Abstract
Hepatocyte growth factor (HGF) and its receptor c-Met have been known as key determinants of growth and angiogenesis in some brain tumors like gliomas, meningiomas, and schwannomas. But little is known about their expression in pituitary adenomas. In this study, the expression of HGF and c-Met in pituitary adenomas of different histology types was investigated by immunohistochemistry, and correlative analysis of their expression with microvessel density (MVD), Ki-67 expression, and other clinicopathologic factors was made. The results showed that the expression of HGF and c-Met exists in 98% (64 of 65) and 92% (60 of 65) pituitary adenomas, respectively, and co-expression of them existed in 91% (59 of 65) adenomas. HGF had significant correlation with MVD (Spearman's correlation coefficient, r = .31, P = .01) and Ki-67 (r = .32, P = .01). c-Met had significant correlation with MVD (r = .30, P = .02) and Ki-67 (r = .38, P = .00). HGF and c-Met expression had no significant correlation with age or extrasellar extension. There were no significant differences in HGF and c-Met expression between pituitary adenomas of different histology types. The results indicate that HGF and c-Met are widely expressed in pituitary adenomas, and their expression correlates with MVD and Ki-67 expression.
Keywords: c-Met, hepatocyte growth factor, pituitary adenoma
Hepatocyte growth factor (HGF) is a multifunctional tumor growth and angiogenic factor. The receptor tyrosine kinase c-Met is the only known receptor for HGF. HGF and c-Met are expressed in human brain tumors like gliomas, meningiomas, and schwannomas, and their expression levels frequently correlate with poor prognosis.1–3 HGF:c-Met signaling pathway has been known to induce tumor cell proliferation, motility, invasion, and promote tumor angiogenesis.4–7 Approaches targeting HGF and c-Met have been proven to inhibit brain tumor growth and angiogenesis as well as tumor HGF and c-Met expression levels.8 So HGF and c-Met pathway targeted treatment may be a promising therapeutic strategy.
Though Kim et al.9 showed Met mRNA expression in functional and nonfunctional pituitary adenomas, little is known about protein expression of HGF and c-Met in pituitary adenomas. Herein, HGF and c-Met protein expression in pituitary adenomas of different histology types were investigated by immunohistochemistry, and correlative analysis of their expression with microvessel density (MVD), Ki-67 expression, and some other clinicopathologic factors was made.
Materials and Methods
About 10% of the neutral buffered formalin and paraffin embedded surgical specimens from 65 patients with pituitary adenomas who were admitted and operated on consecutively from October 2008 to May 2009 at Neurosurgery Department of Provincial Hospital Affiliated to Shandong University were included. Pituitary adenomas were classified according to hormone immunohistochemistry previously done by two pathologists at the pathology department of Provincial Hospital Affiliated to Shandong University. Patients' characteristics can be seen in Table 1.
Table 1.
Expression of HGF, c-Met, Ki-67, MVD, and other clinicopathologic factors in pituitary adenomas of different histology types
| Histology types of pituitary adenomas (number of adenomas) | Sex (male vs female) | Age (mean years) | Number of adenomas with extrasellar invasion | Ki-67 (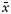 ) ) |
MVD (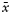 ) ) |
HGF (−/+/++/+++) | c-Met (−/+/++/+++) |
|---|---|---|---|---|---|---|---|
| Simple hormone immunopositive27 | 10/17 | 40 | 20 | 2.7 | 8.9 | 0/10/4/13 | 2/7/3/15 |
| Plurihormone immunopositive28 | 6/22 | 41 | 21 | 3.9 | 9.4 | 1/3/3/21 | 2/2/4/20 |
| Hormone immnonegative10 | 5/5 | 53 | 8 | 3.7 | 10.4 | 0/1/1/8 | 1/1/1/7 |
| Total (65) | 21/44 | 43 | 49 | 3.3 | 9.3 | 1/14/8/42 | 5/10/8/42 |
Abbreviations: HGF, hepatocyte growth factor; MVD, microvessel density. Simple hormone immunopositive adenomas: pituitary adenomas with only 1 kind of hormone immunoreactivity including 10 adrenocorticotropic hormone (ACTH) adenomas, 7 prolactin (PRL) adenomas, 5 growth hormone (GH) adenomas, 4 follicle stimulating hormone (FSH) adenomas, and 1 thyroid stimulating hormone (TSH) adenomas. Plurihormone immunopositive: pituitary adenomas with 2 or more kinds of hormone immunoreactivity including ACTH, PRL, GH, FSH, and TSH. Pituitary adenomas with extrasellar invasion: adenomas which extends beyond its capsule or involves neighboring structures like the dura, bone, blood vessels, and nerves seen from MRI. 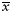 means the mean scores for Ki-67 and MVD in pituitary adenomas of different histology types. −/+/++/+++ refers to the number of adenomas with different immunoreactivity for HGF or c-Met: −, no immunopositive cells; +, <30% of tumor cells are immunopositive; ++, 30%–60% of tumor cells are immunopositive; +++, >60% of tumor cells are immunopositive.
means the mean scores for Ki-67 and MVD in pituitary adenomas of different histology types. −/+/++/+++ refers to the number of adenomas with different immunoreactivity for HGF or c-Met: −, no immunopositive cells; +, <30% of tumor cells are immunopositive; ++, 30%–60% of tumor cells are immunopositive; +++, >60% of tumor cells are immunopositive.
The immunohistochemical study was performed using the strepto-avidin-biotin complex method. Primary antibodies used were as follows: Met (c-28) (rabbit polyclonal, sc-161, 1:50, Santa Cruz Biotechnology); HGFα (H-145) (rabbit polyclonal, sc-7949, 1:100, Santa Cruz Biotechnology); CD34 (BI-3c5) (rabbit polyclonal, sc-19621, 1:100, Santa Cruz Biotechnology); Ki-67 (mouse monoclonal, M7240, 1:150, DAKO). Sections of gliomas were used as a positive control to prove the specificity of the antibodies. In negative control, primary antibodies were replaced by phosphate buffered solution.
Tumor-containing sections of 5 µm thickness were baked at 60°C for 30 minutes, deparaffinized in xylene, and rehydrated in graded concentrations of ethanol. Heat-induced antigen retrieval (10 mM citrate buffer [pH 6.0] at 96°C for 15 minutes in a thermostat-controlled waterbath) was used. Endogenous peroxidase activity was blocked by incubation in 0.3% hydrogen peroxide at 37°C for 15 minutes. Nonspecific binding of primary antibodies was blocked by normal serum from the same species as that of secondary antibodies at 37°C for 25 minutes. Immunostaining involved sequential applications of primary antibody at 4°C overnight, incubation at 37°C for 30 minutes, followed by biotinylated secondary antibodies (Zhongshan Goldenbridge Biotechnology) at 37°C for 30 minutes and strepto-avidin-biotin complex (Zhongshan Goldenbridge Biotechnology) at 37°C for 30 minutes. Diaminobenzidine was used as the enzyme substrate to observe the specific antibody localization, and hematoxylin (not in Ki-67 immunostaining) was used as a nuclear counterstain. In Ki-67 immunostaining, eosin was used as a cytoplasmic counterstain.
Sections were examined and scored for immunoreactivity for HGF, c-Met, MVD, and Ki-67 by an observer who was unaware of the histological diagnoses or clinical features. Scores for HGF and c-Met were recorded as follows: −, no immunopositive cells; +, <30% of tumor cells are immunopositive; ++, 30%–60% of tumor cells are immunopositive; +++, >60% of tumor cells are immunopositive. Scores for Ki-67 were recorded as the number of immunopositive cells per high-power microscope (×400). The number of immunopositive cells under 5 microscopes per slide with the highest cell counts was counted and the average was recorded. MVD was recorded as the number of vessels or clusters of cells immunopositive for CD34 per high-power microscope (×400). Each immunostained cell or cell cluster that was clearly separated from adjacent microvessels was considered as a single countable microvessel. MVD under 5 microscopes per slide with the highest vascular counts were counted and the average was recorded.
Spearman rank correlation analysis (SPSS statistical software, version 11.5) was used in the analysis of correlation between HGF and c-Met expression, MVD, Ki-67 expression, age, sex, and extrasellar extension because the assumption for a parametric test (Kolmogorov–Smirnov test) was invalid except for age and MVD. The Kruskal–Wallis test was used to see whether HGF or c-Met was differentially expressed between adenomas of different histology types. Expression levels of HGF and c-Met in pituitary adenomas of different histology types are recorded in Table 1 and MVD, Ki-67, and patients' age are recorded as mean (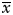 ) in Table 1. The results were reported as being statistically significant if P value was <.05.
) in Table 1. The results were reported as being statistically significant if P value was <.05.
Results
Results showed that HGF and c-Met expression existed in 98% (64 of 65) and 92% (60 of 65) of pituitary adenomas, respectively, and co-expression of HGF and c-Met existed in 91% (59 of 65) of pituitary adenomas. The number of adenomas with different immunoreactivity for HGF and c-Met can be seen in Table 1. There were no significant differences in c-Met or HGF expression between pituitary adenomas of different histology types. HGF and c-met expression in pituitary adenomas can be seen in Figs 1 and 2.
Fig. 1.
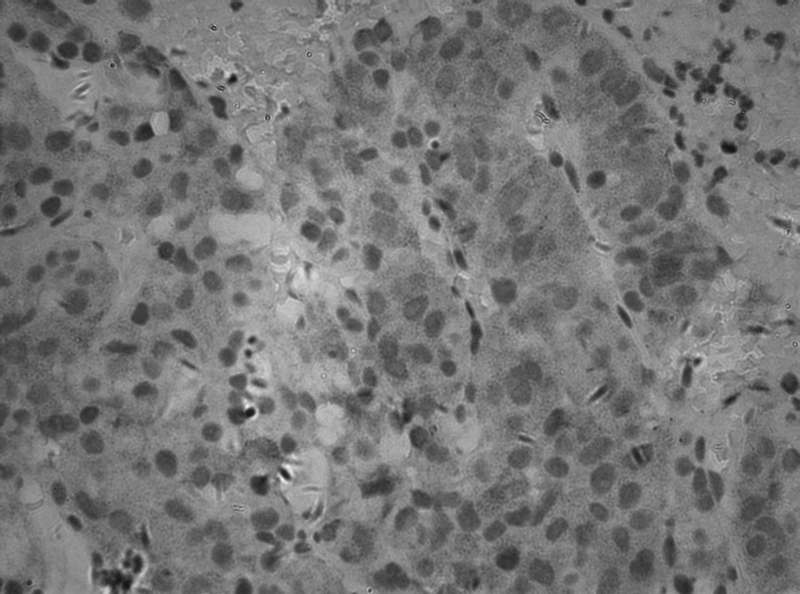
HGF expression in follicle stimulating hormone adenoma (×400).
Fig. 2.
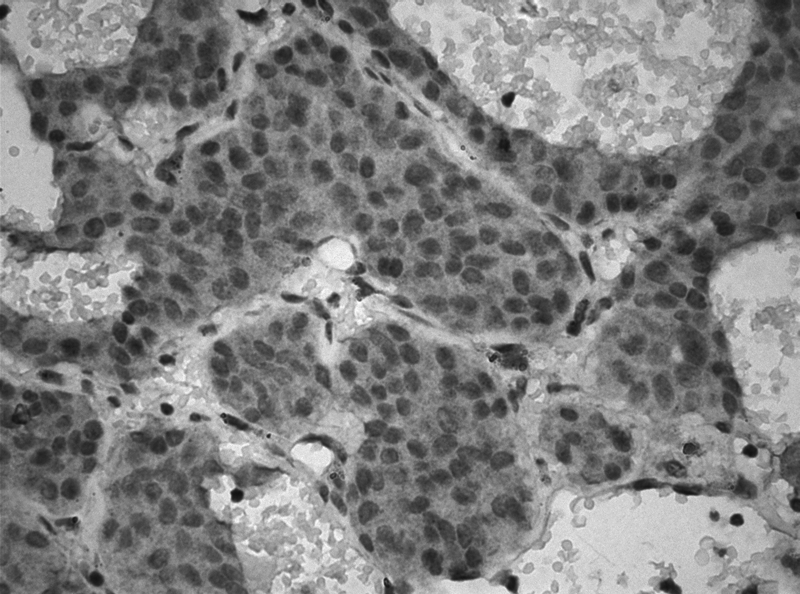
c-Met expression in adrenocorticotropic hormone adenoma (×400).
There was a significant correlation between HGF and c-Met expression (r = .41, P = .00). Both HGF and c-Met expression had significant correlation with MVD and Ki-67, but not with age or extrasellar invasion (for details, see Table 2). c-Met had a correlation with sex (r = .26, P = .04). c-Met expressions in male and female patients were 81% (19 of 21) and 98% (43 of 44), respectively.
Table 2.
Correlational analysis of HGF and c-Met expression with MVD, Ki-67, age, sex, and extrasellar invasion
| Clinicopathological factors | HGF |
c-Met |
||
|---|---|---|---|---|
| r* | p† | r | p | |
| MVD | .31 | 0.01 | .30 | 0.02 |
| Ki-67 | .32 | 0.01 | .38 | 0.00 |
| Age | .05 | 0.70 | −.05 | 0.68 |
| Sex | .13 | 0.32 | .26 | 0.04 |
| Extrasellar invasion | −.03 | 0.79 | −.20 | 0.10 |
Abbreviations: HGF, hepatocyte growth factor; MVD, microvessel density.
*Spearman rank correlation coefficient (r) is considered significant when P < .05.
†Permutation test (p) is used to test the significance of Spearman correlation coefficient.
Discussion
To our knowledge, this is the first study that investigated HGF and c-Met expression in pituitary adenomas. The study shows that HGF and c-Met are widely expressed in pituitary adenomas, and their expression significantly correlate with tumor angiogenic and proliferative factors. This implies that HGF and c-Met may have a role in the angiogenesis and tumorigenesis of pituitary adenomas.
Tumor angiogenesis, the formation of new blood vessels from pre-existing vessels, is essential for tumor growth. Angiogenesis, evaluated as tumor MVD, has significant association with metastasis, poor prognosis, and recurrence in breast, brain, bladder, prostate, and gastric cancers.10–15 HGF and c-Met are involved in various processes of brain tumor angiogenesis, including inducing proliferation and migration of tumor endothelial cells, enhancing vascular endothelial growth factor expression, and inducing endothelial tubule formation and angiogenesis.8 HGF and c-Met expression correlate with angiogenesis in breast, bladder, gastric, and soft tissue tumors.16–19 Inhibitors targeting HGF or c-Met have been shown to inhibit tumor angiogenesis.20,21 The present study shows that HGF and c-Met expression in pituitary tumors significantly correlate with MVD (P < .05). This implies that HGF and c-Met may have a role in pituitary angiogenesis.
HGF and c-Met are involved in the processes of tumorigenesis, including promoting tumor cell proliferation,22,23 invasion,24–26 and metastasis.27 HGF and c-Met expression correlate with tumor growth, invasion, metastasis, and poor prognosis in bladder,28 breast,29 liver,30 and lung31 cancers, and gliomas.32,33 Therapeutic agents targeting HGF and c-Met have been shown to inhibit tumor growth and improve survival.34–36 Ki-67 is a proliferative marker, and its expression correlates with poor prognosis in brain tumors like gliomas, ependymomas, and pituitary adenomas.37–44 The study shows that HGF and c-Met expression in pituitary tumors significantly correlate with Ki-67 expression (P < .05). This implies that HGF and c-Met may have a role in pituitary tumorigenesis.
Most pituitary adenomas are benign, but many of them have aggressive growth and invade important neighboring structures like blood vessels, nerves, dura, and bone, which makes complete surgical resection impossible and tumor recurrence is often observed after surgery. HGF and c-Met expression have no significant differences between invasive and noninvasive adenomas. The result implicates that HGF and c-Met expression in pituitary adenomas may not be related to invasive behaviors.
In the present study, age had no significant correlation with HGF or c-Met, which is in accord with previous results.45–47 c-Met expression had a correlation with sex (r = .26, P = .04), but this is different from the previous results45–47 and the underlying mechanisms are unknown. No significant differences exist in HGF and c-met expression between pituitary adenomas of different histology types. So HGF and c-met expression in pituitary adenomas may not be related to hormone immunoreactivity.
In conclusion, HGF and c-Met are widely expressed in human pituitary adenomas, and their expression levels correlate with angiogenic and proliferative markers, but not with age, extrasellar invasion, or histology types. However, more investigations should be done about their roles in human pituitary adenomas.
Conflict of interest statement. None declared.
Funding
This study was supported by grant 2007GG30002010 from the Science and Technology Department of Shandong Province, China.
References
- 1.Koochekpour S, Jeffers M, Rulong S, et al. Met and hepatocyte growth factor/scatter factor expression in human gliomas. Cancer Res. 1997;57:5391–5398. [PubMed] [Google Scholar]
- 2.Moriyama T, Kataoka H, Kawano H, et al. Comparative analysis of expression of hepatocyte growth factor and its receptor, c-met, in gliomas, meningiomas and schwannomas in humans. Cancer Lett. 1998;124:149–155. doi: 10.1016/s0304-3835(97)00469-2. [DOI] [PubMed] [Google Scholar]
- 3.Arrieta O, Garcia E, Guevara P, et al. Hepatocyte growth factor is associated with poor prognosis of malignant gliomas and is a predictor for recurrence of meningioma. Cancer. 2002;94:3210–3218. doi: 10.1002/cncr.10594. [DOI] [PubMed] [Google Scholar]
- 4.Lamszus K, Schmidt NO, Jin L, et al. Scatter factor promotes motility of human glioma and neuromicrovascular endothelial cells. Int J Cancer. 1998;75:19–28. doi: 10.1002/(sici)1097-0215(19980105)75:1<19::aid-ijc4>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 5.Bowers DC, Fan S, Walter KA, et al. Scatter factor/hepatocyte growth factor protects against cytotoxic death in human glioblastoma via phosphatidylinositol 3-kinase- and AKT-dependent pathways. Cancer Res. 2000;60:4277–4283. [PubMed] [Google Scholar]
- 6.Walter KA, Hossain MA, Luddy C, Goel N, Reznik TE, Laterra J. Scatter factor/hepatocyte growth factor stimulation of glioblastoma cell cycle progression through G(1) is c-Myc dependent and independent of p27 suppression, Cdk2 activation, or E2F1-dependent transcription. Mol Cell Biol. 2002;22:2703–2715. doi: 10.1128/MCB.22.8.2703-2715.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidt NO, Westphal M, Hagel C, et al. Levels of vascular endothelial growth factor, hepatocyte growth factor/scatter factor and basic fibroblast growth factor in human gliomas and their relation to angiogenesis. Int J Cancer. 1999;84:10–18. doi: 10.1002/(sici)1097-0215(19990219)84:1<10::aid-ijc3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 8.Abounader R, Laterra J. Scatter factor/hepatocyte growth factor in brain tumor growth and angiogenesis. Neuro-Oncology. 2005;7:436–451. doi: 10.1215/S1152851705000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim S, Lee B, Kim H, Lee J. Abstracts for the Eighth Congress of the European Association for Neuro-Oncology: Met tyrosine kinase receptor expression in pituitary adenomas. Neuro-Oncology. 2008;10:1134. [Google Scholar]
- 10.Leon SP, Folkerth RD, Black PM. Microvessel density is a prognostic indicator for patients with astroglial brain tumors. Cancer. 1996;77:362–372. doi: 10.1002/(SICI)1097-0142(19960115)77:2<362::AID-CNCR20>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 11.Weidner N, Semple JP, Welch WR, Folkman J. Tumor angiogenesis and metastasis–correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 12.Weidner N, Carroll PR, Flax J, Blumenfeld W, Folkman J. Tumor angiogenesis correlates with metastasis in invasive prostate carcinoma. Am J Pathol. 1993;143:401–409. [PMC free article] [PubMed] [Google Scholar]
- 13.Maeda K, Chung YS, Takatsuka S, et al. Tumor angiogenesis as a predictor of recurrence in gastric carcinoma. J Clin Oncol. 1995;13:477–481. doi: 10.1200/JCO.1995.13.2.477. [DOI] [PubMed] [Google Scholar]
- 14.Bochner BH, Cote RJ, Weidner N, et al. Angiogenesis in bladder cancer: relationship between microvessel density and tumor prognosis. J Natl Cancer Inst. 1995;87:1603–1612. doi: 10.1093/jnci/87.21.1603. [DOI] [PubMed] [Google Scholar]
- 15.Weidner N, Folkman J, Pozza F, et al. Tumor angiogenesis: a new significant and independent prognostic indicator in early-stage breast carcinoma. J Natl Cancer Inst. 1992;84:1875–1887. doi: 10.1093/jnci/84.24.1875. [DOI] [PubMed] [Google Scholar]
- 16.Zhang W, Chu YQ, Ye ZY, Zhao ZS, Tao HQ. Expression of hepatocyte growth factor and basic fibroblast growth factor as prognostic indicators in gastric cancer. Anat Rec (Hoboken) 2009;292:1114–1121. doi: 10.1002/ar.20934. [DOI] [PubMed] [Google Scholar]
- 17.Kuhnen C, Muehlberger T, Honsel M, Tolnay E, Steinau HU, Muller KM. Impact of c-Met expression on angiogenesis in soft tissue sarcomas: correlation to microvessel-density. J Cancer Res Clin Oncol. 2003;129:415–422. doi: 10.1007/s00432-003-0456-4. [DOI] [PubMed] [Google Scholar]
- 18.Garcia S, Dales JP, Charafe-Jauffret E, et al. Overexpression of c-Met and of the transducers PI3K, FAK and JAK in breast carcinomas correlates with shorter survival and neoangiogenesis. Int J Oncol. 2007;31:49–58. [PubMed] [Google Scholar]
- 19.Strohmeyer D, Strauss F, Rossing C, et al. Expression of bFGF, VEGF and c-met and their correlation with microvessel density and progression in prostate carcinoma. Anticancer Res. 2004;24:1797–1804. [PubMed] [Google Scholar]
- 20.Puri N, Khramtsov A, Ahmed S, et al. A selective small molecule inhibitor of c-Met. PHA665752, inhibits tumorigenicity and angiogenesis in mouse lung cancer xenografts. Cancer Res. 2007;67:3529–3534. doi: 10.1158/0008-5472.CAN-06-4416. [DOI] [PubMed] [Google Scholar]
- 21.Matsumoto K, Nakamura T. NK4 gene therapy targeting HGF-Met and angiogenesis. Front Biosci. 2008;13:1943–1951. doi: 10.2741/2813. [DOI] [PubMed] [Google Scholar]
- 22.Lamszus K, Laterra J, Westphal M, Rosen EM. Scatter factor/hepatocyte growth factor (SF/HGF) content and function in human gliomas. Int J Dev Neurosci. 1999;17:517–530. doi: 10.1016/s0736-5748(99)00008-8. [DOI] [PubMed] [Google Scholar]
- 23.Hov H, Tian E, Holien T, et al. c-Met signaling promotes IL-6-induced myeloma cell proliferation. Eur J Haematol. 2009;82:277–287. doi: 10.1111/j.1600-0609.2009.01212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li Y, Lal B, Kwon S, et al. The scatter factor/hepatocyte growth factor: c-met pathway in human embryonal central nervous system tumor malignancy. Cancer Res. 2005;65:9355–9362. doi: 10.1158/0008-5472.CAN-05-1946. [DOI] [PubMed] [Google Scholar]
- 25.Hecht M, Papoutsi M, Tran HD, Wilting J, Schweigerer L. Hepatocyte growth factor/c-Met signaling promotes the progression of experimental human neuroblastomas. Cancer Res. 2004;64:6109–6118. doi: 10.1158/0008-5472.CAN-04-1014. [DOI] [PubMed] [Google Scholar]
- 26.Khoury H, Naujokas MA, Zuo D, et al. HGF converts ErbB2/Neu epithelial morphogenesis to cell invasion. Mol Biol Cell. 2005;16:550–561. doi: 10.1091/mbc.E04-07-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gallego MI, Bierie B, Hennighausen L. Targeted expression of HGF/SF in mouse mammary epithelium leads to metastatic adenosquamous carcinomas through the activation of multiple signal transduction pathways. Oncogene. 2003;22:8498–8508. doi: 10.1038/sj.onc.1207063. [DOI] [PubMed] [Google Scholar]
- 28.Miyata Y, Sagara Y, Kanda S, Hayashi T, Kanetake H. Phosphorylated hepatocyte growth factor receptor/c-Met is associated with tumor growth and prognosis in patients with bladder cancer: correlation with matrix metalloproteinase-2 and -7 and E-cadherin. Hum Pathol. 2009;40:496–504. doi: 10.1016/j.humpath.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 29.Garcia S, Dales JP, Charafe-Jauffret E, et al. Poor prognosis in breast carcinomas correlates with increased expression of targetable CD146 and c-Met and with proteomic basal-like phenotype. Hum Pathol. 2007;38:830–841. doi: 10.1016/j.humpath.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 30.Hida Y, Morita T, Fujita M, et al. Clinical significance of hepatocyte growth factor and c-Met expression in extrahepatic biliary tract cancers. Oncol Rep. 1999;6:1051–1056. doi: 10.3892/or.6.5.1051. [DOI] [PubMed] [Google Scholar]
- 31.Takanami I, Tanana F, Hashizume T, et al. Hepatocyte growth factor and c-Met/hepatocyte growth factor receptor in pulmonary adenocarcinomas: an evaluation of their expression as prognostic markers. Oncology. 1996;53:392–397. doi: 10.1159/000227594. [DOI] [PubMed] [Google Scholar]
- 32.Nabeshima K, Shimao Y, Sato S, et al. Expression of c-Met correlates with grade of malignancy in human astrocytic tumours: an immunohistochemical study. Histopathology. 1997;31:436–443. doi: 10.1046/j.1365-2559.1997.3010889.x. [DOI] [PubMed] [Google Scholar]
- 33.Kong DS, Song SY, Kim DH, et al. Prognostic significance of c-Met expression in glioblastomas. Cancer. 2009;115:140–148. doi: 10.1002/cncr.23972. [DOI] [PubMed] [Google Scholar]
- 34.Guerin C, Luddy C, Abounader R, Lal B, Laterra J. Glioma inhibition by HGF/NK2, an antagonist of scatter factor/hepatocyte growth factor. Biochem Biophys Res Commun. 2000;273:287–293. doi: 10.1006/bbrc.2000.2935. [DOI] [PubMed] [Google Scholar]
- 35.Abounader R, Lal B, Luddy C, et al. In vivo targeting of SF/HGF and c-met expression via U1snRNA/ribozymes inhibits glioma growth and angiogenesis and promotes apoptosis. FASEB J. 2002;16:108–110. doi: 10.1096/fj.01-0421fje. [DOI] [PubMed] [Google Scholar]
- 36.Jin H, Yang R, Zheng Z, et al. MetMAb, the one-armed 5D5 anti-c-Met antibody, inhibits orthotopic pancreatic tumor growth and improves survival. Cancer Res. 2008;68:4360–4368. doi: 10.1158/0008-5472.CAN-07-5960. [DOI] [PubMed] [Google Scholar]
- 37.Kontogeorgos G. Predictive markers of pituitary adenoma behavior. Neuroendocrinology. 2006;83:179–188. doi: 10.1159/000095526. [DOI] [PubMed] [Google Scholar]
- 38.Gejman R, Swearingen B, Hedley-Whyte ET. Role of Ki-67 proliferation index and p53 expression in predicting progression of pituitary adenomas. Hum Pathol. 2008;39:758–766. doi: 10.1016/j.humpath.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 39.Bruna J, Brell M, Ferrer I, Gimenez-Bonafe P, Tortosa A. Ki-67 proliferative index predicts clinical outcome in patients with atypical or anaplastic meningioma. Neuropathology. 2007;27:114–120. doi: 10.1111/j.1440-1789.2007.00750.x. [DOI] [PubMed] [Google Scholar]
- 40.Preusser M, Heinzl H, Gelpi E, et al. Ki67 index in intracranial ependymoma: a promising histopathological candidate biomarker. Histopathology. 2008;53:39–47. doi: 10.1111/j.1365-2559.2008.03065.x. [DOI] [PubMed] [Google Scholar]
- 41.Wolfsberger S, Fischer I, Hoftberger R, et al. Ki-67 immunolabeling index is an accurate predictor of outcome in patients with intracranial ependymoma. Am J Surg Pathol. 2004;28:914–920. doi: 10.1097/00000478-200407000-00011. [DOI] [PubMed] [Google Scholar]
- 42.Rodriguez-Pereira C, Suarez-Penaranda JM, Vazquez-Salvado M, et al. Value of MIB-1 labelling index (LI) in gliomas and its correlation with other prognostic factors. A clinicopathologic study. J Neurosurg Sci. 2000;44:203–210. [PubMed] [Google Scholar]
- 43.Heegaard S, Sommer HM, Broholm H, Broendstrup O. Proliferating cell nuclear antigen and Ki-67 immunohistochemistry of oligodendrogliomas with special reference to prognosis. Cancer. 1995;76:1809–1813. doi: 10.1002/1097-0142(19951115)76:10<1809::aid-cncr2820761020>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 44.Enestrom S, Vavruch L, Franlund B, Nordenskjold B. Ki-67 antigen expression as a prognostic factor in primary and recurrent astrocytomas. Neurochirurgie. 1998;44:25–30. [PubMed] [Google Scholar]
- 45.Lo Muzio L, Leonardi R, Mignogna MD, et al. Scatter factor receptor (c-Met) as possible prognostic factor in patients with oral squamous cell carcinoma. Anticancer Res. 2004;24:1063–1069. [PubMed] [Google Scholar]
- 46.Chen BK, Ohtsuki Y, Furihata M, et al. Overexpression of c-Met protein in human thyroid tumors correlated with lymph node metastasis and clinicopathologic stage. Pathol Res Pract. 1999;195:427–433. doi: 10.1016/S0344-0338(99)80017-X. [DOI] [PubMed] [Google Scholar]
- 47.Sawatsubashi M, Sasatomi E, Mizokami H, Tokunaga O, Shin T. Expression of c-Met in laryngeal carcinoma. Virchows Arch. 1998;432:331–335. doi: 10.1007/s004280050174. [DOI] [PubMed] [Google Scholar]


