusion, our study reveals a strong contribution of NF2 to GBM tumor growth and opens the avenue for new therapeutic approaches for this aggressive and deadly malignancy.
Supplementary Material
Supplementary material is available at Neuro-Oncology Journal online.
Funding
This work was supported by NCI-CA107201 and M. D. Anderson Cancer Center Institutional Research Grant (MMG) and the American Brain Tumor Association (FCM). The DNA sequencing was partially supported by NCI-CA16672.
Supplementary Material
Acknowledgments
We thank S. Momin for help with shRNA testing and G. Fuller for providing the GBM TMA.
Conflict of interest statement. None declared.
References
- 1.Nakada M, Nakada S, Demuth T, Tran NL, Hoelzinger DB, Berens ME. Molecular targets of glioma invasion. Cell Mol Life Sci. 2007;64:458–478. doi: 10.1007/s00018-007-6342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McClatchey AI. Neurofibromatosis. Annu Rev Pathol. 2007;2:191–216. doi: 10.1146/annurev.pathol.2.010506.091940. [DOI] [PubMed] [Google Scholar]
- 3.Hanemann CO. Magic but treatable? Tumours due to loss of merlin. Brain. 2008;131:606–615. doi: 10.1093/brain/awm249. [DOI] [PubMed] [Google Scholar]
- 4.Rouleau GA, Merel P, Lutchman M, et al. Alteration in a new gene encoding a putative membrane-organizing protein causes neuro-fibromatosis type 2. Nature. 1993;363:515–521. doi: 10.1038/363515a0. [DOI] [PubMed] [Google Scholar]
- 5.Trofatter JA, MacCollin MM, Rutter JL, et al. A novel moesin-, ezrin-, radixin-like gene is a candidate for the neurofibromatosis 2 tumor suppressor [erratum appears in Cell 1993;75(4):826] Cell. 1993;72:791–800. doi: 10.1016/0092-8674(93)90406-g. [DOI] [PubMed] [Google Scholar]
- 6.Bretscher A, Edwards K, Fehon RG. ERM proteins and merlin: integrators at the cell cortex. Nat Rev Mol Cell Biol. 2002;3:586–599. doi: 10.1038/nrm882. [DOI] [PubMed] [Google Scholar]
- 7.Georgescu MM, Morales FC, Molina JR, Hayashi Y. Roles of NHERF1/EBP50 in cancer. Curr Mol Med. 2008;8:459–468. doi: 10.2174/156652408785748031. [DOI] [PubMed] [Google Scholar]
- 8.Geiger KD, Stoldt P, Schlote W, Derouiche A. Ezrin immunoreactivity is associated with increasing malignancy of astrocytic tumors but is absent in oligodendrogliomas. Am J Pathol. 2000;157:1785–1793. doi: 10.1016/S0002-9440(10)64816-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tynninen O, Carpen O, Jaaskelainen J, Paavonen T, Paetau A. Ezrin expression in tissue microarray of primary and recurrent gliomas. Neuropathol Appl Neurobiol. 2004;30:472–477. doi: 10.1111/j.1365-2990.2004.00562.x. [DOI] [PubMed] [Google Scholar]
- 10.Turunen O, Wahlstrom T, Vaheri A. Ezrin has a COOH-terminal actin-binding site that is conserved in the ezrin protein family. J Cell Biol. 1994;126:1445–1453. doi: 10.1083/jcb.126.6.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cole BK, Curto M, Chan AW, McClatchey AI. Localization to the cortical cytoskeleton is necessary for Nf2/merlin-dependent epidermal growth factor receptor silencing. Mol Cell Biol. 2008;28:1274–1284. doi: 10.1128/MCB.01139-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Q, Nance MR, Kulikauskas R, et al. Self-masking in an intact ERM-merlin protein: an active role for the central alpha-helical domain. J Mol Biol. 2007;365:1446–1459. doi: 10.1016/j.jmb.2006.10.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pearson MA, Reczek D, Bretscher A, Karplus PA. Structure of the ERM protein moesin reveals the FERM domain fold masked by an extended actin binding tail domain. Cell. 2000;101:259–270. doi: 10.1016/s0092-8674(00)80836-3. [DOI] [PubMed] [Google Scholar]
- 14.Terawaki S, Maesaki R, Hakoshima T. Structural basis for NHERF recognition by ERM proteins. Structure. 2006;14:777–789. doi: 10.1016/j.str.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 15.Hamada K, Shimizu T, Matsui T, Tsukita S, Hakoshima T. Structural basis of the membrane-targeting and unmasking mechanisms of the radixin FERM domain. EMBO J. 2000;19:4449–4462. doi: 10.1093/emboj/19.17.4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Niggli V, Andreoli C, Roy C, Mangeat P. Identification of a phosphatidylinositol-4,5-bisphosphate-binding domain in the N-terminal region of ezrin. FEBS Lett. 1995;376:172–176. doi: 10.1016/0014-5793(95)01270-1. [DOI] [PubMed] [Google Scholar]
- 17.Matsui T, Maeda M, Doi Y, et al. Rho-kinase phosphorylates COOH-terminal threonines of ezrin/radixin/moesin (ERM) proteins and regulates their head-to-tail association. J Cell Biol. 1998;140:647–657. doi: 10.1083/jcb.140.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaw RJ, Paez JG, Curto M, et al. The Nf2 tumor suppressor, merlin, functions in Rac-dependent signaling. Dev Cell. 2001;1:63–72. doi: 10.1016/s1534-5807(01)00009-0. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi K, Yonemura S, Matsui T, Tsukita S. Immunofluorescence detection of ezrin/radixin/moesin (ERM) proteins with their carboxyl-terminal threonine phosphorylated in cultured cells and tissues. J Cell Sci. 1999;112:1149–1158. doi: 10.1242/jcs.112.8.1149. [DOI] [PubMed] [Google Scholar]
- 20.Morales FC, Takahashi Y, Momin S, Adams H, Chen X, Georgescu MM. NHERF1/EBP50 Head-to-tail intramolecular interaction masks association with PDZ domain ligands. Mol Cell Biol. 2007;27:2527–2537. doi: 10.1128/MCB.01372-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Georgescu MM, Kirsch KH, Akagi T, Shishido T, Hanafusa H. The tumor-suppressor activity of PTEN is regulated by its carboxyl-terminal region. Proc Natl Acad Sci USA. 1999;96:10182–10187. doi: 10.1073/pnas.96.18.10182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishihara H, Maeda M, Oda A, et al. DOCK2 associates with CrkL and regulates Rac1 in human leukemia cell lines. Blood. 2002;100:3968–3974. doi: 10.1182/blood-2001-11-0032. [DOI] [PubMed] [Google Scholar]
- 23.Georgescu MM, Kirsch KH, Kaloudis P, Yang H, Pavletich NP, Hanafusa H. Stabilization and productive positioning roles of the C2 domain of PTEN tumor suppressor. Cancer Res. 2000;60:7033–7038. [PubMed] [Google Scholar]
- 24.Wang H, Wang H, Zhang W, Fuller GN. Tissue microarrays: applications in neuropathology research, diagnosis, and education. Brain Pathol. 2002;12:95–107. doi: 10.1111/j.1750-3639.2002.tb00426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reczek D, Berryman M, Bretscher A. Identification of EBP50: A PDZ-containing phosphoprotein that associates with members of the ezrin-radixin-moesin family. J Cell Biol. 1997;139:169–179. doi: 10.1083/jcb.139.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stemmer-Rachamimov AO, Gonzalez-Agosti C, Xu L, et al. Expression of NF2-encoded merlin and related ERM family proteins in the human central nervous system. J Neuropathol Exp Neurol. 1997;56:735–742. [PubMed] [Google Scholar]
- 27.Lau YK, Murray LB, Houshmandi SS, Xu Y, Gutmann DH, Yu Q. Merlin is a potent inhibitor of glioma growth. Cancer Res. 2008;68:5733–5742. doi: 10.1158/0008-5472.CAN-08-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ivetic A, Ridley AJ. Ezrin/radixin/moesin proteins and Rho GTPase signalling in leucocytes. Immunology. 2004;112:165–176. doi: 10.1111/j.1365-2567.2004.01882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gronholm M, Sainio M, Zhao F, Heiska L, Vaheri A, Carpen O. Homotypic and heterotypic interaction of the neurofibromatosis 2 tumor suppressor protein merlin and the ERM protein ezrin. J Cell Sci. 1999;112:895–904. doi: 10.1242/jcs.112.6.895. [DOI] [PubMed] [Google Scholar]
- 30.Meng JJ, Lowrie DJ, Sun H, et al. Interaction between two isoforms of the NF2 tumor suppressor protein, merlin, and between merlin and ezrin, suggests modulation of ERM proteins by merlin. J Neurosci Res. 2000;62:491–502. doi: 10.1002/1097-4547(20001115)62:4<491::AID-JNR3>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 31.Nguyen R, Reczek D, Bretscher A. Hierarchy of merlin and ezrin N- and C-terminal domain interactions in homo- and heterotypic associations and their relationship to binding of scaffolding proteins EBP50 and E3KARP. J Biol Chem. 2001;276:7621–7629. doi: 10.1074/jbc.M006708200. [DOI] [PubMed] [Google Scholar]
- 32.Curto M, McClatchey AI. Nf2/Merlin: a coordinator of receptor signalling and intercellular contact. Br J Cancer. 2008;98:256–262. doi: 10.1038/sj.bjc.6604002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Curto M, McClatchey AI. Ezrin a metastatic detERMinant? Cancer Cell. 2004;5:113–114. doi: 10.1016/s1535-6108(04)00031-5. [DOI] [PubMed] [Google Scholar]
- 34.Hoelzinger DB, Mariani L, Weis J, et al. Gene expression profile of glioblastoma multiforme invasive phenotype points to new therapeutic targets. Neoplasia. 2005;7:7–16. doi: 10.1593/neo.04535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iwadate Y, Sakaida T, Hiwasa T, et al. Molecular classification and survival prediction in human gliomas based on proteome analysis. Cancer Res. 2004;64:2496–2501. doi: 10.1158/0008-5472.can-03-1254. [DOI] [PubMed] [Google Scholar]
- 36.Huang L, Ichimaru E, Pestonjamasp K, et al. Merlin differs from moesin in binding to F-actin and in its intra- and intermolecular interactions. Biochem Biophys Res Commun. 1998;248:548–553. doi: 10.1006/bbrc.1998.9009. [DOI] [PubMed] [Google Scholar]
- 37.Horiguchi A, Zheng R, Shen R, Nanus DM. Inactivation of the NF2 tumor suppressor protein merlin in DU145 prostate cancer cells. Prostate. 2008;68:975–984. doi: 10.1002/pros.20760. [DOI] [PubMed] [Google Scholar]
- 38.Rustgi AK, Xu L, Pinney D, et al. Neurofibromatosis 2 gene in human colorectal cancer. Cancer Genet Cytogenet. 1995;84:24–26. doi: 10.1016/0165-4608(95)00059-3. [DOI] [PubMed] [Google Scholar]
- 39.Ferrari S, Zanella L, Alberghini M, Palmerini E, Staals E, Bacchini P. Prognostic significance of immunohistochemical expression of ezrin in non-metastatic high-grade osteosarcoma. Pediatric Blood Cancer. 2008;50:752–756. doi: 10.1002/pbc.21360. [DOI] [PubMed] [Google Scholar]
- 40.Khanna C, Wan X, Bose S, et al. The membrane-cytoskeleton linker ezrin is necessary for osteosarcoma metastasis. Nat Med. 2004;10:182–186. doi: 10.1038/nm982. [DOI] [PubMed] [Google Scholar]
- 41.Shimizu T, Seto A, Maita N, et al. Structural basis for neurofibromatosis type 2. Crystal structure of the merlin FERM domain. J Bioll Chem. 2002;277:10332–10336. doi: 10.1074/jbc.M109979200. [DOI] [PubMed] [Google Scholar]
- 42.Sherman L, Xu HM, Geist RT, et al. Interdomain binding mediates tumor growth suppression by the NF2 gene product. Oncogene. 1997;15:2505–2509. doi: 10.1038/sj.onc.1201418. [DOI] [PubMed] [Google Scholar]
- 43.Wick W, Grimmel C, Wild-Bode C, Platten M, Arpin M, Weller M. Ezrin-dependent promotion of glioma cell clonogenicity, motility, and invasion mediated by BCL-2 and transforming growth factor-beta2. J Neuros sci. 2001;21:3360–3368. doi: 10.1523/JNEUROSCI.21-10-03360.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.