Abstract
The chemokine CX3CL1 is constitutively expressed in the central nervous system by neurons and astrocytes controlling neuronal survival and neurotransmission. In this work, we analyzed the expression and function of the chemokine CX3CL1 and its receptor, CX3CR1, by human glioma cells. We show that both molecules are expressed on the tumor cell plasma membrane and that soluble CX3CL1 accumulates in the culture supernatants, indicating that the chemokine is constitutively released. We found that CX3CR1 is functional, as all the cell lines adhered to immobilized recombinant CX3CL1 and migrated in response to the soluble form of this chemokine. In addition, the blockade of endogenous CX3CL1 function by means of a neutralizing monoclonal antibody markedly delayed tumor cell aggregation and increased their invasiveness. We also show that CX3CL1 expression is potently modulated by the transforming growth factor-beta1 (TGF-beta1), a key regulator of glioma cell invasiveness. Indeed, both the treatment of glioma cells with recombinant TGF-beta1 and the inhibition of its endogenous expression by siRNA showed that TGF-beta1 decreases CX3CL1 mRNA and protein expression. Overall, our results indicate that endogenously expressed CX3CL1 negatively regulates glioma invasion likely by promoting tumor cell aggregation, and that TGF-beta1 inhibition of CX3CL1 expression might contribute to glioma cell invasive properties.
Keywords: CX3CL1, CX3CR1, glioma, invasion, TGF-beta1
Glioma is the most common primary brain tumor in humans, characterized by a high invasion rate that results in diffuse tumor infiltration throughout the central nervous system (CNS). Malignant glioma frequently overexpress transforming growth factor-beta1 (TGF-beta1) in its mature bioactive form, an important immunoregulatory cytokine that can sustain immune escape, angiogenesis, and favors acquisition of a tumor invasive phenotype.1,2 Glioma cell invasion can be beneficed by TGF-beta1 ability to promote integrin and metalloproteinase (MMP) expression as well as expression of signaling components of the machinery that regulate cell motility.3,4
Chemokines are a family of structurally related leukocyte chemoattractant cytokines that play a central role during inflammation. CX3CL1 (also called fractalkine or neurotactin) is a peculiar member of the chemokine family, both because of its protein structure and its cellular functions: this chemokine can be expressed as a transmembrane protein mediating cell–cell adhesion, and/or as a soluble mediator sustaining chemotaxis.5 In addition, it is becoming increasingly clear that CX3CL1 not only mediates the recruitment/activation of immune cells including monocytes, dendritic cells, and lymphocyte subsets, but can also exert multiple effects on nonimmune cells.6,7 Indeed, in the CNS, CX3CL1 is prevalently expressed by neurons and astrocytes,8–10 and displays a number of functions on microglial cells that contribute to the control of neuronal survival and neurotransmission.11–15
Members of the chemokine family including CCL2, CCL3L1, CXCL8, CXCL16, and CXCL12, and chemokine receptors can be expressed by glioma cells and regulate glioma cell survival, migration, and invasion.16–20
Increasing evidence indicates that CX3CL1 expression in the CNS can regulate tumor growth.21 Nevertheless, its direct effect on glioma cells and the regulation of its functions by glioma cell-produced TGF-beta1 has not been investigated so far.
In this work, we analyzed the expression of the chemokine CX3CL1 by human glioma cells, and the regulation of glioma cell functions mediated by the CX3CL1/CX3CR1 ligand/receptor pair. Our data show that the blockade of CX3CL1 activity provokes a strong increase in glioma cell invasion that correlates with delayed cell aggregation, suggesting that the adhesive properties of CX3CL1 counteract the invasive phenotype. Moreover, we observed that TGF-beta1 negatively controls CX3CL1 production, mostly interfering with CX3CL1 mRNA expression, thus facilitating glioma cell detachment and dispersion.
Materials and Methods
Cell Culture and Transfection
U87MG, T98G, and U251 glioma cell lines were from ATCC (ATTC, Rochville, Maryland) and maintained in complete medium (modified eagle's medium [EMEM], with 10% fetal bovine serum [FBS], 0.15 mM sodium bicarbonate, 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, 1% penicillin–streptomycin, and 2 mM l-glutamine). Glioma primary cell cultures (WHO grade IV) were established from tumor specimens of patients.22 All cell cultures were maintained at 37°C, in a humidified 5% CO2 atmosphere.
U87MG or T98G cells were seeded in a 24 well-plate (8 × 104 cells/well), and transfected with 0.5 µg of plasmid DNA (pSuper vector) encoding TGF-beta1 small interfering RNA or empty vector (kind gift of Michael Weller, University of Tubingen, Tubingen, Germany23) using LipofectAMINE 2000 (Invitrogen Life Tecnologies). Stable clones were selected in complete medium containing 1 µg/mL puromicin (Invitrogen Life Tecnologies) and used for further experiments as bulk culture.
Real-time PCR
The total RNA was extracted using Trizol (Invitrogen Life Tecnologies). One microgram of total RNA was used for cDNA first-strand synthesis in a 25 µL reaction volume.
Real-time PCR was performed using the ABI Prism 7900 Sequence Detection system (Applied Biosystems). cDNA was amplified in triplicate with primers for CX3CL1 (Hs00171086_m1) conjugated with fluorochrome FAM, and beta-actin (4326315E) conjugated with fluorochrome VIC (Applied Biosystems).
The average of the threshold cycles was used to interpolate standard curves and to calculate the transcript amount in samples using the Sequence Detector v1.7 analysis software (Applied Biosystems). mRNA amount of each sample, normalized with beta-actin, was expressed as arbitrary units and referred to untreated cells considered as calibrator.
Immunofluorescence and Flow Cytometry
Two hundred fifty thousand cells/well and 1.2 × 105 cells/well were seeded the day before analysis in 6 well-plates for CX3CR1 and CX3CL1 staining, respectively. Differences in plating conditions were chosen to optimize detection of the molecules on the cell surface; indeed, higher levels of CX3CL1 expression were observed when cells were cultured at low density, although higher cell density was required to detect optimal levels of cell surface CX3CR1. Cells were then detached using PBS containing EDTA 0.5mM, and 2 × 105 cells were stained with specific antibodies: an antihuman CX3CL1 monoclonal antibody (mAb; Clone MA 81506, R&D systems) or isotype control (IC, mouse IgG1, clone MOPC-21, Sigma-Aldrich) at 4°C for 45 minutes. Cells were washed, then stained with Phycoerythrin (PE)-conjugated goat antimouse IgG secondary antibody (Ab; Jackson Immumoresearch Laboratories), for 45 minutes at 4°C. For CX3CR1 staining, cells were incubated with goat serum for 10 minutes at RT to avoid nonspecific binding, and then with PE-conjugate antihuman CX3CR1 mAb (Clone 2A9-1, MBL International) or with equivalent amount of PE-conjugate mouse IgG2b (Pharmingen, Becton Dickinson) for 45 minutes at 4°C. After washing, cells were analyzed by flow cytometry using a FACscalibur (Beckton Dickinson).
Enzyme-linked Immunosorbent Assay
TGF-beta1 concentration in glioma cell line supernatants, generated by 18 hours incubation in serum-free medium and acidified, was evaluated with the commercial sandwich ELISA Kit (Duo Set ELISA Development, R&D systems). Procedures were performed according to manufacturer's instructions.
Soluble CX3CL1 in glioma cell line supernatants, generated with the 18 hours incubation in complete medium, was evaluated with the commercial sandwich ELISA Kit (Duo Set ELISA Development, R&D systems) according to manufacturer's instructions. Working concentrations of capture and detection antibodies were optimized to increase assay sensitivity. Colorimetric analysis was performed using an Elisa reader. Absorbance was obtained by subtracting readings at 540 nm from readings at 450 nm.
Adhesion Assay
Recombinant human CX3CL1 (chemokine domain, Peprotech EC) or bovine serum albumin (BSA; 5 µg/mL) was immobilized on high binding protein 96 well-plates by overnight incubation at 4°C. Optimal CX3CL1 concentration was established in preliminary dose–response experiments. Cells were detached using PBS/EDTA solution, and a single cell suspension was obtained after repeated pipetting. Cells were washed and resuspended in adhesion medium (EMEM, 0.5% BSA) at the concentration of 2.5 × 105 cells/mL, and cell suspension (100 µL) was added in BSA- or CX3CL1-coated wells, and incubated for the indicated time periods. After removal of nonadherent cells by PBS washing, cells were detached by addition of 100 µL of Trypsin/EDTA solution followed by neutralization in 100 µL of FBS-containing medium. Adherent cells were counted by FACS as previously described,24 and adhesion was expressed as the percentage of input cells. The specificity of CX3CL1 mediated adhesion was determined in blocking experiments by incubating the wells before the assay with anti-CX3CL1 or IC mAb (40 µg/mL).
Chemotaxis Assay
Cell migration was assayed using a 48-well microchamber (Neuroprobe, Cabin John, Maryland) as previously described.25 Briefly, cells were detached using PBS/EDTA solution, washed and resuspended in migration medium (MM: EMEM, 1% BSA, 10 mM Hepes) at the concentration of 2 × 105 cells/mL. Cell suspension (50 µL), was added to the top of each well, whereas increasing doses of CX3CL1 in MM were added to the bottom well in triplicate. Polycarbonate filters of 12 µm pore size (Neuro Probe, Inc.) were coated with fibronectin by 2 hours immersion in PBS containing 5 µg/mL fibronectin at 37°C. The duration of the assay was determined in preliminary experiments as the 3 cell lines display different basal migration, with U87MG cells being the most motile on fibronectin. U87MG, U251, and T98G cells were incubated (37°C, 5% CO2) for 3, 6, and 12 hours, respectively. Nonmigrating cells were removed from the upper side by scraping, and the membranes were fixed and stained with a Diff-Quik kit (Dade-Behring). The number of migrating cells was determined by counting 6 fields at 400× magnification. Mean values were calculated, and data are presented as fold variation relative to the migration of cells in the presence of the control vehicle (migration index) ± SD. Each experiment was conducted in triplicate and repeated at least 5 times.
Invasion Assay
Cell invasion was performed using Matrigel coated Transwell (8 µm pore size; BD Biosciences) Transwells were hydrated according to manufacturer's instructions. Cells (4 × 105 in T25 flask) were plated 24 hours before the assay, detached by trypsin, and resuspended in EMEM (1% FBS, 2 mM l-glutamine) at the concentration of 5 × 105 cells/mL. After incubation with the anti-CX3CL1 (40 µg/mL) neutralizing or IC mAb, cell suspension (100 µL) was added to the top of each well whereas complete medium was added to the bottom well. After 18 (U87MG and U251) or 36 hours (T98G) incubation at 37°C, 5% CO2, noninvading cells were removed using a cotton swab whereas invading cells were fixed with Diff-Quik solution. Data were expressed as mean values of 8 randomly selected fields per transwell, counted at 400× magnification. Mean values were calculated, and data were presented as fold variation relative to control (cells incubated with IC mAb). Cells incubated in the presence of IC or in medium behaved similarly.
Slow Aggregation Assay
A slow aggregation assay was performed as follows: after detachment, cells were washed and incubated for 10 minutes in EMEM 0.5% BSA in the presence of an antihuman CX3CL1 (40 µg/mL) or IC (40 µg/mL) mAb. Ten thousand cells were transferred to a semisolid agar gel in a 96-well plate. Evaluation of the aggregation was done at the indicated time periods using an Olympus BX51 microscope. Images were acquired and analyzed using IAAS 2000 software at 40× magnification. No differences in cell aggregation occurred between IC or vehicle treated cells.
Results
Human Glioma Cells Express CX3CL1 and a Functional CX3CR1
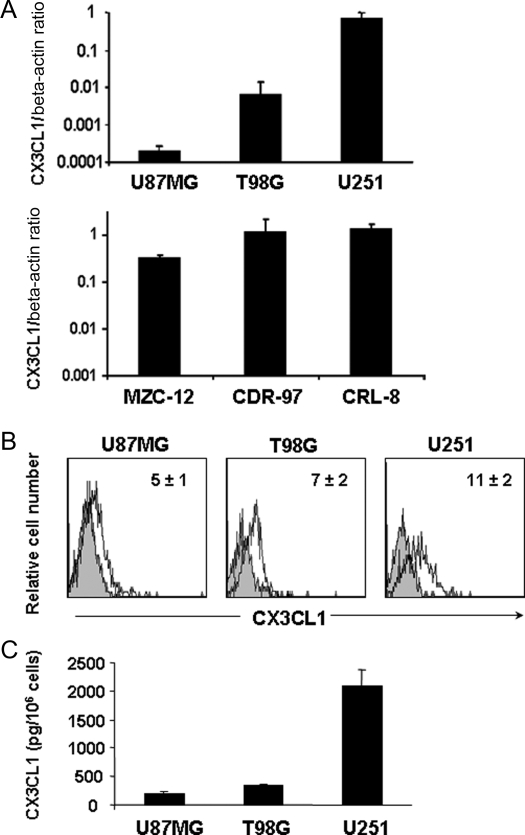
The abundant expression of CX3CL1 in the brain suggests that it can play an important role during glioma progression, and prompted us to analyze the expression of this chemokine by 3 different human glioma cell lines, T98G, U87MG, and U251, and primary glioma cell cultures. To this purpose, we initially analyzed CX3CL1 mRNA expression using real-time PCR analysis. Our results show that CX3CL1 transcript is detectable on all 3 cell lines, with the U251 cell line expressing much higher amounts of CX3CL1 mRNA than the T98G and U87MG cells (Fig. 1, upper panel A). Expression of the CX3CL1 transcript was revealed not only in the established cell lines but also in 3 primary cultures of glioblastoma cells (Fig. 1, lower panel A).
Fig. 1.
CX3CL1 is expressed by human glioma cells. (A) CX3CL1 mRNA was analyzed by RT–PCR on 3 glioma cell lines (upper panel) and 3 primary glioma cell cultures (lower panel). Data are expressed as CX3CL1/beta-actin mRNA ratio, and represent the mean ± SD of 3 independent experiments. (B) Cell surface expression of membrane-bound CX3CL1 on glioma cell lines was revealed by staining with a specific anti-CX3CL1 mAb. Shown are the histogram plot overlays of anti-CX3CL1 mAb staining (empty histogram) against IC mAb (grey histogram). Numbers in the histograms indicate the average ± SD of the geometric mean fluorescence intensity (MFI) of at least 3 independent experiments performed. MFI of control staining was always <3. (C) Soluble CX3CL1 in glioma cell line supernatants after 18 hours culture, analyzed by sandwich ELISA assay. Histograms represent the mean ± SD of at least 5 independent experiments performed.
We then analyzed expression of the membrane-bound form of the chemokine by immunofluorescence and FACS analysis using an anti-CX3CL1 mAb, and we found detectable levels of CX3CL1 on all 3 cell lines (Fig. 1B).
Because of its known role in the regulation of cell–cell interaction, we hypothesized that membrane-bound CX3CL1 on human glioma cell lines contributes to maintaining glioma cell interaction with CX3CR1-expressing cells and regulating glioma positioning into the CNS. On the other hand, CX3CL1 can also be released by glioma cells and thus produce a concentration gradient that may be capable of attracting immune cells. We therefore analyzed whether CX3CL1 was released by human glioma cells by evaluating its levels in the glioma cell supernatants by ELISA assay after an 18 hour culture. Figure 1C shows that detectable amounts of CX3CL1 are released under basal conditions and that, in accordance with mRNA expression, the U87MG and T98G cell lines released much lower amounts of the chemokine (200 ± 50 and 310 ± 42 pg/106 cells, respectively) when compared with U251 (2100 ± 300 pg/106 cells).
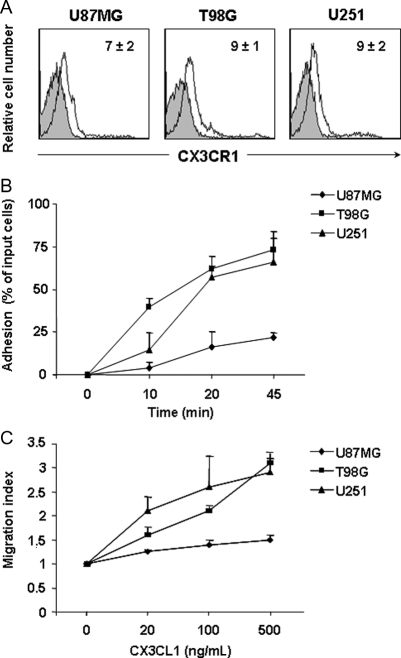
We also analyzed the surface expression of the CX3CL1 receptor, CX3CR1, on T98G, U87MG, and U251 cells by means of immunofluorescence staining followed by flow cytometric analysis, and found evidence of its expression on all 3 glioma cell lines (Fig. 2A). This finding prompted us to study the function of CX3CR1 on these cells. Chemokines can act in a paracrine or autocrine fashion to control tumor growth and invasiveness. We thus evaluated the ability of glioma cell lines to adhere to human recombinant CX3CL1 immobilized on plastic. As shown in Fig. 2B, T98G, U251, and U87MG glioma cells adhered to CX3CL1, and adhesion progressively increased during time. Adhesion to CX3CL1 was specific as shown by a marked reduction of cell adhesion following pretreatment with an anti-CX3CL1 blocking mAb (data not shown).
Fig. 2.
CX3CR1 is functionally expressed on glioma cell lines. (A) CX3CR1 cell surface expression on glioma cell lines was revealed by staining with a specific PE-labeled anti-CX3CR1 mAb. Shown are the histogram plot overlays of anti-CX3CR1 mAb staining (empty histogram) against IC mAb (grey histogram). Numbers in the histograms indicate the average ± SD of the MFI of at least 3 independent experiments performed. MFI of control staining was always <3. (B) Glioma cell adhesion was performed on CX3CL1 immobilized on plastic. Cells were allowed to adhere for 10, 20, and 45 minutes. Adherent cells were collected after repeated washings and quantified as positive events in 60 second acquisitions by flow cytometric analysis. Adhesion to BSA was subtracted and was always <5%. Data are expressed as percent of input cells and represent the mean ± SD of 3 independent experiments. (C) In vitro chemotaxis assay was performed using 48 well-chemotaxis chamber. Increasing doses of CX3CL1 were added in the lower well. Migration was quantified as the average number of 6 randomly selected fields at 400× microscopy magnification. Experiments were performed in triplicate. Results presented are the mean ± SD of 3 independent experiments.
We also analyzed in vitro glioma cell migration in response to increasing doses of CX3CL1. The results shown in Fig. 2C indicate that CX3CL1 causes a dose-dependent induction of directional migration of the 3 cell lines, with a maximum response at 500 ng/mL (migration index, MI = 1.4, 3, and 2.7 for U87MG, T98G, and U251, respectively). The lower level of U87MG cell chemotaxis and adhesion to CX3CL1 with respect to the other cell lines correlates with a higher basal motility of these cells that might reduce their ability to sense a CX3CL1 gradient and to bind to the immobilized chemokine.
Our data indicate that CX3CL1 promotes the adhesion and chemotaxis of CX3CR1-expressing glioma cells in vitro, suggesting that CX3CL1 expressed by other cells in the CNS contributes to the infiltrative behavior of glioma cells in vivo.
Endogenous CX3CL1 Negatively Regulates Glioma Cell Invasiveness
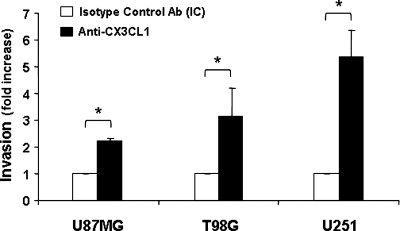
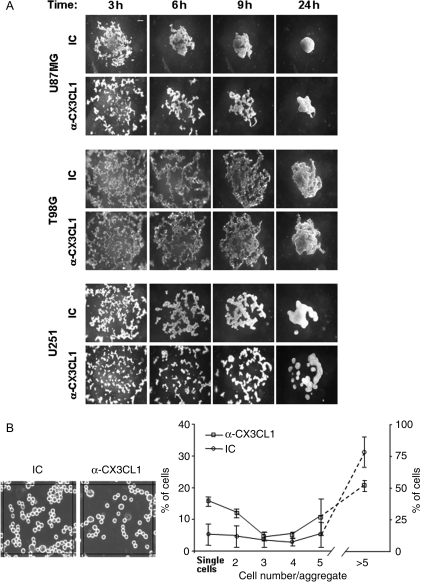
Next, we sought to determine the role of endogenously produced CX3CL1 on glioma cell function. It has been previously demonstrated that glioma-derived CXCL12 and CXCL1 can directly favor the tumor invasion process.26,27 Because both CX3CL1 and CX3CR1 are expressed on glioma cells, we hypothesized that they play an autonomous role on glioma functions. To study the contribution of the CX3CR1/CX3CL1 axis to the invasion process, we analyzed the ability of glioma cells to pass through an extracellular matrix barrier (Matrigel) and direct toward a growing gradient of FBS in the presence of CX3CL1 neutralizing mAb. We observed that all glioma cell lines tested were able to invade a matrigel barrier even though with different kinetics. This difference could be related to the intrinsic basal motility of glioma cells, being T98G slower than the other cell lines (data not shown). Surprisingly, we observed that the disruption of CX3CR1/CX3CL1 interaction by means of an anti-CX3CL1 neutralizing mAb enhanced glioma cell invasion (U87MG 2.1-fold, T98G 3-fold, U251 5-fold), indicating that CX3CL1 inhibits glioma cell invasion (Fig. 3). These results suggest that CX3CL1/CX3CR1 interaction has a pro-adhesive function on glioma cells. To investigate CX3CL1's role on glioma intercellular adhesion, we performed a slow aggregation assay in vitro at different time points. Irrespective of the cell line analyzed, intercellular contacts occurred during time and led to the formation of small aggregates that were progressively integrated in a single cellular mass by 24 hours (Fig. 4A). However, when the assay was performed in the presence of the anti-CX3CL1 neutralizing mAb, we observed a marked delay in U87MG and U251 cell aggregation as compared with IC Ab-treated cells (Fig. 4A). Irrespective of CX3CL1 blockade, all cells formed similar aggregates after 48 hours (data not shown), suggesting that CX3CL1 influences the speed of the aggregation process rather then the final aggregation. The observation that T98G cell aggregation is affected by CX3CL1 blockade only at the earlier time point (3 hours) suggests that this chemokine plays a major role in the initial phase of the T98G cell aggregation process. We thus performed the same assay at earlier times, enumerating free single cells or aggregates containing 2 or more T98G cells. As shown in Fig. 4B, at 1 hour we quantified a greater number of free single cells and a reduced number of aggregates (>5 cells) in the presence of anti-CX3CL1 neutralizing mAb as compared with IC mAb. Similar results were obtained at 2 and 3 hours.
Fig. 3.
CX3CL1 inhibits glioma cell invasion. In vitro invasion assay was performed in 24 matrigel-coated wells. Anti-CX3CL1 mAb (black columns) or IC mAb (white columns) was added together with the cells in the upper chamber. Cell invasion was evaluated at 18 hours for U87MG and U251, and at 36 hours for T98G glioma cells. Invasion was quantified as the average number of cells counted in 10 randomly selected fields at 400× magnification. Experiments were performed in duplicate. Results presented are the mean ± SD of 3 independent experiments and are expressed as fold increase in the invasion capacity of anti-CX3CL1 Ab-treated cells vs that of IC-treated cells. Student's t-test was performed by comparing invasion of IC-treated cells vs anti-CX3CL1 mAb-treated cells. *P < .05. Invasion in the absence or presence of IC mAb was similar.
Fig. 4.
CX3CL1 is involved in glioma cell aggregation. (A) Slow aggregation assay on agar substrate was performed in the presence of 40 µg/mL of anti-CX3CL1 or IC mAb in 96-well plates, at 37°C, 5% CO2 as described in material and methods. Images shown were collected at 40× magnification and indicate a representative experiment of 3 independent experiments performed. Scale bar: 100 µm (B) Aggregation of T98G cells after 1 hour incubation in presence of 40 µg/mL of anti-CX3CL1 or IC mAb. Shown is a representative field (25 000 pixel2) obtained using Image J software. Data were expressed as the percentage of the mean ± SD of the number of single cells or aggregates containing 2 or more cells relative to a total of 500 cells, counted in several central fields.
Collectively these data show that the CX3CL1/CX3CR1 pair interferes with glioma cell invasion, likely by promoting cell aggregation.
TGF-beta1 Regulates CX3CL1 Expression on Glioma Cells
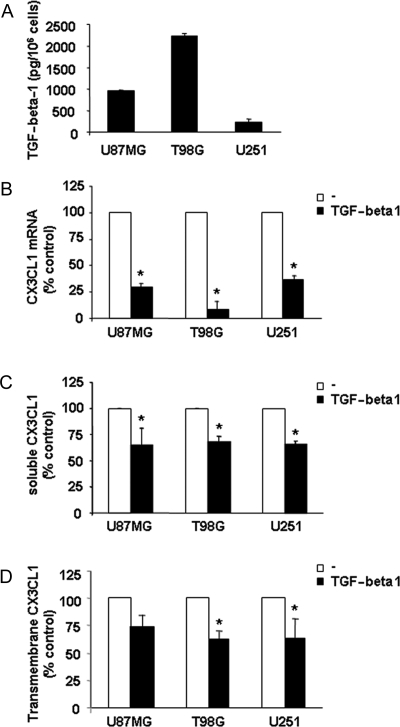
Both TGF-beta1 and its receptors are overexpressed in high grade glioma and influence many aspects of tumor progression including cell migration/invasion, proliferation, and angiogenesis.1 Moreover, TGF-beta1 can regulate chemokine production by bone marrow and cancer cells.28,29 Thus, we investigated whether expression and function of the CX3CL1/CX3CR1 pair in glioma cells could be affected by TGF-beta1. At first, we quantified TGF-beta1 secretion in our cell culture conditions analyzing T98G, U87MG, and U251 cell supernatants by ELISA; TGF-beta1 concentration resulted 2.2 ng/106 cells ± 0.4, 1 ng/106 cells ± 0.2, and 0.2 ng/106 cells ± 0.02, respectively (Fig. 5A).
Fig. 5.
TGF-beta1 down-modulates CX3CL1 expression in glioma cell lines. (A) TGF-beta1 levels were evaluated in the supernatants of glioma cells after 18 hours culture by ELISA assay. Histograms represent the mean ± SD of at least 5 independent experiments performed. (B) CX3CL1 mRNA was analyzed by RT–PCR on 3 glioma cell lines following 18 hours culture with or without (–) recombinant human TGF-beta1 (10 ng/mL) at 37°C, 5% CO2. Data, expressed as the mean ± SD of arbitrary units and derived from 3 independent experiments, were normalized with beta-actin, and referred as percent of untreated cells considered as calibrator. (C) Soluble CX3CL1 (sCX3CL1) levels were evaluated in the supernatants of glioma cells following 18 hours of stimulation with TGF-beta1 (10 ng/mL) by ELISA assay. Histograms represent the percentage of the mean ± SD of CX3CL1 concentration relative to untreated control (–) from at least 4 independent experiments performed. (D) Expression of cell surface-associated CX3CL1 in glioma cell lines following TGFbeta-1 (10 ng/mL) stimulation for 18 hours at 37°C, 5% CO2 detected by immunofluorescence and FACS analysis using a specific anti-CX3CL1 mAb. Histograms represent the percentage of the mean ± SD of CX3CL1 MFI relative to untreated control (–) from at least 4 independent experiments performed. In B, C, and D, Student's t-test was performed by comparing TGF-beta1 treated cell values with untreated cell values. *P < .05.
The evidence that the higher CX3CL1-producing cell line U251 secreted a much lower amount of TGF-beta1 suggests that TGF-beta1 negatively regulates CX3CL1 expression in glioma cells. In accordance, we found that treatment of all 3 cell lines for 18 hours with recombinant human TGF-beta1 led to a strong reduction of CX3CL1 mRNA as compared with the untreated control (Fig. 5B). A similar decrease was observed by analyzing the expression of membrane-bound CX3CL1 and accumulation of the soluble chemokine in the supernatants following TGF-beta1 treatment (Fig. 5C and D).
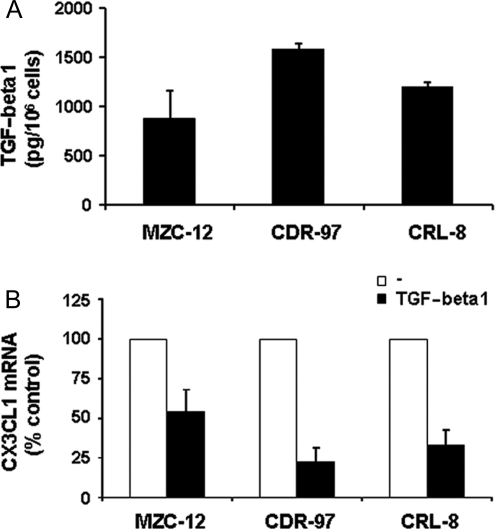
The results obtained with glioma cell lines were also confirmed using primary glioblastoma cell cultures that were found to produce TGF-beta1 and CX3CL1 and to reduce expression of CX3CL1 transcript upon treatment with TGF-beta1 (Fig. 6A and B).
Fig. 6.
TGF-beta1 down-modulates CX3CL1 expression in primary glioma cell cultures. (A) TGF-beta1 levels were evaluated in the supernatants of glioma cells after 18 hours culture by ELISA assay. Histograms represent the mean ± SD of at least 3 independent experiments performed. (B) CX3CL1 mRNA was analyzed by RT–PCR on 3 primary glioma cells following 18 hours culture with or without (–) recombinant human TGF-beta1 (10 ng/mL) at 37°C, 5% CO2. Data are expressed as in Fig. 5B.
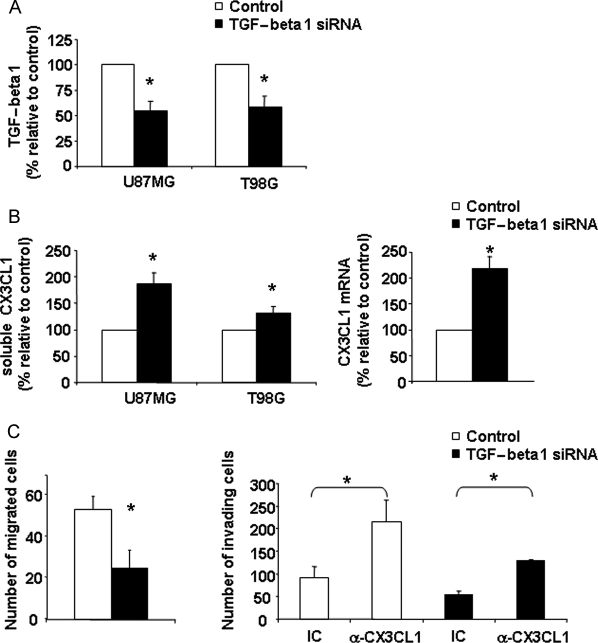
To better clarify the role of endogenous TGF-beta1 on the CX3CL1/CX3CR1 axis affecting glioma cells, we stably transfected T98G and U87MG cell lines with a vector expressing siRNA specific for TGF-beta1, or with an empty vector. ELISA performed on the cell culture supernatant showed that TGF-beta1 production was stably reduced by 50% in U87MG- and T98G-TGF-beta1 siRNA cell transfectants, respectively, as compared with empty vector-transfected cells (Fig. 7A).
Fig. 7.
Increased expression of CX3CL1 and reduced invasiveness in TGFbeta-1 siRNA glioma cells. The release of TGF-beta1 (A) and sCX3CL1 (B, left panel) in supernatants of pSUPER-puro-TGF-beta1 stably transfected U87MG, T98G cells (TGF-beta1 siRNA) or mock transfectants (control) was monitored by ELISA assay. CX3CL1 mRNA was analyzed by RT-PCR in control and TGF-beta1 siRNA T98G cells (B, right panel). The mean ± SD of the concentration (TGF-beta1 and CX3CL1 protein) or arbitrary units (CX3CL1 mRNA) derived from 3 independent experiments and normalized with beta-actin was calculated and results were expressed as percentage of untreated cells. (C, left panel) Control or TGF-beta1 siRNA cells were assayed in migration assay against a gradient of FBS, performed using the 48-well microchamber (C, right panel). Control or TGF-beta1 siRNA cells treated with anti-CX3CL1 or IC mAb were assayed in invasion assay across a matrigel barrier (right panel) as described in material and methods. Student's t-test was performed by comparing control cell values versus TGF-beta1 siRNA cell values except in C, right panel in which IC- vs anti-CX3CL1 Ab-treated cell invasion values were compared. *P < .05.
Soluble CX3CL1 accumulation was markedly increased in the cell culture supernatant of TGF-beta1 siRNA-transfected cells, and this increase was accompanied by a marked induction of CX3CL1 mRNA levels in TGF-beta1 siRNA T98G cells, as shown by RT–PCR analysis (Fig. 7B). These results indicate that the endogenous production of TGF-beta1 by glioma cells negatively regulates cx3cl1 gene expression, and that dampening TGF-beta1 protein levels is sufficient to increase CX3CL1 expression. No differences were found between untransfected and empty vector-transfected cells in terms of TGF-beta1, CX3CL1, or CX3CR1 cell surface expression and functional responses (data not shown).
As expected, standard migration assays revealed that TGF-beta1 siRNA-T98G cells displayed reduced basal motility on fibronectin with respect to vector-transfected cells (Fig. 7, left panel C). This effect correlated with a strong impairment in the invasion capability of TGF-beta1 siRNA tumor cells. Notably, we found that TGF-beta1 siRNA-T98G cell invasion was significantly rescued when cells were incubated with the CX3CL1 blocking mAb, suggesting that the increase of CX3CL1 that occurs because of TGF-beta1 reduction greatly contributes to the inhibition of glioma cell invasion (Fig. 7, right panel D).
Discussion
CX3CL1 is of particular interest in regard to brain tumors because of its abundant and constitutive expression by CNS cells and its role in the neuron-glial cell communication in normal and pathological conditions.8–10,15,30
Herein, our findings demonstrate that CX3CL1 is expressed on both glioma cell lines and primary cell cultures and disclose a previously uncharacterized role of the endogenously expressed chemokine in glioma cell adhesion/invasion. Moreover, our data indicate that TGF-beta1 inhibits CX3CL1 expression by glioma cells with important functional consequences. Indeed, the analysis of CX3CL1 expression following TGF-beta1 glioma cell treatment or on TGF-beta1 siRNA expressing cells demonstrates the existence of an inverse correlation between TGF-beta1 accumulation in glioma cell culture supernatants and CX3CL1 expression. We also showed that reduction of membrane and soluble CX3CL1 expression likely involves modulation of mRNA expression rather than shedding of endogenous CX3CL1. Notably, expression of CX3CR1 on glioma cell plasma membrane is instead increased by TGF-beta1, suggesting that TGF-beta1-dependent reduction of its ligand affects the availability of the receptor on the cell surface (Supplementary Material, Fig. S1). Previous reports showed that migration of immune or tumor cells and their consequent positioning into tissues can be influenced by TGF-beta1, and in some cases this is attributable to its ability to regulate chemokine and/or chemokine receptor expression.28,31 TGF-beta1 is released by glioma cells in large quantities in vitro and in vivo and has been considered central to the malignant progression of glial tumors and to immune dysfunction in patients with glioblastoma. This is because TGF-beta1 promotes tumor angiogenesis, enhances migration and invasion, and inhibits T cell-mediated immune responses. TGF-beta1 action on glioma cell invasion was previously shown to involve the regulation of alphavbeta3 integrin and MMP expression.3,4 To understand how CX3CL1 expression influences glioma cell invasion, we performed invasion assays in the presence of CX3CL1 neutralizing mAb. This set of experiments showed a significant increase of glioma cell invasion when CX3CL1 was neutralized, clearly showing an inhibitory role of CX3CL1 on tumor invasion. Correspondingly, the ability of TGF-beta1 to modulate CX3CL1 expression has important functional consequences on glioma cells as we observed decreased invasiveness of TGF-beta1 silenced T98G cells that was partially reversed by blocking CX3CL1. These observations reveal a peculiar role of CX3CL1 with respect to other chemokines, as previous reports emphasize the positive role of tumor-derived chemokines in cell invasion that was largely attributed to their ability to induce MMP expression and activation.26,27 Indeed, CXCL12 and its receptor, CXCR4, are overexpressed in invading glioma cells, and the blockade of the CXCR4/CXCL12 axis by means of a CXCR4 neutralizing Ab inhibits glioma cell invasion. In addition, glioma cells transduced with CXCL1 are more invasive and tumorigenic in vivo than control cells.
How can CX3CL1 inhibit cellular invasion? A number of pieces evidence indicate that homotypic adhesion can reduce the invasive potential of tumor cells, such as glioma cells.32,33 This suggests that the induction of cell–cell contact by CX3CL1 might prevent the detachment of individual tumor cells from the tumor aggregate that is required for the invasion process. Actually, our observations indicate that the blockade of CX3CL1/CX3CR1 interaction in slow aggregation assays leads to a delayed formation of cell aggregates, underlying an active role for this ligand/receptor pair in the establishment of tumor cell–cell contact. The CX3CL1-mediated inhibitory effect can be attributable to the peculiar structure of this chemokine that directly promotes cell–cell adhesion when expressed as trans-membrane protein. Nonetheless, we cannot exclude that also released CX3CL1 could regulate the aggregation process either in a positive way by enhancing the function of adhesion molecules such as integrins, or in a negative manner by interfering with the membrane-bound chemokine interaction with CX3CR1. In this regard, CX3CL1 cleavage has been involved in the downregulation of inter-cellular adhesive properties and can even result in the detachment of bound cells.7,34 Our data also indicate that glioma cells migrate and adhere in vitro in response to exogenous CX3CL1, suggesting that CX3CL1 distribution in the CNS could affect invasion of tumor cells into neighboring tissues in vivo. In support to this assumption, tissue expression of CX3CL1 has been previously reported to drive localization of other tumor cells in several tissues, including brain.35–37
TGF-beta interferes with antitumor immune responses by inhibiting the maturation of dendritic cells and the activation of T and natural killer (NK) cells.1,23 Previous reports indicate that CX3CL1 in the tumor microenvironment negatively affects progression of several tumors favoring antitumor immune responses.21,38,39 This is achieved also in brain tumors such as neuroblastoma where recruitment of CD8+ T lymphocytes and NK cells strongly depend upon CX3CL1. Our observation that soluble CX3CL1 expression is down-modulated by TGF-beta1 suggests that in addition to suppression of immune cell activation, TGF-beta1-mediated immune evasion is accomplished also through reduction of cell recruitment. In this regard, a recent paper by Liu et al.40 described that the immune response against glioma does not qualitatively change in CX3CR1-deficient mice, and that no differences in immune cell/microglial cell recruitment are present with respect to wild type mice. As the authors used high grade TGF-beta1-producing glioma cells, it is possible that in line with our results these cells display a reduced CX3CL1 expression, and thus the impact of this chemokine on the immune response could be minimal.
In conclusion, our data shed light on a novel mechanism of inhibition of tumor invasion by CX3CL1 and demonstrate that TGF-beta1 promotes tumor invasion also by counteracting CX3CL1 expression. Targeting the TGF-beta1/CX3CL1 balance may thus constitute a useful approach to control glioma progression.
Supplementary Material
Supplementary material is available at Neuro-Oncology Journal online.
Funding
This work was supported by grants of the Italian Association for Cancer Research (AIRC) and of Ministero dell'Università e della Ricerca (MUR: COFIN 40%; 60% FACOLTA'; DM N. 593, 2000).
Acknowledgements
We thank Antonella Calogero (“La Sapienza” University, Rome, Italy) for providing us with glioma primary cells and Francesca Gasparrini for technical suggestions.
Conflict of interest statement. None declared.
References
- 1.Platten M, Wick W, Weller M. Malignant glioma biology: role for TGF-beta in growth, motility, angiogenesis, and immune escape. Microsc Res Tech. 2001;52:401–410. doi: 10.1002/1097-0029(20010215)52:4<401::AID-JEMT1025>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 2.Constam DB, Philipp J, Malipiero UV, ten Dijke P, Schachner M, Fontana A. Differential expression of transforming growth factor-beta 1, -beta 2, and -beta 3 by glioblastoma cells, astrocytes, and microglia. J Immunol. 1992;148:1404–1410. [PubMed] [Google Scholar]
- 3.Platten M, Wick W, Wild-Bode C, Aulwurm S, Dichgans J, Weller M. Transforming growth factors beta(1) (TGF-beta(1)) and TGF-beta(2) promote glioma cell migration via Up-regulation of alpha(V)beta(3) integrin expression. Biochem Biophys Res Commun. 2000;268:607–611. doi: 10.1006/bbrc.2000.2176. [DOI] [PubMed] [Google Scholar]
- 4.Rooprai HK, Rucklidge GJ, Panou C, Pilkington GJ. The effects of exogenous growth factors on matrix metalloproteinase secretion by human brain tumour cells. Br J Cancer. 2000;82:52–55. doi: 10.1054/bjoc.1999.0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bazan JF, Bacon KB, Hardiman G, et al. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385:640–644. doi: 10.1038/385640a0. [DOI] [PubMed] [Google Scholar]
- 6.Nishimura M, Umehara H, Nakayama T, et al. Dual functions of fractalkine/CX3C ligand 1 in trafficking of perforin + /granzyme B+ cytotoxic effector lymphocytes that are defined by CX3CR1 expression. J Immunol. 2002;168:6173–6180. doi: 10.4049/jimmunol.168.12.6173. [DOI] [PubMed] [Google Scholar]
- 7.Ludwig A, Weber C. Transmembrane chemokines: versatile ‘special agents’ in vascular inflammation. Thromb Haemost. 2007;97:694–703. [PubMed] [Google Scholar]
- 8.Schwaeble WJ, Stover CM, Schall TJ, et al. Neuronal expression of fractalkine in the presence and absence of inflammation. FEBS Lett. 1998;439:203–207. doi: 10.1016/s0014-5793(98)01384-2. [DOI] [PubMed] [Google Scholar]
- 9.Hulshof S, van Haastert ES, Kuipers HF, et al. CX3CL1 and CX3CR1 expression in human brain tissue: noninflammatory control versus multiple sclerosis. J Neuropathol Exp Neurol. 2003;62:899–907. doi: 10.1093/jnen/62.9.899. [DOI] [PubMed] [Google Scholar]
- 10.Lindia JA, McGowan E, Jochnowitz N, Abbadie C. Induction of CX3CL1 expression in astrocytes and CX3CR1 in microglia in the spinal cord of a rat model of neuropathic pain. J Pain. 2005;6:434–438. doi: 10.1016/j.jpain.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 11.Bajetto A, Bonavia R, Barbero S, Schettini G. Characterization of chemokines and their receptors in the central nervous system: physiopathological implications. J Neurochem. 2002;82:1311–1329. doi: 10.1046/j.1471-4159.2002.01091.x. [DOI] [PubMed] [Google Scholar]
- 12.Ambrosini E, Aloisi F. Chemokines and glial cells: a complex network in the central nervous system. Neurochem Res. 2004;29:1017–1038. doi: 10.1023/b:nere.0000021246.96864.89. [DOI] [PubMed] [Google Scholar]
- 13.Savarin-Vuaillat C, Ransohoff RM. Chemokines and chemokine receptors in neurological disease: raise, retain, or reduce? Neurotherapeutics. 2007;4:590–601. doi: 10.1016/j.nurt.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cartier L, Hartley O, Dubois-Dauphin M, Krause KH. Chemokine receptors in the central nervous system: role in brain inflammation and neurodegenerative diseases. Brain Res Brain Res Rev. 2005;48:16–42. doi: 10.1016/j.brainresrev.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 15.Lauro C, Di Angelantonio S, Cipriani R, et al. Activity of adenosine receptors type 1 Is required for CX3CL1-mediated neuroprotection and neuromodulation in hippocampal neurons. J Immunol. 2008;180:7590–7596. doi: 10.4049/jimmunol.180.11.7590. [DOI] [PubMed] [Google Scholar]
- 16.Zhou Y, Larsen PH, Hao C, Yong VW. CXCR4 is a major chemokine receptor on glioma cells and mediates their survival. J Biol Chem. 2002;277:49481–49487. doi: 10.1074/jbc.M206222200. [DOI] [PubMed] [Google Scholar]
- 17.Kouno J, Nagai H, Nagahata T, et al. Up-regulation of CC chemokine, CCL3L1, and receptors, CCR3, CCR5 in human glioblastoma that promotes cell growth. J Neurooncol. 2004;70:301–307. doi: 10.1007/s11060-004-9165-3. [DOI] [PubMed] [Google Scholar]
- 18.Nitta T, Allegretta M, Okumura K, Sato K, Steinman L. Neoplastic and reactive human astrocytes express interleukin-8 gene. Neurosurg Rev. 1992;15:203–207. doi: 10.1007/BF00345934. [DOI] [PubMed] [Google Scholar]
- 19.Desbaillets I, Tada M, de Tribolet N, Diserens AC, Hamou MF, Van Meir EG. Human astrocytomas and glioblastomas express monocyte chemoattractant protein-1 (MCP-1) in vivo and in vitro. Int J Cancer. 1994;58:240–247. doi: 10.1002/ijc.2910580216. [DOI] [PubMed] [Google Scholar]
- 20.Ludwig A, Schulte A, Schnack C, et al. Enhanced expression and shedding of the transmembrane chemokine CXCL16 by reactive astrocytes and glioma cells. J Neurochem. 2005;93:1293–1303. doi: 10.1111/j.1471-4159.2005.03123.x. [DOI] [PubMed] [Google Scholar]
- 21.Zeng Y, Jiang J, Huebener N, et al. Fractalkine gene therapy for neuroblastoma is more effective in combination with targeted IL-2. Cancer Lett. 2005;228:187–193. doi: 10.1016/j.canlet.2005.01.057. [DOI] [PubMed] [Google Scholar]
- 22.Calogero A, Lombari V, De Gregorio G, et al. Inhibition of cell growth by EGR-1 in human primary cultures from malignant glioma. Cancer Cell Int. 2004;4:1. doi: 10.1186/1475-2867-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friese MA, Wischhusen J, Wick W, et al. RNA interference targeting transforming growth factor-beta enhances NKG2D-mediated antiglioma immune response, inhibits glioma cell migration and invasiveness, and abrogates tumorigenicity in vivo. Cancer Res. 2004;64:7596–7603. doi: 10.1158/0008-5472.CAN-04-1627. [DOI] [PubMed] [Google Scholar]
- 24.Bernardini G, Sciume G, Bosisio D, Morrone S, Sozzani S, Santoni A. CCL3 and CXCL12 regulate trafficking of mouse bone marrow NK cell subsets. Blood. 2008;111:3626–3634. doi: 10.1182/blood-2007-08-106203. [DOI] [PubMed] [Google Scholar]
- 25.Bernardini G, Spinetti G, Ribatti D, et al. I-309 binds to and activates endothelial cell functions and acts as an angiogenic molecule in vivo. Blood. 2000;96:4039–4045. [PubMed] [Google Scholar]
- 26.Ehtesham M, Winston JA, Kabos P, Thompson RC. CXCR4 expression mediates glioma cell invasiveness. Oncogene. 2006;25:2801–2806. doi: 10.1038/sj.onc.1209302. [DOI] [PubMed] [Google Scholar]
- 27.Zhou Y, Zhang J, Liu Q, et al. The chemokine GRO-alpha (CXCL1) confers increased tumorigenicity to glioma cells. Carcinogenesis. 2005;26:2058–2068. doi: 10.1093/carcin/bgi182. [DOI] [PubMed] [Google Scholar]
- 28.Jung Y, Wang J, Schneider A, et al. Regulation of SDF-1 (CXCL12) production by osteoblasts; a possible mechanism for stem cell homing. Bone. 2006;38:497–508. doi: 10.1016/j.bone.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 29.Bierie B, Chung CH, Parker JS, et al. Abrogation of TGF-beta signaling enhances chemokine production and correlates with prognosis in human breast cancer. J Clin Invest. 2009;119:1571–1582. doi: 10.1172/JCI37480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harrison JK, Jiang Y, Chen S, et al. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci USA. 1998;95:10896–10901. doi: 10.1073/pnas.95.18.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Franitza S, Kollet O, Brill A, et al. TGF-beta1 enhances SDF-1alpha-induced chemotaxis and homing of naive T cells by up-regulating CXCR4 expression and downstream cytoskeletal effector molecules. Eur J Immunol. 2002;32:193–202. doi: 10.1002/1521-4141(200201)32:1<193::AID-IMMU193>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 32.Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–374. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 33.Wheelock MJ, Shintani Y, Maeda M, Fukumoto Y, Johnson KR. Cadherin switching. J Cell Sci. 2008;121:727–735. doi: 10.1242/jcs.000455. [DOI] [PubMed] [Google Scholar]
- 34.Barreiro O, de la Fuente H, Mittelbrunn M, Sanchez-Madrid F. Functional insights on the polarized redistribution of leukocyte integrins and their ligands during leukocyte migration and immune interactions. Immunol Rev. 2007;218:147–164. doi: 10.1111/j.1600-065X.2007.00529.x. [DOI] [PubMed] [Google Scholar]
- 35.Shulby SA, Dolloff NG, Stearns ME, Meucci O, Fatatis A. CX3CR1-fractalkine expression regulates cellular mechanisms involved in adhesion, migration, and survival of human prostate cancer cells. Cancer Res. 2004;64:4693–4698. doi: 10.1158/0008-5472.CAN-03-3437. [DOI] [PubMed] [Google Scholar]
- 36.Andre F, Cabioglu N, Assi H, et al. Expression of chemokine receptors predicts the site of metastatic relapse in patients with axillary node positive primary breast cancer. Ann Oncol. 2006;17:945–951. doi: 10.1093/annonc/mdl053. [DOI] [PubMed] [Google Scholar]
- 37.Marchesi F, Piemonti L, Fedele G, et al. The chemokine receptor CX3CR1 is involved in the neural tropism and malignant behavior of pancreatic ductal adenocarcinoma. Cancer Res. 2008;68:9060–9069. doi: 10.1158/0008-5472.CAN-08-1810. [DOI] [PubMed] [Google Scholar]
- 38.Lavergne E, Combadiere B, Bonduelle O, et al. Fractalkine mediates natural killer-dependent antitumor responses in vivo. Cancer Res. 2003;63:7468–7474. [PubMed] [Google Scholar]
- 39.Ohta M, Tanaka F, Yamaguchi H, Sadanaga N, Inoue H, Mori M. The high expression of Fractalkine results in a better prognosis for colorectal cancer patients. Int J Oncol. 2005;26:41–47. [PubMed] [Google Scholar]
- 40.Liu C, Luo D, Streit WJ, Harrison JK. CX3CL1 and CX3CR1 in the GL261 murine model of glioma: CX3CR1 deficiency does not impact tumor growth or infiltration of microglia and lymphocytes. J Neuroimmunol. 2008;198:98–105. doi: 10.1016/j.jneuroim.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]