Abstract
We conducted a phase I study to determine the safety and recommended phase II dose of enzastaurin (oral inhibitor of the protein kinase C-beta [PKCβ] and the PI3K/AKT pathways) when given in combination with radiation therapy (RT) plus temozolomide to patients with newly diagnosed glioblastoma multiforme or gliosarcoma. Patients with Karnofsky performance status ≥60 and no enzyme-inducing anti-epileptic drugs received RT (60 Gy) over 6 weeks, concurrently with temozolomide (75 mg/m2 daily) followed by adjuvant temozolomide (200 mg/m2) for 5 days/28-d cycle. Enzastaurin was given once daily during RT and adjuvantly with temozolomide; the starting dose of 250 mg/d was escalated to 500 mg/d if ≤1/6 patients had dose-limiting toxicity (DLT) during RT and the first adjuvant cycle. Patients continued treatment for 12 adjuvant cycles unless disease progression or unacceptable toxicity occurred. Twelve patients enrolled. There was no DLT in the first 6 patients treated with 250 mg enzastaurin. At 500 mg, 2 of 6 patients experienced a DLT (1 Grade 4 and 1 Grade 3 thrombocytopenia). The patient with Grade 3 DLT recovered to Grade <1 within 28 days and adjuvant temozolomide and enzastaurin was reinitiated with dose reductions. The other patient recovered to Grade <1 toxicity after 28 days and did not restart treatment. Enzastaurin 250 mg/d given concomitantly with RT and temozolomide and adjuvantly with temozolomide was well tolerated and is the recommended phase II dose. The proceeding phase II trial has finished accrual and results will be reported in 2009.
Keywords: adjuvant therapy, enzastaurin, glioblastoma multiforme, radiation therapy, temozolomide
The standard of care for newly diagnosed glioblastoma multiforme (GBM) or gliosarcoma (GS) includes surgical resection followed by radiation therapy (RT) with concomitant temozolomide, an alkylating agent, followed by adjuvant temozolomide.1 Patients treated with this regimen have a median survival of 14.6 months, and only 1 of 4 patients is alive at 2 years. Currently, all patients receive the same treatment, despite the known molecular heterogeneity of this cancer. In fact, molecular studies have identified numerous alterations in the initiation and progression of brain cancer, and it is expected that this greater understanding of the molecular mechanisms of brain tumors will positively affect prognosis and treatment.2
These genetic alterations also serve as targets for molecularly targeted therapy, and further improvement in survival may be gained by using therapies based on these mechanisms of action.3 Enzastaurin is a serine/threonine kinase inhibitor that selectively targets protein kinase C-beta (PKCβ).4 The PKC family of enzymes is essential to tumor growth, proliferation, and apoptosis.5,6 PKCβ also lies in the signal cascade of vascular endothelial growth factor (VEGF), up-regulated in most GBMs with concomitant overexpression of VEGF receptor.7–9 Inhibition of this pathway by enzastaurin leads to suppression of tumor angiogenesis and growth.10,11 PKC activity is also thought to regulate AKT, an anti-apoptotic protein that is also involved in proliferation in GBM.12,13 Thus, inhibition of the AKT pathway by enzastaurin may lead to decreased cell growth and increased apoptosis.4
Preclinical studies demonstrate the antiproliferative and anti-angiogenic activity of enzastaurin in tumor models, including glioma models.11 In clinical studies, enzastaurin showed promising activity in multiple tumor types and was well tolerated. In the dose-finding study for enzastaurin, no maximum tolerated dose (MTD) was observed up to 700 mg. Based on safety and pharmacokinetic data, a dose of 500 mg/d is recommended. At this dose, the biologically active plasma concentration of 2 µmol/L is achieved at steady state (14 days). Enzastaurin is well tolerated at this dose with no clinically significant Grade 3 or 4 toxicities in healthy volunteers and patients.14–16 Recently, Tabatabai et al.17 evaluated the combination of enzastaurin and radiation in vitro and in vivo, based on inhibition of irradiation-induced VEGF and the anti-invasive properties of PKCβ inhibition. Enzastaurin decreased tumor volume, irradiation-induced tumor satellite formation, upregulation of VEGF expression, and enhanced microvessel density. Enzastaurin thus enhanced the efficacy of RT by preventing unwanted pro-invasive and angiogenic effects and also enhanced temozolomide-induced cell death in GBM cell lines.17 The results from a phase II study in patients with recurrent high-grade gliomas demonstrated that enzastaurin was well tolerated and suggested antitumor activity.18 On the basis of these combined promising data, we conducted a phase I/II study of enzastaurin in patients with newly diagnosed GBM or GS. The objective of the phase I portion of the study reported here was to establish the MTD of enzastaurin, administered concomitantly with RT and temozolomide, followed by adjuvant enzastaurin and temozolomide.
Materials and Methods
Patient Eligibility
Eligibility criteria included patients ≥18 years of age; estimated survival of >12 weeks; Karnofsky performance status ≥60; and a histologically confirmed, newly diagnosed GBM or GS with a biopsy or resection ≤5 weeks prior to treatment. Patients must have recovered from the effects of surgery and had adequate organ function. All patients practiced birth control during and for 3 months after treatment.
Exclusion criteria included prior cranial RT, chemotherapy, or carmustine wafers; any anticoagulant therapy (permitted, if required, after starting treatment with careful monitoring); history of any other cancer (except nonmelanoma skin cancer or carcinoma in situ of the cervix) unless in complete remission without therapy for a minimum of 3 years; any significant medical illnesses that could not be adequately controlled or would compromise the patient's ability to tolerate the treatment; and an electrocardiogram demonstrating clinically significant arrhythmia that was symptomatic or required treatment. Patients must have discontinued enzyme-inducing anti-epileptic drugs ≥2 weeks prior to treatment.
All patients or their designated surrogates signed a consent form approved by the participating institution's ethical review board. The study was conducted in accordance with the Declaration of Helsinki and good clinical practices.
Study Design and Treatment Plan
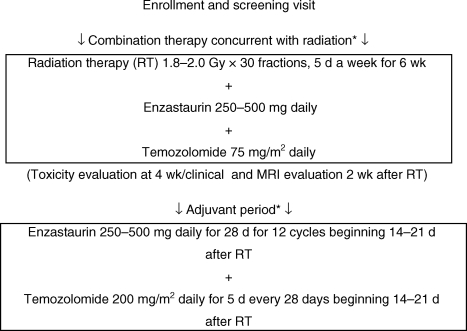
This was a single-institution, phase I, dose-escalation study of enzastaurin administered with temozolomide, during and following RT, in patients with newly diagnosed GBM or GS (Figure 1). Radiotherapy was administered in 1.8–2.0 Gy/d fractions for 5 d/wk (1.8–2.0 Gy × 30 fractions) for 6 weeks. A total of 45.0 Gy was delivered to the clinical tumor volume, consisting of T2-bright edema plus a 2-cm margin, or, if no edema, the contrast-enhancing lesion plus a 2.5-cm margin. An additional boost of 14.4 Gy was delivered to the gross tumor volume, consisting of the contrast-enhancing lesion plus a 1-cm margin. The dose to the optic chiasm and brainstem was limited to ≤54.0 Gy, the dose to one or preferably both retinas was limited to ≤50.0 Gy, and the dose to the cervical spine was limited to ≤45.0 Gy.
Fig. 1.
Study schema for the phase I clinical trial. *Patients also received ondansetron 4–8 mg or granisetron 1–2 mg for prevention of nausea associated with temozolomide.
During RT, patients took 75 mg/m2 oral temozolomide daily, with water, on an empty stomach, followed by a meal and either 250 or 500 mg enzastaurin within 30 minutes after the meal (at the same time each day). Enzastaurin and temozolomide were taken from the night before the first dose of RT to the night before the last dose of RT. At the conclusion of RT, patients took a 14–21 day break from enzastaurin and temozolomide. If ≤1 of the first 6 patients experienced a dose-limiting toxicity (DLT) and neuro-imaging showed no sign of tumor progression, patients completed one adjuvant cycle (28 days) of enzastaurin (250 mg daily) + temozolomide (200 mg/m2/d for 5 days). If there were no DLTs, patients continued subsequent adjuvant enzastaurin + temozolomide at the same doses. Adjuvant treatment was planned for 12 cycles (one cycle = 28 days). Patients were discontinued for tumor progression, unacceptable toxicity, and noncompliance. Treatment could continue beyond 12 cycles at the discretion of the investigator and sponsor.
Dose escalations were planned in 2 cohorts of 6 patients. The maximum tolerated dose (MTD) of enzastaurin was defined as the dose at which <33% patients experienced a DLT. Toxicities were graded according to the National Cancer Institute's Common Terminology Criteria for Adverse Events (CTCAE) version 3.0. DLTs were defined as any of the following events occurring during concurrent RT + enzastaurin + temozolomide or during the first adjuvant cycle that were attributable to enzastaurin or enzastaurin + RT and/or temozolomide: Grade ≥3 thrombocytopenia; Grade 4 anemia or neutropenia; nonhematologic grade ≥3 toxicity; or Grade 4 radiation-induced skin changes.
In the initial cohort, up to 6 patients were to receive 250 mg enzastaurin during concurrent RT + enzastaurin + temozolomide. If ≥2 of the 6 patients experienced DLTs during RT + enzastaurin + temozolomide or during the first adjuvant cycle, then the study would be deemed unsafe and stopped.
If ≤1 of the 6 patients in cohort 1 experienced a DLT during RT and the first adjuvant cycle, up to 6 more patients were entered in cohort 2 at an escalated dose of 500 mg enzastaurin. The timing of evaluations, dosing, and monitoring were the same as those for cohort 1.
Dose Modifications
Dose modifications were based on the expected toxicities for either temozolomide or enzastaurin. Enzastaurin was omitted for any of the following possibly related events: absolute neutrophil count (ANC) <0.5 × 109/L for >7 days, ANC <1.0 × 109/L with fever (temperature of 101°F/38.5°C), platelet count <25 × 109/L, or clinically relevant grade ≥3 nonhematologic toxicity. If the toxicity resolved to Grade ≤1 or the patient's baseline, the patient resumed therapy at 250 mg daily. If not, the patient was discontinued. For Grade ≥3 transaminase elevations that returned to baseline by day 1 of the next cycle, treatment resumed without delay or dose reduction. For patients on 500 mg enzastaurin, the dose was re-escalated to the 500 mg level if the toxicity did not recur after 28 days of therapy at 250 mg.
During RT, temozolomide was reduced to 50 mg/m2/d for Grade ≥3 hematologic toxicity. If a subsequent Grade ≥3 hematologic toxicity occurred after a dose reduction, temozolomide was discontinued for the remainder of RT. If a dose reduction was required during RT, the dose during the first adjuvant cycle was reduced to 150 mg/m2. The dose for subsequent adjuvant cycles was increased to 200 mg/m2 if no Grade ≥3 hematologic toxicity occurred during the first adjuvant cycle.
Temozolomide was delayed in the adjuvant cycles until the following criteria were met: ANC ≥1.5 × 109/L; platelet count ≥100.0 × 109/L; and related nonhematologic toxicities returned to Grade ≤1 (except for alopecia, nausea, and vomiting). If the hematologic criteria were met, temozolomide was reduced to the next lower dose level. If the ANC remained <1.5 × 109/L or the platelet count was <100.0 × 109/L at 4 weeks, despite two dose reductions (to 100 mg/m2), temozolomide was discontinued. Patients who experienced Grade ≥3 toxicity with a dose of 100 mg/m2 discontinued temozolomide, but continued enzastaurin.
If a Grade 5 event occurred during RT or the first adjuvant cycle, then accrual would be suspended.
Patient Evaluations
A complete history, physical, neurological examination, laboratory tests, and baseline MRI or CT scan were done within 14 days of starting treatment. The attending neuro-oncologist assessed pre- and postoperative imaging to determine the extent of resection. Steroid doses had to be stable for 5 days preceding the MRI.
A complete blood count with differential was performed every 2 weeks during RT and in weeks 3 and 4 of each adjuvant cycle of temozolomide. Chemistry was assessed every 4 weeks. Patients had clinical assessment 4 weeks into RT, 2–3 weeks after RT, and at the conclusion of each adjuvant cycle. Radiographic assessments were also performed 2 weeks after RT and every 8 weeks thereafter for the duration of treatment. All evaluable tumor sites were assessed using the same techniques as baseline. The Macdonald Criteria were used to evaluate radiological progression.19
Pharmacokinetics
Plasma samples were collected for pharmacokinetic evaluation of enzastaurin at steady state, when administered either alone (on day 22 of adjuvant cycle 1) or with temozolomide (day 5 of adjuvant cycle 2), at predose, and 2, 4, 6, and 24 hours postdose. Samples were assayed for enzastaurin and its metabolite, LY326020, using high-performance liquid chromatography with tandem mass spectrometry (LC/MS/MS) as previously described (Advion BioSciences, Inc.). Pharmacokinetic parameters were calculated using noncompartmental methods from the plasma concentration–time profiles of enzastaurin and its metabolite with WinNonlin Enterprise 5.0.1 (Pharsight).
Results
Patient Characteristics
From September 2006 through June 2007, 12 patients were enrolled. The characteristics of the 12 patients enrolled in the study are presented in Table 1.
Table 1.
Patient characteristics
| Variable | Enzastaurin 250 mg (n = 6) | Enzastaurin 500 mg (n = 6) | Total (n = 12) |
|---|---|---|---|
| Sex, n | |||
| Female | 1 | 3 | 4 |
| Male | 5 | 3 | 8 |
| Age, n | |||
| >18 and <65 y | 6 | 5 | 11 |
| >65 y | 0 | 1 | 1 |
| Median KPS | 90 | 90 | 90 |
| Neurological functional status, n | |||
| No symptoms, fully active | 2 | 1 | 3 |
| Minor symptoms, fully active | 4 | 5 | 9 |
| Extent of resection, n | |||
| GTR | 3 | 3 | 6 |
| STR | 3 | 3 | 6 |
| MGMT status, n | |||
| Methylated | 3 | 4 | 7 |
| Unmethylated | 3 | 2 | 5 |
Abbreviations: GTR, gross total resection; KPS, Karnofsky performance status; MGMT, O6-methylguanine-DNA methyltransferase; STR, subtotal resection.
Toxicity and MTD
There were no DLTs in the 6 patients treated with enzastaurin 250 mg. Two patients voluntarily discontinued treatment: 1 after 10 adjuvant cycles, who later progressed, and 1 after completing 24 adjuvant cycles. Table 2 lists the clinically relevant drug-related toxicities observed at the 250 mg enzastaurin dose. The most common toxicities were Grade 1 thrombocytopenia, nausea, and constipation. There were no serious adverse events. As of January 2009, the 4 remaining patients in cohort 1 continued on enzastaurin 250 mg/d at ≥22 cycles (Table 3).
Table 2.
Drug-related toxicity by dose levels (NCI CTCAE version 3.0)
| Toxicity | Enzastaurin 250 mg (n = 6) |
Enzastaurin 500 mg (n = 6) |
||
|---|---|---|---|---|
| Grade 1–2 | Grade 3–4 | Grade 1–2 | Grade 3–4 | |
| Thrombocytopenia | 5 | 0 | 1 | 2a,b |
| Lymphopenia | 2 | 3 | 3 | 3 |
| Nausea | 5 | 0 | 4 | 0 |
| Urine discoloration | 2 | 0 | 4 | 0 |
| Leukopenia | 3 | 1 | 3 | 1 |
| Neutropenia | 2 | 0 | 2 | 1a |
| Anorexia | 3 | 0 | 3 | 0 |
| Constipation | 5 | 0 | 4 | 0 |
| Fatigue | 1 | 0 | 5 | 1 |
aOne patient experienced a DLT of Grade 4 thrombocytopenia and grade 4 neutropenia.
bOne patient experienced a DLT of Grade 3 thrombocytopenia.
Table 3.
Summary of pharmacokinetic parameters
| PK parameter | Geometric mean (% CV) |
||
|---|---|---|---|
| Enzastaurin 250 mg |
Enzastaurin 500 mga | ||
| Day 22 of adjuvant cycle 1, enzastaurin alone (n = 6) | Day 5 of adjuvant cycle 2, enzastaurin with temozolomide (n = 6) | Adjuvant cycle 1 (n = 3) | |
| Enzastaurin | |||
| Cmax,ss, nmol/L | 438 (35.6) | 458 (40.7) | 1350 (149) |
| tmax,ssb, h | 3.00 (0.00–5.98) | 4.00 (2.00–4.42) | 4.00 (4.00–6.00) |
| Cav,ssc, nmol/L | 191 (33.7) | 219 (36.2) | 690 (274) |
| CL/F, L/h | 106 (33.7) | 92.1 (36.2) | 58.5 (274) |
| LY326020 | |||
| Cmax,ss, nmol/L | 334 (27.7) | 392 (27.2) | 198 (274) |
| tmax,ssb, h | 4.00 (0.00–4.00) | 4.00 (4.00–14.08) | 4.00 (4.00–6.00) |
| Cav,ssc, nmol/L | 270 (17.1) | 308 (21.6) | 176 (283) |
| MR | 1.42 (35.5) | 1.40 (40.0) | 0.256 (74.6) |
| Total analyte (enzastaurin + LY326020) | |||
| Cmax,ss, nmol/L | 754 (29.5) | 821 (28.5) | 1570 (159) |
| Cav,ssc, nmol/L | 481 (18.4) | 547 (19.6) | 924 (240) |
Abbreviations: MR, ratio of LY326020 AUC0–24,ss/enzastaurin AUC0–24,ss; Cav,ss, steady-state concentration; CL/F, apparent clearance; Cmax,ss, maximum plasma concentration at steady-state; CV, coefficient of variation; MR, metabolic ratio; PK, pharmacokinetic; tmax,ss, time to maximum plasma concentration.
aNo summary data for patients receiving 500 mg in adjuvant cycle 2.
bMedian (range).
cThe average steady-state concentration over one dosing interval (Cav,ss) is reported and is calculated from the AUC0–24,ss divided by the dosing interval (24 h).
In cohort 2 (500 mg enzastaurin), 2 of the 6 patients experienced a DLT during the last week of RT: one patient experienced Grade 4 thrombocytopenia and another had Grade 3 thrombocytopenia. The latter patient recovered to Grade ≤1 thrombocytopenia in less than 28 days and reinitiated adjuvant temozolomide and enzastaurin with dose reductions. The former patient recovered to Grade ≤1 after 28 days and consequently discontinued treatment. Excluding the DLTs, enzastaurin 500 mg resulted in similar clinically relevant drug-related toxicities as enzastaurin 250 (Table 2). Of the remaining 4 patients, 3 eventually discontinued due to progressive disease, whereas 1 patient continued on 500 mg/d enzastaurin (Table 3).
Enzastaurin 500 mg exceeded the MTD because 2 of the 6 patients experienced a DLT at this dose level; thus, enzastaurin 250 mg was the recommended phase II dose.
Pharmacokinetics
Plasma concentration–time data and dosing information (dose date and time) for pharmacokinetic evaluation were available from 9 patients (Table 3). The maximum plasma concentration for enzastaurin was reached in approximately 4 hours after dosing. The steady-state concentration for enzastaurin and LY326020 was similar between cycles 1 and 2 at the 250 mg dose, indicating that temozolomide does not cause any alteration in enzastaurin disposition. The apparent clearance of enzastaurin was 106 and 92.1 L/h in adjuvant cycles 1 and 2, respectively.
Discussion
In this clinical study, we assessed the safety of oral enzastaurin (either 250 or 500 mg) combined with standard RT and temozolomide and adjuvant temozolomide in patients with newly diagnosed GBM or GS. In general, the combined therapy was well tolerated. There were no significant toxicities in patients treated at 250 mg enzastaurin, and the combined therapy was well tolerated by these patients. Conversely, 2 patients treated at 500 mg enzastaurin experienced DLTs of thrombocytopenia, making enzastaurin 250 mg the recommended phase II dose. Although thrombocytopenia has been reported with temozolomide given concurrently with RT,20 the attribution to enzastaurin or the combination of temozolomide and enzastaurin is indistinct.
Since this was a phase I study, efficacy was not a primary endpoint. However, objective radiographic tumor responses were assessed. At each dose level of enzastaurin, 3 patients (6 in total) had objective reduction in evaluable disease. Such radiographic responses may indicate activity of the combination regimen. Radiographic response will be formally assessed in the phase II portion of the trial. Additionally, the phase II trial will evaluate whether magnetic resonance spectroscopic imaging and relative cerebral blood volume imaging change during treatment and if such changes are predictive of tumor progression.
Epigenetic silencing of the MGMT DNA-repair gene by promoter methylation has been found to be an important prognostic factor in patients with GBM.21–23 Prospective validation is required before MGMT methylation can be used for clinical decision-making regarding temozolomide treatment; however, the prognostic significance of MGMT methylation holds promise for tailored therapy according to the molecular profile of an individual's tumor. It is interesting to note that in our study, patients in the 250-mg cohort continued for 10 or more cycles regardless of the methylation of the MGMT promoter (data not shown). The MGMT status and several other potential molecular markers that could affect cellular response to enzastaurin, temozolomide, or RT will be analyzed in the phase II study (the 6 patients in cohort 1 will be included in the phase II analysis).
The pharmacokinetics of enzastaurin and its metabolite, LY326020, were evaluated either alone or in the presence of temozolomide, after RT was completed. As expected, temozolomide had no effect on enzastaurin pharmacokinetics. Exposures of enzastaurin and LY326020 in this study were lower than those observed in a previous phase I study at a similar dose.15 Formerly, it has been shown that there is a 3-fold increase in enzastaurin exposure in the fed state versus the fasted state.24 Although patients were instructed to take enzastaurin with food, the lower exposures could be explained by absorption characteristics in these patients that may be similar to those in the fasted state due to the physiological characteristics of these patients (such as difference in gastric pH, motility, stomach emptying time, or fraction absorbed in the different parts of the GI tract). The variability in enzastaurin exposures in this phase I portion of the study was much lower than seen previously (∼20% in this study when compared with ∼100% in previous single-agent studies).15 Since only 6 patients were evaluated in this portion of the study, however, it is possible that the variability may increase after more patients are enrolled.
In conclusion, the combination of enzastaurin with standard RT and temozolomide and adjuvant temozolomide in patients with newly diagnosed GBM or GS is feasible and well tolerated. The toxicity profile of this regimen is similar to that of temozolomide alone, although enzastaurin with temozolomide may lead to additional hematologic toxicity. The recommended phase II dose is 250 mg daily. The phase II portion of this study recently completed accrual at the University of California in San Francisco and we anticipate publication of results in late 2009. A pharmocogenomics study was performed in conjunction with this phase II study that investigated whether the activity of enzastaurin is enhanced in GBM patients with specific molecular signatures (elevated PKC or PI3K/AKT activity). Additional, ongoing, enzastaurin-based, clinical trials in patients with brain tumors will also provide more information on the antitumor activity of enzastaurin and its possible role in brain tumor therapy.
Funding
The study was sponsored by Eli Lilly and Company.
Acknowledgement
The authors acknowledge Asavari Wagle and Noelle Gasco of Eli Lilly and Company for editorial support.
Conflict of interest statement. None declared.
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. European Organisation for Research Treatment of Cancer Brain Tumor, Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Collins I, Workman P. New approaches to molecular cancer therapeutics. Nat Chem Biol. 2006;2:689–700. doi: 10.1038/nchembio840. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Hegi ME, Gilbert MR, Chakravarti A. Chemoradiotherapy in malignant glioma: standard of care and future directions. J Clin Oncol. 2007;25:4127–4136. doi: 10.1200/JCO.2007.11.8554. [DOI] [PubMed] [Google Scholar]
- 4.Graff JR, McNulty AM, Hanna KR, et al. The protein kinase C beta-selective inhibitor, Enzastaurin (LY317615.HCl), suppresses signaling through the AKT pathway, induces apoptosis, and suppresses growth of human colon cancer and glioblastoma xenografts. Cancer Res. 2005;65:7462–7469. doi: 10.1158/0008-5472.CAN-05-0071. [DOI] [PubMed] [Google Scholar]
- 5.Jarvis WD, Grant S. Protein kinase C targeting in antineoplastic treatment strategies. Invest New Drugs. 1999;17:227–240. doi: 10.1023/a:1006328303451. [DOI] [PubMed] [Google Scholar]
- 6.Donson AM, Banerjee A, Gamboni-Robertson F, Fleitz JM, Foreman NK. Protein kinase C zeta isoform is critical for proliferation in human glioblastoma cell lines. J Neurooncol. 2000;47:109–115. doi: 10.1023/a:1006406208376. [DOI] [PubMed] [Google Scholar]
- 7.Yoshiji H, Kuriyama S, Ways DK, et al. Protein kinase C lies on the signaling pathway for vascular endothelial growth factor-mediated tumor development and angiogenesis. Cancer Res. 1999;59:4413–4418. [PubMed] [Google Scholar]
- 8.Chan AS, Leung SY, Wong MP, et al. Expression of vascular endothelial growth factor and its receptors in the anaplastic progression of astrocytoma, oligodendroglioma, and ependymoma. Am J Surg Pathol. 1998;22:816–826. doi: 10.1097/00000478-199807000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Zhou YH, Tan F, Hess KR, Yung WK. The expression of PAX6, PTEN, vascular endothelial growth factor, and epidermal growth factor receptor in gliomas: relationship to tumor grade and survival. Clin Cancer Res. 2003;9:3369–3375. [PubMed] [Google Scholar]
- 10.Mcnulty AM, Konicek BW, Lynch RL, et al. Enzastaurin (LY317615.HCl) suppresses signaling through the PKC and AKT pathways, inducing apoptosis, suppressing tumor-induced angiogenesis and reducing growth of human cancer xenografts. Proc Amer Assoc Cancer Res. 2006;47:73. (Abstract 1332) [Google Scholar]
- 11.Keyes KA, Mann L, Sherman M, et al. LY317615 decreases plasma VEGF levels in human tumor xenograft-bearing mice. Cancer Chemother Pharmacol. 2004;53:133–140. doi: 10.1007/s00280-003-0713-x. [DOI] [PubMed] [Google Scholar]
- 12.Aeder SE, Martin PM, Soh JW, Hussaini IM. PKC-eta mediates glioblastoma cell proliferation through the AKT and mTOR signaling pathways. Oncogene. 2004;23:9062–9069. doi: 10.1038/sj.onc.1208093. [DOI] [PubMed] [Google Scholar]
- 13.Kawakami Y, Nishimoto H, Kitaura J, et al. Protein kinase C betaII regulates AKT phosphorylation on Ser-473 in a cell type- and stimulus-specific fashion. J Biol Chem. 2004;279:47720–47725. doi: 10.1074/jbc.M408797200. [DOI] [PubMed] [Google Scholar]
- 14.Carducci MA, Musib L, Kies MS, et al. Phase I dose escalation and pharmacokinetic study of enzastaurin, an oral protein kinase C beta inhibitor, in patients with advanced cancer. J Clin Oncol. 2006;24:4092–4099. doi: 10.1200/JCO.2005.05.3447. [DOI] [PubMed] [Google Scholar]
- 15.Rademaker-Lakhai JM, Beerepoot LV, Mehra N, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral protein kinase C beta-inhibitor enzastaurin in combination with gemcitabine and cisplatin in patients with advanced cancer. Clin Cancer Res. 2007;13(15 Pt 1):4474–4481. doi: 10.1158/1078-0432.CCR-06-2912. [DOI] [PubMed] [Google Scholar]
- 16.Welch PA, Sinha VP, Cleverly AL, Darstein C, Flanagan SD, Musib LC. Safety, tolerability, QTc evaluation, and pharmacokinetics of single and multiple doses of enzastaurin HCl (LY317615), a protein kinase C-beta inhibitor, in healthy subjects. J Clin Pharmacol. 2007;47:1138–1151. doi: 10.1177/0091270007304775. [DOI] [PubMed] [Google Scholar]
- 17.Tabatabai G, Frank B, Wick A, et al. Synergistic antiglioma activity of radiotherapy and enzastaurin. Ann Neurol. 2007;61:153–161. doi: 10.1002/ana.21057. [DOI] [PubMed] [Google Scholar]
- 18.Fine HA, Kim L, Royce C, et al. Results from phase II trial of Enzastaurin (LY317615) in patients with recurrent high grade gliomas [abstract] J Clin Oncol. 2005;23(Suppl. 16):1504. [Google Scholar]
- 19.Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 20.Gerber DE, Grossman SA, Zeltzman M, Parisi MA, Kleinberg L. The impact of thrombocytopenia from temozolomide and radiation in newly diagnosed adults with high-grade gliomas. Neuro-Oncol. 2007;9:47–52. doi: 10.1215/15228517-2006-024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 22.Palmisano WA, Divine KK, Saccomanno G, et al. Predicting lung cancer by detecting aberrant promoter methylation in sputum. Cancer Res. 2000;60:5954–5958. [PubMed] [Google Scholar]
- 23.Brandes AA, Tosoni A, Franceschi E, et al. Recurrence pattern after temozolomide concomitant with and adjuvant to radiotherapy in newly diagnosed patients with glioblastoma: correlation with MGMT promoter methylation status. J Clin Oncol. 2009;27:1275–1279. doi: 10.1200/JCO.2008.19.4969. [DOI] [PubMed] [Google Scholar]
- 24.Welch P, Musib L, Darstein C, Baldwin J, Ayan-Oshodi M. Effects of a proton pump inhibitor (lansoprazole) and food on the bioavailability of enzastaurin administered as single oral doses to healthy subjects [abstract] J Clin Oncol. 2007;25(Suppl. 18):14076. [Google Scholar]