Abstract
Convection-enhanced delivery (CED) of cintredekin besudotox (CB) was compared with Gliadel wafers (GW) in adult patients with glioblastoma multiforme (GBM) at first recurrence. Patients were randomized 2:1 to receive CB or GW. CB (0.5 µg/mL; total flow rate 0.75 mL/h) was administered over 96 hours via 2–4 intraparenchymal catheters placed after tumor resection. GW (3.85%/7.7 mg carmustine per wafer; maximum 8 wafers) were placed immediately after tumor resection. The primary endpoint was overall survival from the time of randomization. Prestated interim analyses were built into the study design. Secondary and tertiary endpoints were safety and health-related quality-of-life assessments. From March 2004 to December 2005, 296 patients were enrolled at 52 centers. Demographic and baseline characteristics were balanced between the 2 treatment arms. Median survival was 36.4 weeks (9.1 months) for CB and 35.3 weeks (8.8 months) for GW (P = .476). For the efficacy evaluable population, the median survival was 45.3 weeks (11.3 months) for CB and 39.8 weeks (10 months) for GW (P = .310). The adverse-events profile was similar in both arms, except that pulmonary embolism was higher in the CB arm (8% vs 1%, P = .014). This is the first randomized phase III evaluation of an agent administered via CED and the first with an active comparator in GBM patients. There was no survival difference between CB administered via CED and GW. Drug distribution was not assessed and may be crucial for evaluating future CED-based therapeutics.
Keywords: cintredekin besudotox, convection-enhanced delivery, Gliadel wafers, glioblastoma multiforme, IL13-PE38QQR
Glioblastoma multiforme (GBM) is the most common primary brain tumor in adults, and from the time of recurrence, the median overall survival is 6–8 months. Effective strategies for recurrent GBM have been difficult to develop for several reasons, including inadequate exposure to the therapeutic agent as a result of poor drug delivery across the blood-brain barrier. Local surgical treatment with Gliadel® wafers (GW) has resulted in a small but statistically significant improvement in survival.1
Convection-enhanced delivery (CED) is a loco-regional delivery method that relies on a continuous pressure gradient to administer an infusate containing a therapeutic agent directly into the interstitial space of brain tissue over an interval of a few hours to a few days to bypass the blood-brain barrier and increase drug distribution to the target tissue.2 Several factors influence the injected agent's final distribution volume, including rate and volume of infusion, half-life of the drug, anatomical anisotropy, and surface-binding properties of the drug.3 Preclinical studies have shown clinically significant, topographically targeted, reproducible, and homogeneous distribution of molecules of various sizes.2,4–7 CED of therapeutic agents in malignant glioma has shown promise in preclinical studies and early clinical development.8–13
Cintredekin besudotox (CB), also known as IL13-PE38QQR, is a recombinant chimeric cytotoxin composed of human interleukin-13 (IL13) fused to a truncated, mutated form of Pseudomonas aeruginosa exotoxin A (PE38QQR). This agent targets and kills tumor cells that express the IL13 receptor. The IL13 moiety attaches to the IL13 receptor at the cell surface and facilitates the entry of the exotoxin, which inhibits protein synthesis by adenosine diphosphate riobosylation of elongation factor 2 and also induces caspase-mediated apoptosis.14,15 The majority of malignant glioma cell lines and explants overexpress IL13 receptors.16–20 Furthermore, detection of mRNA and protein for the IL13 receptor chain indicates malignant glioma specificity and a much higher expression than in low-grade or non-neoplastic glia.19 This differential expression provides a specific target for malignant glioma therapy. The concentration of IL13-PE38QQR causing 50% inhibition of protein synthesis (IC50) in glioma cells overexpressing IL13 receptors has been reported to be as low as 0.1 ng/mL or less, creating a wide therapeutic margin.18,21
In a prior phase I study of 120 patients treated with CED of CB, it was shown that catheters placed in the postoperative period as opposed to at the time of tumor resection were far more likely to be located as described by protocol guidelines (79% vs 49%).22 Postoperative catheter placement occurred 1–3 days after tumor resection using the postoperative MRI scan for stereotactic placement planning. In addition, the safety profile was consistent with a comparable population undergoing neurosurgical procedures. The maximum tolerated infusion concentration of CB was 0.5 µg/mL in a volume of 72 mL administered by CED for 96 hours at a rate of 0.750 mL/h. Efficacy evaluation suggested a strong advantage over historical control groups that did not receive GW and led to the design of this study.
The objective of this randomized phase III study was to determine the efficacy, measured by overall survival, of CED of CB compared with GW in GBM patients at first recurrence. As this was an adjuvant to local treatment, the only other approved local treatment was selected as the comparator.
Methods and Materials
All authors contributed to the study design, data analysis, and writing of this manuscript, and all vouch for the data and analysis. Data were gathered by the following authors: S.K., S.C., J.S., M.V., G.B., M.W., M.S., Z.R., J.P., M.P., and C.P.
Cintredekin Besudotox
The full sequence encoding CB was developed by R.K.P. (Tumor Vaccines and Biotechnology Branch, Center for Biologics Evaluation and Research, FDA) and incorporated into a plasmid at Advanced BioScience Laboratories (Kensington, Maryland) and later at Diosynth (Sioux City, Iowa). Escherichia coli transfected with the plasmid were induced and expanded. Protein was purified from inclusion bodies under current good manufacturing practices as described.23
Study Design
This study involved 52 leading neurosurgery sites in the United States, Canada, Europe, and Israel. Two hundred and ninety-six patients were randomized in a 2:1 ratio to receive either postoperative intraparenchymal CB or GW, respectively. Enrollment took place between March 2004 and December 2005.
The investigators and sponsor were blinded to study the results until a protocol-defined (215 deaths) efficacy analysis was performed by an independent data monitoring committee (DMC). An investigator steering committee (also blinded to results) assessed compliance with surgical procedures.
Patient Eligibility Criteria
Adult patients with the first recurrence of GBM were eligible. Tumor specimens from the original surgery were not evaluated for the presence of IL13 receptors. Patients were excluded if the neurosurgeon felt a gross surgical resection would result in an irreparable communication of the resection cavity with the ventricle, based on GW-placement guidelines. Patients who had received either one of the two study drugs, prior brachytherapy, radiosurgery, or any other investigational intracerebral agents were ineligible. Patients signed an Institutional Review Board (IRB)-approved informed consent prior to enrollment. The study was approved by the Federal Drug Administration and IRBs of all participating centers.
Treatment Plan
After randomization, patients underwent gross total resection of their tumor. If randomized to the GW arm, wafers were placed immediately following the resection and MRI was performed within 48 hours of surgery. Patients randomized to treatment with CB underwent a separate procedure to place 2–4 catheters 2–7 days after resection in areas at greatest risk for infiltrating disease (T2-weighted or FLAIR hyperintense signal abnormality or largest white-matter area adjacent to the resection cavity) or in the vicinity of any residual, solid, contrast-enhancing disease. Following the confirmation of appropriate catheter placement on a CT scan, CB infusion was started 24 hours later at a concentration of 0.5 µg/mL and at a total rate of 0.750 mL/h for 96 hours.
Patients were followed with clinical and radiographic assessments every 8 weeks. Toxicity was assessed using the Cancer Therapy Evaluation Program Common Terminology Criteria for Adverse Events, version 3.0. Treatment-emergent adverse events (AEs) were summarized by system organ class, maximum severity, and highest degree of relationship with the study drug.
Catheter Placement
Catheters were placed using either a stereotactic frame or a stereotactic frameless navigation system. Catheter-positioning guidelines were developed from the results of a pilot study demonstrating that the average intraparenchymal volume of distribution ranged from 10 to 15 mm radially from the tip of catheter (Table 1).22 All neurosurgeons attended one off-site and one on-site catheter planning and placement training session. Additionally, each neurosurgeon performed 3 cases of mock catheter planning, and those plans were reviewed and approved by a central review committee. A scoring system was also developed for ongoing study review (Table 1).
Table 1.
Criteria and scoring system for assessment of catheter positioning
| Definition | |
|---|---|
| Criterion | |
| A | Depth ≥25 mm from brain surface or any deep sulcus or from resection cavity wall if placed through the resection cavity |
| B | Catheter tip ≥5 mm from any pial surfaces |
| C | Catheter tip ≥10 mm from the resection cavity walls or any ependymal surfaces |
| Score | |
| 0 | Poor: criterion A not fulfilled (regardless of other criteria) |
| 1 | Fair: criteria A and either B or C fulfilled |
| 2 | Good: all three criteria fulfilled |
Neuro-Imaging Assessment
Postoperative MRI changes may result from catheter placement, infusion, or delayed drug effect24 and can be difficult to distinguish from progression unless a careful review is performed using prior imaging studies with catheter trajectories. A grading system for changes on MRI was developed following early-phase studies with CB, as were guidelines for managing patients with such imaging changes (Table 2).
Table 2.
Management guidelines for MRI changes
| Clinico-radiologic category | Imaging changes grading | MRI changesa | Neurological statusb | Corticosteroids recommendationsc | Suggested follow-up |
|---|---|---|---|---|---|
| I | I | Hyperintense signal abnormality on FLAIR related to catheter tract or tip only; no new contrast-enhancement | No worsening | No change | Scheduled follow-up MRI and clinical assessment |
| IIa | II | Mild contrast-enhancement (<1.0 cm or linear) related to catheter tract or tip | No worsening | Consider resuming or increasing based on imaging features | Repeat clinical assessment in 2–4 wks and MRI in 4–8 wks depending on clinical findings |
| IIb | II | Mild contrast-enhancement (<1.0 cm or linear) related to catheter tract or tip | Worsening | Promptly resume or increase | Repeat MRI and clinical assessment in 2 wks |
| IIIa | III | Moderate contrast-enhancement (1.0–3.0 cm) related to catheter tract or tip | No worsening | Promptly resume or increase | Repeat MRI and clinical assessment in 2 wks |
| IIIb | III | Moderate contrast-enhancement (1.0–3.0 cm) related to catheter tract or tip | Worsening | Promptly resume or increase | Repeat MRI and clinical assessment in 2 wks |
| IV | IV | Extensive contrast-enhancement (>3.0 cm) related to catheter tract or tip, with or without central hypointensity | Worsening | Promptly resume or increase | Repeat MRI and clinical assessment in 2 wks |
aContrast-enhancing lesions diameter include the central hypointensity, if present.
bNeurological symptoms/signs localization have to be related to prior catheter trajectory(ies).
cIncrement, maintenance dose, and duration of treatment are based on clinical findings (eg, neurological symptoms, severity of neurological signs, or interference with activity of daily living) and imaging features (eg, size of the abnormality, severity of mass effect, or proximity to eloquent brain parenchyma).
Statistical Considerations
Three study populations were analyzed: (i) intent-to-treat (ITT) population: all patients randomized to treatment who underwent resection and had histopathologic confirmation of GBM prior to treatment; (ii) efficacy evaluable population: patients in the ITT population who had histopathologic confirmation of recurrent GBM from the central pathology review, underwent resection, and in the CB group received at least 90% of the planned dose of the study drug via the protocol-specified positioning guidelines; and (iii) safety population: all patients who received any study drug.
The primary objective of this study was to compare the overall survival of patients treated with intraparenchymal infusion of CB with that of patients treated with GW. The secondary objective was to assess safety and toxicity. Finally, the tertiary objective was to assess health-related quality-of-life parameters. Two hundred and seventy patients (180 CB, 90 GW) were needed to detect a statistically significant difference in survival at the 2-sided .05 significance level with at least 80% power, when projected median survival is 28 weeks for GW and 42 weeks in CB (50% increase). Up to an additional 30 patients were allowed to be enrolled to account for potential patients who would be randomized but subsequently become ineligible based on postrandomization histopathological criteria for GBM confirmation or other reasons. An interim efficacy analysis was planned at 160 deaths and the protocol-specified event efficacy analysis was planned at 215 deaths. Demographics and baseline characteristics were studied by Cochran–Mantel–Haenszel for categorical variables and ANOVA for continuous baseline variables. A stratified log-rank test (center, categorized Karnofsky Performance Status [KPS], categorized age) and Cox proportional hazard analyses were used to determine efficacy endpoints, and survival curves were compared by the Kaplan–Meier method.
Survival was defined as the number of days from the date of randomization to the date of death or last known alive date. Cox proportional hazard analyses were performed to further compare treatments adjusting for prognostic factors identified by the backward selection procedure. Prespecified potential prognostic factors included age, KPS score, time from original diagnosis, tumor size, extent of resection, and prior systemic glioma treatment. Treatment and pooled center were retained in the model. Hazard ratios and associated 2-sided 95% confidence intervals under the Cox proportional hazard analyses framework were obtained.
Interim Safety and Futility Analyses
In addition to the interim analysis for efficacy, the DMC performed 2 prespecified interim analyses for futility after 50 and 100 deaths were reported. Conditional power calculations were performed both under the null and alternative hypotheses using the “stochastic curtailment” method. At these time points, the DMC recommended continuing the study as planned.
Results
Efficacy Analysis
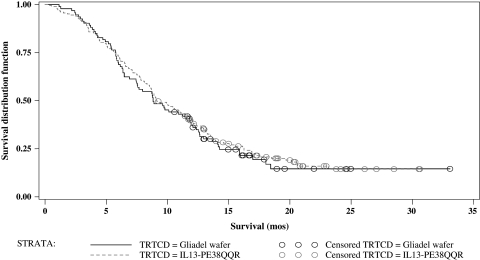
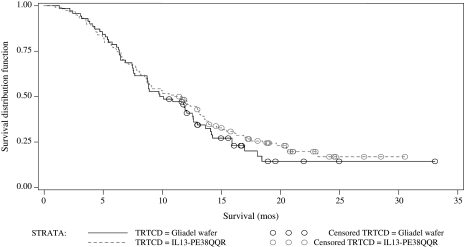
A total of 296 patients were randomized; 192 to CB and 104 to GW. Patients' demographics are shown in Table 3. There was no statistical difference in patient characteristics between the 2 study groups. A total of 276 patients (183 in CB and 93 in GW arms) actually underwent resection and had histopathologic confirmation of GBM (ITT group). The cut-off date for the safety and efficacy analyses was December 8, 2006, which corresponded to the time at which the prespecified milestone of 215 deaths was reached. Two hundred and sixty-nine patients were available for safety analysis (ie, they received any study drug) and 188 were available for efficacy analysis. Median survival for the ITT population was 36.4 weeks (9.1 months) for the CB group and 35.3 weeks (8.8 months) for the GW group (P = .476; hazard ratio 0.89; 95% CI = 0.67–1.18). Kaplan–Meier survival curves are presented in Fig. 1. Median survival in the efficacy evaluable population was 45.3 weeks (11.3 months) for CB and 39.8 weeks (10 months) for GW (P = .310; hazard ratio 0.81; 95% CI = 0.67–1.18; Fig. 2).
Table 3.
Demographic and baseline characteristics for patients in the PRECISE study (ITT population)
| Parameter | Summary type | Treatment group |
Total (n = 276) | P value | |
|---|---|---|---|---|---|
| Cintredekin besudotox (n = 183) | Gliadel wafer (n = 93) | ||||
| Gender | .534 | ||||
| Female | n (%) | 62 (34) | 28 (30) | 90 (33) | |
| Male | n (%) | 121 (66) | 65 (70) | 186 (67) | |
| Age category (y) | .506 | ||||
| < 55 | n (%) | 85 (46) | 47 (51) | 132 (48) | |
| ≥ 55 | n (%) | 98 (54) | 46 (49) | 144 (52) | |
| Age (y) | .609 | ||||
| Mean (SD) | 54.8 (11.23) | 54.3 (10.75) | 54.7 (11.06) | ||
| Median | 56 | 54 | 55 | ||
| Minimum | 18 | 22 | 18 | ||
| Maximum | 76 | 81 | 81 | ||
| Ethnicity | .285 | ||||
| Missing | n (%) | 6 (3) | 3 (3) | 9 (3) | |
| Hispanic or Latino | n (%) | 6 (3) | 1 (1) | 7 (3) | |
| Not Hispanic or Latino | n (%) | 171 (93) | 89 (96) | 260 (94) | |
| Race | .278 | ||||
| Missing | n (%) | 5 (3) | 2 (2) | 7 (3) | |
| Asian | n (%) | 1 (1) | 2 (2) | 3 (1) | |
| Black or African American | n (%) | 1 (1) | 2 (2) | 3 (1) | |
| Native Hawaiian or Other Pacific Islander | n (%) | 2 (1) | 0 | 2 (1) | |
| Other | n (%) | 3 (2) | 0 | 3 (1) | |
| White | n (%) | 171 (93) | 87 (94) | 258 (93) | |
| KPS (actual score) | .607 | ||||
| 70 | n (%) | 25 (14) | 12 (13) | 37 (13) | |
| 80 | n (%) | 43 (23) | 16 (17) | 59 (21) | |
| 90 | n (%) | 78 (43) | 46 (49) | 124 (45) | |
| 100 | n (%) | 37 (20) | 19 (20) | 56 (20) | |
| Screening KPS (summary statistics) | .361 | ||||
| Mean (SD) | 86.9 (9.46) | 87.7 (9.22) | 87.2 (9.37) | ||
| Median | 90 | 90 | 90 | ||
| Minimum | 70 | 70 | 70 | ||
| Maximum | 100 | 100 | 100 | ||
| Screening KPS category | .85 | ||||
| < 80 | n (%) | 25 (14) | 12 (13) | 37 (13) | |
| ≥ 80 | n (%) | 158 (86) | 81 (87) | 239 (87) | |
| Handedness | .52 | ||||
| Ambidextrous | n (%) | 1 (1) | 0 | 1 (0) | |
| Left | n (%) | 19 (10) | 10 (11) | 29 (11) | |
| Right | n (%) | 163 (89) | 81 (87) | 244 (88) | |
| Unknown | n (%) | 0 | 2 ( 2) | 2 (1) | |
| Time from initial diagnosis to study resection (wks) | .346 | ||||
| Mean (SD) | 41.2 (36.31) | 42.3 (33.04) | 41.6 (35.18) | ||
| Median | 32.14 | 30.43 | 31.5 | ||
| Minimum | 12.7 | 2.1 | 2.1 | ||
| Maximum | 290 | 210 | 290 | ||
| Prior systemic glioma treatment | .494 | ||||
| No | n (%) | 33 (18) | 20 (22) | 53 (19) | |
| Yes | n (%) | 150 (82) | 73 (78) | 223 (81) | |
| Largest tumor diameter (cm) | .019* | ||||
| Mean (SD) | 3.7 (1.52) | 4.3 (1.55) | 3.9 (1.56) | ||
| Median | 3.7 | 4.2 | 3.9 | ||
| Minimum | 1 | 1 | 1 | ||
| Maximum | 8.2 | 8.9 | 8.9 | ||
Abbreviations: SD, standard deviation; KPS, Karnofsky Performance Score.
*Not significant for longest perpendicular diameter.
Fig. 1.
Kaplan–Meier estimate of overall survival for all ITT patients. Treatment with IL13-PE38QQR: Total (censored) = 183 (37); Median (95% CI) = 36.4 (34.14–45.57). Treatment with Gliadel wafer: Total (censored) = 93 (20); Median (95% CI) = 35.3 (29.86–47.29). CB compared with Gliadel wafer hazard ratio (2-sided; 95% CI): 0.89 (0.67–1.18), P value = 0.416.
Fig. 2.
Kaplan–Meier estimate of overall survival for all efficacy evaluable (EE) patients. Treatment with IL13-PE38QQR: Total (censored) = 118 (28); Median (95% CI) = 45.3 (34.71–52.57). Treatment with Gliadel wafer: Total (censored) = 70 (16); Median (95% CI) = 39.8 (34.86–50.43). CB compared with Gliadel wafer hazard ratio (2-sided; 95% CI): 0.81 (0.58–1.14), P value = 0.234.
Safety Analysis
Overall, the 2 treatment groups showed similar safety profiles. Although the incidence of AEs of severity grade ≥3, SAEs, and AEs resulting in death or study drug discontinuation was slightly higher in the CB group, these differences were not statistically significant (Table 4) with the exception of thromboembolic complications. Vascular disorders was the only system organ class for which the incidence of grade ≥3 AEs was significantly higher in patients treated with CB (P < .001). This difference appears to be predominantly related to a higher incidence of pulmonary embolism in the CB group (8% vs 1%, P = .014), resulting in death in 2 patients (1%) treated with CB. There were non-reversible AEs of severity grade ≥3 that were found to be slightly higher in either treatment group, but the difference was not statistically significant (Table 4).
Table 4.
Adverse events
| Incidence of adverse events in safety population | ||||
|---|---|---|---|---|
| Variable | Treatment group |
P value | ||
| Cintredekin besudotox (n = 177)> | Gliadel wafer (n = 92) | |||
| Adverse events severity grade ≥3 (n [%]) | 149 (84) | 71 (77) | >.05 (NS) | |
| Serious adverse events (n [%]) | 111 (63) | 47 (51) | >.05 (NS) | |
| Adverse events resulting in death (n [%]) | 34 (19) | 13 (14) | >.05 (NS) | |
| Adverse events resulting in study drug discontinuation (n [%]) | 6 (3) | 2 (2) | >.05 (NS) | |
| Summary of total number of nonreversible (defined as under observation, residual sequelae, death, or no resolution at the time of death) adverse events grade ≥3 most frequently reported out of all adverse events | ||||
| Preferred term | System organ class | IL-13 (n= 3239) | Gliadel wafer (n= 1103) | Total (n= 4342) |
| Aphasia | Nervous system disorders | 39 (1.2%) | 18 (1.6%) | 57 (1.3%) |
| Hemiparesis | Nervous system disorders | 28 (0.8%) | 16 (1.5%) | 44 (1.0%) |
| Deep vein thrombosis | Vascular disorders | 16 (0.5%) | 2 (0.2%) | 18 (0.4%) |
| Monoparesis | Nervous system disorders | 15 (0.5%) | 2 (0.2%) | 17 (0.4%) |
| Headache | Nervous system disorders | 13 (0.4%) | 3 (0.3%) | 16 (0.4%) |
| Hemiplegia | Nervous system disorders | 11 (0.3%) | 5 (0.5%) | 16 (0.4%) |
| Gait disturbance | General disorders and administration site conditions | 10 (0.3%) | 5 (0.5%) | 15 (0.3%) |
| Brain edema | Nervous system disorders | 10 (0.3%) | 2 (0.2%) | 12 (0.3%) |
| Coordination abnormal | Nervous system disorders | 9 (0.3%) | 2 (0.2%) | 11 (0.3%) |
| Mental status changes | Psychiatric disorders | 9 (0.3%) | 1 (0.1%) | 10 (0.2%) |
Subsequent Therapy at Progression
Although no formal impact analyses were performed, secondary antitumor therapies were administered for progression and were well-balanced between the 2 treatment groups. Therapies included craniotomy, radiation therapies, cytotoxic agents, biological/cytostatic agents, and investigational agents. Following administration of GW or CB, approximately 43% of patients received additional antitumor therapies, 56% underwent no further treatment, and 1% had no data available.
Discussion
The PRECISE study is the largest surgically based randomized controlled study using CED in patients with recurrent GBM. Although well-tolerated, there was no survival advantage of CB administered via CED compared with GW. Several factors are critical to the therapeutic success of administering an agent via CED for brain-tumor patients.25 First is the specificity and antitumor effect of the agent itself. Patients in the current trial were not selected for participation based on their level of IL13 receptor expression because previous studies reported that IL13 receptor is overexpressed in a large percentage of GBM samples and testing for receptor expression in fine-needle biopsy samples for intracranial tumors is challenging due to heterogeneity in target expression. Variation in the level of IL13 receptor expression, however, exists among different GBM samples and between sites within an individual tumor,16,19,26,27 and this may have contributed to the inadequate antitumor effect.
Several parameters affect the agent's volume of distribution via CED, including catheter configuration, infusion rate and volume, and catheter positioning.25 Therefore, neurosurgeons were trained in protocol-compliant positioning of catheters and a steering committee monitored the catheter-placement procedures and resolved any difficulties encountered by investigators. Despite these measures, only 68% of catheters were positioned in accordance with protocol guidelines, indicating limited suitability of the equipment used. The post hoc analysis of patients with more than 2 catheters with a positioning score of 2 showed a slight but insignificant increase in survival. Investigator experience has been shown to be an important factor for technically challenging procedures.28 For local delivery techniques such as CED, it is critical that operator-dependent factors are standardized.
Ultimately, the most important factor in evaluating CED is whether the agent is distributed to the targeted region in sufficient concentrations to have a therapeutic effect. To determine this, one needs to be aware of where residual tumor is located and whether the agent reached the target. In this study, optimal catheter positioning was used as a surrogate for agent distribution; however, this relationship has not been prospectively validated using real-time imaging techniques. Ongoing studies combining imaging agents (eg, radiolabeled or gadolinium-based coinfusates) with mathematical modeling programs will provide guidance for optimal infusion parameters.29–32 The more commonly used MRI contrast agent Gd-DTPA also has been safely infused via CED into the human brain to track delivery of therapeutic molecules.33,34 We await the more routine application of this technology in patients to assess its utility. A study in a rodent model using magnetic nanoparticles to visualize high-viscosity infusates via MRI has shown reliable real-time imaging of distribution.35 Another recent study on optimization of cannula placement for infusions into the primate putamen used an image-guided system allowing real-time visualization of infusates, which may allow neurosurgeons to alter the parameters or, if necessary, terminate the infusion.36 The application of these novel technologies and further research into optimal catheter placement will perhaps enhance CED in future clinical trials.
The safety profile of CB observed in this trial is supported by data from 3 early-phase studies in patients with recurrent supratentorial malignant glioma.22 There were no differences in the CB or GW arms except for pulmonary embolism likely related to the prolonged hospital stay for the infusion procedure in the CED group. The majority of toxicities seen were in the expected range of a similar population of patients undergoing neurosurgical procedures and receiving corticosteroids.37,38 Procedural-related adverse effects of bacterial meningitis seen in CB-treated patients are likely due to the additional surgical procedure (stereotactic catheter placement) and externalized catheters being in place for up to 6 days; neurological symptoms such as seizures in CB-treated patients may be related to the presence of an intraparenchymal device or CB itself; and the depressed level of consciousness/mental-status change in CB-treated patients may be related to a variety of processes (eg, cerebral edema, CNS infection, epileptic events, or metabolic disorders). These findings are relevant to future planning of clinical trials evaluating intratumoral and intraparenchymal delivery of agents via CED.
Protocol management guidelines (Table 2) were critical in early recognition and management of clinical and neuroimaging changes, as corticosteroids appear to help stabilize and reverse both symptoms and imaging changes. CB-related imaging changes make it challenging to use MRI alone to assess the response to treatment in cases of subtotal resection and to determine tumor progression.24 Repeat biopsy for histopathological diagnosis remains the definitive method for differentiating treatment-related changes from recurrent or progressive tumor, but metabolic imaging modalities such as MR spectroscopy, MR perfusion studies, and positron emission tomography are assuming more importance in determining the nature of these changes.
Finally, it should be noted that the median survival in the efficacy evaluable group was 45 weeks for the CB patients and was well over the anticipated 42 weeks on which the statistical analysis plan was based and on which the study was powered, assuming a published historical control of 28 weeks for GW. The increase in the actuarial median survival in the GW control arm emphasizes that efficacy trials need a concurrent control because the clinical environment in which a study is conducted may have changed considerably as seen in the PRECISE trial, where the control arm had an almost 40% improved survival compared with prior experience. This could possibly be influenced as a result of better surgical techniques, more efficacious salvage treatment regimens, and supportive management as well as optimal patient selection.
Conclusion
This is the first completed randomized phase III evaluation of an agent administered via CED in GBM patients using an active comparator. Although reasonably well-tolerated, there was no survival difference between CB administered via CED and GW. Drug distribution was not assessed and should be incorporated into future trials of CED-based therapeutics.
Funding
Funding for this study was provided by NeoPharm, Inc.
Acknowledgments
The authors are grateful to Ilona Garner, Department of Neurological Surgery, UCSF, for editing this manuscript. The findings and conclusions in this article have not been formally disseminated by the Food and Drug Administration and should not be construed to represent any Agency determination or policy.
The following institutions and investigators participated in the trial: City of Hope Medical Center, Duarte, California (Behnam Badie); Ohio State University Medical Center, Columbus, Ohio (Antonio Chiocca); H. Lee Moffitt Cancer Center, Tampa, Florida (Frank Vrinis); University of Hamburg-Eppendorf, Hamburg, Germany (M.W.); University of Schleswig–Holstein, Lubeck, Germany (Maximillian H. Mehdorn); Sheba Medical Center, Tel Hashomer, Israel (Zvi Cohen); Tel Aviv Sourasky Medical Center, Tel Aviv, Israel (Z.R.); The Cleveland Clinic Foundation, Cleveland, Ohio (G.B.); University of Colorado Hospital-Medical School, Aurora, Colorado (Kevin Lillehei); Hospital of the University of Pennsylvania, Philadelphia, Pennsylvania (Kevin Judy); Baylor College of Medicine, Houston, Texas (Pamela New); Emory University, Atlanta, Georgia (Jeffrey Olson); Henry Ford Health Systems, Detroit, Michigan (Tom Mikkelsen); University of Alabama at Birmingham, Birmingham, Alabama (James Markert); Wake Forest University Health Sciences, Winston-Salem, North Carolina (Stephen Tatter); John Hopkins Hospital, Baltimore, Maryland (Jon Weingart); The University of Texas M. D. Anderson Cancer Center, Houston, Texas (Fredrick Lang); Yale University School of Medicine, New Haven, Connecticut (J.P.); University of California–San Francisco, San Francisco, California (M.P.); Memorial Sloan Kettering Cancer Center, New York, New York (Lauren Abrey); Duke University Medical Center, Durham, North Carolina (David Reardon); Carolina Neurosurgery and Spine Associates, Charlotte, North Carolina (Anthony Asher); Dana Farber Cancer Institute, Boston, Massachusetts (Peter Black); Northwestern Medical Faculty Foundations, Inc., Chicago, Illinois (James Chandler); Evanston Northwestern Healthcare, Evanston, Illinois (Nina Paleologos); University of Wisconsin Hospital and Clinics, Madison, Wisconsin (John Kuo); Cedar Sinai Medical Center, Neurosurgical Institute, Los Angeles, California (John Yu); CINN at Rush University Medical Center, Chicago, Illinois (Richard Byrne); Medical University of South Carolina, Charleston, South Carolina (Sunil Patel); University of Virginia Health Systems, Charlottesville, Virginia (M.S.); Florida Hospital Neuroscience Institute, Orlando, Florida (Edward Pan); University of California–Los Angeles, Los Angeles, California (Timothy Cloughesy); Weill Cornell Medical College, Department of Neurological Surgery, New York, New York (Theodore Schwartz); University of Chicago Medical Center, Chicago, Illinois (Macieij Lesniak); Columbia University Medical Center, New York, New York (Jeffrey Bruce); University of Southern California, Los Angeles, California (Thomas Chen); Montreal Neurological Institute and Hospital, Montreal, Quebec, Canada (Rolando Del Maestro); Cancer Care Manitoba, Winnipeg, Manitoba, Canada (David Eisenstat); Royal University Hospital, Saskatoon, Saskatchewan, Canada (Daryl Fourney); Toronto Western Hospital, Toronto, Ontario, Canada (Abhijit Guha); University of Utah, Salt Lake City, Utah (Randy L. Jensen); London Regional Cancer Program, London, Ontario, Canada (David MacDonald); Walter Mackenzie Health Sciences Center, Edmonton, Alberta, Canada (Vivek Mehta); University Hospital Groningen, Groningen, The Netherlands (Jan Jakob A. Mooij); Sunnybrook and Women's College Health Sciences Centre, Toronto, Ontario, Canada (Farhad Pirouzmand); Institute of Neurological Sciences, Southern General Hospital, Glasgow, United Kingdom (Laurence T. Dunn); Technische Universitat Dresden Klinic und Poliklinik fur Neuochirugie, Dresden, Germany (Gabriele Schackert); Ludwig-Maximilians-Universitat Munchen, Munich, Germany (Roland Goldbrunner); Mayo Clinic, Rochester, Minnesota (Joon Uhm); University of Heidelberg, Heidelberg, Germany (Andreas Unterberg); Erasmus University Medical Center, Rotterdam, The Netherlands (John G. Wolbers); Rabin Medical Center, Petah Tikva, Israel (Zvi Harry Rappaport); Calgary Health Region, Calgary, Alberta, Canada (Mark Hamilton); Baptist Memorial Hospital, Memphis, Tennessee (Bruce Frankel); West Virginia University, Robert C. Byrd Health Sciences Center, Morgantown, West Virginia (Warren Boling); St Louis University, St Louis, Missouri (Richard Bucholz); University of Texas Southwestern Medical Center, Dallas, Texas (Bruce Mickey); Oregon Health and Science University, Portland, Oregon (Johhny Delashaw Jr); Virginia Mason Clinic, Seattle, Washington (Lynne Taylor).
Conflict of interest statement. R.K.P. reports a Cooperative Research Development Agreement (CRADA) between the Food and Drug Administration and NeoPharm Inc., and he reports having a patent on the therapeutic agent described here, along with the NIH and Val-Chum. Rights are assigned to Secretary of the Department of Health and Human Services. M.V. reports serving as a consultant and receiving consulting fees for NeoPharm, Inc., Eisai, and Schering Plough and lecture fees from Schering Plough and Genentech.
References
- 1.Brem H, Piantadosi S, Burger PC, et al. Placebo-controlled trial of safety and efficacy of intraoperative controlled delivery by biodegradable polymers of chemotherapy for recurrent gliomas. The Polymer-brain Tumor Treatment Group. Lancet. 1995;345:1008–1012. doi: 10.1016/s0140-6736(95)90755-6. [DOI] [PubMed] [Google Scholar]
- 2.Bobo RH, Laske DW, Akbasak A, Morrison PF, Dedrick RL, Oldfield EH. Convection-enhanced delivery of macromolecules in the brain. Proc Natl Acad Sci USA. 1994;91:2076–2080. doi: 10.1073/pnas.91.6.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen MY, Lonser RR, Morrison PF, Governale LS, Oldfield EH. Variables affecting convection-enhanced delivery to the striatum: a systematic examination of rate of infusion, cannula size, infusate concentration, and tissue-cannula sealing time. J Neurosurg. 1999;90:315–320. doi: 10.3171/jns.1999.90.2.0315. [DOI] [PubMed] [Google Scholar]
- 4.Laske DW, Morrison PF, Lieberman DM, et al. Chronic interstitial infusion of protein to primate brain: determination of drug distribution and clearance with single-photon emission computerized tomography imaging. J Neurosurg. 1997;87:586–594. doi: 10.3171/jns.1997.87.4.0586. [DOI] [PubMed] [Google Scholar]
- 5.Lieberman DM, Laske DW, Morrison PF, Bankiewicz KS, Oldfield EH. Convection-enhanced distribution of large molecules in gray matter during interstitial drug infusion. J Neurosurg. 1995;82:1021–1029. doi: 10.3171/jns.1995.82.6.1021. [DOI] [PubMed] [Google Scholar]
- 6.Morrison PF, Chen MY, Chadwick RS, Lonser RR, Oldfield EH. Focal delivery during direct infusion to brain: role of flow rate, catheter diameter, and tissue mechanics. Am J Physiol. 1999;277:R1218–R1229. doi: 10.1152/ajpregu.1999.277.4.R1218. [DOI] [PubMed] [Google Scholar]
- 7.Morrison PF, Laske DW, Bobo H, Oldfield EH, Dedrick RL. High-flow microinfusion: tissue penetration and pharmacodynamics. Am J Physiol. 1994;266:R292–R305. doi: 10.1152/ajpregu.1994.266.1.R292. [DOI] [PubMed] [Google Scholar]
- 8.Kawakami K, Kawakami M, Kioi M, Husain SR, Puri RK. Distribution kinetics of targeted cytotoxin in glioma by bolus or convection-enhanced delivery in a murine model. J Neurosurg. 2004;101:1004–1011. doi: 10.3171/jns.2004.101.6.1004. [DOI] [PubMed] [Google Scholar]
- 9.Kunwar S. Convection enhanced delivery of IL13-PE38QQR for treatment of recurrent malignant glioma: presentation of interim findings from ongoing phase 1 studies. Acta Neurochir Suppl. 2003;88:105–111. doi: 10.1007/978-3-7091-6090-9_16. [DOI] [PubMed] [Google Scholar]
- 10.Lidar Z, Mardor Y, Jonas T, et al. Convection-enhanced delivery of paclitaxel for the treatment of recurrent malignant glioma: a phase I/II clinical study. J Neurosurg. 2004;100:472–479. doi: 10.3171/jns.2004.100.3.0472. [DOI] [PubMed] [Google Scholar]
- 11.Sampson JH, Akabani G, Archer GE, et al. Progress report of a Phase I study of the intracerebral microinfusion of a recombinant chimeric protein composed of transforming growth factor (TGF)-alpha and a mutated form of the Pseudomonas exotoxin termed PE-38 (TP-38) for the treatment of malignant brain tumors. J Neurooncol. 2003;65:27–35. doi: 10.1023/a:1026290315809. [DOI] [PubMed] [Google Scholar]
- 12.Weaver M, Laske DW. Transferrin receptor ligand-targeted toxin conjugate (Tf-CRM107) for therapy of malignant gliomas. J Neurooncol. 2003;65:3–13. doi: 10.1023/a:1026246500788. [DOI] [PubMed] [Google Scholar]
- 13.Weber F, Asher A, Bucholz R, et al. Safety, tolerability, and tumor response of IL4-Pseudomonas exotoxin (NBI-3001) in patients with recurrent malignant glioma. J Neurooncol. 2003;64:125–137. doi: 10.1007/BF02700027. [DOI] [PubMed] [Google Scholar]
- 14.Kawakami M, Kawakami K, Puri RK. Intratumor administration of interleukin 13 receptor-targeted cytotoxin induces apoptotic cell death in human malignant glioma tumor xenografts. Mol Cancer Ther. 2002;1:999–1007. [PubMed] [Google Scholar]
- 15.Kawakami M, Kawakami K, Puri RK. Tumor regression mechanisms by IL-13 receptor-targeted cancer therapy involve apoptotic pathways. Int J Cancer. 2003;103:45–52. doi: 10.1002/ijc.10778. [DOI] [PubMed] [Google Scholar]
- 16.Debinski W, Gibo DM, Hulet SW, Connor JR, Gillespie GY. Receptor for interleukin 13 is a marker and therapeutic target for human high-grade gliomas. Clin Cancer Res. 1999;5:985–990. [PubMed] [Google Scholar]
- 17.Debinski W, Miner R, Leland P, Obiri NI, Puri RK. Receptor for interleukin (IL) 13 does not interact with IL4 but receptor for IL4 interacts with IL13 on human glioma cells. J Biol Chem. 1996;271:22428–22433. doi: 10.1074/jbc.271.37.22428. [DOI] [PubMed] [Google Scholar]
- 18.Debinski W, Obiri NI, Powers SK, Pastan I, Puri RK. Human glioma cells overexpress receptors for interleukin 13 and are extremely sensitive to a novel chimeric protein composed of interleukin 13 and pseudomonas exotoxin. Clin Cancer Res. 1995;1:1253–1258. [PubMed] [Google Scholar]
- 19.Joshi BH, Plautz GE, Puri RK. Interleukin-13 receptor alpha chain: a novel tumor-associated transmembrane protein in primary explants of human malignant gliomas. Cancer Res. 2000;60:1168–1172. [PubMed] [Google Scholar]
- 20.Liu H, Jacobs BS, Liu J, et al. Interleukin-13 sensitivity and receptor phenotypes of human glial cell lines: non-neoplastic glia and low-grade astrocytoma differ from malignant glioma. Cancer Immunol Immunother. 2000;49:319–324. doi: 10.1007/s002620000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Husain SR, Joshi BH, Puri RK. Interleukin-13 receptor as a unique target for anti-glioblastoma therapy. Int J Cancer. 2001;92:168–175. doi: 10.1002/1097-0215(200102)9999:9999<::aid-ijc1182>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 22.Kunwar S, Prados MD, Chang SM, et al. Direct intracerebral delivery of cintredekin besudotox (IL13-PE38QQR) in recurrent malignant glioma: a report by the Cintredekin Besudotox Intraparenchymal Study Group. J Clin Oncol. 2007;25:837–844. doi: 10.1200/JCO.2006.08.1117. [DOI] [PubMed] [Google Scholar]
- 23.Joshi BH, Puri RK. Optimization of expression and purification of two biologically active chimeric fusion proteins that consist of human interleukin-13 and Pseudomonas exotoxin in Escherichia coli. Protein Expr Purif. 2005;39:189–198. doi: 10.1016/j.pep.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 24.Parney IF, Kunwar S, McDermott M, et al. Neuroradiographic changes following convection-enhanced delivery of the recombinant cytotoxin interleukin 13-PE38QQR for recurrent malignant glioma. J Neurosurg. 2005;102:267–275. doi: 10.3171/jns.2005.102.2.0267. [DOI] [PubMed] [Google Scholar]
- 25.Vogelbaum MA. Convection enhanced delivery for treating brain tumors and selected neurological disorders: symposium review. J Neurooncol. 2007;83:97–109. doi: 10.1007/s11060-006-9308-9. [DOI] [PubMed] [Google Scholar]
- 26.Jarboe JS, Johnson KR, Choi Y, Lonser RR, Park JK. Expression of interleukin-13 receptor alpha2 in glioblastoma multiforme: implications for targeted therapies. Cancer Res. 2007;67:7983–7986. doi: 10.1158/0008-5472.CAN-07-1493. [DOI] [PubMed] [Google Scholar]
- 27.Saikali S, Avril T, Collet B, et al. Expression of nine tumour antigens in a series of human glioblastoma multiforme: interest of EGFRvIII, IL-13Ralpha2, gp100 and TRP-2 for immunotherapy. J Neurooncol. 2007;81:139–148. doi: 10.1007/s11060-006-9220-3. [DOI] [PubMed] [Google Scholar]
- 28.Reznick RK. Teaching and testing technical skills. Am J Surg. 1993;165:358–361. doi: 10.1016/s0002-9610(05)80843-8. [DOI] [PubMed] [Google Scholar]
- 29.Fiandaca MS, Forsayeth JR, Dickinson PJ, Bankiewicz KS. Image-guided convection-enhanced delivery platform in the treatment of neurological diseases. Neurotherapeutics. 2008;5:123–127. doi: 10.1016/j.nurt.2007.10.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krauze MT, Forsayeth J, Park JW, Bankiewicz KS. Real-time imaging and quantification of brain delivery of liposomes. Pharm Res. 2006;23:2493–2504. doi: 10.1007/s11095-006-9103-5. [DOI] [PubMed] [Google Scholar]
- 31.Sampson JH, Brady ML, Petry NA, et al. Intracerebral infusate distribution by convection-enhanced delivery in humans with malignant gliomas: descriptive effects of target anatomy and catheter positioning. Neurosurgery. 2007;60 doi: 10.1227/01.NEU.0000249256.09289.5F. ONS89–ONS98; discussion ONS98–ONS99. [DOI] [PubMed] [Google Scholar]
- 32.Sampson JH, Raghavan R, Brady ML, et al. Clinical utility of a patient-specific algorithm for simulating intracerebral drug infusions. Neuro-Oncology. 2007;9:343–353. doi: 10.1215/15228517-2007-007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lonser RR, Schiffman R, Robison RA, et al. Image-guided, direct convective delivery of glucocerebrosidase for neuronopathic Gaucher disease. Neurology. 2007;68:254–261. doi: 10.1212/01.wnl.0000247744.10990.e6. [DOI] [PubMed] [Google Scholar]
- 34.Voges J, Reszka R, Gossmann A, et al. Imaging-guided convection-enhanced delivery and gene therapy of glioblastoma. Ann Neurol. 2003;54:479–487. doi: 10.1002/ana.10688. [DOI] [PubMed] [Google Scholar]
- 35.Perlstein B, Ram Z, Daniels D, et al. Convection-enhanced delivery of maghemite nanoparticles: increased efficacy and MRI monitoring. Neuro-Oncology. 2008;10:153–161. doi: 10.1215/15228517-2008-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yin D, Valles FE, Fiandaca MS, et al. Optimal region of the putamen for image-guided convection-enhanced delivery of therapeutics in human and non-human primates. Neuroimage. 2009 doi: 10.1016/j.neuroimage.2009.08.069. doi:10.1016/j.neuroimage.2009.08.069. Epub ahead of print September 15, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fadul C, Wood J, Thaler H, Galicich J, Patterson RH, Jr, Posner JB. Morbidity and mortality of craniotomy for excision of supratentorial gliomas. Neurology. 1988;38:1374–1379. doi: 10.1212/wnl.38.9.1374. [DOI] [PubMed] [Google Scholar]
- 38.Sawaya R, Hammoud M, Schoppa D, et al. Neurosurgical outcomes in a modern series of 400 craniotomies for treatment of parenchymal tumors. Neurosurgery. 1998;42 doi: 10.1097/00006123-199805000-00054. 1044–1055; discussion 1055–1056. [DOI] [PubMed] [Google Scholar]