Abstract
We conducted a prospective Phase II study of high-dose methotrexate (HD-MTX) and rituximab with deferred whole brain radiotherapy in patients with newly diagnosed B-cell primary central nervous system lymphoma with a primary objective of evaluating progression-free survival (PFS). Forty patients (25 men; 15 women), ages 18–93 years (median 61.5), were treated. All patients received biweekly HD-MTX/rituximab (8 g/m2/dose; 375 mg/m2/dose) for 4–6 cycles (induction) and following best radiographic response, with every 4 weeks HD-MTX (8 g/m2/dose) for 4 cycles (maintenance). Neurological and neuroradiographic evaluation were performed every 4 weeks during induction therapy and every 8 weeks during maintenance therapy. All patients were evaluable. A total of 303 cycles of HD-MTX (median 8 cycles; range 4–10) was administered. HD-MTX/rituximab-related toxicity included 16 grade 3 adverse events in 13 patients (32.5%). Following induction, 8 patients (20%) demonstrated progressive disease and discontinued therapy; 32 patients (80%) demonstrated a partial (8/40; 20%) or complete (24/40; 60%) radiographic response. At the conclusion of maintenance therapy (6–10 months of total therapy), 28 patients (70%) demonstrated either a partial (1/28) or complete (27/28) response. Overall, survival of these 28 patients ranged from 11 to 80 months (median 33.5). Survival in the entire cohort ranged from 6 to 80 months with an estimated median of 29 months. Overall, PFS ranged from 2 to 80 months (median 21.0). HD-MTX/rituximab and deferred radiotherapy demonstrated similar or better efficacy similar to other HD-MTX-only regimens and reduced time on therapy on average to 6 months.
Keywords: deferred whole brain irradiation, high-dose methotrexate, primary CNS lymphoma, rituximab, up-front therapy
Primary central nervous system lymphomas (PCNSLs) are uncommon primary brain tumors and represent 1–2% of all brain tumors.1–26 PCNSLs occur in both immunocompetent and immunocompromized patients, especially in patients following organ transplantation and in patients with acquired immune deficiency syndrome.1,5 The clinical presentation of patients with PCNSL is a reflection of the tumor topography within the central nervous system and is most commonly 1 of several cerebral syndromes, for example, raised intracranial pressure, stroke, or encephalopathy.1–26 Notwithstanding initial response to therapy, the majority21,27,28 of patients with PCNSL recur.21,27,28
Still controversial is the most appropriate initial treatment of newly diagnosed PCNSL. The majority of oncologists advocate a chemotherapy platform utilizing high-dose methotrexate (HD-MTX) with or without whole brain radiotherapy (WBI).1–26 This report is a prospective Phase II trial utilizing the combination of HD-MTX and rituximab with deferred WBI in 40 immunocompetent adults with newly diagnosed B-cell PCNSL. Rituximab, a humanized anti-CD20 monoclonal antibody, is effective in a variety of B-cell lymphomas and may enter the brain in the context of a newly diagnosed B-cell PCNSL with associated blood–brain barrier disruption. This background provides the rationale for using HD-MTX and rituximab for newly diagnosed PCNSL.
Patients and Methods
The prospective Phase II study was performed at the University of Southern California, Norris Cancer Center, Los Angeles, California and the University of Florida, H Lee Moffitt Cancer Center and Research Institute, Tampa, Florida. The trial commenced in January 2000 and closed in July 2007. The study was conducted without industry support; approval and funding for off-label use of rituximab was obtained from the patient's insurance carrier. Patients were apprised of the non-standard of care treatment and agreed to rituximab treatment after disclosure of potential risks and benefits. Approval of the protocol and informed consent by the university human investigation committee was obtained. Signed informed consent was obtained from each subject.
Objectives and Endpoints
The primary objective was to determine the efficacy of HD-MTX/rituximab-only in the treatment of newly diagnosed adults with PCNSL. The primary endpoint was median progression-free survival (PFS). Secondary endpoints included overall survival (OS), time to progression, and response. Toxicity was evaluated in all eligible patients receiving at least 1 cycle of HD-MTX/rituximab.
Eligibility
Patients were required to have a histologically proven B-cell PCNSL that was newly diagnosed and previously untreated. Patients were required to have radiographically measurable intracranial disease wherein the tumor was bi-dimensionally measurable (at least 1 cm × 1 cm) by cranial contrast-enhanced magnetic resonance imaging (MRI). Pregnant or lactating women were not permitted to participate. Patients of child bearing potential were required to implement adequate contraceptive measures during participation in this study. Patients must have had a Karnofsky performance status greater than or equal to 50 and a life expectancy greater than 3 months.
Adequate hematologic, renal, and hepatic functions were required and were defined by the following: absolute granulocyte count >1500 per dL or white blood cell count >4000 per dL, platelet count >100 000 per dL, total bilirubin level <1.8 mg/dL, transaminase level <4 times the upper limit of normal, and creatinine concentration <1.8 mg/dL (or creatinine clearance greater than or equal to 30 mL/minute).
All patients were aware of the neoplastic nature of their disease and willingly agreed to participate after being informed of the procedures to be used, experimental nature of the therapy, alternatives, potential benefits, side effects, risk, and discomforts. Patients with acquired immunodeficiency syndrome or postorgan transplant were not eligible. No serious concurrent medical illnesses or active infection could be present that would jeopardize the ability of the patient to receive HD-MTX/rituximab therapy. Patients could not have an active concomitant malignancy except skin cancer (squamous cell or basal cell). Patients were required to be 18 years of age or older.
Drug Schedule
HD-MTX was administered to all patients at 1 of 2 dose levels based on an estimated creatinine clearance (8 g/m2 if the estimated creatinine clearance >60 mL/minute or 4 g/m2 if creatinine clearance <60 mL/minute). HD-MTX was administered intravenously over 6 hours on a single day every other week. Concurrent dexamethasone was permitted for control of neurologic signs and symptoms. HD-MTX was administered with ondansetron premedication and following prechemotherapy hydration. All patients received a similar HD-MTX treatment protocol, including aggressive hydration (sterile water + 150 mEq NaHCO/L + 20 mEq KCl/L administered at 150 mL/hour) to maintain urine output ≥100 mL/hour and urinary pH > 7 before administration of HD-MTX and throughout the elimination phase until serum methotrexate levels decreased to ≤0.1 µM/L. Serum methotrexate levels were obtained every 24 hours following initiation of HD-MTX.
HD-MTX was administered at 8 g/m2/dose intravenously over 6 hours every 2 weeks during induction (a minimum of 4 cycles) and every 4 weeks during maintenance. Before each cycle of HD-MTX, calculated creatinine clearance was determined using a contemporary serum creatinine. For patients with calculated creatinine clearance <60 mL/minute, the dose of HD-MTX was decreased by 50%.
In addition, all patients received oral folinic acid (10 mg/m2) every 6 hours beginning 18 hours after completion of the HD-MTX infusion. The dose of folinic acid was altered as a function of serum methotrexate levels obtained at 24, 48, and 72 hours after the beginning of the HD-MTX infusion. Serum creatinine levels were obtained simultaneously with serum methotrexate levels. Additionally, serum creatinine and methotrexate levels were obtained >72 hours after each dose of the HD-MTX, at the physician's discretion. Intravenous hydration and folinic acid was discontinued and patients were discharged to home once serum methotrexate levels reached ≤0.1 µM/L. All patients were given instructions to maintain oral hydration >1.5 L/m2/24 hours for 3 consecutive days upon discharge. As well, all patients were given discharge folinic acid (leucovorin calcium 25 mg per os every 6 hours for 2 days).
Patients received 375 mg/m2 of rituximab (Rituxan, Biogen-Idec Corporation) intravenously every other week alternating with HD-MTX. Rituximab was administered as an outpatient infusion treatment without premedication or hydration.
An induction cycle of therapy was operationally defined as 14 days, during which HD-MTX was administered on Day 1 and rituximab was administered once between Days 7 and 10. Treatment cycles with HD-MTX/rituximab were repeated every 14 days from Day 1 provided that all hematologic toxicity from the previous cycle had resolved to grade 2 or less, and all nonhematologic toxicity had recovered to either grade 1 or less. If recovery had not occurred by Day 14, the subsequent cycle of HD-MTX was delayed until these criteria were met. All toxicities due to HD-MTX/rituximab therapy were rated according to the NCI Common Toxicity Criteria (version 3.0).
No HD-MTX or rituximab dose escalations were permitted. Dose reduction for HD-MTX toxicity was by 50% in patients with grade ≥4 toxicity. Patients having grade ≥3 toxicity of any type after 1 dose reduction discontinued HD-MTX.
Method of Evaluation
Laboratory tests (complete blood counts, complete metabolic panel, and calculated creatinine clearance) were obtained weekly, neurologic examination was performed every 2 weeks, and contrast-enhanced cranial MR was performed after every 2 cycles of HD-MTX/rituximab until best response and thereafter every 8 weeks. Neuroradiographic response criteria as defined by Macdonald et al.29 were used.
In patients with a partial response (PR) after 4 cycles of HD-MTX/rituximab, 2 additional cycles of HD-MTX/rituximab were administered, following which patients were assessed again as described. Following induction HD-MTX/rituximab (4–6 cycles), patients were treated with 4 cycles of monthly HD-MTX. Patients with stable disease or progressive disease (PD) after 4 cycles of HD-MTX/rituximab were removed from the study and were offered alternative therapy.
PFS and OS were defined as the time from the first day of treatment with HD-MTX until progression (PFS) or death (OS). Patients were removed from the study if there was PD, development of unacceptable toxicity, patient refusal, or noncompliance with protocol requirements.
Experimental Design and Statistical Methods
The primary objective was to determine whether HD-MTX/rituximab and deferred radiotherapy could significantly delay progression in patients with newly diagnosed B-cell PCNSL. Historical values were obtained from analysis of the RTOG whole brain radiotherapy trial of patients with newly diagnosed PCNSL, in which the 12-month PFS was 31%.19 The hypotheses tested were H0: P ≤ P0 vs H1: P ≥ P1, where P is the probability of remaining alive and progression free at 12 months, with a Type I error α ≤ 0.05 and a Type II error β ≤ 0.20. For PCNSL, P0 was set at .30 and P1 at .50, looking for an improvement of 0.20. The current study was designed to accrue 40 PCNSL patients.34,35 For PCNSL patients, success was defined as observing more than 20 of 40 patients alive and progression free at 12 months (yielding α = 0.03 and β = 0.21). The median survival, time to progression, and the associated 95% confidence intervals were computed. Kaplan–Meier plots were constructed to display the estimated probabilities of OS and time to progression.
Results
Study Population
Forty patients (25 men; 15 women), ages 18–93 years (median 61.5), with newly diagnosed B-cell PCNSL (original pathology reviewed and confirmed in all cases by the participating institutions) were treated with HD-MTX/rituximab (Table 1). Histopathology was determined by stereotactic biopsy in 29 patients, resective surgery in 9, cerebrospinal fluid (CSF) flow cytometry in 1, and vitrectomy in 1 patient. Notwithstanding resective surgery in 9 patients, residual disease was measurable (median tumor volume 18 cubic centimeters) in all patients.
Table 1.
PCNSL: adjuvant therapy with high-dose methotrexate and rituximab
| Patient | Gender/age (y) | Tumor location | Surgery | KPS |
Adjuvant therapy: HD-MTX + rituximab; cycles (best response) | Maintenance therapy: HD-MTX; cycles (best response) | Progression-free survival (mo) | Salvage therapy |
Survival (mo) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Therapy | Cycles/response/duration (mo) | |||||||||
| 1 | F/93 | R parietal | Bx | 70 | 4 (CR) | 4 (SD) | 18 | HD-MTX | 6/CR/5 | 23 |
| 2 | F/50 | L temporal | STR | 70 | 4 (CR) | 4 (SD) | 56+ | 56+ | ||
| 3 | F/72 | R parietal | Bx | 100 | 4 (PD) | 2 | TMZ | 6/PR/8 | 10 | |
| 4 | F/18 | Corpus callosum | Bx | 50 | 6 (PR) | 4 (CR) | 62+ | 62+ | ||
| 5 | M/52 | Corpus callosum | Bx | 100 | 6 (PR) | 4 (CR) | 60+ | 60+ | ||
| 6 | F/79 | Corpus callosum | Bx | 80 | 4 (CR) | 4 (SD) | 10 | 12 | ||
| 7 | F/72 | R basal ganglia | Bx | 70 | 4 (CR) | 2 (PD) | 6 | TMZ | 1/PD/1 | 8 |
| 8 | M/48 | Corpus callosum | Bx | 90 | 4 (PD) | 2 | PCV | 4/CR/38+ | 38+ | |
| 9 | F/56 | R frontal | STR | 90 | 6 (PR) | 4 (SD) | 24 | TMZ WBI | 4/SD/6 36 Gy/PR/8 | 40 |
| 10 | F/65 | R frontal; L frontal | Bx | 90 | 4 (PD) | 2 | PCV | 4/PR/18 | 22 | |
| 11 | M/58 | R parietal; R midbrain | Bx | 50 | 4 (CR) | 4 (SD) | 76+ | 76+ | ||
| 12 | F/53 | L caudate | Bx | 90 | 4 (CR) | 4 (SD) | 68+ | 68+ | ||
| 13 | M/50 | R frontal; Corpus callosum | Bx | 90 | 4 (PD) | 2 | PCV WBI | 1/PD/2 36 Gy/PR/12 | 18 | |
| 14 | M/43 | Corpus callosum | Bx | 80 | 6 (PR) | 4 (CR) | 80+ | 80+ | ||
| 15 | M/44 | L frontal | Bx | 100 | 4 (PD) | 2 | PCV WBI | 5/PR/10 36 Gy/PR/8 | 25 | |
| 16 | M/51 | L frontal | STR | 100 | 6 (PR) | 4 (CR) | 78+ | 78+ | ||
| 17 | M/82 | R frontal | Bx | 90 | 6 (PR) | 4 (CR) | 14 | PCV | 4/PR/10 | 28 |
| 18 | M/57 | R orbital; L frontal | Vitrectomy | 90 | 4 (CR) Orbital RT | 4 (SD) | 56+ | 56+ | ||
| 19 | M/76 | L frontal | Bx/VPS | 70 | 4 (CR) | 4 (SD) | 21 | 24 | ||
| 20 | M/44 | Hypothalamus | Bx | 100 | 4 (CR) | 4 (SD) | 47+ | 47+ | ||
| 21 | M/85 | R frontal | STR | 70 | 4 (CR) | 2 (PD) | 4 | TMZ | 2/PD/2 | 9 |
| 22 | F/64 | R frontal | STR | 90 | 4 (PD) | 2 | WBI | 36 Gy/PR/4 | 8 | |
| 23 | F/63 | R parietal | STR | 80 | 6 (PR) | 4 (CR) | 24 | TMZ WBI | 2/PD/2 36 Gy/PR/6 | 34 |
| 24 | F/74 | L parietal; Corpus callosum | Bx | 90 | 4 (CR) | 4 (SD) | 36 | 38 | ||
| 25 | F/80 | L temporal; Corpus callosum | Bx | 60 | 4 (PD) | 2 | WBI | 36 Gy/PR/6 | 9 | |
| 26 | M/52 | R occipital; L frontal | Bx | 70 | 4 (CR) | 4 (SD) | 39+ | 39+ | ||
| 27 | M/82 | Bi-temporal | Bx | 90 | 4 (CR) | 4 (SD) | 29 | 31 | ||
| 28 | M/80 | R frontal | STR | 80 | 4 (CR) | 4 (SD) | 30 | 33 | ||
| 29 | M/81 | Subarachnoid | CSF | 70 | 4 (CR) | 4 (SD) | 7 | WBI | 36 Gy/PR/3 | 11 |
| 30 | F/71 | R occipital | Bx | 90 | 4 (CR) | 4 (SD) | 24 | 27 | ||
| 31 | M/57 | L parietal; L cerebellum | Bx | 50 | 6 (PR) | 2 (PD) | 5 | TMZ WBI | 2/PD/2 36 Gy/PR/20 | 29 |
| 32 | F/60 | R thalamus | Bx | 70 | 6 (CR) | 4 (SD) | 38+ | 38+ | ||
| 33 | M/54 | Ventricular | Bx | 50 | 4 (CR) | 1 (PD) | 3 | TMZ | 7/PR/8 | 13 |
| 34 | M/80 | L frontal; L parietal | Bx | 90 | 6 (CR) | 4 (SD) | 15 | 18 | ||
| 35 | M/73 | L parietal; L cerebellum | Bx | 90 | 6 (CR) | 4 (SD) | 20 | 22 | ||
| 36 | M/53 | L frontal; L parietal | STR | 80 | 4 (CR) | 4 (CR) | 32+ | 32+ | ||
| 37 | M/48 | R frontal; R cerebellum; L cingulated | Bx | 90 | 6 (CR) | 4 (CR) | 28+ | 28+ | ||
| 38 | M/72 | R frontal | STR | 60 | 4 (CR) | 4 (CR) | 14 | TMZ | 2/PD/2 | 18 |
| 39 | M/19 | Hypothalamus | Bx/VPS | 70 | 4 (CR) | 4 (CR) | 20+ | 20+ | ||
| 40 | M/71 | Corpus callosum | Bx | 70 | 4 (PD) | 2 | TMZ | 2/PD/2 | 6 | |
Abbreviation: M, male; F, female; R, right; L, left; Bx, biopsy; STR, subtotal resection; VPS, ventriculoperitoneal shunt; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; +, alive and disease free; TMZ, temozolomide; WBI, whole brain irradiation; PCV, procarbazine, CCNU, vincristine; Gy, gray.
Patients presented at the time of tumor diagnosis with the following signs and symptoms: altered mental status (n = 19), increased intracranial pressure as manifested by increasing headache (n = 12), progressive hemiparesis (n = 12), new onset seizures (n = 9), new onset headaches (n = 9), and visual acuity deterioration (n = 2). Patient performance status using the Karnofsky scale ranged from 50–100 (median 80) at the time of diagnosis and initiation of HD-MTX/rituximab therapy. Tumor locations are delineated in Tables 1 and 2. Pathology (reviewed by a panel of 2 neuropathologists) showed all tumors to be B-cell lymphomas by WHO criteria (in 38 patients with evaluable tissue, diffuse large B-cell histology).
Table 2.
Characteristics of study patients
| Variables | Number patients | Percent |
|---|---|---|
| Total patients | 40 | 100 |
| Age | ||
| ≥50 | 33 | 82.5 |
| ≥70 | 17 | 42.5 |
| Median (range) | 61.5 | (18–93) |
| Sex | ||
| Male | 25 | 62.5 |
| Female | 15 | 37.5 |
| Location of tumor | ||
| Frontal | 18 | 45 |
| Temporal | 3 | 7.5 |
| Parietal | 9 | 22.5 |
| Corpus callosum | 9 | 22.5 |
| Deep gray nuclei | 5 | 12.5 |
| Occipital | 2 | 5 |
| Cerebellum | 3 | 7.5 |
| Brainstem | 1 | 2.5 |
| Subarachnoid/ventricular | 2 | 5 |
| Multilobar | 12 | 30 |
| Extent of initial surgery | ||
| Subtotal resection | 9 | 22.5 |
| Biopsy | 29 | 72.5 |
| Vitrectomy | 1 | 2.5 |
| Response to up-front chemotherapy | ||
| Complete response | 24 | 60 |
| Partial response | 8 | 20 |
| Progressive disease | 8 | 20 |
| Progression-free survival to up-front chemotherapy | ||
| Median (range) | 21 mo | 2–78 mo |
| Salvage therapy at recurrence | ||
| Temozolomide | 9 | 22.5 |
| Procarbazine, CCNU, Vincristine | 5 | 12.5 |
| HD-MTX | 1 | 2.5 |
| Whole brain irradiation | 8 | 20 |
| Response to salvage therapy | ||
| Complete response | 2 | 9 |
| Partial response | 12 | 67 |
| Progressive disease | 4 | 22 |
| Progression-free survival to salvage therapy | ||
| Median (range) | 6 mo | (1 to 38+ mo) |
| Overall survival | ||
| Median (range) | 29 mo | (6 to 80+ mo) |
| Alive and disease free | 20 | 50 |
| ≤50 y of age | 7/9 | 78 |
| >50 y of age | 8/31 | 26 |
| >60 y of age | 0/20 | 0 |
Treatment is shown in Tables 1 and 2. All patients were treated with HD-MTX/rituximab following tissue diagnosis and clinical staging (serum HIV titer, CSF flow cytometry and cytology, slit lamp examination of the vitreal cavity, neuraxis MRI, CT of the chest, abdomen and pelvis, and body FDG-PET).30 No patient was HIV positive and body CT and FDG-PET were negative in all patients. Seven patients (17.5%) had positive CSF flow cytometry of whom CSF cytology was positive in 4 (10%). No patient had a positive CSF cytology and negative CSF flow cytometry. CSF contamination by PCNSL was considered present if either flow cytometry or cytology were positive. A single patient (2.5%) had a positive slit lamp examination and underwent vitrectomy demonstrating a B-cell lymphoma.
All patients were treated with HD-MTX/rituximab, and no patient discontinued therapy for noncompliance or unacceptable toxicity. HD-MTX/rituximab began with a median time to initiation following tissue diagnosis of 1 week with a range of 3 days to 3 weeks. A total of 303 cycles of HD-MTX were administered (induction and maintenance). A minimum of 4 cycles of HD-MTX was administered to each patient with a median of 8 cycles (range 4–10). HD-MTX was administered at the prescribed dose in all but 4 patients (2 at onset with a creatinine clearance less than 60 mL/minute and 2 during treatment due to renal toxicity). During induction, patients received in alternate weeks, rituximab. A total of 184 cycles of rituximab were administered (median 4; range 4–6). No other anti-lymphoma agents aside from dexamethasone were utilized during the study. Oral dexamethasone was used concurrently in all patients and was increased in 8 patients with clinical disease progression and lack of response to HD-MTX/rituximab. An additional 6 patients with disease progression required re-initiation of dexamethasone therapy. The dexamethasone dose was decreased in 32 patients with a neuroradiographic response and as patient clinical status permitted.
Eighteen patients (45%) received an alternative chemotherapy (temozolomide 9; PCV 5) following progression or failure to respond to HD-MTX/rituximab (Tables 1 and 2). One patient was retreated with HD-MTX. Nine patients received radiotherapy, 1 at treatment onset (orbital radiation) and 8 as salvage therapy for disease progression (in all, whole brain irradiation given as a total dose of 36 Gy in 20 fractions).
Toxicity
Toxicity was recorded for all grades for all patients by type using the NCI common toxicity criteria (version 3.0). Table 3 lists all grade 2–5 toxicity observed with each figure representing the sum of the highest grade of toxicity attained, per toxicity, per cycle for all patients. A total of 303 treatment cycles of HD-MTX (184 in conjunction with rituximab) were administered of which there were 16 grade 3 adverse events (AEs) in 13 patients (32.5%) and 2 grade 4 AEs. There were no grade 5 AEs, treatment-related transfusions, episodes of febrile neutropenia, or treatment-related deaths. Two patients developed the HD-MTX-related acute renal failure (1 during first cycle of HD-MTX and the other during cycle #3 of HD-MTX) requiring treatment with β-carboxypeptidase according to NCI guidelines.31 Both patients made an uneventful recovery and were retreated (following a median delay of 24 days for recovery of creatinine clearance) with HD-MTX (initially at 50% dose reduction and subsequent doses at 8 g/m2) without difficulty.
Table 3.
High-dose methotrexate/rituximab: toxicity
| Toxicity | Grade 2 | Grade 3 | Grade 4 | Grade 5 | Total |
|---|---|---|---|---|---|
| Anemia | 1 | 2 | 0 | 0 | 3 |
| Fatigue | 6 | 1 | 0 | 0 | 7 |
| Hepatic | 2 | 1 | 0 | 0 | 3 |
| Hyperglycemia | 6 | 2 | 0 | 0 | 8 |
| Neutropenia without fever | 1 | 4 | 0 | 0 | 5 |
| Nausea | 2 | 0 | 0 | 0 | 2 |
| Renal | 13 | 4 | 2 | 0 | 19 |
| Thrombophlebitis | 0 | 2 | 0 | 0 | 2 |
| Totals | 31 | 16 | 2 | 0 | 49 |
Response
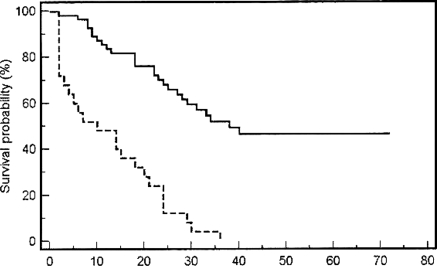
All patients were assessable for response and survival (Tables 1 and 2). Following induction with 4–6 cycles of HD-MTX/rituximab, 8 patients (20%) demonstrated PD and discontinued therapy. Thirty-two patients (80%) demonstrated a partial (8/40; 20%) or complete (24/40; 60%) radiographic response to HD-MTX/rituximab induction. Six of the 8 patients (75%) with a PR to induction HD-MTX/rituximab converted to a complete response with maintenance HD-MTX. Four patients (10%) progressed during maintenance HD-MTX. At the conclusion of induction and maintenance HD-MTX (6–8 months of total therapy), 28 patients (70%) demonstrated either a partial (1/28) or complete (27/28) response. The OS of these 28 patients ranged from 11 to 80 months (median 33.5). At the conclusion of HD-MTX/rituximab, the Karnofsky performance status ranged from 50 to 90 with a median of 80 in the entire study group. The PFS following HD-MTX/rituximab treatment ranged from 2 to 80 months (Fig. 1), with an estimated median of 21.0 (95% CI: 13.83, 28.17) months. Patients who failed to respond (8 patients), progressed while on maintenance (4 patients), or after HD-MTX/rituximab (6 patients) were offered alternative (temozolomide 9 patients, PCV 5; whole brain irradiation 8, re-challenge with HD-MTX 1; 1 salvage therapy 13 patients, 2 therapies 5) or supportive therapy. Response to alternative therapy included 2 patients (11%) with a complete response and 12 patients (67%) with a PR. PFS to salvage therapy ranged from 1 to 38 months with a median of 6 months. One patient of the 18 that received salvage therapy remains alive and disease free. Survival in the entire cohort ranged from 6 to 80 months with an estimated median of 29.0 (95% CI: 19.72, 38.28) months (Fig. 1). The probability of disease-free survival in patients less than 50 years was 86% (6/7), in patients 50–59 years 33% (4/12), in patients 60–69 years 25% (1/4), and in patients greater than 70 years 0% (0/17). All but 15 patients have died, and all deaths were directly attributable to the effects of the progressive intracranial tumor. In the 15 surviving patients, all treated with HD-MTX/rituximab and no WBI, no cognitive decline was documented. However, formal neuropsychological testing was not prospectively performed.
Fig. 1.
Progression free and overall survival for patients with newly diagnosed primary CNS lymphoma treated with high-dose methotrexate and rituximab.
Discussion
Still controversial is the preferred treatment of newly diagnosed adults with PCNSL.1–26 In large part, this controversy reflects the paucity of multi-institution trials and the relative failure of adjuvant WBI without chemotherapy as initial treatment of PCNSL (median survival 11.6 months, as demonstrated in the multicenter RTOG 8315 trial).19,25 In addition, the late neurotoxic consequences of WBI (cognitive impairment), particularly in patients >60 years of age, the majority of patients with PCNSL have dampened enthusiasm for up-front treatment of PCNSL with radiotherapy.1,4,5,10,13,15,16,22 Consequently and increasingly, PCNSL is treated with deferred radiotherapy and primary chemotherapy. Seminal studies by Hochberg and later by DeAngelis, Abrey, and Batchelor have provided the basis for inclusion of HD-MTX in most treatment regimens of PCNSL.1,4,6,7,14 However, the dose of methotrexate has varied from 3.5 to 8 g/m2 reflecting in part institutional philosophies of whether to use single agent or multiagent chemotherapy treatment regimens. Additionally, the response to single-agent HD-MTX has varied widely. Batchelor reported a combined complete response (52%) and PR (22%) rate to single-agent HD-MTX in the NABTT 96-07 trial (a total of 25 patients) of 74% with a median PFS of 12.8 months and OS of 32 months.4,13 In contrast, Herrlinger for the German Cancer Society (NOA-3 trial) using a similar regimen of single agent HD-MTX in 37 patients reported a combined complete response and PR rate of 29.7% and a median PFS of 13.7 months.15 How to reconcile these similar studies is difficult and may in part reflect the difference in the duration of induction therapy (8 vs 6 biweekly cycles of HD-MTX). The current study, similar to the German trial, utilized a shorter induction (4–6 cycles of HD-MTX/rituximab) and yet demonstrated an 80% response rate (60% complete response) and 21-month median PFS. Twelve- and 24-month survival curve probabilities are 80% and 60%, respectively, for the present study vs 55% and nearing 40% in the NABTT 96-07 trial.4,13 This improvement in both response rate and PFS likely reflects the addition of rituximab to HD-MTX. Therefore, an advantage of the present trial appears to be an improvement in PFS (the main study objective) relative to other HD-MTX regimens only.
NABTT is currently conducting a single agent trial of rituximab in patients with recurrent PCNSL and has reported in an abstract a 30% response rate. Also not clear from the limited literature is the duration of maintenance therapy with HD-MTX. The NABTT protocol utilized 11 cycles of monthly HD-MTX (following 2 further cycles of biweekly HD-MTX after achieving a complete response) as contrasted with the present study which utilized 4 cycles of monthly HD-MTX. Therefore, the current regimen permits a shortening of the total duration of treatment relative to the NABTT HD-MTX regimen (6 vs. 14 months). Increasingly in oncology there is an emphasis on shorter duration of adjuvant therapy as recently demonstrated for systemic lymphoma and lung cancer. In addition, the current regimen of HD-MTX/rituximab appears to shorten the interval during induction to best response from 6 (as determined in the NABTT trial) to 4 cycles (as in the present study).
At present, there remains lack of agreement on whether single-agent HD-MTX is as efficacious as multiagent regimens including dose dense chemotherapy and peripheral stem cell transplantation.1,2,4–6,8–10,12,13,15–17,20–22,24 In addition, there has been renewed interest in utilizing WBI, though at reduced and response-based dose, as part of up-front therapy, either intercalated with chemotherapy as in the DeAngelis regimen or as consolidation in the Italian regimen.8–11
Equally important in the management of patients with PCNSL is the use of salvage therapy at the time of tumor recurrence.32–37 The value of re-challenge with HD-MTX has been demonstrated in patients with an initial response to HD-MTX and in whom a long disease-free interval (6 to 12+ months) is achieved. Additionally, other chemotherapies for example PCV, temozolomide, topotecan, and dose dense chemotherapy and peripheral stem cell transplantation have been used successfully as salvage therapy with primarily palliative intent. Finally, the value of WBI in patients with recurrent PCNSL and previously treated with primary up-front chemotherapy has been demonstrated. Most of these modalities were used in the present study and in aggregate resulted in a median PFS of 6 months. Clearly needed are more effective and durable salvage therapies for recurrent or refractory PCNSL. Perhaps increasing the use of high-dose chemotherapy and peripheral stem cell transplantation following a complete response with salvage therapy, as is used for recurrent systemic non-Hodgkin's lymphoma, would provide improved outcome in patients with recurrent PCNSL.
In conclusion, there is no standard therapy for newly diagnosed patients with PCNSL and consequently there is a need to treat patients on clinical trials. Importantly, multicenter trials are needed to better define adjuvant treatment. In young patients, treatment is with curative intent, whereas in older patients, treatment is palliative and designed to minimize neurotoxicity. The combined use of HD-MTX and rituximab as used in this study may obviate early WBI permitting radiotherapy to be deferred until PCNSL progression or eliminated altogether in patients with a durable and likely curative response to first-line therapy. Importantly, the present regimen of HD-MTX/rituximab recapitulates the best objective response seen in other HD-MTX-only regimens, accelerates the time to complete response, improves PFS (the main study outcome measure) compared with HD-MTX-only regimens and permits reduction in total time on therapy.
Conflict of interest statement. M.C.C. collected data and M.C.C. and S.K.J. analyzed data.
References
- 1.Abrey LE, Yahalom J, DeAngelis LM. Treatment of primary CNS lymphoma: the next step. J Clin Oncol. 2000;18:3144–3150. doi: 10.1200/JCO.2000.18.17.3144. [DOI] [PubMed] [Google Scholar]
- 2.Abrey LE, Moskowitz CH, Mason WP, et al. Intensive methotrexate and cytarabine followed by high-dose chemotherapy with autologous stem-cell rescue in patients with newly diagnosed primary CNS lymphoma. J Clin Oncol. 2001;21:4151–4156. doi: 10.1200/JCO.2003.05.024. [DOI] [PubMed] [Google Scholar]
- 3.Bataille B, Delwail V, Menet E, et al. Primary intracerebral malignant lymphoma: a report of 248 cases. J Neurosurg. 2000;92:261–266. doi: 10.3171/jns.2000.92.2.0261. [DOI] [PubMed] [Google Scholar]
- 4.Batchelor TT, Carson K, O'Neill A, et al. NABTT CNS Consortium: the treatment of primary central nervous system lymphoma (PCNSL) with methotrexate and deferred radiotherapy - NABTT 96–07. J Clin Oncol. 2003;21:1044–1049. doi: 10.1200/JCO.2003.03.036. [DOI] [PubMed] [Google Scholar]
- 5.Batchelor T, Loeffler JS. Primary CNS lymphoma. J Clin Oncol. 2006;24:1281–1288. doi: 10.1200/JCO.2005.04.8819. [DOI] [PubMed] [Google Scholar]
- 6.DeAngelis LM, Yahalom J, Thaler HT, Kher U. Combined modality therapy for primary CNS lymphoma. J Clin Oncol. 1992;10(4):635–643. doi: 10.1200/JCO.1992.10.4.635. [DOI] [PubMed] [Google Scholar]
- 7.DeAngelis LM, Seiferheld W, Schold SC, Fisher B, Schultz CJ. Combination chemotherapy and radiotherapy for primary central nervous system lymphoma: radiation therapy oncology group 93–100. J Clin Oncol. 2002;20:4643–4648. doi: 10.1200/JCO.2002.11.013. [DOI] [PubMed] [Google Scholar]
- 8.Ferreri AJ, Dell'Oro S, Foppoli M, et al. MATLLDE regimen followed by radiotherapy is an active regimen against primary CNS lymphoma. Neurology. 2006;66:1435–1438. doi: 10.1212/01.wnl.0000210464.94122.e1. [DOI] [PubMed] [Google Scholar]
- 9.Ferreri AJ, Reni M, Pasini F, et al. A multicenter study of treatment of primary CNS lymphoma. Neurology. 2002;59:1513–1520. doi: 10.1212/wnl.58.10.1513. [DOI] [PubMed] [Google Scholar]
- 10.Freilich RJ, Delattre JY, Monjour A, DeAngelis LM. Chemotherapy without radiation therapy as initial treatment for primary CNS lymphoma in older patients. Neurology. 1996;46:435–439. doi: 10.1212/wnl.46.2.435. [DOI] [PubMed] [Google Scholar]
- 11.Fisher BJ, Seiferheld W, Chultz C, et al. Secondary analysis of RTOG 9310:an intergroup Phase II combined modality treatment of primary central nervous system lymphoma with chemotherapy and hyperfractionated radiotherapy. J Neuro Oncol. 2005;74(2):201–205. doi: 10.1007/s11060-004-6596-9. [DOI] [PubMed] [Google Scholar]
- 12.Gavrilovic IT, Hormgo A, Yahalom J, et al. Long-term follow-up of high dose methotrexate based therapy with or without whole brain irradiation for newly diagnosed primary CNS lymphoma. J Clin Oncol. 2006;24:4570–4574. doi: 10.1200/JCO.2006.06.6910. [DOI] [PubMed] [Google Scholar]
- 13.Gerstner ER, Carson KA, Grossman SA, Batchelor TT. Long-term outcome in PCNSL patients treated with high-dose methotrexate and deferred radiation. Neurology. 2008;70:401–403. doi: 10.1212/01.wnl.0000300671.37279.0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glass J, Gruber ML, Cher L, et al. Preirradiation methotrexate chemotherapy of primary central nervous system lymphoma: long term outcome. J Neurosurg. 1994;81:188–195. doi: 10.3171/jns.1994.81.2.0188. [DOI] [PubMed] [Google Scholar]
- 15.Herrlinger U, Schabert M, Brugger W, et al. German Cancer Society Neuro-Oncology Working group NOA-03 multicenter trial of single agent methotrexate for primary central nervous system lymphoma. Ann Neurol. 2002;51:247–252. doi: 10.1002/ana.10102. [DOI] [PubMed] [Google Scholar]
- 16.Hoang-Xuan K, Tallandier L, Chinot O, Soubeyran P, Bogdhan U, Hildebrand J. Chemotherapy alone as initial treatment for primary CNS lymphoma in patients older than 60 years: a Multicenter Phase II study (26952) of the European Organization for research and treatment of Cancer brain tumor group. J Clin Oncol. 2003;21:2726–2731. doi: 10.1200/JCO.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 17.Illerhaus G, Marks R, Ihorst G, et al. High-dose chemotherapy with autologous stem cell transplantation and hyperfractionated radiotherapy as first-line treatment of primary CNS lymphoma. J Clin Oncol. 2006;24:3865–3870. doi: 10.1200/JCO.2006.06.2117. [DOI] [PubMed] [Google Scholar]
- 18.Lachance DH, O'Neill BP, MacDonald DR, Jaeckle K, Witzig T, Li C. Primary leptomeningeal lymphoma: report of 9 cases, diagnosis with immunocytochemical analysis, and review of the literature. Neurology. 1991;41:95–100. doi: 10.1212/wnl.41.1.95. [DOI] [PubMed] [Google Scholar]
- 19.Nelson DF, Martz KL, Bonner H, et al. Non-Hodgkin's lymphoma of the brain: can high dose, large volume radiation therapy improve survival? Report on the prospective trial by Radiation Therapy Oncology Group (RTOG): RTOG 8315. Int J Rad Oncol Biol Phys. 1992;23:9–17. doi: 10.1016/0360-3016(92)90538-s. [DOI] [PubMed] [Google Scholar]
- 20.Neuwelt EA, Goldman DL, Dahlborg SA, et al. Primary CNS lymphoma treated with osmotic blood-brain barrier disruption: prolonged survival and preservation of cognitive function. J Clin Oncol. 1991;9(9):1580–1590. doi: 10.1200/JCO.1991.9.9.1580. [DOI] [PubMed] [Google Scholar]
- 21.Pels H, Schmidt-Wolf IG, Glasmacher A, et al. Primary central nervous system lymphoma: results of a pilot and phase II study of systemic and intraventricular chemotherapy with deferred radiotherapy. J Clin Oncol. 2003;21:4489–4495. doi: 10.1200/JCO.2003.04.056. [DOI] [PubMed] [Google Scholar]
- 22.Plotkin SR, Betensky RA, Hochberg FH, et al. Treatment of primary central nervous system lymphoma with sequential courses of high-dose methotrexate. Clin Cancer Res. 2004;10:5643–5646. doi: 10.1158/1078-0432.CCR-04-0159. [DOI] [PubMed] [Google Scholar]
- 23.Poortmans PMP, Kluin-Nelemans HC, Haaxma-Reiche H, et al. High-dose methotrexate based chemotherapy followed by consolidating radiotherapy in non-AIDS-related primary central nervous system lymphoma: european organization for research and treatment of cancer Lymphoma Group Phase II trial 20962. J Clin Oncol. 2003;21:4483–4488. doi: 10.1200/JCO.2003.03.108. [DOI] [PubMed] [Google Scholar]
- 24.Shah GD, Yahalom J, Correa DD, et al. Combined immunochemotherapy with reduced whole brain radiotherapy for newly diagnosed primary CNS lymphoma. J Clin Oncol. 2007;25(30):4730–4735. doi: 10.1200/JCO.2007.12.5062. [DOI] [PubMed] [Google Scholar]
- 25.Shibamoto Y, Ogino H, Hassegawa M, Suzuki K, Nishio M, Fujii T. Results of radiation monotherapy for primary central nervous system lymphoma in the 1990s. Int J Radiat Oncol Biol Phys. 2005;62:809–813. doi: 10.1016/j.ijrobp.2004.12.043. [DOI] [PubMed] [Google Scholar]
- 26.Velasquez WS. Primary central nervous system lymphoma. J Neuro-Oncol. 1994;20:177–185. doi: 10.1007/BF01052727. [DOI] [PubMed] [Google Scholar]
- 27.Balmaceda C, Gaynor JJ, Sun M, Gluck J, DeAngelis L. Leptomeningeal tumor in primary central nervous system lymphoma: recognition, significance, and implications. Ann Neurol. 1995;38:202–209. doi: 10.1002/ana.410380212. [DOI] [PubMed] [Google Scholar]
- 28.Chamberlain MC, Kormanik P, Glantz M. Recurrent primary central nervous system lymphoma complicated by lymphomatous meningitis. Oncology Reports. 1998;5:521–523. doi: 10.3892/or.5.2.521. [DOI] [PubMed] [Google Scholar]
- 29.MacDonald DR, Cascino TL, Schold SC, Cairncross JG. Response criteria for phase 2 studies of supratentorial malignant glioma. J Clin Oncol. 1990;8:1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 30.Abrey LE, Batchelor TT, Ferreri AJ, et al. Report of an international workshop to standardize baseline evaluation and response criteria for primary CNS lymphoma. J Clin Oncol. 2005;23:5034–5043. doi: 10.1200/JCO.2005.13.524. [DOI] [PubMed] [Google Scholar]
- 31.Widemann BC, Balis FM, Murphy RF, et al. Carboxypeptidase-G2, thymidine, and leucovorin rescue in cancer patients with methotrexate-induced renal dysfunction. J Clin Oncol. 1997;15(5):2125–2134. doi: 10.1200/JCO.1997.15.5.2125. [DOI] [PubMed] [Google Scholar]
- 32.Abrey LE, Ben-Porat L, Panageas KS, et al. Primary central nervous system lymphoma: the Memorial-Sloan Kettering Cancer Center prognostic model. J Clin Oncol. 2006;24:5711–5715. doi: 10.1200/JCO.2006.08.2941. [DOI] [PubMed] [Google Scholar]
- 33.Fischer L, Thiel E, Klasen H-A, et al. Prospective trial on topotecan salvage therapy in primary CNS lymphoma. Ann Oncol. 2006;17:1141–1145. doi: 10.1093/annonc/mdl070. [DOI] [PubMed] [Google Scholar]
- 34.Herrlinger U, Brugger W, Bamberg M, Kuker W, Dichgans J, Weller M. PCV salvage chemotherapy for recurrent primary CNS lymphoma. Neurology. 2000;54:1707–1709. doi: 10.1212/wnl.54.8.1707. [DOI] [PubMed] [Google Scholar]
- 35.Hottinger AF, DeAngelis LM, Yahalom J, Abrey L. Salvage whole brain radiotherapy for recurrent or refractory primary CNS lymphoma. Neurology. 2007;69:1178–1182. doi: 10.1212/01.wnl.0000276986.19602.c1. [DOI] [PubMed] [Google Scholar]
- 36.Reni M, Zaja F, Mason W, et al. Temozolomide as salvage treatment in primary brain lymphoma. Br J Cancer. 2007;96:864–867. doi: 10.1038/sj.bjc.6603660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soussain C, Suzan F, Hoang-Xuan K, et al. Results of intensive chemotherapy followed by hematopoetic stem-cell rescue in 22 patients with refractory or recurrent primary CNS lymphoma or intraocular lymphoma. J Clin Oncol. 2001;19:742–749. doi: 10.1200/JCO.2001.19.3.742. [DOI] [PubMed] [Google Scholar]