Abstract
Patients with glioblastoma (GBM) exhibit profound systemic immune defects that affect the success of conventional and immune-based treatments. A better understanding of the contribution of the tumor and/or therapy on systemic immune suppression is necessary for improved therapies, to monitor negative effects of novel treatments, to improve patient outcomes, and to increase understanding of this complex system. To characterize the immune profile of GBM patients, we phenotyped peripheral blood and compared these to normal donors. In doing so, we identified changes in systemic immunity associated with both the tumor and dexamethasone treated tumor bearing patients. In particular, dexamethasone exacerbated tumor associated lymphopenia primarily in the T cell compartment. We have also identified unique tumor and dexamethasone dependent altered monocyte phenotypes. The major population of altered monocytes (CD14+HLA-DRlo/neg) had a phenotype distinct from classical myeloid suppressor cells. These cells inhibited T cell proliferation, were unable to fully differentiate into mature dendritic cells, were associated with dexamethasone-mediated changes in CCL2 levels, and could be re-created in vitro using tumor supernatants. We provide evidence that tumors express high levels of CCL2, can contain high numbers of CD14+ cells, that tumor supernatants can transform CD14+HLA-DR+ cells into CD14+HLA-DRlo/neg immune suppressors, and that dexamethasone reduces CCL2 in vitro and is correlated with reduction of CCL2 in vivo. Consequently, we have developed a model for tumor mediated systemic immune suppression via recruitment and transformation of CD14+ cells.
Keywords: dexamethasone, glioblastoma, immune suppression, monocytes, myeloid suppressor cells
Despite recent advances in therapy for glioblastoma (GBM, WHO Grade IV malignant glioma), the prognosis for these patients remains dismal and new treatments are urgently needed. Current standard of care includes tumor resection, followed by radiation and concurrent chemotherapy resulting in a median overall survival of ∼15 months.1 Many novel therapies are now targeting the immune system using anticancer vaccines or chemotherapeutics that promote antitumor immunity.2–4 However, continued improvement of immune mediated therapies requires detailed characterization of functional immunity in GBM patients.
GBM-related systemic immunosuppression has been well documented. However, many studies have only investigated deficiencies of individual components of immunity. These include reduced T lymphocyte number and function,5–7 defective receptor expression on monocytes,8,9 defective dendritic cell (DC) function,8 elevated immunosuppressive regulatory T cells (Tregs),10 and altered cytokine secretion by tumors.9,11 Although mechanisms of local immunosuppression have focused on tumor secreted factors, there are few models to explain how these factors translate into the profound systemic immunosuppression noted in GBM. Complicating this problem is the confounding effect of chemotherapy, surgery, and radiation on immunity. Dexamethasone (DEX) is commonly used in GBM to control cerebral edema and relieve its associated symptoms. DEX and other glucocorticoids are well-known immunosuppressants used to reduce T cell responses in transplant patients and patients with severe autoimmune disease.12–16 Yet evidence suggests that DEX use may negatively impact GBM patient prognosis.17 For example, DEX in combination with radiation therapy has been reported to drop CD4 counts to less than 200 cells/mm3.18 Overall, it is clear that many factors contribute to loss of normal immunity in GBM patients.
We report here details of cellular and cytokine immune profiles of GBM patients. We have identified a novel mechanism of immunosuppression in GBM associated with the presence of CD14+HLA-DRlo/neg monocytes whose level is exacerbated during DEX treatment. Interestingly, these immunosuppressive monocytes have previously been described as poor prognostic markers for patients with sepsis19,20 and may represent another population of myeloid derived suppressor cells (MDSCs). On the basis of our findings, we propose a model of tumor recruitment of monocytes, their transformation into immunosuppressive CD14+HLA-DRlo/neg monocytes, and the role of DEX in mobilization of these immunosuppressive cells.
Patients, Materials, and Methods
GBM Patients and Normal Donors
Human samples were collected from 37 GBM patients (26 newly diagnosed and 11 recurrent) and 15 normal donors after signed informed consent performed under approval of the Mayo Clinic Institutional Review Board. Patients enrolled in the study had a Karnofsky performance score >60, histopathologically confirmed newly diagnosed or recurrent GBM, and were acceptable candidates for surgery. Patients with coexisting medical illness likely to impact their immune status (such as active infection, unexplained febrile illness, acquired or congenital immunodeficiency, autoimmune disease, or treated with immune therapies) were excluded from the study. Patients were given DEX only if symptomatic from their tumor or the surrounding edema. Patients were treated with the minimum dose required to ameliorate their symptoms and tapered off the medication as quickly as tolerated. GBM patient samples were collected at Mayo Clinic Rochester and Mayo Clinic Jacksonville from August 2006 to March 2008. Twenty-six patients were immunophenotyped and samples from an additional 9 newly diagnosed and 2 recurrent patients were used in functional studies. Samples from Jacksonville were shipped at ambient temperature overnight and analyzed within 24 hours. For newly diagnosed and recurrent patients, peripheral blood was collected perioperatively and prior to any radiation or chemotherapy. Recurrent patients had not had any treatments for at least 8 weeks. For some patients, tumor samples were collected, minced with a scalpel, and cultured in DMEM (Invitrogen) supplemented with 10% fetal calf serum (Cellgro/Mediatech, Inc).
Immune-Phenotyping of Peripheral Blood from Normal Donors and GBM Patients
Leukocytes were analyzed by direct antibody staining of whole blood and analyzed by flow cytometry. The protocol for whole blood staining was adapted from Appay et al.21 Where indicated, 50 µL of whole blood was added and stained in Trucount™ tubes according to manufacturer's directions (BD Biosciences). Data were acquired on a BD FACSCalibur flow cytometer (Becton Dickinson) that was calibrated the day of use and analyzed with Cell Quest and Multiset (Becton Dickinson) software. The antibodies used for these studies are listed in Supplementary Material, Table S1.
Cell Isolation and Ex Vivo Culture of CD14+ Monocytes
Peripheral blood mononuclear cells (PBMC) were isolated by density gradient centrifugation (Lymphoprep, MP Biomedicals LLC). Monocytes and T cells were isolated from PBMC by incubation with anti-CD14, anti-CD3, or Pan T cell immunomagnetic beads, respectively (Miltenyi Biotec), per manufacturer's protocol, and selected using the AutoMACS Separator (Miltenyi Biotec). For autologous T cell proliferation assays, monocytes were selected with pan-monocyte immunomagnetic beads (Miltenyi) and subsequently enriched for HLA-DRlo/neg cells by incubation with anti-HLA-DR immunomagnetic beads (Miltenyi) and negative selection with AutoMACS. CD14+ cells were plated in polystyrene flasks at 3 × 106 cells/mL and cultured in X-Vivo (Lonza) plus 1% human AB serum (Sigma), 2800 IU/mL GM-CSF (Berlex Laboratories), 1000 IU/mL IL-4 (R&D Systems), and 1× penicillin/streptomycin (P/S; Invitrogen) for 3 days. Immature DCs were then matured with 1000 IU/mL TNF-α (R&D Systems) and 1 µg/mL PGE2 (Sigma) for 2 days. DCs were analyzed as previously described.22
Allogeneic Mixed Lymphocyte Reaction
Pan T cells (1 × 105 total per well pooled from 3 normal donors) were seeded in 96 round-bottom well plate (CoStar Corning Inc.) in RPMI-1640 (Invitrogen) with 10% fetal bovine serum (FBS; Mediatech) and 1× P/S (Invitrogen) with varying ratios of monocytes. Pan T cells incubated with 2 µL of anti-CD3/CD28 beads (Dynabeads, Invitrogen Dynal AS) were used as positive control. Cells were cultured for 72 hours with tritiated thymidine added 18 hours before collection.
Polyclonal Autologous T Cell Proliferation Assay
Freshly isolated T cells were resuspended in PBS at 1 × 107 cells/mL and incubated with CFSE stock solution (Renovar) for 10 minutes at 37°C. Cells were washed twice and resuspended in RPMI-1640 with 10% FBS and 1% P/S to stabilize the CFSE staining. After a final wash step, CFSE labeled T cells were cultured in 24-well plates (CoStar Corning Inc.) at 1 × 106 cells/well with varying amounts of monocytes and anti-CD3/28 beads at 20 µL per 106 T cells. For control conditions, CFSE unlabeled T cells were cultured alone and CFSE labeled T cells were cultured alone with and without anti-CD3/CD28 beads. After 4 days, cells were harvested, divided into 2 groups, and incubated for 10 minutes at room temperature with APC-conjugated anti-CD3 (eBioscience). After antibody staining, cells were washed, resuspended in PBS with propidium iodide (Renovar), and analyzed by flow cytometry.
Tumor Supernatant Studies
Normal human astrocytes (NHA, StemCell Technologies), primary GBM tumor cells, or GBM tumor cell lines (ATCC) were cultured at 1 × 106 cells/mL in DMEM (Invitrogen) and 10% FBS, 1% P/S (Invitrogen). Culture supernatant was collected after 72 hours, centrifuged at 800 × g for 10 minutes, and stored at 4°C until use. Monocytes from healthy donors were isolated as described above and cultured for 72 hours at 3 × 106 cells/mL in either control media or control media and 50% tumor supernatant with and without DEX. DMEM with 10% FBS and 1% P/S was used as control media. Cells were collected after 72 hours, stained with anti-HLA-DR PerCP, and analyzed by flow cytometry. For allogeneic mixed lymphocyte reaction (MLR), cells were collected after 72 hours, assessed for viability with Trypan blue, and cocultured with allogeneic pan-T cells as previously described at 1:10 monocyte to T cell ratio for 72 hours. Tritiated thymidine was added 18 hours prior to collection. For generation of mature DCs, TNF-alpha (1100 units/mL) and PGE2 (1 µg/mL) were added after the initial 72 hours of culture, and the cells were incubated for another 48 hours. Cells were collected at the end of culture, stained for anti-CD80 FITC and anti-CD83 PE, and analyzed by flow cytometry.
Enzyme Linked Immunosorbent Assays and Cytokine Analyses
Enzyme linked immunosorbent assay (ELISA) kits for MIF-1, TGF-β, ILT3, VEGF, OPG, and CCL2, IP-10, and DcR3 were purchased from R&D Systems and performed according to the manufacturer's instructions. All other cytokines were analyzed with the Beadlyte® Human 22-plex Multi-Cytokine Detection System (Upstate Biotechnology). Culture supernatants were analyzed for lactate concentration with Lactate Assay Kit II (BioVision).
Immunohistochemistry
H&E slides were reviewed by a pathologist from the Pathology Core of the Mayo Clinic Brain Cancer SPORE and corresponding blocks were chosen for appropriate representative tissue. The region of the block to be “cored” was circled on an H&E stained slide and 3 (0.6 mm) cores were used to build a tissue microarray (TMA) containing, respectively, 45 and 48 newly diagnosed grade 3 and 4 astrocytomas and controls using the Beacher ATA-27 microarray constructor. Matched slides were then stained with a Prestige® polyclonal antihuman CD14 antibody (Sigma-Aldrich) and antihuman CCL2 (R&D Systems) processed in the Mayo Clinic Tissue and Cell Molecular Analysis Laboratory. There were multiple samples of each tumor on the array. Intensity of staining was scored from 0 to 3 and the overall tumor staining determined by the average of the scorable tissue from each tumor. To determined correlative staining between CD14 and CCL2 the staining in each sample was compared with the identical samples on the other array with the correlation done between all matching samples.
Statistical Analyses
Values between groups of data were tested for statistical significance using the 2-tailed Student t test for unpaired samples. Correlation analysis was performed using Pearson correlation with the coefficient of correlation (r2) calculated using Prism, version 4.0 software (GraphPad Software). The significance level was set at probability of significance set at less than .05 with specific calculated P values provided when applicable.
Results
Systemic Immunosuppression in GBM Patients
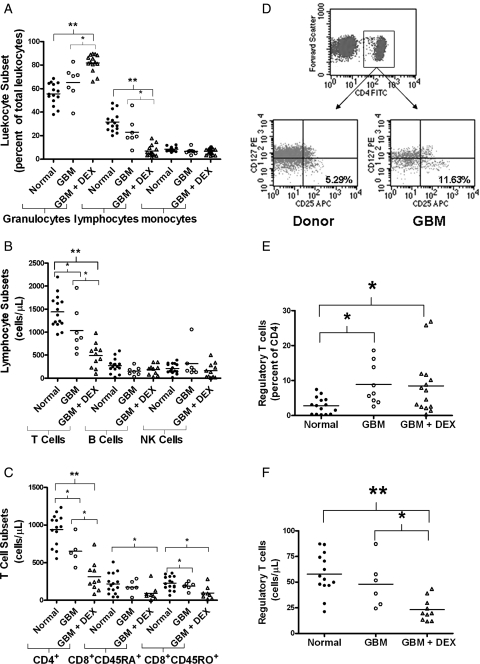
We analyzed the immune phenotypes on peripheral whole blood collected from 26 patients with GBM (17 newly diagnosed and 9 recurrent patients). Immune phenotypes from 15 age-matched normal donors were used for controls. GBM patients were subgrouped by DEX use where indicated. GBM patients with no current DEX treatment had similar ratios of granulocytes, lymphocytes, and monocytes compared with that of normal donors (Fig. 1A; numerical values and P values are listed in Supplementary Material, Tables 2 and 3). However, those patients treated with DEX had more granulocytes, fewer lymphocytes, but similar monocytes compared with untreated GBM patients or normal donors. The lymphopenia was mainly a reduction of T cells (cells/μL whole blood) as we saw no difference between B cells and NK cell numbers (Fig. 1B). The CD4 count within the T cell population was significantly lower in GBM patients and was further reduced with DEX treatment (GBM + DEX; Fig. 1C). GBM + DEX patients exhibited significantly lower naïve and memory CD8 T cells per microliter than healthy donors, but untreated GBM patients only had a difference in memory T cells compared with healthy donors (Fig. 1C). We found the percentage of immunosuppressive regulatory T cells (Tregs, CD4+CD25+CD127lo 23 with representative gating shown in Fig. 1D) within the CD4 population significantly higher in GBM patients compared with controls, and this effect was similar with patients receiving DEX (Fig. 1E). Despite the increase in percentage of circulating Tregs in both GBM and GBM + DEX patients, DEX treatment significantly lowered the absolute count of Tregs in circulation (Fig. 1F). These results demonstrate that GBM patients have profound T cell deficiencies with lymphopenia in the CD4 compartments and a relative increase in circulating Tregs. DEX treatment exacerbates specific aspects of this deficit, namely a lymphopenia concentrated within the T cell population without significantly increasing the ratio of circulating Tregs.
Fig. 1.
Systemic immune suppression in GBM patients is exacerbated by DEX. Analysis of peripheral blood immunophenotypes from normal donors (normal; filled circles) GBM patients (open circles) or GBM patients on DEX (GBM + DEX; triangles) by flow cytometry. (A) Percentage of major leukocyte subsets (granulocytes, lymphocytes, and monocytes) by gating on size and granularity (forward scatter vs side scatter). (B) Absolute counts (cells per microliter) of CD45+ lymphocytes including T cells (CD3+), B cells (CD3−CD19+), NK cells (CD3−CD16+CD56+). (C) Absolute counts (cells per microliter) of CD4+, CD45RA+ naïve, and CD45RO+ memory CD8+ cells. (D) Gating strategy for CD4+/CD25+/CD127lo Regulatory T cells. Representative dot plots are shown. CD4 positive lymphocytes were further subgrouped for CD25 and CD127. Regulatory T cells were reported by calculating them as a percent of total CD4 cells (E) and an absolute number (F) after multiplying the percent of Tregs per CD4 population by the number of CD4 cells counted per microliter in (C). Line in each column represents the mean with comparisons between sets indicated by brackets with * representing P < .05 and **P < .0001.
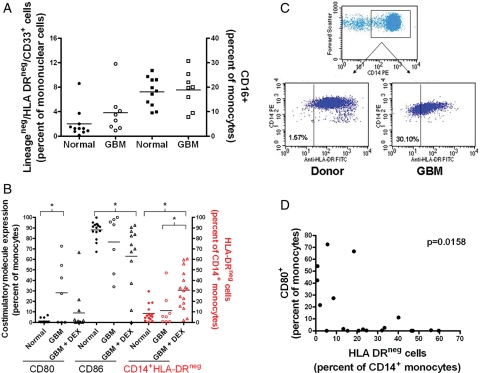
We also studied the expression of costimulatory and antigen presenting molecules on monocytes, the frequency of MDSCs (lineagenegHLA-DRnegCD33+,24 CD14+HLA-DRlo/neg,25,26 and CD16+ monocytes27). GBM patients and healthy donors had similar levels of LineagenegHLA-DRnegCD33+ MDSCs and CD16+ monocytes (Fig. 2A). We have previously shown that LineagenegHLA-DRnegCD33+ MDSCs, when calculated based on the percent of Lineageneg cells were significantly increased in GBM patients.28 In the current study, we additionally found changes in expression of costimulatory and antigen presenting molecules on monocytes. Monocytes from healthy donors were CD80−/loCD86hi. The phenotype of the monocytes in the periphery changed in GBM patients and was dependent of DEX treatment. GBM patients, not on DEX, had a higher frequency of CD80+ monocytes (Fig. 2B), while those on DEX were more likely to have a reduced frequency of CD86+. We also observed a profound increase in the frequency of CD14+ monocytes with low to negative expression of HLA-DR in DEX-treated patients (30.7% ± 4.6%, GBM + DEX patients; 11.3% ± 5.6%, DEX-naive GBM patients; 8.5% ± 2.1%, healthy donors; Fig. 2B in red and dot plots in Fig. 2C). We saw similar results when measuring the mean fluorescence intensity of monocyte HLA-DR expression in the cohort (data not shown). Although CD80 expression was not changed with DEX treatment, we did find an inverse correlation between CD80 expression and HLA-DR expression (Fig. 2D), suggesting distinct abnormal monocyte populations. We also found a positive correlation between the frequencies of CD14+HLA-DRlo/neg monocytes and circulating Tregs (Supplementary Material, Fig. S1). To summarize, abnormal monocyte phenotypes in GBM patients include CD14+HLA-DR+CD80+ or CD14+HLA-DRlo/negCD80−monocytes.
Fig. 2.
DEX contributes to abnormal monocyte phenotype in GBM. Monocytes from whole blood were phenotyped by flow cytometry. (A) Percentage of Lineageneg /HLA-DRneg/CD33+ MDSCs as a percentage of total mononuclear cells and CD16+ monocytes as a percentage of total monocytes in normal donors (filled squares) and GBM patients (open squares). (B) CD80 and CD86 costimulatory molecule expression as a percentage of the cells in the monocyte forward and side scatter gate. Percentage of CD14+/HLA-DRneg monocytes vs total CD14+ monocytes (in red). Normal donors are shown as filled circles, GBM patients not receiving DEX treatment as open circles, and those patients receiving DEX as open triangles. (C) Dot plots showing HLA-DR gating strategy. Gated monocytes (FSC vs SSC) were plotted as FSC vs CD14. CD14+ gated monocytes were plotted against HLA-DR. (D) Correlation between CD80 expression on monocytes and HLA-DR negative monocytes. Lines represent the mean with comparisons in brackets where * indicates significant difference (P < .05).
CD14+ HLA-DRLo/Neg Monocytes in GBM Patients Are Immunosuppressive
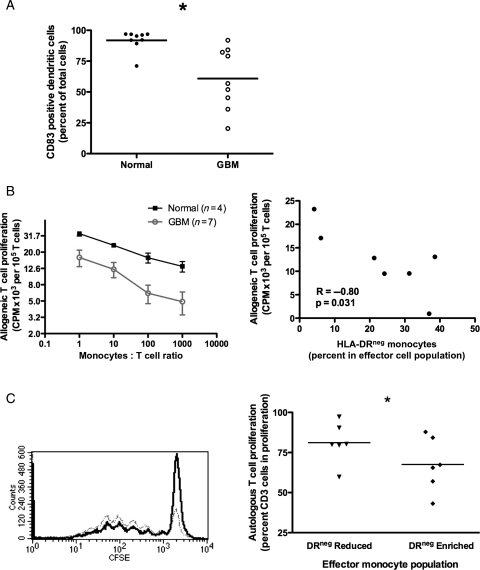
We analyzed the efficiency of differentiation of CD14+ monocytes into mDC using a standard in vitro DC culture method.29,30 CD14+ monocytes from GBM patients were deficient in their ability to differentiate into mDC (60.8% ± 8.2% CD83+ GBM; 91.8% ± 2.8% normals, n = 9, P = .003; Fig. 3A). Compared with healthy donors, CD14+ monocytes from GBM patients were less capable of stimulating T cell proliferation (Fig. 3B). The observed reduction in T cell proliferation was correlated with increased ratio of HLA-DRlo/neg monocytes (Fig. 3B, right panel for 1:10 monocyte:T cell ratio). Therefore, CD14+ monocytes from GBM patients are defective in direct T cell stimulation and DC differentiation capacity, and they retain their immunosuppressive phenotype in vitro.
Fig. 3.
CD14+HLA-DRlo/neg monocytes suppress immune function. (A) Generation of mature DCs is decreased from monocytes of GBM patients (open circles) compared with that of healthy donors (filled circles). (B) Monocytes from GBM patients (circles) have decreased capacity to stimulate T cell proliferation compared with healthy donors (squares) in MLR (left panel). This reduced capacity is inversely correlated with the percent of HLA-DRlo/neg monocytes (right panel; 1:10 monocytes:T cell ratio). (C) HLA-DRlo/neg enriched monocytes from healthy donors (black) inhibited autologous T cell proliferation compared with the HLA-DRlo/neg reduced monocyte population (Gray; Left panel, histogram of T cell CFSE intensity from one donor). Percent of T cells going into proliferation from three healthy donors is shown on the right panel (* P < .05 for all panels).
Due to severe lymphopenia in GBM patients, we examined the effect of HLA-DRlo/neg monocyte on autologous T cell proliferation in healthy donors. The ratio of proliferating T cells was reduced when cultured with autologous HLA-DRlo/neg enriched monocytes (67.5% ± 6.8% vs 81.2% ± 5.2%, n = 6, P = .002, Fig. 3C). Thus, the HLA-DRlo/neg cells of nontumor bearing hosts are capable of suppressing autologous T cell proliferation. Similarly, these cells isolated from healthy donors were less likely to differentiate to mDC than HLA-DR+ cells (data not shown).
Tumor Derived Factors Mediate HLA-DR Expression on Monocytes
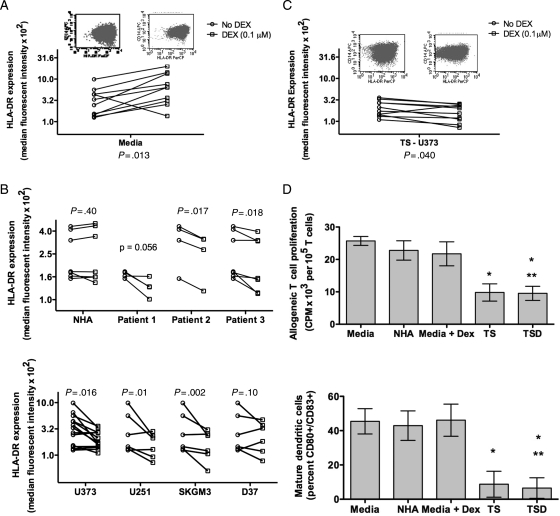
To determine the source of DEX mediated loss of HLA-DR on monocytes, we incubated monocytes from healthy donors with typical plasma levels of DEX. Surprisingly, we found that DEX increased the expression of HLA-DR (Fig. 4A). We have previously shown that DEX can inhibit DC maturation in vitro, inducing altered phenotypes.31 However, those studies did not investigate the HLA-DR phenotype. The data suggest that DEX alone may not be sufficient to generate the high levels of HLA-DRlo/neg monocytes seen in GBM patients. Further studies will be necessary to determine the contribution of DEX to monocyte phenotype in vivo.
Fig. 4.
GBM and DEX-derived effect on monocytes from healthy donors. (A) HLA-DR expression on monocytes increased after culture in media with DEX (square) compared with media alone (circle). (B) Compared with culture with media alone (circle), HLA-DR expression on monocytes were not changed with supernatants (square) from NHA (top panel) but decreased with supernatants from cultures of patients' primary tumors (top panel) and GBM cell lines (bottom panel). (C) Culture with supernatant of U373 with DEX further decreased monocytes HLA-DR expression. (D) Monocytes cultured in supernatants from U373 (TS) or U373 with 0.1 µM DEX (TSD) have decreased capacity to stimulate T cell proliferation in MLR compared with those cultured in media alone, media with 0.1 µM DEX, or NHA supernatant (top panel). Mature DC generation is shown on the bottom panel for the same supernatant conditions (for both panels, n = 3; * P < .05 between labeled condition and Media; ** P < .05 between labeled condition and Media + DEX.).
It has previously been shown that tumor supernatants can produce CD33+HLA-DR−Lin− MDSC from normal monocytes.28 To determine if tumor derived factors may cause loss of DR expression, monocytes from healthy donors were cultured with cell-free supernatants from NHA, primary GBM cell cultures, or GBM tumor cell lines. Supernatants from NHA did not alter HLA-DR expression but monocyte HLA-DR expression was reduced from cultures with supernatants from 2 of the 3 primary tumors and 3 of the 4 GBM tumor cell lines (Fig. 4B). The changes were not associated with culture dependent lactic acid production (data not shown). Thus, some primary tumor cultures and cell lines secrete factors sufficient to initiate the loss of monocyte HLA-DR expression in vitro. We repeated these experiments adding DEX to the media during tumor culture. Culture supernatants from DEX treated tumor cells further reduced normal monocyte HLA-DR expression compared with supernatant from tumor cultures without DEX (P = .04) and to DEX treatment alone (P < .01; Fig. 4C). Thus, tumor derived factors alone were capable of converting CD14+HLA-DR+ cells to CD14+HLA-DRlo/neg cells and this conversion was increased with DEX incubation with the tumor cells. It should be noted that the effect observed with DEX required careful titration of the drug as too much DEX caused the increase in DR expression seen in the monocytes alone. Therefore, additional work is required to fully understand the interactions between DEX, tumor, and monocytes.
Finally, we tested the function of in vitro generated CD14+HLA-DRlo/neg cells in analogous assays used for our GBM samples. Cells cultured with media, NHA supernatants, or media with DEX stimulated T cell proliferation and mDC differentiation similarly. Monocytes cultured in tumor cell culture supernatant or supernatants from tumor cell cultures treated with DEX were reduced in their capacity to stimulate allogeneic T cell proliferation and to generate mDC (Fig. 4D). The reduction in functional capacity was comparable to that observed in monocytes from GBM patients. We did not observe functional differences between cells treated with tumor or tumor plus DEX supernatants, likely due to the profound inhibition of the tumor supernatants alone. Our data strongly suggest that some tumors are capable of altering the phenotype of HLA-DR+ monocytes to HLA-DRlo/neg, that these effects can be exacerbated by tumor treatment with DEX, and that the changes induced in these monocytes translate into persistently impaired monocyte function. However, further work needs to be done to fully understand the combination of tumor-derived factors and their interaction with DEX.
Cytokines and Chemokines in GBM Patient Plasma
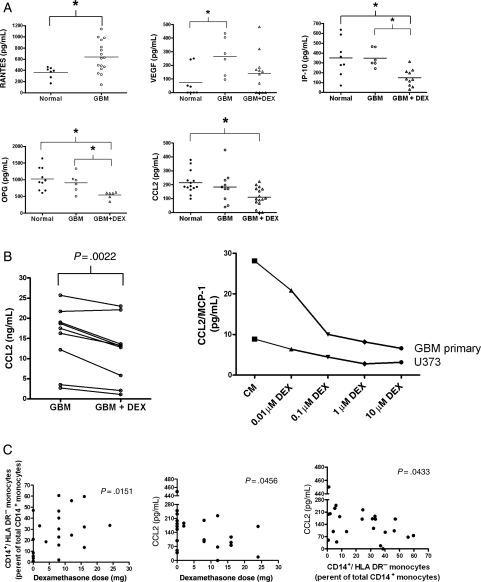
We hypothesized that soluble, tumor-derived immune suppressive molecules responsible for the loss of DR expression would be detectable in the plasma of GBM patients. We analyzed the plasma levels of 27 cytokines and chemokines. The soluble factors that were undetectable or not differentially expressed between healthy donors and GBM patients are listed in Supplementary Material, Table S4. Notably absent were cytokines with known immune suppressive qualities such as IL-10, TGF-β, IL-4, and IL-6. However, we noted altered levels of cytokines and chemotatic chemokines involved in recruitment of monocytes and macrophages and pro-angiogenic factors. Factors increased in GBM patients included RANTES/CCL5 (all GBM patients in this group) and VEGF (Fig. 5A and Supplementary Material, Table S5). DEX treatment decreased plasma levels of IP-10/CXCL10, OPG, and CCL2.
Fig. 5.
Secretion of monocyte-affecting cytokines and growth factors are altered by tumor derived factors and DEX. (A) Plasma samples of peripheral whole blood were analyzed for cytokines and growth factors using the 22-Plex (Upstate) kit or ELISA. Cytokines with significant differences are shown. Normals donors are represented in filled circles, GBM patients in open circles, and GBM patients receiving DEX in open triangles. For RANTES samples, all GBM patients were grouped together. (B) Analysis of cell culture supernatants from primary and established GBM cell lines grown in the presence or absence in DEX. Each point to point connection represents paired samples prior to or after treatment with 0.1 µM DEX for 72 hours. One primary GBM cell line and one established GBM cell line, U373, were also subjected to various doses of DEX treatment. A representative experiment is shown. (C) Correlation between DEX dose, CD14+HLA-DRlo/neg monocytes, and plasma CCL2. * indicates significant difference (P < .05).
Supernatants of cell cultures were analyzed for these cytokines from four glioma cell lines and five primary GBM cell cultures in the presence and absence of 0.1 µM DEX. DEX treatment decreased CCL2 by an average of 22% across all cell lines and did so in a dose dependent manner (Fig. 5B). DEX treatment did not significantly change the levels of OPG, IP-10, and VEGF. These data show a correlation with DEX treatment and tumor CCL2 secretion. Changes in CCL2 secretion in DEX-treated tumor cell lines are consistent with changes seen in plasma of DEX treated patients. CCL2 plasma concentrations were inversely correlated with DEX dose (Fig. 5C). As CCL2 is one of the most potent chemoattractants of monocytes,32–34 we hypothesized that decrease in CCL2 plasma level would correlate with decreased tumor recruitment of monocytes and increased release of monocytes from tumor microenvironment. On the basis of in vitro evidence that tumor secreted factors can decrease HLA-DR expression on monocytes, we also hypothesized that the percentage of CD14+HLA-DRlo/neg monocytes would be increased in circulation with decreased plasma CCL2. Indeed, we found that the rise in percentage of CD14+HLA-DRlo/neg monocytes in DEX-treated GBM patients was correlated to increasing DEX dose and decreasing CCL2 level (Fig. 5C). Therefore, the in vitro DEX effect on tumor secretion of CCL2 is consistent with observed changed in patient plasma CCL2 level following DEX treatment; and the reduction in plasma CCL2 concentration is accompanied by an increase in percentage of CD14+ HLA-DRlo/neg monocytes.
CD14+ Monocytes in the Tumor Microenvironment
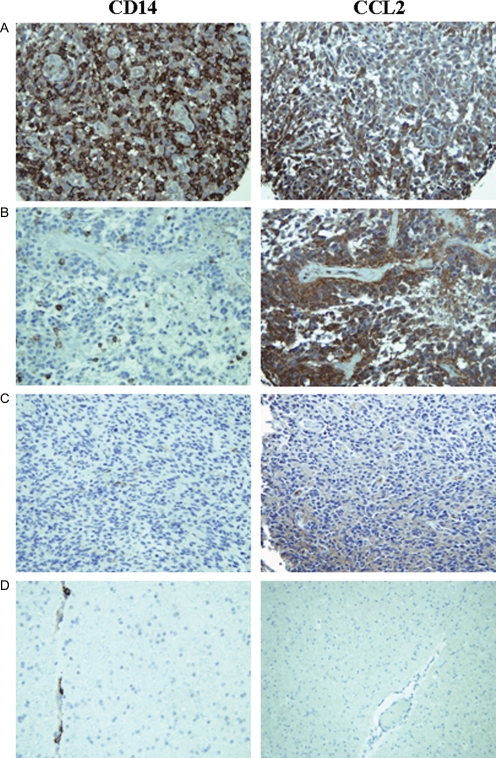
Our finding of high CCL2 secretion in certain primary GBM cell lines suggests that tumors are able to recruit monocytes to the tumor microenvironment. Our data also suggests a mechanism of generating CD14+HLA-DRlo/neg cells via local access to tumor-secreted factors. Thus, we hypothesized that GBM tumors should contain CD14+ cells and their presence reflects the presence of CCL2 in tumor microenvironment. Figure 6A–C show examples of the kind of staining observed for CD14 and CCL2. Staining was specific for these proteins: there was very little staining in nonmalignant gliosis (Fig. 6D); CD14 staining was on the surface of monocytes/microglia; and CCL2 staining was found in the cytoplasm of tumor cells (Supplementary Material, Fig. S2). In the samples we tested representing tumors from 80 patients, the number of CD14+ cells ranged from less than 1% of total cells to more than 50%. Most of the tumors demonstrated high level of staining (47.5% or 38 tumor specimens) with the remainder split between tumors with medium and low CD14 staining (26.3%; or 21 tumors staining at each level). The level of CD14 staining correlated significantly (P < .0001) to the identical tumor samples similarly stained and scored tumors for CCL2.
Fig. 6.
CD14+ monocytes are found in GBM tumors and their presence correlates to CCL2 expression by tumors. TMAs spotted with GBM tumors were stained with an antihuman CD14 antibody and an identical array stained with CCL2 antibody. (A) An example of a GBM tumor showing high CD14 staining, high CCL2 staining. (B) An example of a GBM tumor showing moderate CD14 staining and high CCL2 staining. (C) An example of a GBM tumor from showing both low CD14 and CCL2 staining. (D) CD14 and CCL2 staining of human brain gliosis.
Discussion
Understanding the source and consequences of systemic immune suppression in GBM should lead to improved prognosis for patients. Many conventional antitumor therapies such as chemotherapy, radiation, and surgery contribute to suppressed immunity and confound attempts to understand the source of systemic immune suppression. Investigations using tumor cell lines or animal models often focus on contributions of single factors to immune suppression. Reported GBM-mediated immune defects include reduced T cell and CD4 populations, elevated Treg cell populations, and altered monocyte phenotypes6,8–10 with at least one example of a failure to differentiate into mDC.8 However, few studies have looked at the tumor and tumor/therapy combinations and the resulting immune profile (phenotype, secretome, and function). We have identified tumor and therapy specific influences on immunity. Tumor mediated systemic immune suppression was observed in the number of circulating T cells, monocyte phenotype and circulating growth factor, and chemokine levels.
Our data shows that DEX exacerbates some aspects of tumor initiated immune suppression. For example, DEX-treated GBM patients become more lymphopenic with higher populations of granulocytes, and altered costimulatory molecule expression on monocytes. In addition, DEX treatment also induced specific changes in plasma levels of many cytokines and a large increase in the frequency of an abnormal population of circulating monocytes characterized by CD14+HLA-DRlo/neg.
MDSCs have been associated with tumor-mediated immune suppression.24,35–37 In mouse models, MDSCs are CD11b+/Gr-1+ mononuclear cells and shown to suppress antitumor immunity by inhibiting effector T cell function, proliferation, and/or upregulating T regulatory cells.24,37–39 In humans, MDSCs comprise a heterogeneous population of immature and mature myelomonocytic cells characterized by several different phenotypic markers including LineagenegHLA-DRnegCD33+,35 CD34+/CD33+/CD15neg,40 and CD14loCD16+.27 We have reported that LineagenegHLA-DRnegCD33+ MDSCs measured as a percent of Lineageneg accumulate in peripheral blood in GBM patients.28 As measured here, this population was not elevated in our current cohort and did not appear to be affected by DEX treatment. The monocyte phenotype (CD14+HLA-DRlo/neg) that we describe here has been identified in melanoma25 hepatocellular carcinoma.26 Unlike these studies, however, we commonly found GBM patients with more than one-third of their circulating monocytes with a loss of HLA-DR expression. This myeloid phenotype is of particular interest in that CD14+HLA-DRlo/neg are markers of immune suppression in nonmalignant pathologies. For example, CD14+HLA-DRlo/neg cells are clinically useful in predicting the outcomes of patients with sepsis,19,20 acute pancreatitis,41,42 and liver failure.43 In all cases, CD14+HLA-DRlo/neg monocyte presence was positively correlated with a poorer prognosis and/or higher mortality. Reduction in HLA-DR expression on monotyces has been previously observed in GBM patients8,9 but the functional immunosuppressive consequences of these HLA-DRlo/neg cells were not examined. CD14+HLA-DRlo/neg monocytes were not the only abnormal monocyte phenotype we observed in GBM. However, HLA-DRlo/neg monocytes are likely powerful suppressors of systemic immunity given that they persist after elimination of the induction signal, resist DC differentiation, and inhibit T cell stimulation.
DEX can have independent effects on the immune response including inhibiting DC maturation and altering some myeloid functions. In patients suffering from septic shock, both endogenous cortisol and glucocorticoids down regulates HLA-DR transcription by decreasing the class II transactivator A mRNA levels.44 However, our data suggest that in short term cultures at plasma concentrations, DEX alone is not singularly responsible for the development of the CD14+HLA-DRlo/neg phenotype. We found that cell-free tumor supernatants were capable of generating the CD14+HLA-DRlo/neg phenotype and that tumor cultures treated with DEX enhanced this response. Thus, our data argues for an indirect immune suppressive effect of DEX via tumor mediated mechanisms.
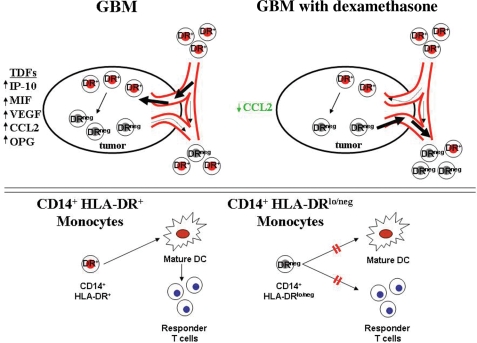
Tumor mediated generation of CD14+HLA-DRlo/neg cells likely requires juxtaposition of monocytes with tumor. It is likely that many tumor secreted cytokines and growth factors are involved in the recruitment and conversion of normal monocytes into CD14+HLA-DRlo/neg monocytes. We identified altered plasma levels of RANTES, VEGF, CCL2, IP-10, all of which are migration-inducing molecules for attracting monocytes to the tumor microenvironment.45 Of these, CCL2 is one of the most potent molecules for monocyte migration46,47 and has been associated with tumor progression and recruitment of MDSCs to breast, gastric, and ovarian tumors.48 CCL2 mediated CD14+ monocyte migration to the brain has recently been confirmed as part of the normal biology of the generation of brain microglia.49 We have demonstrated that tumor secreted factors can decrease HLA-DR expression on normal monocytes and that this effect can be exacerbated by DEX treatment of tumor (Fig. 4). We have identified a correlation between the presence of CD14+ cells in the tumor and high levels of tumor derived CCL2 (Fig. 6), decreasing CCL2 levels with increasing DEX dose, and decreasing CCL2 levels and increasing numbers of circulating CD14+HLA-DRlo/neg monocytes (Fig. 5). On the basis of these data, we propose a model of tumor mediated monocyte recruitment and transformation into DRlo/neg suppressive monocytes in human GBM tumors (Fig. 7). Subsequently, the CD14+HLA-DRlo/neg monocytes inhibit the adaptive immune response by preventing mDCs differentiation and indirect inhibition of T cell responses. DEX acts on multiple parts of this pathway. Although we cannot rule out long-term effects of DEX in vivo, we have no evidence that at the concentrations we use, DEX can directly reduce DR expression on the monocytes. However, at these concentrations DEX did enhance tumor-mediated reduction of HLA-DR on monocytes. More importantly, DEX was associated with reduced tumor expression and plasma CCL2 suggesting release of monocytes from the tumor and attenuating the migratory signal of monocytes to the tumor. These cells spread to the periphery promoting an environment of systemic immunosuppression. This model remains to be fully tested to determine the actual role of each of these components within this system.
Fig. 7.
Proposed model of GBM induced immunosuppression by CD14+HLA-DRlo/neg monocytes. In GBM, tumor derived factors like CCL2 recruit CD14+ cells to the tumor environment whereby they are exposed to tumor derived factors and other immunosuppressive cytokines. Subsequently, CD14+HLA-DR+ monocytes become CD14+HLA-DRlo/neg monocytes. DEX down-regulates specific cytokine expression within the tumor (like CCL2), thus inhibiting the recruitment of monocytes to the tumor or releasing of CD14+HLA-DRlo/neg monocytes from the tumor microenvironment into the bloodstream (upper panel). Under normal conditions, CD14+HLA-DR+ monocytes differentiate into mature DCs and activate responder T cells. CD14+HLA-DRlo/neg monocytes are unable to fully differentiate into mature DCs and also directly inhibit T cell responses.
We found that some GBM cell lines did not convert CD14+HLA-DR+ monocytes to CD14+HLA-DRlo/neg monocytes (Fig. 5C) and that some patients receiving DEX did not have abnormal levels of CD14+HLA-DRneg monocytes (Fig. 2B). These observations would suggest that our model only applies to those GBM tumors that are capable of converting normal monocytes into immunosuppressive monocytes. As such, further studies will be required to determine the mechanism(s) of this process. The presence of monocytes in the GBM tumors has additional implications for the biology of GBM. For example, increased monocyte recruitment to the tumor leads to increased angiogenesis50,51 and alterations in blood brain barrier permeability resulting in cerebral edema.52,53
With the limited yet promising results of Phase I clinical trials involving various immunotherapies and vaccines for GBM, understanding the mechanisms of immunological defects in these patients can identify strategies to improve therapy. As monocytes are an important intermediary for DC based vaccines, CD14+HLA-DRlo/neg monocytes may be both a novel prognostic biomarker and an important suppressor to overcome in designing vaccine therapies.
Supplementary Material
Supplementary material is available at Neuro-Oncology online.
Funding
This work was supported by the BRAIN Cancer SPORE Project P50 CA 108961. Y.L. is supported in part by the Gerstner Family Foundation Career Development Award in Individualized Medicine.
Acknowledgements
This research was presented in two abstracts at the American Association for Cancer Research Annual Meeting (April 12–16, 2008, San Diego, CA). We thank Deb Sprau and Sue Steinmetz for their assistance as clinical coordinators, The Brain Cancer SPORE for providing TMA slides, Dr. Caterina Giannini, and Amanda Rynearson for subsequent analysis of TMAs, and the Tissue and Cellular Molecular Analysis (TACMA) Lab for their assistance in staining the TMAs. We would also like to thank Dr. Stanimir Vuk-Pavlovic for his support of space, equipment, and advice to this work.
Conflict of interest statement. None declared.
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Emens LA, Jaffe EM. Leveraging the activity of tumor vaccines with cytotoxic chemotherapy. Cancer Res. 2005;65:8059–8064. doi: 10.1158/0008-5472.CAN-05-1797. [DOI] [PubMed] [Google Scholar]
- 3.Gustafson MP, Knutson KL, Dietz AB. Therapeutic vaccines for malignant brain tumors. Biologics. 2008;2(4):753–761. doi: 10.2147/btt.s3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haynes NM, van der Most RG, Lake RA, Smyth MJ. Immunogenic anti-cancer chemotherapy as an emerging concept. Curr Opin Immunol. 2008;20:1–13. doi: 10.1016/j.coi.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Brooks WH, Roszman TL, Mahaley MS, Woosley RE. Immunobiology of primary intracranial tumors: II. Analysis of lymphocyte subpopulations in patients with primary brain tumors. Clin Exp Immunol. 1977;29:61–66. [PMC free article] [PubMed] [Google Scholar]
- 6.Rapp M, Ozcan Z, Steiger HJ, Wernet P, Sabel MC, Sorg RV. Cellular immunity of patients with malignant glioma: prerequisites for dendritic cell vaccination immunotherapy. J Neurosurg. 2006;105:41–50. doi: 10.3171/jns.2006.105.1.41. [DOI] [PubMed] [Google Scholar]
- 7.Roszman T, Elliott L, Brooks W. Modulation of T-cell function by gliomas. Immunol Today. 1991;12:370–374. doi: 10.1016/0167-5699(91)90068-5. [DOI] [PubMed] [Google Scholar]
- 8.Ogden AT, Horgan D, Waziri A, et al. Defective receptor expression and dendritic cell differentiation of monocytes in glioblastomas. Neurosurgery. 2006;59:902–910. doi: 10.1227/01.NEU.0000233907.03070.7B. [DOI] [PubMed] [Google Scholar]
- 9.Woiciechowsky C, Asadullah K, Nestler D, et al. Diminished monocytic HLA-DR expression and ex vivo cytokine secretion capacity in patients with glioblastoma: effect of tumor extirpation. J Neuroimmunol. 1998;84:164–171. doi: 10.1016/s0165-5728(97)00236-1. [DOI] [PubMed] [Google Scholar]
- 10.Fecci PE, Mitchell DA, Whitesides JF, et al. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006;66(6):3294–3302. doi: 10.1158/0008-5472.CAN-05-3773. [DOI] [PubMed] [Google Scholar]
- 11.Zou JP, Morford LA, Chougnet C, et al. Human glioma-induced immunosuppression involves soluble factor(s) that alters monocyte cytokine profile and surface markers. J Immunol. 1999;162:4882–4892. [PubMed] [Google Scholar]
- 12.Ashwell JD, Lu FWM, Vacchio MS. Glucocorticoids in T cell development and function. Annu Rev Immunol. 2000;18:309–345. doi: 10.1146/annurev.immunol.18.1.309. [DOI] [PubMed] [Google Scholar]
- 13.Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004;351:2715–2729. doi: 10.1056/NEJMra033540. [DOI] [PubMed] [Google Scholar]
- 14.Migita K, Eguchi K, Kawabe Y, et al. Apoptosis induction in human peripheral blood T lymphocytes by high-dose steroid therapy. Transplantation. 1997;63:583–587. doi: 10.1097/00007890-199702270-00017. [DOI] [PubMed] [Google Scholar]
- 15.Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids-new mechanisms for old drugs. N Engl J Med. 2005;353:1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 16.Tornatore KM, Reed K, Venuto R. 24-Hour immunologic assessment of CD4+ and CD8+ lympocytes in renal transplant recipients receiving chronic methylprednisolone. Clin Nephrol. 1995;44:290–298. [PubMed] [Google Scholar]
- 17.Hohwieler Schloss M, Freidberg SR, Heatley GJ, Lo TCM. Glucocorticoid dependency as a prognostic factor in radiotherapy for cerebral gliomas. Acta Oncol. 1989;28:51–55. doi: 10.3109/02841868909111181. [DOI] [PubMed] [Google Scholar]
- 18.Hughes MA, Parisi M, Grossman S, Kleinberg L. Primary brain tumors treated with steroids and radiotherapy:low CD4 counts and risk of infection. Int J Radiat Oncol Biol Phys. 2005;62:1423–1426. doi: 10.1016/j.ijrobp.2004.12.085. [DOI] [PubMed] [Google Scholar]
- 19.Lekkou A, Karakantza M, Mouzaki A, Kalfarentzos F, Gogos CA. Cytokine production and monocyte HLA-DR expression as predictors of outcome for patients with community-acquired sever infections. Clin Diagn Lab Immunol. 2004;11:161–167. doi: 10.1128/CDLI.11.1.161-167.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monneret G, Lepape A, Viorin N, et al. Persisting low monocyte human leukocyte antigen-DR expression predicts mortality in septic shock. Intensive Care Med. 2006;32:1175–1183. doi: 10.1007/s00134-006-0204-8. [DOI] [PubMed] [Google Scholar]
- 21.Appay V, Reynard S, Voelter V, Romero P, Speiser DE, Leyvraz S. Immuno-monitoring of CD8+ T cells in whole blood versus PBMC samples. J Immunol Methods. 2006;309:192–199. doi: 10.1016/j.jim.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 22.Dietz AB, Bulur PA, Emery RL, et al. A novel source of viable peripheral blood mononuclear cells from leukoreduction system chambers. Transfusion. 2006;46(12):2083–2089. doi: 10.1111/j.1537-2995.2006.01033.x. [DOI] [PubMed] [Google Scholar]
- 23.Hartigan-O'Conner DJ, Poon C, Sinclair E, McCune JM. Human CD4+ regulatory T cells express lower levels of the IL-7 receptor alpha chain (CD127), allowing consistent identification and sorting of live cells. J Immunol Methods. 2007;319:41–52. doi: 10.1016/j.jim.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 24.Serafini P, Borello I, Bronte V. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Semin Cancer Biol. 2006;16:53–65. doi: 10.1016/j.semcancer.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Filipazzi P, Valenti R, Huber V, et al. Identification of a new subset of myeloid suppressor cells in pheripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. 2007;25:2546–2553. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 26.Hoechst B, Ormandy LA, Ballmaier M, et al. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4 + CD25 + Foxp3+ T cells. Gastroenterology. 2008;135:234–243. doi: 10.1053/j.gastro.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 27.Ziegler-Heitbrock L. The CD14 + CD16+ blood monocytes: their role in infection and inflammation. J Leukoc Biol. 2007;81:584–592. doi: 10.1189/jlb.0806510. [DOI] [PubMed] [Google Scholar]
- 28.Rodrigues J, Gonzalez G, Zhang L, et al. Normal human monocytes exposed to glioma cells acquire myeloid derived suppressor cell-like properties. Neuro-Oncology. doi: 10.1093/neuonc/nop023. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dietz AB, Bulur PA, Erickson MR, et al. Optimizing preparation of normal dendritic cells and bcr-abl+ mature dendritic cells derived from immunomagnetically purified CD14+ cells. J Hematother. 2000;9(1):95–101. doi: 10.1089/152581600319676. [DOI] [PubMed] [Google Scholar]
- 30.Jonuleit H, Kuhn U, Muller G, et al. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol. 1997;27(12):3135–3142. doi: 10.1002/eji.1830271209. [DOI] [PubMed] [Google Scholar]
- 31.Matasic R, Dietz AB, Vuk-Pavlovic S. Dexamethasone inhibits dendritic cell maturation by redirecting differentiation of a subset of cells. J Leukoc Biol. 1999;66(6):909–914. doi: 10.1002/jlb.66.6.909. [DOI] [PubMed] [Google Scholar]
- 32.Jiang Y, Beller DI, Frendl G, Graves DT. Monocyte chemoattractant protein-1 regulates adhesion molecule expression and cytokine production in human monocytes. J Immunol. 1992;148:2423–2428. [PubMed] [Google Scholar]
- 33.Matsushima K, Larsen CG, DuBois GC, Oppenheim JJ. Purification and characterization of a novel monocyte chemotactic and activating factor produced by a human myelomonocytic cell line. J Exp Med. 1989;169:1485–1490. doi: 10.1084/jem.169.4.1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoshimura T, Robinson EA, Tanaka S, Appella E, Kuratsu J, Leonard EJ. Purification and amino acid analysis of two human glioma-derived monocyte chemoattractants. J Exp Med. 1989;169:1449–1459. doi: 10.1084/jem.169.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fricke I, Mirza N, Dupont J, et al. Vascular endothelial growth factor-trap overcomes defects in dendritic cell differentiation but does not improve antigen-specific immune responses. Clin Cancer Res. 2007;13:4840–4848. doi: 10.1158/1078-0432.CCR-07-0409. [DOI] [PubMed] [Google Scholar]
- 36.Gabrilovich DI, Bronte V, Chen SH, et al. The terminology issue for myeloid-derived suppressor cells. Cancer Res. 2006;67:425. doi: 10.1158/0008-5472.CAN-06-3037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nagaraj S, Gabrilovich DI. Myeloid-derived supressor cells. Adv Exp Med Biol. 2007;601:213–223. doi: 10.1007/978-0-387-72005-0_22. [DOI] [PubMed] [Google Scholar]
- 38.Marigo I, Dolcetti L, Serafini P, Zanovello P, Bronte V. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol Rev. 2008;222:162–179. doi: 10.1111/j.1600-065X.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- 39.Rodriguez PC, Ochoa AC. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives. Immunol Rev. 2008;222:180–191. doi: 10.1111/j.1600-065X.2008.00608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Almand B, Clark JI, Nikitina E, et al. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166:678–689. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 41.Ho Y-P, Sheen IS, Chiu C-T, Wu C-S, Lin C-Y. A strong association between down-regulation of HLA-DR expression and the late mortality in patients with severe acute pancreatitis. Am J Gastroenterol. 2006;101(5):1117–1124. doi: 10.1111/j.1572-0241.2006.00495.x. [DOI] [PubMed] [Google Scholar]
- 42.Mentula P, Kylanpaa-Back ML, Kemppainen E, et al. Decreased HLA (human leukocyte antigen)-DR expression on peripheral blood monocytes predicts the development of organ failure in patients with acute pancreatitis. Clin Sci. 2003;105:409–417. doi: 10.1042/CS20030058. [DOI] [PubMed] [Google Scholar]
- 43.Antoniades CG, Berry PA, Davies ET, et al. Reduced monocyte HLA-DR expression: a novel biomarker of disease severity and outcome in acetaminophen-induced acute liver failure. Hepatology. 2006;44:34–43. doi: 10.1002/hep.21240. [DOI] [PubMed] [Google Scholar]
- 44.Le Tulzo Y, Pangault C, Amiot L, et al. Monocyte human leukocyte antigen-DR transcriptional downregulation by cortisol during septic shock. Am J Respir Crit Care Med. 2004;169(10):1144–1151. doi: 10.1164/rccm.200309-1329OC. [DOI] [PubMed] [Google Scholar]
- 45.Dirkx AE, Oude Egbrink MGA, Wagstaff J, Griffioen AW. Monocyte/macrophage infiltration in tumors: modulators of angiogenesis. J Leukoc Biol. 2006;80:1183–1196. doi: 10.1189/jlb.0905495. [DOI] [PubMed] [Google Scholar]
- 46.Desbaillets I, Tada M, de Tribolet N, Diserens AC, Hamou MF, Van Meir EG. Human astrocytomas and glioblastomas express monocyte chemoattractant protein-1 (MCP-1) in vivo and in vitro. Int J Cancer. 1994;58:240–247. doi: 10.1002/ijc.2910580216. [DOI] [PubMed] [Google Scholar]
- 47.Leung SY, Wong MP, Chung LP, Chan AS, Yuen ST. Monocyte chemoattractant protein-1expression and macrophage infiltration in gliomas. Acta Neuropathol. 1997;93:518–527. doi: 10.1007/s004010050647. [DOI] [PubMed] [Google Scholar]
- 48.Huang B, Lei Z, Zhao J, et al. CCL2/CCR2 pathway mediates recruitment of myeloid suppressor cells to cancers. Cancer Lett. 2007;252:86–92. doi: 10.1016/j.canlet.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 49.Mildner A, Schmidt H, Nitsche M, et al. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat Neurosci. 2007;10:1544–1553. doi: 10.1038/nn2015. [DOI] [PubMed] [Google Scholar]
- 50.Hong KH, Ryu J, Han KH. Monocyte chemoattractant protein-1-induced angiogenesis is mediated by vascular endothelial growth factor-A. Blood. 2005;105:1405–1407. doi: 10.1182/blood-2004-08-3178. [DOI] [PubMed] [Google Scholar]
- 51.Niu J, Azfer A, Zhelyabovska O, Fatma S, Kolattukudy PE. Monocyte chemotactic protein (MCP)-1 promotes angiogenesis via a novel transcription factor, MCP-1-induced protein (MCPIP) J Biol Chem. 2008;283:14542–14551. doi: 10.1074/jbc.M802139200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dimitrijevic OB, Stamatovic SM, Keep RF, Andjelkovic AV. Absence of the chemokine receptor CCR2 protects against cerebral ischemia/reperfusion injury in mice. Stroke. 2007;38:1345–1353. doi: 10.1161/01.STR.0000259709.16654.8f. [DOI] [PubMed] [Google Scholar]
- 53.Stamatovic SM, Stamatovic SM, Shakui P, Keep RF, et al. Monocyte chemoattractant protein-1 regulation of blood-brain barrier permeability. J Cereb Blood Flow Metab. 2005;25:593–606. doi: 10.1038/sj.jcbfm.9600055. [DOI] [PubMed] [Google Scholar]