Abstract
Adjuvant whole-brain radiation therapy (WBRT) after resection of single brain metastases remains controversial. Despite a phase III trial to the contrary, clinicians often withhold WBRT after resection of single brain metastases based on the argument that available evidence does not inform regarding treatment of all patients, such as those with radioresistant tumors. However, there is limited information about whether subpopulations benefit equally from WBRT after resection. Therefore, we undertook a retrospective study to determine the clinical, radiographic, and histologic features that influenced the effectiveness of adjuvant WBRT. We reviewed 358 patients with newly diagnosed, single brain metastases, who underwent resection, of which 142 (40%) received adjuvant WBRT and 216 (60%) did not. Median follow-up was 60.1 months. There were multiple tumor histologies, including 197 (55%) "radiosensitive" and 161 (45%) "radioresistant" tumors. Compared with observation, WBRT significantly reduced recurrence both locally (HR = 0.58; 95% CI 0.35–0.98, P = .04) and at distant brain sites (HR = 0.43, 95% CI 0.30–0.61, P < .001). Multivariate analyses demonstrated that withholding WBRT was an independent predictor of local and distant recurrence. For local recurrence, tumors with a maximum diameter of ≥3 cm that did not receive adjuvant WBRT had an increased risk of recurring locally (HR = 3.14, 95% CI 1.02–9.69, P = .05). For distant recurrence, patients whose primary disease was progressing and who did not receive WBRT had an increased risk of distant recurrence (HR = 2.16, 95% CI 1.01–4.66, P = .05). There was no effect of WBRT based on tumor type. Adjuvant WBRT significantly reduces local and distant recurrences in subsets of patients, particularly those with metastases >3 cm or with active systemic disease.
Keywords: brain metastases, surgical resection, whole-brain radiation therapy
The routine use of adjuvant whole-brain radiation therapy (WBRT) after resection of single brain metastases remains an area of intense investigation in neuro-oncology. In a landmark phase III trial of patients with single brain metastases, Patchell et al. demonstrated that surgical resection followed by WBRT resulted in a statistically significant reduction in recurrence at the surgical site (local recurrence) and at other sites in the brain (distant recurrence) compared with surgery alone.1 Despite this class I evidence favoring adjuvant WBRT, many oncologists still withhold WBRT after resection of single brain metastases based at least in part on the premise that the results of Patchell et al. are not necessarily applicable to all patients because the full clinical spectrum of patients was not represented in the trial. For example, most patients enrolled in the trial of Patchell et al. had lung and breast cancers, which are often considered to be relatively sensitive to radiotherapy, whereas melanomas, which are more resistant to radiotherapy, were under represented.1,2 Thus, many oncologists argue that given the risk of radiation-induced dementia3,4 a more selective application of WBRT is preferable to routine administration of WBRT. However, there is limited information guiding clinicians on the extent to which particular populations of patients, such as those with relatively radioresistant tumors, may or may not benefit from WBRT after resection of single metastases.
To begin to define subsets of patients who are more likely than other patients to benefit from adjuvant WBRT after surgical resection of single brain metastases, we analyzed the outcomes of 358 patients with single brain metastases originating from a wide range of primary sites, all of whom were treated with microsurgical resection without or with standard dose adjuvant WBRT (30 Gy). The primary goal of this study was to evaluate the influence of various clinical, radiographic, and histologic features on the effectiveness of adjuvant WBRT in delaying tumor progression and prolonging survival in patients with a single brain metastasis.
Methods
Patient Selection
The database of the Department of Neurosurgery, The University of Texas M. D. Anderson Cancer Center was searched for patients who underwent surgical resection of newly diagnosed, single brain metastases from June 1993 to April 2003. Patients were excluded if they were ≤16 years old, had a KPS score <70, had >1 brain metastasis or leptomeningeal disease, or had undergone prior cranial irradiation. The institutional review board approved the study.
Data Review
The following data were reviewed: age, sex, KPS score, RTOG-RPA class,5 interval between diagnosis of the primary and brain metastasis, primary tumor type, systemic disease activity (no evidence of cancer outside the brain, stable, or progressing), tumor functional grade,6 pre- and postoperative tumor volume,7 administration of adjuvant WBRT, location and treatment of brain tumor recurrences, vital status, and duration of follow-up. At the M. D. Anderson Cancer Center Brain Tumor Center, patients are tracked prospectively with neuro-imaging every 4–12 weeks. At each visit, providers record treatments, changes in clinical status, and tumor recurrences. Most data were obtained in real time and entered into the database prospectively. When prospective data were unavailable, primary sources were reviewed retrospectively.
Radiosensitivity Grouping
Lung, breast, testicular, gastrointestinal, gynecologic, head and neck, lymphoma, and prostate cancers were classified as radiosensitive. Melanomas, sarcomas, kidney, thyroid, and genitourinary cancers were classified as radioresistant. Exceptions were pancreatic adenocarcinoma (gastrointestinal); uterine malignant mullerian or trophoblastic carcinoma primaries (gynecologic), which were classified as radioresistant; and rhabdomyosarcoma, Ewing's sarcoma, and a choriocarcinoma of unknown origin, which were classified as radiosensitive.
Adjuvant WBRT
Whole-brain radiation therapy was defined as adjuvant when it was (i) given postoperatively before the development of local or distant brain tumor progression and (ii) designated in the record as being specifically planned as adjuvant. Whole-brain radiation therapy was administered as 30 Gy in 10–15 fractions.
Outcome Measures
The endpoints were tumor recurrence and survival. Recurrence was classified as local (regrowth at the site of surgery) or distant (new brain tumors away from the surgery site).
Statistical Analysis
Differences in the distribution of discrete characteristics between the WBRT and observation groups were tested using χ2 or Fisher exact tests. Continuous and ordinal variables were tested using Student's t-test. For tumor recurrence endpoint analyses, follow-up was censored at the time of WBRT given for the treatment of a recurrence site (eg, follow-up for local recurrence was censored at the time of WBRT that was administered for the treatment of a distant recurrence). Overall survival and recurrence-free survival (RFS) times were assessed by the Kaplan–Meier method. Because adjuvant WBRT was administered at varying times after surgery, the adequacy of using Kaplan–Meir plots in the presence of a time-dependent entity was evaluated using the method of Therneau and Grambsch.8 Accordingly, any patient crossing from the observation group to WBRT status had 2 observations, one for the postoperative time elapsed without WBRT and another for the time following administration of WBRT. In this study, time from surgery to WBRT was short compared with the time from surgery to tumor recurrence/death. Therefore, treating WBRT as a baseline variable in the Kaplan–Meier plots and extrapolating the medians from these plots was a reasonable approach. The proportions of local and distant recurrences and their 95% confidence intervals (CIs), adjusted for competing events, were obtained using the cumulative incidence method of Prentice et al.9 Patients who did not show recurrence were censored at the time of last brain imaging. The Cox proportional hazards method was used to estimate the rates of survival and rates of local or distant recurrences for the different groups and to compute rates adjusted for other covariates. Interactions between adjuvant WBRT and the various covariates, including tumor type and radiosensitivity, were assessed. In these analyses, adjuvant WBRT was analyzed as a time-dependent covariate. A two-sided P value of ≤0.05 was considered significant. SPSS 15.0, Stata 7.0, and NCSS 2007 were used for these analyses.
Results
Patient Population
We identified 358 eligible patients (Table 1) who underwent resections of single brain metastases, with 97% having no evidence of contrast enhancement on the postoperative MRI and 3% having minimally detectable enhancement that was judged not to be tumor. Thirty-six percent of patients had no evidence of systemic disease (NED) at the time of surgery. A wide range of tumor types were represented, including 105 patients (29%) with lung cancer, 75 (21%) with melanoma, 71 (20%) with renal cell carcinoma, 39 (11%) with breast cancer, 20 (6%) with gastrointestinal cancer, 12 (3%) with sarcomas, 12 (3%) with gynecologic cancer, 9 (2.5%) with testicular cancer, 6 (2%) with head and neck cancer, and 9 (2.5%) with other cancer types (Table 1).
Table 1.
Patient and tumor characteristics
| Characteristic | All patients (n = 358) | Adjuvant WBRT groupa (n = 142) | Observation group (n = 216) | P value* |
|---|---|---|---|---|
| Sex, n (%) | ||||
| Male | 208 (58) | 67 (32) | 141 (68) | 0.001 |
| Female | 150 (42) | 75 (50) | 75 (50) | |
| Age, median (range), y | 55 (17–91) | 54 (18–79) | 55 (17–91) | 0.50 |
| ≥55 | 177 (49) | 69 (39) | 108 (61) | 0.79 |
| <55 | 181 (51) | 73 (40) | 108 (60) | |
| RPA, n (%) | ||||
| I | 171 (48) | 76 (44) | 95 (56) | 0.08 |
| II | 187 (52) | 66 (35) | 121 (65) | |
| Evidence of systemic disease, n (%) | ||||
| Yes | 230 (64) | 69 (30) | 161 (70) | <0.001 |
| No | 128 (36) | 73 (57) | 55 (43) | |
| Primary cancer, n (%) | ||||
| Lung | 105 (29) | 65 (62) | 40 (38) | <0.001 |
| Breast | 39 (11) | 27 (69) | 12 (31) | <0.001 |
| Melanoma | 75 (21) | 23 (31) | 52 (69) | 0.07 |
| Kidney | 71 (20) | 1 (1) | 70 (99) | <0.001 |
| Gastrointestinal | 20 (6) | 9 (45) | 11 (55) | 0.62 |
| Otherb | 48 (13) | 17 (35) | 31 (65) | 0.42 |
| Relative tumor radiosensitivityc, n (%) | ||||
| Sensitive | 197 (55) | 117 (59) | 80 (41) | <0.001 |
| Resistant | 161 (45) | 25 (16) | 136 (84) | |
| Interval from diagnosis of primary to brain metastasis, median (range), months | 19 (0–399) | 19 (0–337) | 18 (0–399) | 0.17 |
| <6 | 77 (22) | 25 (32) | 52 (68) | 0.15 |
| ≥6 | 281 (78) | 117 (42) | 164 (58) | |
| Brain tumor location, n (%) | ||||
| Supratentorial | 292 (82) | 112 (38) | 180 (62) | 0.29 |
| Infratentorial | 66 (18) | 30 (45) | 36 (55) | |
| Tumor functional grade, n (%) | ||||
| I (noneloquent) | 128 (36) | 52 (41) | 76 (59) | 0.33 |
| II (near-eloquent) | 145 (40) | 62 (43) | 83 (57) | |
| III (eloquent) | 85 (24) | 28 (33) | 57 (67) | |
| Brain tumor maximal diameter, pre-op median (range), cm | ||||
| >3 | 87 (24) | 44 (51) | 43 (49) | 0.017 |
| ≤3 | 271 (76) | 98 (36) | 173 (64) | |
Abbreviation: WBRT, whole-brain radiation therapy; RPA, recursive partitioning analysis.
aMedian time from surgery to adjuvant WBRT in the adjuvant WBRT treatment group was 0.6 months (0.1–4.1 months).
bOne case each with lung, ovarian, and prostate cancer were grouped with sarcoma for a total of 12 cases of sarcoma. Other primary cases included gynecologic nonsarcoma cases (12); testicular cancer (9); lymphoma (2); carcinoma of prostate (3); ureter (1); thyroid (2); larynx (2); tonsils (2); soft palate (1); tongue (1); unknown primary (1).
cSee Table 2 for a grouping of sensitivities to radiotherapy.
*P values in bold denote statistical significance.
Of the 358 patients, 142 (40%) were in the WBRT group and 216 (60%) were in the observation group (Table 1). The median time to WBRT was 19 days, and 80% of patients (114 of 142) received WBRT within 1 month of surgical resection. In 24 cases (17%), WBRT was administered 1–2 months after resection; in 4 cases, it was given 2.5–4.1 months after surgery.
There were no significant differences between the WBRT group and the observation group with respect to age, RTOG-RPA class, interval between diagnosis of the primary tumor and brain metastasis, brain tumor location, or tumor functional grade (Table 1). However, there were significant differences between the groups in terms of sex, the status of the systemic disease, the type of primary cancer, the relative tumor radiosensitivity, and preoperative tumor size (Table 1). Interestingly, 57% of patients who had NED received adjuvant WBRT; yet, only 30% of patients with evidence of systemic disease received it. Additionally, patients with tumors with a maximal diameter of >3 cm were more likely to receive WBRT (51%) than were patients with tumors of ≤3 cm (36%). Whether patients received WBRT also depended on the type of primary cancer. For lung cancer and breast cancer, which are considered radiosensitive, most patients (64%) received adjuvant WBRT (Tables 1 and 2). In contrast, for melanoma and renal cell carcinoma, which are considered radioresistant, only 16% received WBRT. Likewise, only 25 (16%) of the 161 tumors classified as radioresistant, and only 1 (1%) patient with renal cell carcinoma received adjuvant WBRT. In contrast, 117 (59%) of the 197 patients classified as having radiosensitive tumors received WBRT (Table 2).
Table 2.
Tumors considered to be radioresistant or radiosensitive
| Tumor type | Total | Adjuvant WBRT group | Observation group |
|---|---|---|---|
| Radioresistant (n = 161) | |||
| Melanoma | 75 | 23 | 52 |
| Kidney | 71 | 1 | 70 |
| Gastrointestinala | 1 | 0 | 1 |
| Sarcoma/histiocytoma | 9 | 0 | 9 |
| Gynecologic (nonsarcoma)b | 2 | 1 | 1 |
| Other primaryc | 3 | 0 | 3 |
| Radiosensitive (n = 197) | |||
| Lung (nonsarcoma) | 105 | 65 | 40 |
| Breast | 39 | 27 | 12 |
| Gastrointestinal | 19 | 9 | 10 |
| Sarcomad | 3 | 1 | 2 |
| Gynecologic | 10 | 4 | 6 |
| Testicular | 9 | 2 | 7 |
| Head and neck | 6 | 4 | 2 |
| Unknown primarye | 1 | 1 | 0 |
| Other primaryf | 5 | 4 | 1 |
Abbreviation: WBRT, whole-brain radiation therapy (adjuvant).
aPancreatic adenocarcinoma.
bMullerian and trophoblastic carcinomas.
cThyroid (n = 2); ureter (n = 1).
dRhabdomyosarcoma, Ewing's sarcomas.
eChoriocarcinoma.
fProstate (n = 3); lymphoma (n = 2).
Survival
At the time of last follow-up, 289 (81%) of the 358 study patients had died. The median duration of follow-up for the 69 living patients was 53.3 months (range, 0.6–156.1 months); thus more than half of the surviving patients were monitored for nearly 5 years. The median survival time for the group was 12.5 months (95% CI, 10.9–14.0 months).
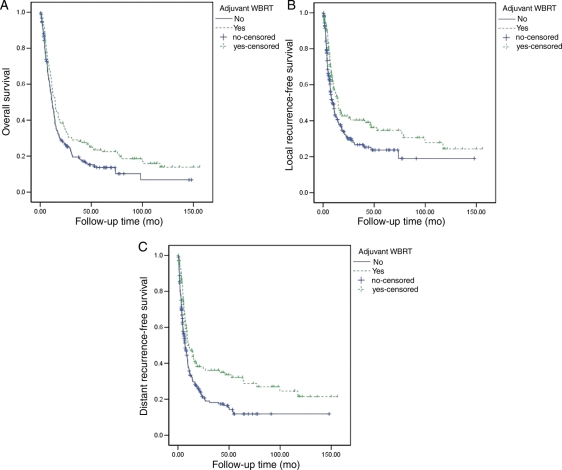
When the survival of all 358 patients was analyzed, there was a statistically significant increase in survival in the WBRT group compared with the observation group in the Cox univariate analysis (HR = 0.77; 95% CI, 0.61–0.98; P = .03). In the Kaplan–Meier analysis, the median survival was 14.7 months for the WBRT group (95% CI, 12.0–17.5 months) and 11.7 months for the observation group (95% CI, 9.8–13.6 months; P = .02) (Fig. 1A). This protective effect of adjuvant WBRT was observed within the well-represented tumor types (lung, breast, melanoma, and gastrointestinal) when each was analyzed separately, and within the radiosensitive and radioresistant tumor groups, when they were also analyzed separately, although in these analyses the effect of WBRT failed to reach statistical significance.
Fig. 1.
(A) Kaplan–Meier plots of overall survival comparing patients who received adjuvant whole-brain radiation therapy (WBRT) with those who did not. The median overall survival was 14.7 months for the adjuvant WBRT group (95% CI, 12.0–17.5 months) and 11.7 months for the observation group (95% CI, 9.8–13.6 months; P = .02). (B) Kaplan–Meier plots of local recurrence-free survival comparing patients who received adjuvant WBRT with those who did not. The median local RFS was 14.8 months in the WBRT group (95% CI, 10.4–19.1 months) compared with 9.2 months in the observation group (95% CI, 6.7–11.7 months; P = .006). (C) Kaplan–Meier plots of distant RFS comparing patients who received adjuvant WBRT with those who did not. The median distant RFS was 10.1 months for the adjuvant WBRT group (95% CI, 5.5–14.7 months) and 7.0 months for the observation group (95% CI, 5.3–8.7 months, P < .001).
Adjuvant WBRT was not a significant independent predictor of survival in the multivariate Cox proportional hazards model, which included the variables given in Table 1 (HR = 0.89; 95% CI, 0.68–1.18; P = .42). Instead, significant predictors of shorter survival were age ≥55 years, evidence of systemic disease (progressing or stable on therapy), radioresistant histology (primarily melanoma), infratentorial location, interval of <6 months from primary diagnosis to brain tumor diagnosis, and preoperative tumor size >3 cm in maximal diameter (Table 3).
Table 3.
Significant multivariate predictors of survival, all patients
| Characteristic | Hazard ratioa | 95% Confidence interval | P value |
|---|---|---|---|
| Age, y | |||
| ≥55 | 1.40 | 1.11–1.77 | 0.004 |
| <55b | 1.00 | — | — |
| Evidence of systemic disease | |||
| Yes | 1.52 | 1.18–1.96 | 0.001 |
| Nob | 1.00 | — | — |
| Relative tumor radiosensitivity | |||
| Resistant | 1.33 | 1.04–1.69 | 0.02 |
| Sensitiveb | 1.00 | — | — |
| Interval from primary diagnosis to brain tumor diagnosis, months | |||
| <6 | 1.37 | 1.03–1.85 | 0.033 |
| ≥6b | 1.00 | — | — |
| Brain tumor location | |||
| Infratentorial | 1.49 | 1.10–2.00 | 0.009 |
| Supratentorialb | 1.00 | — | — |
| Brain tumor maximal diameter, pre-op, cm | |||
| >3 | 1.37 | 1.04–1.80 | 0.026 |
| ≤3b | 1.00 | — | — |
aHazard ratio refers to the risk of death from any cause per unit time.
bReferent (ie, group others are compared with).
Because patients with renal cell carcinomas were unevenly distributed between the WBRT and observation groups, a similar multivariate analysis was undertaken excluding these patients. In this analysis of 287 patients, adjuvant WBRT was still not an independent predictor of survival (data not shown).
Tumor Recurrence
Local Recurrence
Among the 358 patients, there were 67 (19%) local recurrences. The proportion of local recurrences adjusting for competing events9 was 24% (95% CI, 20%–30%). Based on a Kaplan–Meier analysis, the median local RFS of the whole group was 11.1 months (95% CI, 8.3–13.9 months).
We first compared the crude incidence of local recurrence of the observation group with that of the WBRT group. Local recurrence occurred in 21 (15%) of the 142 patients in the WBRT group and in 46 (21%) of the 216 patients in the observation group. When we adjusted for competing events using the cumulative incidence method,9 the proportions of local recurrences were 20% (95% CI, 13%–29%) and 27% (95% CI, 21%–36%) in the WBRT and observation groups, respectively. Based on univariate Kaplan–Meier analyses, the median local-RFS was 14.8 months in the WBRT group (95% CI, 10.4–19.1 months) compared with 9.2 months in the observation group (95% CI, 6.7–11.7 months; P = .006; Fig. 1B). The hazard ratio for local recurrence in the Cox proportional hazards univariate analysis was 0.58 (95% CI, 0.35–0.98; P = .04), indicating a significant effect of adjuvant WBRT.
Multivariate Cox analyses were performed to identify independent predictors of local recurrence in the 358 patients using the variables given in Table 1. Age and WBRT were the only significant predictors of an increased risk of local recurrence (Table 4). Patients aged ≥55 years had a >2-fold increased risk for local recurrence compared with patients aged <55. More importantly, withholding WBRT was a statistically significant independent predictor of local recurrence. However, the effect was seen only in patients with large tumors. Specifically, patients with tumors with a diameter of >3 cm maximal who did not receive adjuvant WBRT had a 3-fold increased risk of local recurrence compared with all other patients (Table 4). In contrast, there was no interaction between WBRT and tumor type or tumor radiosensitivity. Thus, the reduction in local recurrence by adjuvant WBRT was not statistically significantly different among patients with different primaries, or when radioresistant tumors as a group were compared with the radiosensitive ones.
Table 4.
Significant multivariate predictors of local and distant recurrences, all patients
| Characteristic | Hazard ratiob | 95% Confidence interval | P value |
|---|---|---|---|
| Local recurrencea | |||
| Age, y | |||
| ≥55 | 2.18 | 1.32–3.57 | 0.002 |
| <55c | 1.00 | — | — |
| Adjuvant WBRT and brain tumor maximal diameter, pre-op | |||
| No adjuvant WBRT and brain tumor maximal diameter >3 cm | 3.14 | 1.02–9.69 | 0.05 |
| Otherc | 1.00 | — | — |
| Distant recurrenced | |||
| Relative tumor radiosensitivity | |||
| Resistant | 1.61 | 1.14–2.27 | 0.007 |
| Sensitivec | 1.00 | — | — |
| Adjuvant WBRT and systemic cancer status | |||
| No adjuvant WBRT and evidence of systemic disease | 2.16 | 1.01–4.66 | 0.05 |
| Otherc | 1.00 | — | — |
Abbreviation: WBRT, whole-brain radiation therapy.
aThere were 67 local control failures in the whole group: 21 in the WBRT group and 46 in the observation group.
bHazard ratio refers to the risk of local recurrence per unit time.
cReferent (ie, group others are compared with).
dThere were 156 distant control failures in the whole group: 42 in the WBRT group and 114 in the observation group.
Distant Recurrence
Among the 358 patients, there were 156 (44%) distant recurrences. The proportion of distant recurrences adjusting for competing events was 58% (95% CI, 52% − 65%). Kaplan–Meier analysis showed that the median distant-RFS for the whole group was 8.7 months (95% CI, 7.3–10.0 months).
When distant recurrence was compared in the WBRT and observation groups using crude incidence measures, 42 (30%) of the 142 patients in the adjuvant WBRT group and 114 (53%) of the 216 patients in the observation group recurred at distant brain sites. The proportions of distant recurrences adjusting for competing events were 39% (95% CI, 31%–51%) and 72% (95% CI, 64%–80%) in the WBRT and observation groups, respectively. The Kaplan–Meier analysis of distant-RFS showed that the median distant-RFS was 10.1 months for the adjuvant WBRT group (95% CI, 5.5–14.7 months) and 7.0 months for the observation group (95% CI, 5.3–8.7 months, P < .001; Fig. 1C). The hazard ratio for distant recurrence in the Cox univariate analysis was 0.43 (95% CI, 0.30–0.61; P < .001), indicating a significant WBRT effect.
In multivariate Cox analyses, tumor type and WBRT were independent predictors of distant recurrence (Table 4). Patients with tumors categorized as radioresistant (mainly melanoma) had a 1.6-fold increased risk of distant recurrence compared with those having radiosensitive tumors (mainly lung, breast, and gastrointestinal metastases). More importantly, withholding WBRT was associated with an increased risk of distant recurrence. However, this effect of WBRT was only seen in patients with evidence of systemic disease. Specifically, patients with evidence of systemic disease who did not receive WBRT had a >2-fold increased risk of distant recurrence (HR = 2.2; 95% CI, 1.01–4.7; P = .05) compared with all other patients (Table 4). Importantly, there was no apparent differential effect of WBRT on suppression of distant recurrence for tumors of different types or radiosensitivities. For both local and distant recurrence, similar results were obtained when patients with renal cell carcinoma were excluded from the multivariate Cox analyses.
Recurrence of Specific Tumor Types
To further decipher the role of adjuvant WBRT, we assessed the local and distant recurrences for lung cancer and melanoma separately. We chose these tumors because lung cancer is considered radiosensitive, whereas melanoma is considered radioresistant, and relatively large numbers of study patients had them.
For both tumor types, administration of WBRT reduced the risk of local recurrence. For lung cancer, WBRT resulted in a 20% reduction in the risk of local recurrence and for melanoma, WBRT resulted in a 68% reduction, although these effects were not statistically significant. The univariate HR for adjuvant WBRT for patients with lung cancer was 0.63 (95% CI, 0.27–1.43; P = .27) and for patients with melanoma, it was 0.27 (95% CI, 0.03–2.24; P = .23; Table 5). Further analyses supported the prior conclusion that the effects of WBRT on local recurrence in both cancer types were most significant with large tumors. Similarly, for both tumor types, WBRT significantly reduced the risk of distant recurrence. The univariate HR for adjuvant WBRT for patients with lung cancer was 0.40 (95% CI, 0.20–0.80; P = .009) and, for patients with melanoma, it was 0.42 (95% CI, 0.21–0.83; P = .01; Table 5). Further analyses also supported the prior conclusion that the effects of WBRT in both groups were significant in cases with evidence of systemic disease. Thus, regardless of whether the tumor was radiosensitive (lung) or radioresistant (melanoma), the effects of adjuvant WBRT were similar.
Table 5.
Impact of adjuvant whole-brain treatment (WBRT) on survival, local recurrence, and distant recurrence in patients with a lung cancer or melanoma primary
| Primary type | No. of patients |
Hazard ratioa | 95% Confidence interval | P value | ||
|---|---|---|---|---|---|---|
| Whole group | WBRT group | Observation group | ||||
| Deaths, total/n | ||||||
| Lung | 105/83 | 65/52 | 40/31 | 0.82 | 0.52–1.29 | 0.39 |
| Melanoma | 75/63 | 23/21 | 52/42 | 0.75 | 0.44–1.28 | 0.29 |
| Local recurrence, total/n | ||||||
| Lung | 105/23 | 65/13 | 40/10 | 0.63 | 0.27–1.43 | 0.27 |
| Melanoma | 74b/8 | 22/1 | 52/7 | 0.27 | 0.03–2.24 | 0.23 |
| Distant recurrence, total/n | ||||||
| Lung | 105/33 | 65/14 | 40/19 | 0.40 | 0.20–0.80 | 0.009 |
| Melanoma | 74b/47 | 22/11 | 52/36 | 0.42 | 0.21–0.83 | 0.01 |
aUnadjusted hazard ratio. The referent or comparison group in this analysis is the observation group (group not receiving adjuvant WBRT). A hazard ratio <1 indicates a protective effect of WBRT (significant or otherwise) against the outcome of interest (death and the local and distant recurrences).
bOne patient with melanoma had no clinical follow-up after receiving his adjuvant WBRT and was therefore dropped out of the recurrence analysis.
Discussion
In this analysis, we show that withholding WBRT after resection of single brain metastases significantly increased local recurrence in patients with large tumors (>3 cm in maximal diameter) and increased distant recurrence in patients with evidence of active systemic disease. Our results also indicate that the histology and presumed radiosensitivity of the metastases were not associated with WBRT effects. These results suggest that there are some groups of patients for whom adjuvant WBRT after resection of single brain metastases may be more beneficial than others.
There has been an increasing desire to develop a personalized approach to the management of patients with brain metastases, particularly regarding the administration of WBRT, the side effects of which are not inconsequential. Clearly, the randomized trial of Patchell et al.1 provided important evidence supporting the use of adjuvant WBRT after resection of single brain metastases as the recurrence rate of patients receiving WBRT in this trial was significantly reduced compared with those in whom WBRT was withheld. However, because this randomized study overwhelmingly enrolled patients with lung and breast cancer and was not designed to determine whether clinical or radiographic features, such as tumor size, influenced the conclusions, debate has continued regarding whether adjuvant WBRT is needed in all patients after resection of single brain metastases. In addition, Chang et al.4 recently showed that a more selective application of WBRT after stereotactic radiosurgery (SRS) may improve outcomes. In this context, our analysis of 358 patients with a wide range of clinical, radiographic, and histologic features provides evidence, albeit retrospective, that there are populations of patients who may benefit from WBRT compared with other populations. Specifically, as in the phase III trial of Patchell et al.,1 we show that withholding WBRT was a statistically significant independent predictor of local and distant recurrence. However, because of the large sample size and diversity of the study cohort, we were also able to show that there was a statistical interaction between WBRT and specific clinical variables. For local recurrence, there was an interaction between WBRT and tumor size, such that tumors with a maximal diameter of >3 cm that did not receive adjuvant WBRT had an increased risk of recurring locally compared with tumors with a diameter of <3 cm. For distant recurrence, there was an interaction between WBRT and the status of the systemic disease, such that patients with active systemic disease who did not receive WBRT had an increased risk of distant recurrence compared with patients without evidence of systemic disease. Therefore, our results extend the findings of Patchell et al.1 because they indicate that the beneficial effects of WBRT may depend on certain clinical variables that have heretofore not been considered important, namely the size of the resected tumor and the status of the systemic disease. Clearly, phase III trials will be needed to verify the impact of these variables on the effects of WBRT.
An interesting result of our study is that adjuvant WBRT significantly reduced recurrences regardless of the histology of the tumor or its presumed radiosensitivity. This finding is important because many clinicians base their decision to withhold or administer WBRT on the histologic type and the presumed responsiveness of that tumor to radiation. The validity of this pattern of practice has not been adequately studied heretofore and largely stems from anecdotal observations.10–15 Although the retrospective series of Smalley et al.,13 DeAngelis et al.,14 and Maiuri et al.15 included patients with brain metastases from 6 to 8 categories of primary tumors, these studies did not compare patients receiving or not receiving WBRT according to tumor type. In the randomized study of Patchell et al.,1 each arm of the study contained only 1 melanoma patient and an unspecified (but small) number of patients with renal cell cancer, and so the influence of tumor type on outcome could not be ascertained. In this context, we show, for the first time, that the effects of adjuvant WBRT do not seem to be influenced by tumor type or tumor radiosensitivity. Although patients with radioresistant tumors fared worse than patients with more sensitive tumors, adjuvant WBRT improved the outcomes in both of these groups. Separate analyses of lung cancer (a common radiosensitive tumor) and melanoma (a common radioresistant tumor) supported this result. Therefore, our data suggest that it may not be justified to withhold WBRT based on the presumed radioresistance of the primary tumor and that other factors, such as tumor size and status of the systemic disease, may be more important. Prospective clinical trials focusing on individual tumor types will be required to resolve this issue.
The finding that withholding WBRT in patients with tumors of ≥3 cm in maximal diameter increased the risk of local recurrence is a logical result in terms of tumor growth patterns and surgical methods. Larger tumors are probably more likely to have areas of invasion that are missed at surgery. In addition, larger tumors are more likely to be removed piecemeal rather than en bloc and thus to have their borders violated, thereby increasing the risk of spillage of tumor cells. Indeed, previous work from our group has shown that en bloc resections result in reduced rates of leptomeningeal disease compared with piecemeal resections, presumably due to the lack of violation of the tumor wall.16 It is also logical that withholding WBRT in patients who have evidence of active systemic disease significantly increases the risk of distant recurrence compared with patients who have no evidence of systemic disease. Indeed, patients with active systemic disease are probably more likely to have high numbers of circulating tumor cells that can metastasize to the brain, forming microscopic deposits that require WBRT for control of growth. The numbers of circulating tumor cells in patients who have NED is probably negligible, thereby reducing the chance for developing new brain metastases and lessening the impact of WBRT.
Although adjuvant WBRT was associated with lower rates of recurrence, the effect on survival was less evident in our study despite the large numbers of patients. Adjuvant WBRT resulted in a statistically significant improvement in overall survival in the univariate analysis; however, this effect was lost in the multivariate analysis. A positive effect of adjuvant WBRT on survival has notoriously been difficult to demonstrate.1,17,18 In the randomized study of Patchell et al., WBRT did not significantly alter survival.1 Likewise, in a phase III study of SRS with or without WBRT, Aoyoma et al.17 were also unable to demonstrate a significant survival advantage of adjuvant WBRT. However, these trials were not sufficiently powered for a survival endpoint. In a recent commentary, Patchell et al.18 pointed out that to show a survival benefit from withholding WBRT after surgery would require approximately 2250 patients. This need for such large cohorts results from the clinical complexity of patients with systemic cancer and brain metastases. In addition, many patients cross over from the observation arm to the WBRT arm after recurrence, negating the differences between the 2 groups in survival analyses. Nevertheless, this lack of effect on survival does not mitigate the effectiveness of WBRT on reducing recurrence.
The results of this study shed light on possible strategies for administering WBRT after resection of a single brain metastasis, or at least provide parameters for stratification in future prospective clinical trials. Specifically, our results suggest that patients with evidence of active systemic disease should probably receive WBRT, regardless of tumor size, because of the need to reduce distant recurrence in the brain, which is common in this group of patients. For patients with active systemic disease and tumors with a maximal diameter of >3 cm, adjuvant WBRT will also provide the added benefit of reducing the chance of developing local recurrence. For patients who have no evidence of systemic disease, clinicians might consider withholding WBRT if the maximal diameter of the resected tumor is <3 cm because the risk of local and distant recurrence in this group is low. For patients who have NED and have a tumor with a maximal diameter of >3 cm, our data would support administering adjuvant WBRT. Alternatively, because these patients (NED with a resected tumor with a diameter of >3 cm) are mainly at risk for local recurrence, stereotactic irradiation of the resection cavity, which is increasingly being reported as an effective strategy for controlling local recurrence,19,20 may provide the same reduction in local recurrence as WBRT while avoiding the adverse effects of WBRT on the normal brain.
Of course, any recommendations for treatment must be viewed with caution because the study presented here is a retrospective investigation and, therefore, suffers from all the limitations of this type of analysis. Although the data were prospectively collected and validated in real-time, and are therefore highly reliable, patients were not randomized to receive WBRT, nor were they stratified for specific variables. Partiality in the application of WBRT could have skewed the results. Although the statistical analyses were designed to address the differences between the WBRT and the observation groups, these could not control for all the known biases, and unrecognized biases could also exist. Furthermore, although the cohort of patients represents the largest number of single metastases analyzed to date for the effects of adjuvant WBRT, and although there was a good distribution of patients who received WBRT (40%) compared with those who did not (60%), the number of patients in the radioresistant group who received WBRT was low (16%). This bias against administering WBRT in this group of patients could have also influenced the results. In addition, the classification of different histologic tumor types into radiosensitive and radioresistant groups was based on the impression of the radiation oncologists who participated in this study, and one could argue that these classifications were not precise, particularly for the less common tumors. Nevertheless, the separate analysis of melanoma (which is usually agreed to be relatively resistant to radiation) and lung cancer (which is usually considered to be radiosensitive) supports the conclusions of the larger cohort, despite the smaller numbers of patients. Finally, the impact of WBRT on neuro-cognitive function was not assessed in this study. Considering recent clinical trials of SRS and WBRT, which showed that WBRT decreased recurrence, but deleteriously affected neuro-cognition,4 there is a pressing need to evaluate objectively the cognitive effects of WBRT after surgery. By selectively applying WBRT as suggested by the results of the present study, only those patients who will maximally benefit from WBRT will be exposed to its risk. Clearly, well-designed phase III trials will be needed to validate the results presented herein.
Conflict of interest statement. None declared.
Funding
This work was supported by grant from the Elias Family Fund, the McCollough Family Fund, and the Gene Pennebaker Brain Cancer Fund. The authors thank Lei Feng for statistical advice, Stephanie Jenkins for assisting with the preparation of the manuscript, and David M. Wildrick, Ph.D., for editorial assistance.
References
- 1.Patchell RA, Tibbs PA, Regine WF, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. J Am Med Assoc. 1998;280:1485–1489. doi: 10.1001/jama.280.17.1485. [DOI] [PubMed] [Google Scholar]
- 2.Peacock KH, Lesser GJ. Current therapeutic approaches in patients with brain metastases. Curr Treat Options Oncol. 2006;7:479–489. doi: 10.1007/s11864-006-0023-8. [DOI] [PubMed] [Google Scholar]
- 3.DeAngelis LM, Delattre JY, Posner JB. Radiation-induced dementia in patients cured of brain metastases. Neurology. 1989;39:789–796. doi: 10.1212/wnl.39.6.789. [DOI] [PubMed] [Google Scholar]
- 4.Chang EL, Wefel JS, Hess KR, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10:1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 5.Gaspar LE, Scott C, Murray K, Curran W. Validation of the RTOG recursive partitioning analysis (RPA) classification for brain metastases. Int J Radiat Oncol Biol Phys. 2000;47:1001–1006. doi: 10.1016/s0360-3016(00)00547-2. [DOI] [PubMed] [Google Scholar]
- 6.Sawaya R, Hammoud M, Schoppa D, et al. Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery. 1998;42:1044–1056. doi: 10.1097/00006123-199805000-00054. [DOI] [PubMed] [Google Scholar]
- 7.Shi WM, Wildrick DM, Sawaya R. Volumetric measurement of brain tumors from MR imaging. J Neurooncol. 1998;37:87–93. doi: 10.1023/a:1005944724470. [DOI] [PubMed] [Google Scholar]
- 8.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. 1st ed. New York: Springer; 2000. [Google Scholar]
- 9.Prentice RL, Kalbfleisch JD, Peterson AV, Jr, Flournoy N, Farewell VT, Breslow NE. The analysis of failure times in the presence of competing risks. Biometrics. 1978;34:541–554. [PubMed] [Google Scholar]
- 10.Dosoretz DE, Blitzer PH, Russell AH, Wang CC. Management of solitary metastasis to the brain: the role of elective brain irradiation following complete surgical resection. Int J Radiat Oncol Biol Phys. 1980;6:1727–1730. doi: 10.1016/0360-3016(80)90260-6. [DOI] [PubMed] [Google Scholar]
- 11.Hagen NA, Cirrincione C, Thaler HT, DeAngelis LM. The role of radiation therapy following resection of single brain metastasis from melanoma. Neurology. 1990;40:158–160. doi: 10.1212/wnl.40.1.158. [DOI] [PubMed] [Google Scholar]
- 12.Armstrong JG, Wronski M, Galicich J, Arbit E, Leibel SA, Burt M. Postoperative radiation for lung cancer metastatic to the brain. J Clin Oncol. 1994;12:2340–2344. doi: 10.1200/JCO.1994.12.11.2340. [DOI] [PubMed] [Google Scholar]
- 13.Smalley SR, Schray MF, Laws ER, Jr, O'Fallon JR. Adjuvant radiation therapy after surgical resection of solitary brain metastasis: association with pattern of failure and survival. Int J Radiat Oncol Biol Phys. 1987;13:1611–1616. doi: 10.1016/0360-3016(87)90154-4. [DOI] [PubMed] [Google Scholar]
- 14.DeAngelis LM, Mandell LR, Thaler HT, et al. The role of postoperative radiotherapy after resection of single brain metastases. Neurosurgery. 1989;24:798–805. doi: 10.1227/00006123-198906000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Maiuri F, Iaconetta G, Gangemi M. Brain metastses: a survey of the surgical treatment of 240 patients. Cancer. 1998;11:76–81. [Google Scholar]
- 16.Suki D, Abouassi H, Patel AJ, Sawaya R, Weinberg JS, Groves MD. Comparative risk of leptomeningeal disease after resection or stereotactic radiosurgery for solid tumor metastasis to the posterior fossa. J Neurosurg. 2008;108:248–257. doi: 10.3171/JNS/2008/108/2/0248. [DOI] [PubMed] [Google Scholar]
- 17.Aoyama H, Shirato H, Tago M, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. J Am Med Assoc. 2006;295:2483–2491. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 18.Patchell RA, Regine WF, Renschler M, et al. Comments about the prospective randomized trial by Aoyama et al. Surg Neurol. 2006;66:459–460. doi: 10.1016/j.surneu.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 19.Jagannathan J, Yen CP, Ray DK, et al. Gamma Knife radiosurgery to the surgical cavity following resection of brain metastases. J Neurosurg. 2009;111:431–438. doi: 10.3171/2008.11.JNS08818. [DOI] [PubMed] [Google Scholar]
- 20.Mathieu D, Kondziolka D, Flickinger JC, et al. Tumor bed radiosurgery after resection of cerebral metastases. Neurosurgery. 2008;62:817–823. doi: 10.1227/01.neu.0000316899.55501.8b. (Discussion 823–824) [DOI] [PubMed] [Google Scholar]