Abstract
Tumor necrosis factor–related apoptosis-inducing ligand (TRAIL/Apo2 L) preferentially induces apoptosis in human tumor cells through its cognate death receptors DR4 or DR5, thereby being investigated as a potential agent for cancer therapy. Here, we applied fully human anti-human TRAIL receptor monoclonal antibodies (mAbs) to specifically target one of death receptors for TRAIL in human glioma cells, which could also reduce potential TRAIL-induced toxicity in humans. Twelve human glioma cell lines treated with several fully human anti-human TRAIL receptor mAbs were sensitive to only anti-DR5 mAbs, whereas they were totally insensitive to anti-DR4 mAb. Treatment with anti-DR5 mAbs exerted rapid cytotoxicity and lead to apoptosis induction. The cellular sensitivity was closely associated with cell-surface expression of DR5. Expression of c-FLIPL, Akt, and Cyclin D1 significantly correlated with sensitivity to anti-DR5 mAbs. Primary cultures of glioma cells were also relatively resistant to anti-DR5 mAbs, exhibiting both lower DR5 and higher c-FLIPL expression. Downregulation of c-FLIPL expression resulted in the sensitization of human glioma cells to anti-DR5 mAbs, whereas overexpression of c-FLIPL conferred resistance to anti-DR5 mAb. Treatment of tumor-burden nude mice with the direct agonist anti-DR5 mAb KMTR2 significantly suppressed growth of subcutaneous glioma xenografts leading to complete regression. Similarly, treatment of nude mice bearing intracerebral glioma xenografts with KMTR2 significantly elongated lifespan without tumor recurrence. These results suggest that DR5 is the predominant TRAIL receptor mediating apoptotic signals in human glioma cells, and sensitivity to anti-DR5 mAbs was determined at least in part by the expression level of c-FLIPL and Akt. Specific targeting of death receptor pathway through DR5 using fully human mAbs might provide a novel therapeutic strategy for intractable malignant gliomas.
Keywords: c-FLIPL, glioblastoma, monoclonal antibody, TRAIL, TRAIL-R2/DR5
Malignant glioma, the most frequent primary intrinsic neoplasm arising in the central nervous system, remains incurable despite multimodal intensive treatments comprising maximum surgical resection, radiotherapy, and chemotherapy. Prognosis of patients with glioblastoma multiforme (GBM), the most malignant WHO grade IV glioma, has been dismal, with median survival time being only 12–15 months from initial diagnosis.1 Chemotherapy only gives rise to a mild survival benefit using temozolomide given concomitantly with radiotherapy followed by adjuvant administration in patients with GBM;2 thus novel therapeutic strategies which could exert robust tumoricidal activity have been required.
One such approach is to directly activate apoptosis pathways in tumor cells. Chemotherapy and ionizing irradiation trigger apoptosis by provoking the mitochondria-mediated “intrinsic” apoptosis pathway, which is regulated by the Bcl-2 family members and molecules involved in the downstream apoptosome.3 Defects in the mitochondrial pathway may contribute to resistance to these conventional therapies. Activation of “death receptors” through oligomerization by their cognate trimeric ligands can also induce tumor cell apoptosis through the “extrinsic” pathway.4 Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) (also called Apo2 L) is a member of the TNF superfamily which includes FasL (CD95 L) and TNF-α. TRAIL binds to its cognate death receptors, DR4 (TRAIL-R1)5 and DR5 (Killer/TRAIL-R2/TRICK2)6,7 containing a death domain (DD) in their cytoplasmic domain, and is capable of inducing rapid apoptosis through the activation of caspases, a family of cysteine proteases, in tumor cells of diverse origins, but not in most normal cells including astrocytes in vitro,8–13 thereby being investigated as a potential agent for cancer therapy.14
Besides death-inducing TRAIL receptors DR4 and DR5, there are 3 other receptors, DcR1 (TRID/TRAIL-R3),6,7 DcR2 (TRUNDD/TRAIL-R4),15,16 and osteoprotegerin17 acting as decoy receptors by competing with DR4 or DR5 for binding to TRAIL. DR4 and DR5 transcripts are expressed in some glioma cells, while at a low level in the brain.5,7,11,18 Activation of DR4 and/or DR5 causes recruitment of adaptor molecules, preferentially fas-associated death domain protein (FADD), through their DD interaction, which in turn activates the initiator caspase-8 or -10 through their death effector domains (DEDs) (formation of death-inducing signaling complex; DISC).19 This leads to direct activation of the effector caspase-3 or -7 and subsequent progression of irreversible cell death processes. TRAIL may also activate intrinsic mitochondrial apoptosis pathways to variable extents through caspase-8-mediated cleavage of cross-talk protein Bid, where cytochrome c release from mitochondria leads to the formation of the “apoptosome” with Apaf-1, dATP, and procaspase-9, resulting in the activation of an initiator caspase-9 and subsequent progression of the caspase cascade.20,21 Molecules acting on these pathways may confer resistance to TRAIL-induced apoptosis when inadequately expressed. These include reduced expression of FADD, caspase-8, as well as increased expression of TRAIL decoy receptors and intrinsic apoptosis inhibitors, c-FLICE inhibitory protein (FLIP), XIAP, Bcl-2 family members, PEA-15, upregulated Akt and nuclear factor kappa B (NF-κB) signaling, and caspase mutations.22–31
In human glioma cells, a recombinant soluble form of TRAIL (sTRAIL) induces rapid and significant apoptosis, and its cytotoxic activity can be further enhanced by combination use of chemotherapeutic agent cisplatin or ionizing irradiation.9,32 Importantly, systemic administration of human sTRAIL has shown a reasonably safety profile in mice or nonhuman primates,8,33 and furthermore, treatment with the untagged, trimeric sTRAIL has shown no cytotoxicity on human hepatocytes or keratinocytes.34,35 The potential clinical use of sTRAIL has been investigated in clinical trials (Phase II) (Genentech homepage).
Alternative approaches to activate the TRAIL pathway include the use of specific agonistic antibodies36,37 that exploit the receptor discriminating specificity and prolonged bioavailability of IgG. Monoclonal antibodies (mAbs) are now widely applied clinically for cancer therapy; for instance, rituximab, an anti-CD20 mAb for CD20-positive non-Hodgkin B-cell lymphomas, trastuzumab, an anti-Her2/ErbB2 mAb for breast cancers, and bevacizumab, an anti-VEGF mAb for colorectal cancers. Among several types of recent therapeutic mAbs, fully human mAbs have a discrete advantage that they would evade the potential immunological response against the introduced antibody, compared with other mAbs carrying mouse sequences, which could be still immunogenic as fully mouse mAbs. We have generated fully human mAbs to DR4 or DR5 (IgG class), which are specifically bound to the ectodomain of the receptors,38 thereby potently induced the death of cancer cells in vitro.38 A direct agonist mAb to DR5, KMTR2, could suppress the growth of human colon cancer xenografts in vivo without antibody crosslinking,39 independent of host effector function.
These observations prompted us to test whether a fully human anti-TRAIL receptor mAb could effectively induce glioma cell death. Here, we show that DR5 is the predominant TRAIL receptor, which is expressed at the cell surface and mediates apoptotic signals in human glioma cells. Sensitivity of human glioma cells to anti-DR5 mAbs might be determined at least in part by the expression level of c-FLIPL and Akt. Anti-DR5 mAbs exert antitumor effects both in vitro and in vivo. Our results suggest that specific targeting of the death receptor pathway through DR5 using a fully human mAbs might provide a novel therapeutic strategy for intractable malignant gliomas.
Materials and Methods
Reagents
Soluble human recombinant FLAG-TRAIL was prepared as previously described9 and was stored at −80°C. Anti-FLAG monoclonal antibody (mAb) M2 was obtained from Sigma.
Monoclonal Antibodies
Fully human anti-human DR4 mAb, B12, and anti-DR5 mAb, E11, H48, and KMTR2, were described elsewhere.38,39 These mAbs, except KMTR2, require crosslinking with anti-human IgG to be fully active apoptosis inducers. KMTR2 clusters DR5 on the cell surface, thereby inducing apoptosis with or without crosslinking.39
Cells
The human glioma cell lines used were described previously32 and were cultured as described.40 A tumorigenic subpopulation of the T98G cell line, designated T98SQ1, was generated by re-culturing in dish of a T98G xenograft that was established in a nude mouse. Normal human astrocytes (NHA) were purchased from Cambrex (Walkersville, MD), and cultivated in AGM medium per the manufacturer's recommendation. Primary cultures of glioma cells were established by transferring tumor tissues in DMEM growth medium supplemented with 10% FBS immediately after resection, thereafter they were mechanically dispersed and plated into T75 flasks and incubated in DMEM supplemented with 10% FBS, 2 mM glutamine, 100 U/mL penicillin, and 100 µg/mL streptomycin in a humidified atmosphere of 5% CO2 at 37°C. The culture medium was changed every 3–4 days. When cells reached subconfluence, they were passaged using trypsin digestion. Malignant gliomas were surgically removed at Kyorin University Hospital. Patient material was obtained with informed consent and approval from the Institutional Ethics Committee.
Plasmids and Transfection
The plasmid encoding human c-FLIPL and c-FLIPS, pCR3.V64-Met-Flag-FLIPL and pCR3.V62-Met-Flag-FLIPS, respectively, were kind gifts from Dr. Jurg Tschopp (University of Lausanne). The empty vector pCR3.neo was generated by removing an EcoRI fragment from the pCR3.V62-Met-Flag-FLIPS plasmid. Cells were transfected with these plasmids using the calcium phosphate precipitation method and selected in the presence of G418 (Gibco/BRL) to establish G418-resistant subclones, including those expressing high levels of c-FLIPL.
Western Blotting
Whole cell lysates were prepared in RIPA buffer and were subjected to Western blot analyses as previously described.40 Proteins on the polyvinylidene difluoride (PVDF) membranes were probed with antibodies against DR5 (polyclonal, R&D Systems), DR4 (polyclonal, BD PharMingen), DcR1 (Imgenex), DcR2 (polyclonal, Imgenex), FADD (monoclonal, BD Transduction), c-FLIP (monoclonal, ALEXIS Biochemicals), caspase-8 (polyclonal, BD PharMingen), caspase-9 (monoclonal, Trevigen), caspase-3, cleaved caspase-3 (Cell Signaling Technology), XIAP (BD Transduction Laboratories), Apaf-1 (Transduction Laboratories), Akt (Cell Signaling), Survivin (Cell Signaling), Cyclin D1 (NeoMarkers), Bax (Ab-1, NeoMarkers), Bak (Ab-1, Oncogene), Bid (polyclonal, BD PharMingen), Bcl-2 (Ab-1, NeoMarkers), Bcl-XL (polyclonal, BD Transduction), poly(ADP-ribose) polymerase (PARP) (C2-10; Enzyme System Products), and detected by chemiluminescence and quantified (LAS 1000, Fuji). Loading of lysates on membranes was evaluated by β-actin blot.
Flow Cytometry
For the detection of cell surface TRAIL receptors, cells (∼1 × 106 cells/sample) were washed with PBS containing 1% FBS and 0.05% NaN3 and were stained with phycoerythrin (PE)-labeled mouse monoclonal anti-human DR4 or DR5 (eBioscience) on ice for 1 hour. After washing, cells were analyzed by on flow cytometry (FACScan, BD).38 For apoptosis assays, cells were plated overnight and treated for 48 hours. Cells were then collected, fixed in 4% paraformaldehyde and permeabilized in 0.1% Triton-X in 0.1% sodium citrate solution, followed by incubation with terminal deoxynucleotidyl transferase-mediated nick end labeling (TUNEL) solution (Roche) at 37°C for 1 hour. TUNEL-positive cells stained with fluorescein were analyzed by flow cytometry using Cell Quest software.
Growth Inhibition Assays
Cytotoxicity was evaluated using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) survival assays as described.32 Briefly, cells were plated at 1 × 104 cells/well in 96-well microtiter plates overnight. Cells were then treated with 200 µL fresh medium containing drugs, cultured for 48 hours followed by an additional 4 hours with 250 µg/mL MTT, and analyzed using a microplate reader (Molecular Devices). The effects of treatment are expressed as percentage of growth inhibition using untreated cells as the uninhibited control.
TUNEL Assays
Apoptotic cell death was determined by TUNEL assays using In Situ Cell Death Detection Kits (Roche) as described.40
siRNA Treatment
A double-stranded siRNA oligonucleotide mixture against c-FLIPL was purchased from Dharmacon (siGENOME SMARTpool; Dharmacon RNA Technologies). siRNA for nonsilencing control (nontargeting siRNA) (siCtl) is an irrelevant siRNA with random nucleotides and no known specificity. c-FLIPL-siRNA (0.1 µM) or nontargeting siRNA (0.1 µM) was transfected into glioma cells using DharmaFECT, and the cells were used for experiments 24–72 hours after transfection.
In Vivo Study
Human glioma cells (2 × 106 cells) were suspended in 0.1 mL PBS and injected subcutaneously into the right flank of 4- to 5-week-old female nude mice of BALB/CA background (Saitama Experimental Animals Supply, Co. Ltd.). For the treatment of the established xenografts, the tumors were permitted to establish and grow for 20 days (tumor volume ∼120 mm3). For intracerebral stereotactic inoculation, 5 × 105 glioma cells in 5 µL of PBS were inoculated into the right corpus striatum of the mouse brain as described.40 Either anti-DR5 mAb (5 mg/kg) or control nonspecific human IgG (DNP) was administered i.p. daily for 3 consecutive days. The growth of tumors was measured as described.41 Systemic toxicity of the treatments was assessed by change in body weight and by organ inspection at autopsy. Mice were sacrificed by CO2 inhalation when they became moribund. All animal procedures were approved by the Animal Care and Use Committee of the Kyorin University Faculty of Medicine.
Statistical Analysis
The data were analyzed for significance by Mann–Whitney's U-test or Student's t-test. Correlation was analyzed using Spearman's rank correlation test. Survival of mice bearing intracerebral xenografts was calculated according to the Kaplan–Meier method, and differences in survival were evaluated with the log-rank test. All statistical analyses were done using the statistical package SPSS 17.0J (SPSS, Inc.).
Results
Fully Human Anti-human DR5 mAbs Induce Apoptosis in Human Glioma Cells
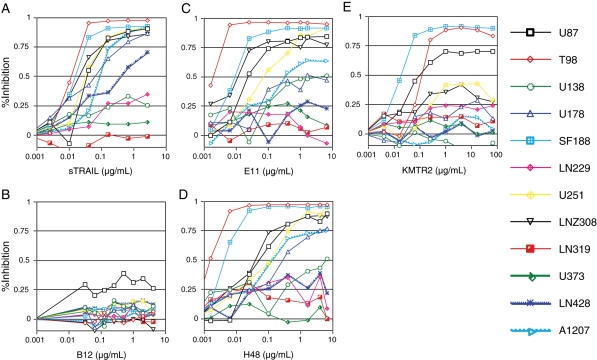
We first sought to determine whether specific targeting of cell surface TRAIL receptors by anti-TRAIL receptor mAbs could induce cytotoxic effects in 12 human glioma cell lines. The anti-TRAIL receptor mAbs, B12 or E11, H48, and KMTR2, have been previously shown to bind specifically to their cognate receptor, either DR4 (B12) or DR5 (E11, H48, and KMTR2), respectively.38,39 Soluble FLAG-tagged TRAIL (sTRAIL) with crosslinkers effectively killed the majority of human glioma cell lines with IC50 values lower than 0.1 µg/mL. Similarly, treatment with either anti-DR5 mAb E11 or H48 resulted in significant cytotoxicity in the presence of crosslinking anti-human IgG antibody in 8 of 12 glioma cell lines (Fig. 1). This was accompanied by changes in morphology typical of apoptotic cell death, which was confirmed by DNA fragmentation detected by TUNEL assays (Fig. 2). Furthermore, treatment with anti-DR5 mAbs lead to cleavage and activation of caspase-8, -9, and -3, as well as Bid, which results in the cleavage of PARP, an intrinsic substrate for executioner caspase-3 (data not shown). The cytotoxicity was abrogated in the presence of TRAIL-neutralizing DR5-Fc (data not shown), suggesting that the binding of antibody to DR5 is essential for the antibody-mediated effects.
Fig. 1.
Cytotoxic effects of soluble TRAIL (A) and fully human mAbs against DR4 (B12, B) and DR5 (E11, C; H48, D; KMTR2, E) in a panel of human glioma cell lines. Cells were treated for 48 hours with either sTRAIL, B12, E11, or H48 in the presence of antibodies for crosslinking, or KMTR2 in the absence of crosslinkers, at doses indicated at the bottom of each panel. Cytotoxicity was determined by MTT assays. Results were reproduced in 2 or more independent experiments; values are expressed as the mean of triplicate wells; bars, SD.
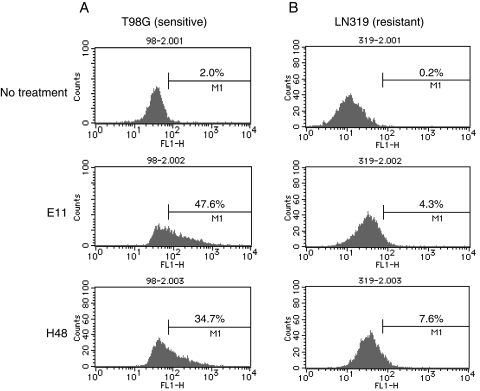
Fig. 2.
Fully human anti-DR5 mAbs induced apoptosis in human glioma cells. TRAIL-sensitive T98G (A) and TRAIL-resistant LN319 (B) cells were treated with or without anti-DR5 mAbs E11 or H48 (0.1 µg/mL). After 48 hours treatment, cells were fixed, permeabilized, and stained for TUNEL, followed by flow cytometry analysis. The percentage of TUNEL-positive cells (fluorescein-positive) is indicated on each graph.
The profiles of cell lines sensitive to anti-DR5 mAbs were identical to that to sTRAIL. IC50 values of sensitive cells, such as T98G, SF188, LNZ308, U87MG, and U251MG, were also lower than 0.1 µg/mL (Table 1). KMTR2 induced cell death effectively in T98G, SF188, and U87MG cells, even in the absence of crosslinkers. In contrast, glioma cells were totally insensitive to treatment with anti-DR4 mAb B12 (Fig. 1). These results suggest that TRAIL induces apoptosis predominantly through the DR5-mediated pathway but not DR4 in human glioma cells.
Table 1.
IC50 values for TRAIL and anti-TRAIL receptor mAbs in human glioma cell lines
| Cell line | sTRAIL | E11 | H48 | B12 |
|---|---|---|---|---|
| U87 | 0.036 | 0.024 | 0.064 | H |
| T98 | 0.013 | 0.002 | 0.001 | H |
| U138 | H | 4 | 6 | H |
| U178 | 0.079 | 1.519 | 0.319 | H |
| SF188 | 0.019 | 0.011 | 0.005 | H |
| LN229 | H | H | H | H |
| U251 | 0.035 | 0.083 | 0.129 | H |
| LNZ308 | 0.0252 | 0.014 | 0.049 | H |
| LN319 | H | H | H | H |
| U373 | H | H | H | H |
| LN428 | 0.46 | H | H | H |
| A1207 | 0.106 | 0.014 | 0.147 | H |
| (µg/mL) | H: >2.56 | H: >6.4 | H: >6.4 | H: >4.0 |
Anti-DR5 mAbs Did Not Affect the Viability of NHA
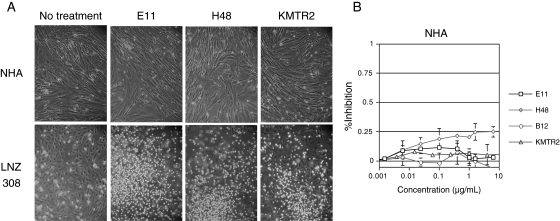
We next examined the effects of the treatments on NHA. Treatments with anti-DR5 mAbs, E11, H48, and KMTR2, and anti-DR4 mAb B12 did not cause cytotoxicity in these cells, even at a high concentration (10 µg/mL), whereas treatment of LNZ308 cells, a positive control of the treatment, did show the expected cytotoxicity (Fig. 3), indicating that NHA are insensitive to the mAb treatment.
Fig. 3.
Fully human anti-DR5 mAbs do not affect viability of NHA. (A) Photomicrographs showing anti-DR5 mAb-induced cytotoxicity in LNZ308 cells, but not in NHA. (B) MTT assay for quantifying cytotoxicity induced by various mAbs against DR4 or DR5 in NHA. Cells were treated with either anti-DR5 mAbs E11, H48, and KMTR2, or anti-DR4 mAb B12 at doses indicated at the bottom of each panel for 48 hours and then were subjected to MTT assays. LNZ308 cells were treated as a positive control for the assay. The experiment was repeated 4 times with the similar results.
Molecular Determinants of Sensitivity to Anti-DR5 mAbs
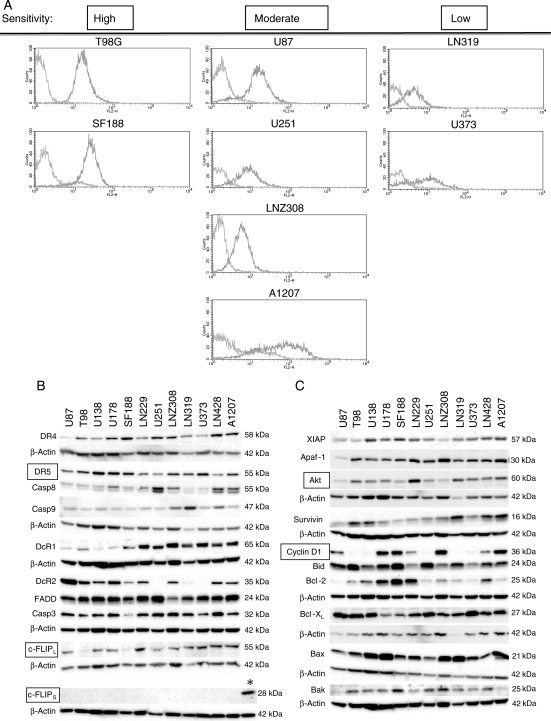
We next sought to determine which key molecules working in the apoptosis-inducing pathways are responsible for sensitivity to anti-DR5 mAb in human glioma cells. As we have demonstrated that the proapoptotic TRAIL-specific receptor DR5 was upregulated by both DNA damaging chemotherapeutic drugs and ionizing irradiation, thereby mediating enhancing TRAIL sensitivity in human glioma cells,9,32 we examined the cell surface expression of DR5 by flow cytometry as well as its whole cellular expression by Western blot. The DR5 cell surface expression level, but not its whole cellular expression level, was weakly associated with the sensitivity of glioma cells to anti-DR5 mAbs (E11: P = .145, H48: P = .118, Spearman's rank correlation) (Fig. 4A and Table 1).
Fig. 4.
Expression levels of apoptosis-related molecules in human glioma cell lines. (A) DR5 cell-surface expression determined by flow cytometry analysis. Cultured cells were washed and reacted with PE-labeled anti-DR5 antibody, followed by flow cytometry analysis. Glioma cells with high sensitivity to anti-DR5 mAbs tended to have high cell-surface expression of DR5, whereas those with low sensitivity were associated with low expression. (B and C) Western blot analyses showing expression levels of various apoptosis-related molecules. Human glioma cells were harvested for whole cell lysates, which were then subjected to Western blotting. Expression levels of c-FLIPL, Akt, and cyclin D1 significantly correlated with sensitivity of these cell lines to treatment with anti-DR5 mAbs. Intrinsic expression of c-FLIPs was undetectable in all cell lines tested, whereas exogenous expression of c-FLIPs was identified in T98G.FLIPs cells (indicated as *). The molecules and their molecular sizes are shown on the left and right sides on each panel, respectively.
Among molecules downstream to DR5, the expression of an intrinsic apoptosis inhibitor c-FLIPL was almost undetectable in highly sensitive T98G and SF188 glioma cell lines, and its expression level significantly correlated with sensitivity to anti-DR5 mAbs (E11: P = .003, H48: P = .006, sTRAIL: P = .008 Spearman's rank correlation) (Fig. 4B). In contrast, expression of the alternative spliced form c-FLIPS was undetectable in all 12 human glioma cell lines tested (positive control of the Western blot was T98G.FLIPs cells). FADD, another key molecule in DISC, and Bcl-2 family molecules, such as Bcl-XL, Bax, Bak, and Bid were irrelevant to the sensitivity. Expression of IAP proteins, other cellular apoptosis inhibitors, did not associated with anti-DR5 mAb sensitivity, either. However, the expression of Akt/PKB, which could contribute to tumor cell proliferation and survival, significantly correlated with the sensitivity (E11: P = .014, H48: P = .017). Furthermore, the expression level of cyclin D1 showed a correlation with the sensitivity as well (E11: P = .045, H48: P = .028) (Fig. 4C). Among the molecules which were found to be significantly correlated with sensitivity to anti-DR5 mAbs, only the expression of Akt and c-FLIPL showed a significant correlation (P = .017).
Involvement of c-FLIPL Expression in Sensitivity to Anti-DR5 mAb
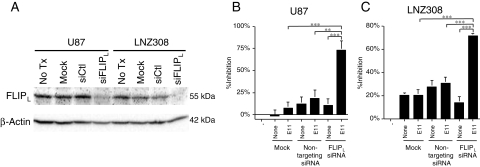
As the expression of c-FLIPL, a key regulator at the DISC, significantly correlated with sensitivity to anti-DR5 mAbs in human glioma cells, we downregulated c-FLIPL expression by using an siRNA specific to human c-FLIPL mRNA to determine its role in resistance to anti-DR5 mAbs. Transfection of c-FLIPL siRNA resulted in a significant decrease of c-FLIPL expression at the protein level in both U87MG and LNZ308 cells (Fig. 5A). Although c-FLIPL downregulation per se did not affect cell viability, E11 treatment induced robust cell death in those cells with downregulated c-FLIPL, but not in control siRNA treated cells (P < .001, t-test) (Fig. 5B).
Fig. 5.
Downregulation of c-FLIPL expression sensitizes human glioma cells to anti-DR5 mAb. (A) Western blot showing reduced expression of c-FLIPL protein upon treatment with siRNA against c-FLIPL (siFLIPL) in U87MG and LNZ308 cells. Cells were transfected with either nontargeting siRNA (siCtl) or siFLIPL and were harvested for the preparation of total lysates after being cultured for 48 hours. Twenty micrograms of total lysate was size fractionated in a 10% SDS–polyacrylamide gel, transferred to a PVDF membrane and reacted with a monoclonal antibody against human c-FLIPL. The β-Actin blot demonstrates the loading of lysate in each lane. No Tx, no treatment. (B and C) Enhanced anti-DR5 mAb-induced cytotoxicity by the suppression of c-FLIPL expression. U87MG (B) or LNZ308 (C) cells were transfected with either siCtl or siFLIPL, followed by treatment with or without E11 at a sublethal concentration (0.01 µg/mL) for 24 hours and then were subjected to the MTT assay. Similar results were obtained when treated with KMTR2. ***P < .001, **P < .01 (Student's t-test).
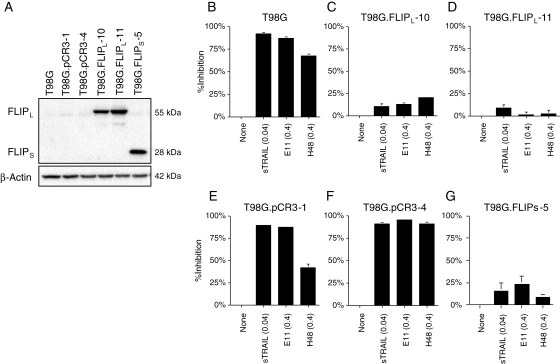
Alternatively, T98G cells lacking c-FLIPL expression were introduced with the c-FLIPL expression vector pCR.FLAG-c-FLIPL, and stable sublines overexpressing c-FLIPL were obtained (Fig. 6A). As expected, overexpression of c-FLIPL resulted in remarkable suppression of cytotoxic effects induced by either E11, H48, or sTRAIL when compared with control cells with empty vector (pCR3) transfection (Fig. 6B–F). These results suggested that c-FLIPL expression confers, at least in part, resistance to anti-DR5 mAbs in human glioma cells, consistent with the good negative correlation observed between the c-FLIPL expression level and anti-DR5 mAb sensitivity in the panel of human glioma cell lines. Similar results were obtained when T98G cells were forced to overexpress c-FLIPs by transfection with the c-FLIPs expression vector (T98G.c-FLIPs-5, Fig. 6G).
Fig. 6.
Overexpression of c-FLIPL confers resistance to anti-DR5 mAb in human glioma cells. (A) Western blot showing high expression of either c-FLIPL or c-FLIPS in T98G.FLIPL-10 and -11, or T98G.FLIPs-5 cells transfected with pCR3.V64-Met-Flag-FLIPL, or pCR3.V62-Met-Flag-FLIPS, respectively, whereas undetectable levels of c-FLIPL and c-FLIPS in parental T98G and its subclones T98G.pCR3-1 and -4 cells transfected with the empty vector. (B–G) Suppression of cytotoxic effects by the treatment with anti-DR5 mAbs and sTRAIL in both T98G.FLIPL and T98G.FLIPS cells, but not in T98G.pCR3 cells. Cells were treated with either sTRAIL (0.04 µg/mL), E11 (0.4 µg/mL), or H48 (0.4 µg/mL) for 48 hours and then were subjected to MTT assay.
Treatment of Animals Carrying Established Tumor Xenografts with the Anti-DR5 mAbs Causes In Vivo Tumor Regression
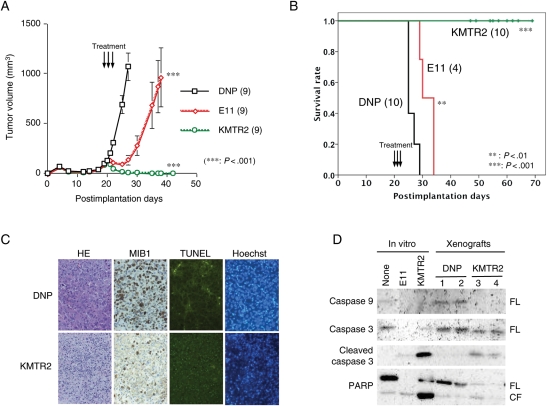
We next determined the effect of the anti-DR5 mAb treatment against established tumors. Mice bearing subcutaneous LNZ308 tumor xenografts were treated systemically with E11 for 5 consecutive days as described in the Materials and Methods. The tumors carried by mice treated with E11 grew slower and remained smaller after a course of treatment than those in animals treated with the vehicle control or with the mock control (DNP) (DNP vs. E11: P < .05, Mann–Whitney's U-test) (data not shown). As E11 requires crosslinking by effector molecules such as anti-immunoglobulin antibodies for its full apoptotic activity and in in vivo conditions such molecules and/or cells involved in crosslinking are presumably limited to the complement component C1q and Fc receptors present on most immune effector cells,42,43 we applied another anti-DR5 mAb KMTR2, which has been shown to directly activate apoptosis independent of host effector function.39 The growth of LNZ308 subcutaneous tumor xenografts were suppressed slightly more by the treatment with KMTR2 (DNP vs. KMTR2: P < .01) than by E11 (data not shown). To confirm the possibility that KMTR2 has better antitumor effects in vivo, we next tested the efficacy of these mAbs to TRAIL-sensitive T98SQ1 xenografts. Mice bearing subcutaneous T98SQ1 tumor xenografts were treated systemically with either E11, KMTR2, or DNP for only 3 days. E11 treatment resulted in the suppression of tumor growth, and even tumor shrinkage for a short period of time. However, KMTR2 treatment lead to complete tumor regression in all mice treated (Fig. 7A). This striking antitumor effect by KMTR2 was associated with massive apoptosis induction accompanied with a reduced proliferative activity in tumor cells, demonstrated by TUNEL assays and MIB-1 immunostainings, respectively (Fig. 7C). In KMTR2-treated tumors, caspase activation was also detected (Fig. 7D).
Fig. 7.
Tumor regression by treatment with anti-DR5 mAb KMTR2 in vivo. (A) Growth suppression and tumor regression of established T98SQ1 xenografts by fully human anti-DR5 mAbs, E11 and KMTR2, respectively, in vivo. Nude mice (9 per each group) were injected subcutaneously with 2 × 106 T98SQ1 cells and were allowed to establish tumors. From postimplantation Day 20, mice were treated with either anti-DR5 mAb E11 or KMTR2 (5 mg/kg) or control non-specific human IgG (DNP), administered i.p. daily for 3 consecutive days. Square, control DNP; diamond, E11; circle, KMTR2. Data are shown as the mean ± SE. ***P < .001 (DNP vs E11; DNP vs KMTR2). The experiment was repeated independently 2 times with similar results. (B) Effect of fully human anti-DR5 mAb treatment on survival of mice bearing intracerebral T98SQ1 xenografts. Nude mice were injected intracerebrally with 5 × 105 T98SQ1 cells and were treated from postimplantation Day 20 for 3 consecutive days as described in (A). Mice treated with KMTR2 were sacrificed at various time points and were found to carry no intracerebral tumor at any points. ***P < .001 (DNP vs KMTR2), **P = .003 (DNP vs E11) (log-rank test). The experiment was repeated independently 3 times with similar results. (C) Decreased proliferative activity (MIB-1 index) and increased apoptosis induction (TUNEL positivity) by KMTR2 treatment of T98SQ1 cells in xenografts. Nude mice with established subcutaneous xenografts derived from T98SQ1 cells were treated with either KMTR2 or DNP i.p. for 2 days and were sacrificed on the next day. Tumor tissues were harvested and subjected to either MIB-1 staining or TUNEL assay. (D) Treatment with KMTR2 induces multiple caspase activation in established T98SQ1 xenografts. Tumor lysates were prepared as described in (C) and were subjected to Western blot analyses. Caspase-9, caspase-3, and PARP, a substrate of activated caspase-3, were significantly cleaved upon treatment with KMTR2 but not by control DNP. FL, full length; CL, cleaved form.
We also examined the potency of the anti-DR5 mAb treatments for brain tumors using intracerebral xenograft models. Mice stereotactically inoculated in the brain with T98SQ1 cells were treated with either E11, KMTR2, or DNP for 3 consecutive days. The KMTR2 treatment significantly extended the survival of all mice bearing intracerebral xenografts when compared with either DNP or E11 (P < .001) (Fig. 7B). There was only minimum survival benefit by the E11 treatment. Notably, all mice treated with KMTR2 showed no tumor burden when sacrificed at various time points beyond 25 days after treatment, whereas all of those treated with DNP died of tumor within 10 days after treatment. Similar but less survival elongation was observed by these mAbs in mice bearing LNZ308 brain tumor xenografts (data not shown). Body weight of host mice did not significantly change by any types of treatments. These results suggest that anti-DR5 mAbs, especially direct agonist KMTR2, also have potent antitumor effects on brain tumors in vivo.
Association of Relative Resistance of Primary Culture Glioma Cells to Anti-DR5 mAbs with Altered Expression of DR5 and c-FLIPL
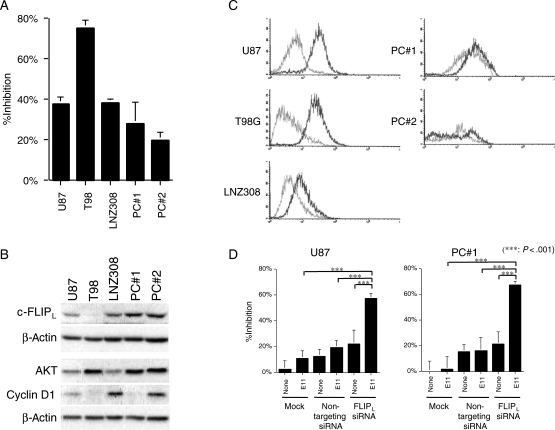
As established cell lines may not always represent original characteristics of primary tumors due to long-term culture conditions, we utilized cultures of primary GBM cells at short-term passage numbers and examined their sensitivity to anti-DR5 mAbs in vitro. Primary cultured cells derived from 2 independent glioma tissues did not respond as well to E11 as did the sensitive glioma cell lines by MTT assays (Fig. 8A). These primary cultured cells exhibited higher amounts of c-FLIPL expression (Fig. 8B) as well as lower levels of cell surface DR5 expression (Fig. 8C), both of which correlated with their sensitivity to the mAb. Furthermore, siRNA-mediated downregulation of c-FLIPL expression resulted in the enhancement of E11 sensitivity in the primary cultured cells (Fig. 8D), suggesting that the altered expression of these key molecules may, at least in part, account for the resistance.
Fig. 8.
Association of relative resistance of primary cultures of glioma cells to anti-DR5 mAbs with altered expression of DR5 and c-FLIPL. (A) Primary glioma cells were less sensitive to anti-DR5 mAb than sensitive glioma cell lines. Cells were treated with E11 (0.1 µg/mL) for 48 hours and then were subjected to the MTT assay. The experiment was repeated 3 times with similar results. (B) Western blot analyses showing expression levels of c-FLIPL, Akt, and cyclin D1 in human glioma cell lines and primary glioma cells. Whole cell lysates were subjected to Western blotting and serially probed with the specific antibodies indicated. High expression of c-FLIPL is observed in primary glioma cells. (C) DR5 cell-surface expression determined by flow cytometry analysis as described in Fig. 4. Primary culture glioma cells tended to have lower cell-surface expression of DR5 than the established GBM cell lines with high sensitivity to E11. (D) siRNA-mediated targeting of c-FLIPL enhances the sensitivity of primary glioma cells to anti-DR5 mAb. Cells were treated as described in Fig. 5 (E11 at 0.01 µg/mL) and then were subjected to the MTT assay. ***P < .001 (Student's t-test). The experiment was repeated 2 times with similar results.
Discussion
One of the major reasons for the poor prognosis of patients with malignant gliomas is derived from the resistance of these tumors to the chemotherapeutic drugs currently used. Induction of apoptosis through death receptor-mediated intracellular signaling is an intriguing anticancer strategy, especially because of involving signaling pathways directly activating the death executing caspase cascade rather than the mitochondrial damage through which most anticancer therapies operate.44 Activation of the TRAIL-mediated death signal, among other death-inducing factors, has been intensively investigated both experimentally and clinically because of its advantage as cancer therapeutics due to preferential targeting of tumor cells but not normal cells. sTRAIL, however, binds to all TRAIL receptors including the death-inducing receptors DR4 and DR5, as well as decoy receptors that do not mediate death signals, rendering its therapeutic spectrum and effects wider or more unpredictable than targeting an individual receptor specifically. Furthermore, some versions of recombinant sTRAIL have been shown to induce differential hepatocyte toxicity.8,37,38,45 This potential limitation led to the development of proapoptotic mAbs against individual TRAIL receptors,38,39,46,47 because mAbs restrict the therapeutic target to tumors with a distinct receptor expression profile. The potential therapeutic advantages of using mAb over recombinant sTRAIL to target TRAIL receptors include a longer half-life in vivo, a higher affinity for the target receptor, and no decoy receptor engagement, and they may provide a mechanism to induce long-term, tumor-specific T-cell memory that prevents tumor recurrence.48
Here, we show that the fully human mAbs to human DR5, E11, H48, and KMTR2, induced significant cytotoxicity as well as tumor regression in most of human glioma cells through rapid apoptotic induction but did not cause cell death in normal astrocytes. In contrast, anti-DR4 mAb B12, which had been shown to have a potential to affect hepatocyte viability38 did not induce cell death in all human glioma cell lines, providing support for targeting individual TRAIL receptor by mAbs. In addition, our results that the temozolomide/nitrosourea-resistant T98G and SF188 human glioma cells were highly susceptible to anti-DR5 mAbs suggest that specific targeting of TRAIL receptors could be a meaningful alternative therapeutic strategy to overcome drug resistance.49 Indeed, agonistic anti-TRAIL receptor mAbs have been currently under intensive investigation, including mapatumumab (HGS-ETR1, anti-human DR4 mAb), lexatumumab (HGS-ETR2, anti-human DR5 mAb), and MD5-1 (anti-mouse DR5 mAb). The former 2 mAbs have been tested in Phase 1 clinical trials in patients with systemic malignancy, exhibiting excellent safety profiles.36,50–52 Anti-mouse DR5 mAb MD5-1 could also be administered safely without inducing hepatotoxicity either alone or in combination with histone deacetylase (HDAC) inhibitors in mice.53
Our findings that human glioma cells were only susceptible to anti-DR5 mAbs but not to anti-DR4 mAb and that their sensitivity to sTRAIL strongly correlated with those to anti-DR5 mAbs suggest that DR5 is the major TRAIL receptor that transduces the death signal in human glioma cells. This might be attributable to the pattern of cell surface expression of TRAIL receptors. Protein expression of both DR4 and DR5 was detectable in all human glioma cell lines tested by Western blot analysis using whole cell lysates, whereas only DR5 was identified to be expressed at the cell surface by flow cytometry. Furthermore, the expression level of DR5 at the cell surface was associated with the sensitivity of glioma cells to anti-DR5 mAb treatment. The reason for the lack of DR4 cell surface expression is yet unclear, leaving room for further investigation including protein trafficking or degradation. The DR5 predilection for TRAIL death signaling in human glioma cells appears to be in agreement with the preclinical experiments, which utilized TRAIL mutants that selectively bind either DR4 or DR5 and have suggested that DR5 might be the more potent receptor for ligand-induced apoptosis of many cancer cell types.54
Among human glioma cell lines, some disclosed resistance to anti-DR5 mAb treatment. One of the putative determinants for TRAIL sensitivity includes the expression of the decoy receptors against TRAIL. However, this might not be the case in glioma like other cancers as suggested previously,55 as we did not see any correlation between the sensitivity to anti-DR5 mAbs and the expression of intrinsic TRAIL decoy receptors. Rather, the glioma cell sensitivity to anti-DR5 mAbs were significantly correlated with expression levels of intracellular intrinsic apoptosis suppressors c-FLIPL and Akt/PKB. c-FLIP contains a DED, can interact with DED of both FADD and pro-caspase-8 and block upstream caspase activation at the DISC level.56,57 c-FLIP has thus been implicated in the resistance of cancer cells to apoptosis and is upregulated in some cancer types including Hodgkin's lymphoma, and ovarian and colon carcinomas.58 c-FLIP is expressed as 2 alternative splice forms, FLIP short (FLIPs) and FLIP long (FLIPL). Both isoforms have been reported to regulate TRAIL sensitivity in a variety of human tumor cell lines.22,56,58–60 In the glioma cell lines tested, c-FLIPL expression was nearly absent in T98G and SF188 cells, both of which were the most sensitive to anti-DR5 mAbs. Furthermore, gene transfer of c-FLIPL into T98G cells resulted in acquired resistance to anti-DR5 mAb treatment. Alternatively, siRNA-mediated downregulation of c-FLIPL expression markedly sensitized c-FLIPL expressing glioma cells to anti-DR5 mAbs. These results suggest the expression level of c-FLIPL as an important molecular determinant, at least in part, of resistance to anti-DR5 mAbs in human glioma cells. Primary cultured glioma cells were also shown to be resistant to TRAIL, which was associated with weak DR5 expression.61 Accordingly, our results show that the primary glioma cells tended to be less sensitive to anti-DR5 mAb than sensitive established cell lines, which was well correlated with higher amounts of c-FLIPL expression as well as lower levels of cell surface DR5 expression. Similar to results with the established GBM cell lines, siRNA treatment of c-FLIPL resulted in the enhancement of E11 sensitivity in the primary culture cells, providing a further rationale to target c-FLIPL to increase the anti-DR5 mAb sensitivity. Panner et al.62 reported that c-FLIPs is one of the major intracellular molecules that regulate death signals driven by TRAIL treatment in human glioma cells, as c-FLIPs also targets and inhibits the DISC function. In the human glioma cell lines tested, however, c-FLIPs protein expression was undetectable using several specific antibodies, one of which, albeit, clearly identified exogenously expressed c-FLIPs protein in T98G cells (Figs 4B and 6A), which also conferred resistance to anti-DR5 mAb (Fig. 6G). Although the reason for lack of c-FLIPs detection at the protein level is not clear, our data indicate that c-FLIPL could also play a pivotal role in regulating the sensitivity of human glioma cells to anti-DR5 mAb.
In addition to c-FLIPL, protein expression levels of both Akt and Cyclin D1 correlated significantly with the sensitivity of these cell lines to anti-DR5 mAb treatments. Interestingly, there was significant correlation between the expressions of c-FLIPL and Akt (P = .017). Akt plays a key role in transducing survival signals from receptor tyrosine kinases including epidermal growth factor receptor (EGFR) and has been shown to be upregulated in many high-grade gliomas. Panner et al.62 reported that Akt signaling upregulates the expression level of c-FLIPs through activation of its downstream target mTOR, suggesting a potential connection between Akt and c-FLIPL status in glioma cells. Overexpression of Cyclin D1 was shown to be associated with the reduced colonization activity by TRAIL in a breast cancer cell line, which had increased expression of DR5.63 However, the significance of Cyclin D1 expression in the regulation of anti-DR5 mAb sensitivity remains to be elucidated, as there has been no definite evidence of Cyclin D1 transactivation of DR5 expression in human glioma cells.
Treatment of mice bearing human glioma xenografts with anti-DR5 mAbs exerted significant antitumor effects in vivo. E11 monotherapy resulted in reduced tumor volume and suppression of tumor growth, compared with control treatments. However, the response of tumors to E11 remained partial, and there were no complete response and no durable tumor regression obtained. In contrast, monotherapy with another anti-DR5 mAb, KMTR2, exhibited a marked tumor shrinkage leading to complete disappearance and durable remission without recurrence of both subcutaneous and intracranial xenografts derived from TRAIL-sensitive human glioma cells. E11 exists as a monomer, thus requiring an antibody crosslinking to effectively activate death signals that stem from TRAIL receptors. In the athymic mouse body, anti-human IgG (isotype of E11) antibodies are almost lacking, and there are no cytotoxic T cells. In this sense, the cytotoxic action by E11 may be restricted to immune systems except T cells or complement-dependent cytotoxicity which is mediated through the activation of complement system by binding to the DR5–E11 complex and thus could be less effective in inducing robust tumor cell death in athymic mice. On the other hand, even in the milieu where T cells are defective, KMTR2 has a potential to bind to and directly multimerize DR5 leading to the activation of death signals. As a result, KMTR2 was very efficacious in killing tumor cells in vivo compared with E11, demonstrating complete response and durable remission in the animal models. This finding is of great advantage in the application of the anti-TRAIL receptor mAb therapy to human glioma, providing a basis for further testing of its efficacy using spontaneous glioma models or in nonhuman primates. The administration of therapeutic mAbs to intraparenchymal neoplastic lesions in the brain might be limited by antibody penetration into the tumor. Macromolecules such as mAbs can, however, cross the blood–brain barrier (BBB) in clinically relevant concentrations in tumors with contrast enhancement, as seen in high-grade gliomas, which is indicative of having a disturbed BBB. Likewise, systemic administration of an mAb specific to EGFR was shown to effectively target and eradicate the established gliomas overexpressing EGFR in the nude mouse brain.64 Another strategy to localize mAbs in the glioma, especially in the region of tumor cell infiltration where BBB is assumed intact, could be obtained by convection-enhanced delivery (CED). Saito et al65 have shown that CED of sTRAIL resulted in the enhanced survival of mice bearing glioma in the brain in combination with temozolomide. Administration of pseudomonas end toxin conjugated to IL-13 (cintredekin besudotox) to patients with glioma by CED has already been tested under clinical trials.66 Furthermore, combining mAb therapy with other therapeutic modalities such as chemotherapy, radiotherapy, molecular-targeted therapy, or toxins has been shown to be more effective than antibody alone.67–69 Indeed, combination of the anti-DR5 mAbs with cisplatin or HDAC inhibitors synergistically enhanced the cytotoxic effects in human glioma cells (unpublished observations; Frew et al.53) and will be further investigated to potentially overcome resistance to anti-DR5 mAb therapy.
Funding
This work was supported in part by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (17591529 to M.N.).
Acknowledgments
The authors thank Dr J. Tschopp for a gift of c-FLIP constructs and S. Matsushima for technical assistance on flow cytometry.
Conflict of interest statement: None declared.
References
- 1.The committee of Brain Tumor Registry of Japan. Report of brain tumor registry of Japan (1969–1996) Neurol Med Chir (Tokyo). 2003;43:34–96. (suppl) [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Guchelaar HJ, Vermes A, Vermes I, Haanen C. Apoptosis: molecular mechanisms and implications for cancer chemotherapy. Pharm World Sci. 1997;19:119–125. doi: 10.1023/a:1008654316572. [DOI] [PubMed] [Google Scholar]
- 4.Ashkenazi A. Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nat Rev Cancer. 2002;2:420–430. doi: 10.1038/nrc821. [DOI] [PubMed] [Google Scholar]
- 5.Pan G, O'Rourke K, Chinnaiyan AM, et al. The receptor for the cytotoxic ligand TRAIL. Science. 1997;276:111–113. doi: 10.1126/science.276.5309.111. [DOI] [PubMed] [Google Scholar]
- 6.Pan G, Ni J, Wei YF, Yu G, Gentz R, Dixit VM. An antagonist decoy receptor and a death domain-containing receptor for TRAIL [see comments] Science. 1997;277:815–818. doi: 10.1126/science.277.5327.815. [DOI] [PubMed] [Google Scholar]
- 7.Sheridan JP, Marsters SA, Pitti RM, et al. Control of TRAIL-induced apoptosis by a family of signaling and decoy receptors [see comments] Science. 1997;277:818–821. doi: 10.1126/science.277.5327.818. [DOI] [PubMed] [Google Scholar]
- 8.Ashkenazi A, Pai RC, Fong S, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Invest. 1999;104:155–162. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagane M, Pan G, Weddle JJ, Dixit VM, Cavenee WK, Huang HJ. Increased death receptor 5 expression by chemotherapeutic agents in human gliomas causes synergistic cytotoxicity with tumor necrosis factor-related apoptosis-inducing ligand in vitro and in vivo. Cancer Res. 2000;60:847–853. [PubMed] [Google Scholar]
- 10.Pitti RM, Marsters SA, Ruppert S, Donahue CJ, Moore A, Ashkenazi A. Induction of apoptosis by Apo-2 ligand, a new member of the tumor necrosis factor cytokine family. J Biol Chem. 1996;271:12687–12690. doi: 10.1074/jbc.271.22.12687. [DOI] [PubMed] [Google Scholar]
- 11.Rieger J, Naumann U, Glaser T, Ashkenazi A, Weller M. APO2 ligand: a novel lethal weapon against malignant glioma? FEBS Lett. 1998;427:124–128. doi: 10.1016/s0014-5793(98)00409-8. [DOI] [PubMed] [Google Scholar]
- 12.Wiley SR, Schooley K, Smolak PJ, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 13.Song JH, Bellail A, Tse MC, Yong VW, Hao C. Human astrocytes are resistant to Fas ligand and tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis. J Neurosci. 2006;26:3299–3308. doi: 10.1523/JNEUROSCI.5572-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnstone RW, Frew AJ, Smyth MJ. The TRAIL apoptotic pathway in cancer onset, progression and therapy. Nat Rev Cancer. 2008;8:782–798. doi: 10.1038/nrc2465. [DOI] [PubMed] [Google Scholar]
- 15.Degli-Esposti MA, Dougall WC, Smolak PJ, Waugh JY, Smith CA, Goodwin RG. The novel receptor TRAIL-R4 induces NF-kappaB and protects against TRAIL-mediated apoptosis, yet retains an incomplete death domain. Immunity. 1997;7:813–820. doi: 10.1016/s1074-7613(00)80399-4. [DOI] [PubMed] [Google Scholar]
- 16.Marsters SA, Sheridan JP, Pitti RM, et al. A novel receptor for Apo2 L/TRAIL contains a truncated death domain. Curr Biol. 1997;7:1003–1006. doi: 10.1016/s0960-9822(06)00422-2. [DOI] [PubMed] [Google Scholar]
- 17.Emery JG, McDonnell P, Burke MB, et al. Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J Biol Chem. 1998;273:14363–14367. doi: 10.1074/jbc.273.23.14363. [DOI] [PubMed] [Google Scholar]
- 18.Frank S, Köhler U, Schackert G, Schackert HK. Expression of TRAIL and its receptors in human brain tumors. Biochem Biophys Res Commun. 1999;257:454–459. doi: 10.1006/bbrc.1999.0493. [DOI] [PubMed] [Google Scholar]
- 19.Almasan A, Ashkenazi A. Apo2 L/TRAIL: apoptosis signaling, biology, and potential for cancer therapy. Cytokine Growth Factor Rev. 2003;14:337–348. doi: 10.1016/s1359-6101(03)00029-7. [DOI] [PubMed] [Google Scholar]
- 20.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 21.Younes A, Kadin ME. Emerging applications of the tumor necrosis factor family of ligands and receptors in cancer therapy. J Clin Oncol. 2003;21:3526–3534. doi: 10.1200/JCO.2003.09.037. [DOI] [PubMed] [Google Scholar]
- 22.Xiao C, Yang BF, Asadi N, Beguinot F, Hao C. Tumor necrosis factor-related apoptosis-inducing ligand-induced death-inducing signaling complex and its modulation by c-FLIP and PED/PEA-15 in glioma cells. J Biol Chem. 2002;277:25020–25025. doi: 10.1074/jbc.M202946200. [DOI] [PubMed] [Google Scholar]
- 23.Vogler M, Durr K, Jovanovic M, Debatin KM, Fulda S. Regulation of TRAIL-induced apoptosis by XIAP in pancreatic carcinoma cells. Oncogene. 2007;26:248–257. doi: 10.1038/sj.onc.1209776. [DOI] [PubMed] [Google Scholar]
- 24.Khanbolooki S, Nawrocki ST, Arumugam T, et al. Nuclear factor-kappaB maintains TRAIL resistance in human pancreatic cancer cells. Mol Cancer Ther. 2006;5:2251–2260. doi: 10.1158/1535-7163.MCT-06-0075. [DOI] [PubMed] [Google Scholar]
- 25.Cheng J, Hylander BL, Baer MR, Chen X, Repasky EA. Multiple mechanisms underlie resistance of leukemia cells to Apo2 Ligand/TRAIL. Mol Cancer Ther. 2006;5:1844–1853. doi: 10.1158/1535-7163.MCT-06-0050. [DOI] [PubMed] [Google Scholar]
- 26.Yang LQ, Fang DC, Wang RQ, Yang SM. Effect of NF-kappaB, survivin, Bcl-2 and Caspase3 on apoptosis of gastric cancer cells induced by tumor necrosis factor related apoptosis inducing ligand. World J Gastroenterol. 2004;10:22–25. doi: 10.3748/wjg.v10.i1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang XD, Borrow JM, Zhang XY, Nguyen T, Hersey P. Activation of ERK1/2 protects melanoma cells from TRAIL-induced apoptosis by inhibiting Smac/DIABLO release from mitochondria. Oncogene. 2003;22:2869–2881. doi: 10.1038/sj.onc.1206427. [DOI] [PubMed] [Google Scholar]
- 28.Chawla-Sarkar M, Bae SI, Reu FJ, Jacobs BS, Lindner DJ, Borden EC. Downregulation of Bcl-2, FLIP or IAPs (XIAP and survivin) by siRNAs sensitizes resistant melanoma cells to Apo2 L/TRAIL-induced apoptosis. Cell Death Differ. 2004;11:915–923. doi: 10.1038/sj.cdd.4401416. [DOI] [PubMed] [Google Scholar]
- 29.Fulda S, Meyer E, Debatin KM. Inhibition of TRAIL-induced apoptosis by Bcl-2 overexpression. Oncogene. 2002;21:2283–2294. doi: 10.1038/sj.onc.1205258. [DOI] [PubMed] [Google Scholar]
- 30.Kandasamy K, Srivastava RK. Role of the phosphatidylinositol 3′-kinase/PTEN/Akt kinase pathway in tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in non-small cell lung cancer cells. Cancer Res. 2002;62:4929–4937. [PubMed] [Google Scholar]
- 31.Hopkins-Donaldson S, Bodmer JL, Bourloud KB, Brognara CB, Tschopp J, Gross N. Loss of caspase-8 expression in neuroblastoma is related to malignancy and resistance to TRAIL-induced apoptosis. Med Pediatr Oncol. 2000;35:608–611. doi: 10.1002/1096-911x(20001201)35:6<608::aid-mpo25>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 32.Nagane M, Cavenee WK, Shiokawa Y. Synergistic cytotoxicity through the activation of multiple apoptosis pathways in human glioma cells induced by combined treatment with ionizing radiation and tumor necrosis factor-related apoptosis-inducing ligand. J Neurosurg. 2007;106:407–416. doi: 10.3171/jns.2007.106.3.407. [DOI] [PubMed] [Google Scholar]
- 33.Walczak H, Miller RE, Ariail K, et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo [see comments] Nat Med. 1999;5:157–163. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- 34.Lawrence D, Shahrokh Z, Marsters S, et al. Differential hepatocyte toxicity of recombinant Apo2 L/TRAIL versions. Nat Med. 2001;7:383–385. doi: 10.1038/86397. [DOI] [PubMed] [Google Scholar]
- 35.Qin J, Chaturvedi V, Bonish B, Nickoloff BJ. Avoiding premature apoptosis of normal epidermal cells. Nat Med. 2001;7:385–386. doi: 10.1038/86401. [DOI] [PubMed] [Google Scholar]
- 36.Gajewski TF. On the TRAIL toward death receptor-based cancer therapeutics. J Clin Oncol. 2007;25:1305–1307. doi: 10.1200/JCO.2006.09.9804. [DOI] [PubMed] [Google Scholar]
- 37.Ichikawa K, Liu W, Zhao L, et al. Tumoricidal activity of a novel anti-human DR5 monoclonal antibody without hepatocyte cytotoxicity. Nat Med. 2001;7:954–960. doi: 10.1038/91000. [DOI] [PubMed] [Google Scholar]
- 38.Mori E, Thomas M, Motoki K, et al. Human normal hepatocytes are susceptible to apoptosis signal mediated by both TRAIL-R1 and TRAIL-R2. Cell Death Differ. 2004;11:203–207. doi: 10.1038/sj.cdd.4401331. [DOI] [PubMed] [Google Scholar]
- 39.Motoki K, Mori E, Matsumoto A, et al. Enhanced apoptosis and tumor regression induced by a direct agonist antibody to tumor necrosis factor-related apoptosis-inducing ligand receptor 2. Clin Cancer Res. 2005;11:3126–3135. doi: 10.1158/1078-0432.CCR-04-1867. [DOI] [PubMed] [Google Scholar]
- 40.Nagane M, Coufal F, Lin H, Bögler O, Cavenee WK, Huang HJS. A common mutant epidermal growth factor receptor confers enhanced tumorigenicity on human glioblastoma cells by increasing proliferation and reducing apoptosis. Cancer Res. 1996;56:5079–5086. [PubMed] [Google Scholar]
- 41.Nagane M, Narita Y, Mishima K, et al. Human glioblastoma xenografts overexpressing a tumor-specific mutant epidermal growth factor receptor sensitized to cisplatin by the AG1478 tyrosine kinase inhibitor. J Neurosurg. 2001;95:472–479. doi: 10.3171/jns.2001.95.3.0472. [DOI] [PubMed] [Google Scholar]
- 42.Chuntharapai A, Dodge K, Grimmer K, et al. Isotype-dependent inhibition of tumor growth in vivo by monoclonal antibodies to death receptor 4. J Immunol. 2001;166:4891–4898. doi: 10.4049/jimmunol.166.8.4891. [DOI] [PubMed] [Google Scholar]
- 43.Xu Y, Szalai AJ, Zhou T, et al. Fc gamma Rs modulate cytotoxicity of anti-Fas antibodies: implications for agonistic antibody-based therapeutics. J Immunol. 2003;171:562–568. doi: 10.4049/jimmunol.171.2.562. [DOI] [PubMed] [Google Scholar]
- 44.Kaufmann SH, Earnshaw WC. Induction of apoptosis by cancer chemotherapy. Exp Cell Res. 2000;256:42–49. doi: 10.1006/excr.2000.4838. [DOI] [PubMed] [Google Scholar]
- 45.Zheng SJ, Wang P, Tsabary G, Chen YH. Critical roles of TRAIL in hepatic cell death and hepatic inflammation. J Clin Invest. 2004;113:58–64. doi: 10.1172/JCI200419255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Georgakis GV, Li Y, Humphreys R, et al. Activity of selective fully human agonistic antibodies to the TRAIL death receptors TRAIL-R1 and TRAIL-R2 in primary and cultured lymphoma cells: induction of apoptosis and enhancement of doxorubicin- and bortezomib-induced cell death. Br J Haematol. 2005;130:501–510. doi: 10.1111/j.1365-2141.2005.05656.x. [DOI] [PubMed] [Google Scholar]
- 47.Pukac L, Kanakaraj P, Humphreys R, et al. HGS-ETR1, a fully human TRAIL-receptor 1 monoclonal antibody, induces cell death in multiple tumour types in vitro and in vivo. Br J Cancer. 2005;92:1430–1441. doi: 10.1038/sj.bjc.6602487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takeda K, Yamaguchi N, Akiba H, et al. Induction of tumor-specific T cell immunity by anti-DR5 antibody therapy. J Exp Med. 2004;199:437–448. doi: 10.1084/jem.20031457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nagane M, Asai A, Shibui S, Nomura K, Matsutani M, Kuchino Y. Expression of O6-methylguanine-DNA methyltransferase and chloroethylnitrosourea resistance of human brain tumors. Jpn J Clin Oncol. 1992;22:143–149. [PubMed] [Google Scholar]
- 50.Hotte SJ, Hirte HW, Chen EX, et al. A phase 1 study of mapatumumab (fully human monoclonal antibody to TRAIL-R1) in patients with advanced solid malignancies. Clin Cancer Res. 2008;14:3450–3455. doi: 10.1158/1078-0432.CCR-07-1416. [DOI] [PubMed] [Google Scholar]
- 51.Plummer R, Attard G, Pacey S, et al. Phase 1 and pharmacokinetic study of lexatumumab in patients with advanced cancers. Clin Cancer Res. 2007;13:6187–6194. doi: 10.1158/1078-0432.CCR-07-0950. [DOI] [PubMed] [Google Scholar]
- 52.Tolcher AW, Mita M, Meropol NJ, et al. Phase I pharmacokinetic and biologic correlative study of mapatumumab, a fully human monoclonal antibody with agonist activity to tumor necrosis factor-related apoptosis-inducing ligand receptor-1. J Clin Oncol. 2007;25:1390–1395. doi: 10.1200/JCO.2006.08.8898. [DOI] [PubMed] [Google Scholar]
- 53.Frew AJ, Lindemann RK, Martin BP, et al. Combination therapy of established cancer using a histone deacetylase inhibitor and a TRAIL receptor agonist. Proc Natl Acad Sci USA. 2008;105:11317–11322. doi: 10.1073/pnas.0801868105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kelley RF, Totpal K, Lindstrom SH, et al. Receptor-selective mutants of apoptosis-inducing ligand 2/tumor necrosis factor-related apoptosis-inducing ligand reveal a greater contribution of death receptor (DR) 5 than DR4 to apoptosis signaling. J Biol Chem. 2005;280:2205–2212. doi: 10.1074/jbc.M410660200. [DOI] [PubMed] [Google Scholar]
- 55.Rohn TA, Wagenknecht B, Roth W, et al. CCNU-dependent potentiation of TRAIL/Apo2 L-induced apoptosis in human glioma cells is p53-independent but may involve enhanced cytochrome c release. Oncogene. 2001;20:4128–4137. doi: 10.1038/sj.onc.1204534. [DOI] [PubMed] [Google Scholar]
- 56.Irmler M, Thome M, Hahne M, et al. Inhibition of death receptor signals by cellular FLIP [see comments] Nature. 1997;388:190–195. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
- 57.Krueger A, Schmitz I, Baumann S, Krammer PH, Kirchhoff S. Cellular FLICE-inhibitory protein splice variants inhibit different steps of caspase-8 activation at the CD95 death-inducing signaling complex. J Biol Chem. 2001;276:20633–20640. doi: 10.1074/jbc.M101780200. [DOI] [PubMed] [Google Scholar]
- 58.Kataoka T. The caspase-8 modulator c-FLIP. Crit Rev Immunol. 2005;25:31–58. doi: 10.1615/critrevimmunol.v25.i1.30. [DOI] [PubMed] [Google Scholar]
- 59.Ricci MS, Jin Z, Dews M, et al. Direct repression of FLIP expression by c-myc is a major determinant of TRAIL sensitivity. Mol Cell Biol. 2004;24:8541–8555. doi: 10.1128/MCB.24.19.8541-8555.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang BF, Xiao C, Roa WH, Krammer PH, Hao C. Calcium/calmodulin-dependent protein kinase II regulation of c-FLIP expression and phosphorylation in modulation of Fas-mediated signaling in malignant glioma cells. J Biol Chem. 2003;278:7043–7050. doi: 10.1074/jbc.M211278200. [DOI] [PubMed] [Google Scholar]
- 61.Rieger J, Frank B, Weller M, Wick W. Mechanisms of resistance of human glioma cells to Apo2 ligand/TNF-related apoptosis-inducing ligand. Cell Physiol Biochem. 2007;20:23–34. doi: 10.1159/000104150. [DOI] [PubMed] [Google Scholar]
- 62.Panner A, James CD, Berger MS, Pieper RO. mTOR controls FLIPs translation and TRAIL sensitivity in glioblastoma multiforme cells. Mol Cell Biol. 2005;25:8809–8823. doi: 10.1128/MCB.25.20.8809-8823.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhou Q, Fukushima P, DeGraff W, et al. Radiation and the Apo2 L/TRAIL apoptotic pathway preferentially inhibit the colonization of premalignant human breast cells overexpressing cyclin D1. Cancer Res. 2000;60:2611–2615. [PubMed] [Google Scholar]
- 64.Mishima K, Johns TG, Luwor RB, et al. Growth suppression of intracranial xenografted glioblastomas overexpressing mutant epidermal growth factor receptors by systemic administration of monoclonal antibody (mAb) 806, a novel monoclonal antibody directed to the receptor. Cancer Res. 2001;61:5349–5354. [PubMed] [Google Scholar]
- 65.Saito R, Bringas JR, Panner A, et al. Convection-enhanced delivery of tumor necrosis factor-related apoptosis-inducing ligand with systemic administration of temozolomide prolongs survival in an intracranial glioblastoma xenograft model. Cancer Res. 2004;64:6858–6862. doi: 10.1158/0008-5472.CAN-04-1683. [DOI] [PubMed] [Google Scholar]
- 66.Vogelbaum MA, Sampson JH, Kunwar S, et al. Convection-enhanced delivery of cintredekin besudotox (interleukin-13-PE38QQR) followed by radiation therapy with and without temozolomide in newly diagnosed malignant gliomas: phase 1 study of final safety results. Neurosurgery. 2007;61:1031–1037. doi: 10.1227/01.neu.0000303199.77370.9e. discussion 1037–1038. [DOI] [PubMed] [Google Scholar]
- 67.Green MC, Murray JL, Hortobagyi GN. Monoclonal antibody therapy for solid tumors. Cancer Treat Rev. 2000;26:269–286. doi: 10.1053/ctrv.2000.0176. [DOI] [PubMed] [Google Scholar]
- 68.Trail PA, Bianchi AB. Monoclonal antibody drug conjugates in the treatment of cancer. Curr Opin Immunol. 1999;11:584–588. doi: 10.1016/s0952-7915(99)00012-6. [DOI] [PubMed] [Google Scholar]
- 69.Pietras RJ, Pegram MD, Finn RS, Maneval DA, Slamon DJ. Remission of human breast cancer xenografts on therapy with humanized monoclonal antibody to HER-2 receptor and DNA-reactive drugs. Oncogene. 1998;17:2235–2249. doi: 10.1038/sj.onc.1202132. [DOI] [PubMed] [Google Scholar]