Abstract
Phosphatidyl-inositol-3 kinases (PI3Ks) constitute a family of intracellular lipid kinases that are frequently hyperactivated in glioblastoma. The PI3K complex links growth factor signaling with cellular proliferation, differentiation, metabolism, and survival. Mammalian target of rapamycin (mTOR) acts both as a downstream effector and upstream regulator of PI3K, thus highlighting its importance in glioblastoma. This review highlights laboratory and clinical evidence of mTOR's role in glioblastoma. Mechanisms of escape from mTOR inhibition are also discussed, as well as future clinical strategies of mTOR inhibition.
Keywords: glioblastoma, mTOR, PI3K
Introduction: mTOR Signaling in Glioblastoma
Glioblastoma is the most common malignant primary brain tumor in adults and one of the most lethal of all cancers.1 Glioblastomas invade the surrounding brain, making complete surgical excision highly improbable. They are also among the most radiation therapy- and chemotherapy-resistant cancer types, with a median survival duration of 12–15 months after initial diagnosis.2 Thus, new therapeutic approaches are needed.
Mammalian target of rapamycin (mTOR), a key mediator of phosphatidyl-inositol-3-kinase (PI3K) signaling, has emerged as a compelling molecular target in glioblastoma patients, although clinical efforts to target mTOR have not been successful. Here, we outline the evidence demonstrating that mTOR is a compelling molecular target in glioblastoma. We focus on the role of mTOR in PI3K signaling and its larger role in metabolic cellular programs in glioblastoma. We summarize the current clinical status of mTOR inhibition, paying particular attention to resistance through studying glioblastoma patients treated with the mTORC1 complex inhibitor rapamycin in phase I and II clinical trials. We describe how the study of glioblastoma patients has increased our understanding of the complexity of mTOR signaling. Finally, we describe new strategies for targeting mTOR in glioblastoma patients.
Constitutive PI3K Pathway Activation Is a Hallmark of Glioblastoma
PI3Ks are a family of highly conserved intracellular lipid kinases that regulate cellular proliferation, differentiation, metabolism, and survival.3 Class IA PI3Ks are activated by growth factor receptor tyrosine kinases (RTKs), either directly or through interaction with the insulin receptor substrate family of adaptor molecules.3 This activity results in the production of phosphatidyl-inositol-3,4,5-trisphospate (PIP3), a critical regulator of the serine/threonine kinase Akt, which links growth factor signaling with cellular growth, proliferation, metabolism, and survival.3,4
Tightly regulated PI3K signaling is essential for normal development, whereas persistently activated PI3K signaling is associated with the development of cancer.5 PI3K-activating mutations are found in nearly all patients with glioblastoma,6,7 as indicated by the phopshorylation of key signaling proteins in the PI3K pathway.8 EGFR amplification is detected in 45% of glioblastoma patients, providing one common route to PI3K pathway activation. EGFR activating mutations are also commonly detected in EGFR-amplified glioblastomas (Fig. 1).9–11 EGFRvIII, the most common EGFR mutation (occurring in 20%–30% of glioblastomas), results from genomic deletion of exons 2–7.12,13 It lacks a ligand-binding domain, yet it is persistently activated and fails to be internalized normally, resulting in constitutive signaling with a preferential effect on PI3K activity in relation to the wild-type receptor.8,14 Other RTKs also contribute to PI3K pathway activation, including c-MET and PDGFRα, both of which can be co-activated in EGFR-amplified tumors1 and confer resistance to EGFR inhibitors by providing an alternative route for maintaining PI3K signal flux.15
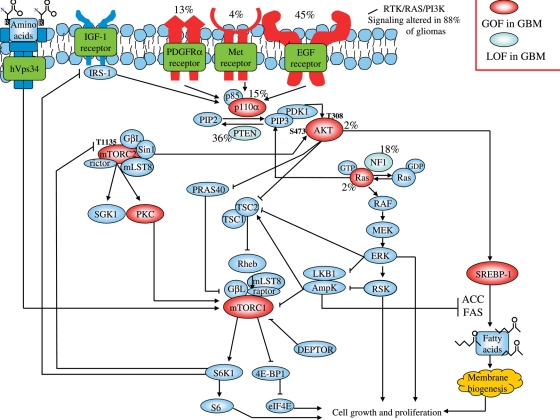
Fig. 1.
mTORC plays a key role in integrating signal transduction and metabolic pathways in glioblastoma. Schematic representation shows pathways that regulate or are regulated by mTOR signaling in glioblastoma, with the known frequency of molecular alterations in these genes.6,32,67,77
Amplification or activating mutations of the catalytic and regulatory subunits of PI3K also contribute to PI3K pathway activation in up to 10% of glioblastomas.6 Loss of the phosphatase and tensin homolog deleted on chromosome 10 (PTEN) tumor suppressor protein, the major negative regulator of the PI3K pathway that antagonizes PI3K signaling by dephosphorylating PIP3, occurs in more than 50% of glioblastomas and is a third mechanism of constitutive PI3K pathway activation.8,16 In addition, NF1 loss, either through genetic inactivation6 or enhanced proteosomal degradation,17 is a relatively common event in glioblastoma that can lead to enhanced PI3K pathway activity.18 Therefore, multiple PI3K-activating genetic lesions occur in nearly all glioblastoma patients, highlighting the biological importance of this pathway.
Consistent with the results of analyses of patient tumor tissues, mouse genetic models indicate that constitutive PI3K pathway activation may be required for malignant glioma formation and progression.19 In these models, constitutive PI3K signaling cooperates with alterations in other signaling pathways that are also commonly activated in glioblastoma patients, including those involving RAS/MEK/ERK, p53, and pRB.20–22,78,79 Other mouse genetic models demonstrate alternative routes towards malignant glioma formation that involve PI3K hyperactivation, including mutant EGFR in combination with Ink4a/arf.23 Further, PTEN loss cooperates with pRB loss to promote glioma formation and progression.24,25 Perhaps most impressively, concurrent alterations of the same pathways identified in human glioblastomas through gene mutation analysis, including NF1 loss, p53 loss, and PTEN loss (including haploinsufficiency), promote the formation and progression of malignant gliomas. Thus, mouse genetic models directly complement human tumor analyses by suggesting a causative role for persistent PI3K pathway activation and the accompanying mTOR signaling in the development and progression of malignant gliomas.
mTOR as a Therapeutic Target in Glioblastoma
mTOR acts through the canonical PI3K pathway via 2 distinct complexes, each characterized by different binding partners that confer distinct activities. In complex with PRAS40, raptor, and mLST8/GbL, mTOR acts as a downstream effector of PI3K/Akt signaling, linking growth factor signals with protein translation, cell growth, proliferation, and survival. This complex is known as mTORC1. In complex with rictor, mSIN1, protor, and mLST8 (mTORC2), mTOR acts as an upstream activator of Akt.26 The recently identified negative regulator of mTOR, DEPTOR, is a component of both the mTORC1 and mTORC2 complexes.27 Upon growth factor receptor-mediated activation of PI3K, Akt is recruited to the membrane through the interaction of its pleckstrin homology domain with PIP3, thus exposing its activation loop and enabling phosphorylation at threonine 308 (Thr308) by the constitutively active phosphoinositide-dependent protein kinase 1 (PDK1).28–30 For maximal activation, Akt is also phosphorylated by mTORC2, at serine 473 (Ser473) of its C-terminal hydrophobic motif. Akt activates mTORC1 through inhibitory phosphorylation of TSC2, which along with TSC1, negatively regulates mTORC1 by inhibiting the Rheb GTPase, a positive regulator of mTORC1. mTORC1 has 2 well-defined substrates, p70S6K (referred to hereafter as S6K1) and 4E-BP1, both of which critically regulate protein synthesis.30 Thus, mTORC1 is an important downstream effector of PI3K, linking growth factor signaling with protein translation and cellular proliferation.31 Surprisingly, an Akt-independent, PKCα-dependent mechanism that links PI3K with mTORC1 in glioblastoma has also been described.32 In a serial cell line transformation model, mTORC1 signaling through S6K1 was required for malignant glioma formation, highlighting its functional importance.33
The compelling nature of mTORC1 as a glioma target, coupled with the relative efficacy of the mTORC1 complex inhibitor rapamycin and its derivatives in preclinical mouse glioblastoma models,34–36 has generated considerable hope for more effective targeted therapy for malignant glioma patients. However, a number of factors suggest that rapamycin-based treatments are not the best choice for effectively targeting mTOR in patients. First, mTORC1 plays a dual role as a positive regulator of PI3K/Akt signaling from growth factor receptors and a negative regulator of PI3K pathway activation when signal flux through PI3K is high. This ensures homeostatic regulation of this critical signal in healthy cells.26 mTORC1 impairs PI3K activation in growth factor-stimulated cells by downregulating IRS 1 and 2 and PDGFR.37–39 Its role in regulating PI3K signaling downstream of EGFR is less well understood, although the results of a recent study suggest that an activated EGFRvIII allele enhances feedback activation of PI3K signaling in glioblastoma cells treated with rapamycin.40 Derepression of mTORC1-mediated feedback with rapamycin could result in more rapid clinical progression in glioblastoma patients, as described below.41 The results of a recent study suggest that mTORC1 has rapamycin-insensitive, kinase-dependent activity.42 Further, rapamycin and its derivatives do not consistently target mTORC2, whose function and importance is discussed below.27 Therefore, the recent development of mTOR kinase-specific inhibitors offers a whole new set of therapeutic options that will need to be evaluated clinically.26
mTORC2 signaling in glioblastoma is less well understood than that in mTORC1. mTORC2, which is activated by PI3K, phosphorylates Akt on serine 473 (Ser473) of its C-terminal hydrophobic motif, thereby promoting maximal Akt activity. mTORC2 also activates additional kinases, including serum glucocorticoid-induced protein kinase and PKCα, all of which may play important roles in regulating cellular proliferation and growth. mTORC2 activity has been shown to be elevated in glioma cell lines and clinical isolates, and rictor overexpression has been shown to enhance motility and promote glioma cell proliferation in vitro.43 Further, in a recent Drosophila model of gliomagenesis promoted by constitutive coactivation of EGFR-Ras-Akt, mTORC2 activity was required for glial proliferation. The results of these studies suggest that mTORC2 signaling is essential for growth in the context of enhanced signal flux through the PI3K pathway.44 Future studies using both genetic and pharmacological approaches will be needed to better understand the role of mTORC2 signaling in malignant glioma. Clearly, elucidating the molecular circuitry that underlies mTORC1 and mTORC2 signaling will be very important for developing more effective glioblastoma treatments. In fact, an enriched picture of these signaling networks began to emerge during the first rapamycin-based clinical trials. Below, we discuss the results of these trials, highlighting efforts to study mechanisms of resistance in glioma patients treated with rapamycin.
Targeting the EGFR/PI3K/mTOR Signaling Pathway in Glioma Patients: Lessons Learned
A number of phase I and II single-agent clinical trials of rapamycin (and its analogue CCI-779) have been conducted in malignant glioma. In addition, EGFR tyrosine kinase inhibitor and EGFR tyrosine kinase inhibitor/rapamycin analogue clinical trials have been reported.41,45–48 Although results with these agents have been disappointing, a considerable amount has been learned from the study of treated tumor tissue, suggesting more effective treatment strategies.
Initial results with the EGFR tyrosine kinase inhibitors gefitinib and erlotinib suggest relatively low response rates of 10%–15%.46,49 These results are somewhat difficult to reconcile with the perceived importance of the EGFR target. Our research group demonstrated that constitutively active mutant EGFRvIII expression sensitizes tumors to EGFR inhibitors in vitro and clinically, but only if the PTEN tumor suppressor protein is intact. In fact, loss of PTEN inhibition of downstream PI3K signaling has been shown to be a critical promoter of up-front resistance to EGFR inhibitors.45 Haas-Kogan et al.46 demonstrated, in vitro and in glioma patients, that high levels of EGFR, coupled with low levels of activated Akt (a critical effector of PI3K signaling), are associated with a favorable response. The results of these studies demonstrate that the intact regulation of PI3K signaling is critical for an effective response to EGFR; similar results were also found in a human serially passaged xenograft model of glioblastoma50 and in other cancer types.51
Maintained signal flux through PI3K, whether through PTEN loss or RTK coactivation, is a common mechanism of EGFR inhibitor resistance,15 and mTORC1 appears to be its critical effector.32,52 Preclinical studies demonstrated that dual EGFR/mTOR inhibition was effective at targeting EGFR-activated PTEN deficient tumors;52–54 however, 2 small studies (one of everolimus plus gefitinib and the other of rapamycin and erlotinib) in patients with recurrent malignant glioma failed to demonstrate durable responses.48,55 Similarly, phase II studies of single-agent rapalogs in recurrent glioblastoma multiforme have failed to demonstrate clinical efficacy.56,57
A number of possible explanations exist for the clinical ineffectiveness of rapamycin and its analogues, whether as monotherapy or in combination with EGFR kinase inhibitors.49,56 Typically, when a new cancer drug enters clinical trials, it is developed empirically by first defining the maximum tolerated dose and then assessing clinical activity across a range of diseases. This approach may not be adequate for determining optimal dose or assessing efficacy of target inhibition when using a drug similar to rapamycin. First, it is anticipated that targeted agents, such as rapamycin, will be effective primarily in patients whose tumors are dependent on the molecule being targeted. For example, the results of work from multiple investigators58–63 suggest that activation of PI3K signaling through PTEN loss sensitizes tumor cells to rapamycin in preclinical models, although other pathways that modulate sensitivity have been described.31 This suggests that it may be possible to stratify patients for treatment on the basis of PTEN status. Second, for targeted agents that inhibit the activity of specific signaling pathways, such as the mTORC1/S6K1/S6 signaling axis, assays to assess the adequacy of pathway inhibition need to be incorporated into the design, interpretation, and implementation of clinical trials. Furthermore, tumor tissue must be accessed at relevant time points during treatment, which presents additional challenges for clinical trial design.
Salvage surgical resection is often part of treatment for glioblastoma patients who experience relapse after standard up-front therapy. This clinical scenario presents opportunities for trials that include molecular-based selection criteria. In collaboration with Drs Tim Cloughesy, Charles Sawyers, Ingo Mellinghoff, and colleagues, we conducted a small pilot phase I and II neoadjuvant clinical trial of rapamycin in patients with relapsed PTEN-negative glioblastoma. Rapamycin was orally administered to patients prior to a scheduled tumor resection, with the primary goals of defining the dose required for mTOR target inhibition and assessing potential anti-proliferative effects on tumor cells. PTEN status in tumor tissue was determined at initial clinical presentation and biopsy evaluation. After relapse, patients with PTEN-deficient tumors at biopsy received a 10-day course of rapamycin at 1 of 3 dosages (2, 5, or 10 mg/day b.i.d.), followed by surgical excision. Intratumor drug levels, mTORC1 signaling (as measured by S6 phosphorylation), and cellular proliferation were measured and compared between both surgical procedures in rapamycin-treated patients and in a set of similarly matched “control” patients with relapsed glioblastoma who had not undergone rapamycin treatment.
Intratumoral rapamycin concentrations sufficient to inhibit mTOR in vitro were achieved in all patients, even at the lowest dose. However, the magnitude of mTORC1 inhibition in tumor cells varied significantly from patient to patient, irrespective of dose (10%–80% inhibition of S6 phosphorylation). Reduced tumor cell proliferation (as measured by Ki67 staining) in vivo was significantly related to the degree of inhibition of mTORC1 signaling. mTORC1 inhibition of more than 50% resulted in significantly inhibited tumor cell proliferation; lower inhibition did not result in a cytostatic response. Significantly, ex vivo-cultured tumor cells from rapamycin-“resistant” patients were highly sensitive to the drug, suggesting that rapamycin resistance is not cell autonomous but rather due to a failure of the drug to fully access its target in vivo.
Pathway Cross-Talk and Feedback Loops in Patients
To determine whether rapamycin treatment promotes tumor cell growth in patients by repressing the negative feedback loop for attenuating PI3K signaling,64,65 patients were given rapamycin after surgery and monitored for disease progression. Strikingly, rapamycin treatment led to Akt activation in 7 of 14 patients, presumably due to the loss of negative feedback, which was associated with significantly shorter time-to-progression during postsurgical rapamycin maintenance therapy.41 Thus, understanding the complex role of mTOR in regulating signal transduction and cellular metabolism (as discussed below) is critical to developing more effective mTOR-targeted therapies.
Dual PI3K/mTOR and a Role for mTOR/Erk Inhibition
The finding of PI3K pathway reactivation after rapamycin treatment suggests that dual PI3K/mTOR inhibitors function by preventing PI3K signaling reactivation and more effectively target TORC2 (and TORC1) signaling. A dual PI3K/mTOR inhibitor (PI-103) was shown to be efficacious at blocking the growth of glioblastoma cells in vitro and in vivo, independent of PTEN status.53 The combination of this inhibitor and erlotinib or gefitinib also resulted in a significant benefit in EGFR-activated tumor cells.54 A number of dual PI3K/mTOR inhibitors are being evaluated in early clinical trials, including NVP-BEZ235, which has shown efficacy against a panel of glioblastoma cell lines.66 Somewhat surprisingly, in preclinical glioblastoma models, dual PI3K/mTOR inhibitors have failed to promote tumor cell apoptosis.66 Investigations are underway to determine whether these dual PI3K/mTOR inhibitors will promote apoptosis when used in conjunction with other pathway-targeted agents, cytotoxic chemotherapies, or radiation therapy.
The remarkable plasticity of tumor cells and their capacity for rewiring was recently demonstrated in studies that found mTORC1 inhibition leads to ERK pathway activation through a PI3K-dependent mechanism.12 The results of these studies indicate that dual PI3K/mTOR inhibitors may also suppress ERK signaling activation. A similar “biopsy-treat-biopsy” strategy41 to that described above may be required to assess the effect of dual PI3K/mTOR inhibitors on PI3K, mTORC1, mTORC2, Akt, and ERK signaling and assess their clinical benefits. Such studies will help determine the use of these agents, alone or in combination with other pathway-targeted agents, cytotoxic therapies, or radiation therapy.
mTOR at the Interface of Signal Transduction and Cellular Metabolism
Up to this point, studies of mTOR have focused on its role as a key effector and regulator of PI3K signaling. However, mTOR also plays a critical role in integrating cellular metabolism with signal transduction. Sarah Kozma's and George Thomas' research groups demonstrated that class 3 PI3K (vps34) provides an amino acid-sensing mechanism to activate mTORC1 signaling in a process that is entirely independent of class I PI3K and its canonical signaling pathway.67 This observation indicates that mTOR is a key node for integrating growth factor signaling with cellular metabolism. It also raises the possibility that class 3 PI3K signaling to mTORC1 could promote escape from mTOR or dual class I PI3K/mTOR inhibitors. Further studies are needed to address the clinical relevance of class 3 PI3K signaling to mTOR in glioblastoma patients and to assess its contribution towards promoting clinical resistance to RTK/PI3K/mTOR inhibitors.
mTORC1 has also emerged as a critical effector downstream of the tumor suppressor liver kinase B1 (LKB1, also referred to as STK11). The germline loss of LKB1 is responsible for Peutz-Jeghers inherited cancer syndrome, and its sporadic loss has been detected in a variety of cancers.68 LKB1 is thought to suppress tumors by negatively regulating mTORC1 signaling via the central metabolic switch, AMP-activated protein kinase (AMPK). The LKB1-AMPK pathway acts as a metabolic checkpoint that arrests cell growth when nutrients are scarce. LKB1 phosphorylates and activates AMPK, which in turn negatively regulates mTORC1 signaling through the activation of TSC2 and the direct inhibitory phosphorylation of the mTOR binding partner raptor.69–71 Further, in tumors caused by LKB1 loss,68,72 mTOR may play a key role downstream of LKB1/AMPK metabolic signaling.
Other classic growth factor signaling pathways may also interact with LKB1/AMPK. Recent studies have demonstrated that the B-RAF V600E mutation, a common hyperactivating mutation in melanoma and other cancer types,73–75 forms a complex with Erk and LKB1 that promotes phosphorylation and inactivation of LKB1 by ERK, thus functionally suppressing LKB1's ability to activate AMPK and activating mTORC1.76 Strikingly, LKB1 that lacks V600E BRAF phosphorylation sites (s325 and s428) inhibits V600E-driven proliferation, suggesting that the LKB1/AMPK axis is a critical mediator of oncogenic RAF signaling.76
The role of LKB1/AMPK signaling and the importance of mTORC1 as LKB1/AMPK's mediator in malignant gliomas remain to be elucidated. A recent study by our research group demonstrated that the AMPK agonist AICAR is very effective at blocking the growth of EGFR-activated glioblastomas, both in vitro and in vivo.40 Surprisingly, this was only partially mediated by inhibition of mTORC1 signaling. Rather, the striking anti-growth effect of AICAR on EGFR-activated tumor cells was mediated primarily by inhibiting lipogenesis. Understanding how AMPK regulates lipogenesis and how it interacts with mTORC1 signaling, as well as with EGFR signaling through the PI3K/Akt and RAS/ERK pathways, may prove important for developing more effective treatment strategies.77
EGFR Regulation of Lipogenesis
Rapidly dividing cancer cells require a supply of fatty acids for the formation of new cellular membranes. In addition, fatty acids may be very important for regulating signal transduction, and they provide an alternative energy source for the cell. It appears that de novo synthesis of fatty acids is the preferred route for cancer cells. This suggests that an important link exists between cancer progression and fatty acid synthesis and that targeting fatty acid synthesis could be an effective way to block cancer growth. However, the molecular circuitry linking oncogenes and tumor suppressor proteins and the pathways they regulate, along with the metabolic changes in cancer cells, are not well understood. An improved understanding of the relationships between cancer-associated genes and cellular metabolism could lead to new cancer treatments.
We recently demonstrated that EGFR signaling promotes activation of the transcriptional regulator of fatty acid synthesis, SREBP-1.77,80 This was first discovered in glioblastoma tissue samples from patients treated with the dual EGFR/Her2 kinase inhibitor lapatinib. We compared tissue samples before and after lapatinib treatment and found that tissue that exhibited a response to lapatinib, as demonstrated by a decrease in p-EGFR, showed a concomitant decrease in SREBP-1 staining. Expanding on this observation through the use of a series of glioblastoma cell lines, we observed that EGFR signaling promoted cleavage and nuclear translocation of SREBP-1, with increased transcriptional activation of its downstream target fatty acid synthase and increased amounts of intracellular fatty acids.
Most importantly, we found that abundant EGFR signaling makes glioblastoma cells more dependent on fatty acid synthesis. As a consequence, interruption of fatty acid synthesis, either by blocking SREBP-1 cleavage or by blocking the activity of the fatty acid synthase enzyme, causes massive apoptotic cell death in tumors with abundant EGFR signaling but not in those with little EGFR signaling. Surprisingly, this phenomenon was independent of mTORC1, although its pathway dependence on mTORC2 has yet to be explored.
Concluding Thoughts
Because it is a key mediator of PI3K signaling and an integrator of signal transduction and cellular metabolism, mTOR represents an attractive therapeutic target for glioblastoma. Despite the relative clinical failure of rapamycin and its derivatives, an enormous amount has been learned from studying mTOR signaling in well-controlled experimental glioma models and rapamycin and its analogues in phase I and II clinical trials. However, a number of challenging questions have been raised. Do we fully understand the importance of mTORC2 signaling in malignant gliomas, and is dependence on this pathway enhanced in the context of persistent PI3K signaling? What is the role of class III PI3K signaling to mTORC1 in malignant gliomas, and does this pathway play a role in promoting resistance to growth factor receptor and class I PI3K targeted therapies? To what extent is mTORC1 the critical effector of LKB1/AMPK signaling in malignant gliomas, and if so, is AMPK's dependence on mTORC1 mediated by cell context? Will true mTOR kinase inhibitors prove to be more effective than rapamycin and its derivatives? Will PTEN status matter? Will dual PI3K/mTOR inhibitor treatments be needed, and will these agents suppress feedback activation of ERK signaling, or will their efficacy depend on the concurrent use of ERK inhibitors? Will it be possible to develop better AMPK agonists that cross the blood-brain barrier, and will they be effective at blocking mTORC1 signaling and glioblastoma tumor growth? Answers to these questions, along with emerging data about the underlying molecular circuitry and the development of true mTOR kinase inhibitors, will result in an array of new treatment approaches. Perhaps most importantly, well-designed clinical trials are needed in which tissue can be accessed at relevant time points to analyze pathway activation biomarkers.
Conflict of interest statement. None declared.
Funding
This work was supported by the National Institute for Neurological Disorders and Stroke [NS050151], National Cancer Institute [CA119347 and CA108633], the Ben and Catherine lvy Foundation, and the Lya and Harrison Latta Endowed Chair of Pathology and Laboratory Medicine at the University of California, Los Angeles.
References
- 1.Furnari FB, Fenton T, Bachoo RM, et al. Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev. 2007;21:2683–2710. doi: 10.1101/gad.1596707. doi:10.1101/gad.1596707. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. doi:10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 3.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. doi:10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 4.Chalhoub N, Zhu G, Zhu X, Baker SJ. Cell type specificity of PI3K signaling in Pdk1- and Pten-deficient brains. Genes Dev. 2009;23:1619–1624. doi: 10.1101/gad.1799609. doi:10.1101/gad.1799609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vivanco I, Sawyers CL. The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489–501. doi: 10.1038/nrc839. doi:10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 6.The Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. doi:10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parsons DW, Jones S, Zhang X, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. doi:10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choe G, Horvath S, Cloughesy TF, et al. Analysis of the phosphatidylinositol 3'-kinase signaling pathway in glioblastoma patients in vivo. Cancer Res. 2003;63:2742–2746. [PubMed] [Google Scholar]
- 9.Huang HS, Nagane M, Klingbeil CK, et al. The enhanced tumorigenic activity of a mutant epidermal growth factor receptor common in human cancers is mediated by threshold levels of constitutive tyrosine phosphorylation and unattenuated signaling. J Biol Chem. 1997;272:2927–2935. doi: 10.1074/jbc.272.5.2927. [DOI] [PubMed] [Google Scholar]
- 10.Montgomery RB, Moscatello DK, Wong AJ, Cooper JA, Stahl WL. Differential modulation of mitogen-activated protein (MAP) kinase/extracellular signal-related kinase kinase and MAP kinase activities by a mutant epidermal growth factor receptor. J Biol Chem. 1995;270:30562–30566. doi: 10.1074/jbc.270.51.30562. [DOI] [PubMed] [Google Scholar]
- 11.Wikstrand CJ, McLendon RE, Friedman AH, Bigner DD. Cell surface localization and density of the tumor-associated variant of the epidermal growth factor receptor, EGFRvIII. Cancer Res. 1997;57:4130–4140. [PubMed] [Google Scholar]
- 12.Carracedo A, Ma L, Teruya-Feldstein J, et al. Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest. 2008;118:3065–3074. doi: 10.1172/JCI34739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshimoto K, Dang J, Zhu S, et al. Development of a real-time RT-PCR assay for detecting EGFRvIII in glioblastoma samples. Clin Cancer Res. 2008;14:488–493. doi: 10.1158/1078-0432.CCR-07-1966. doi:10.1158/1078-0432.CCR-07-1966. [DOI] [PubMed] [Google Scholar]
- 14.Huang PH, Mukasa A, Bonavia R, et al. Quantitative analysis of EGFRvIII cellular signaling networks reveals a combinatorial therapeutic strategy for glioblastoma. Proc Natl Acad Sci USA. 2007;104:12867–12872. doi: 10.1073/pnas.0705158104. doi:10.1073/pnas.0705158104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stommel JM, Kimmelman AC, Ying H, et al. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science. 2007;318:287–290. doi: 10.1126/science.1142946. doi:10.1126/science.1142946. [DOI] [PubMed] [Google Scholar]
- 16.Ermoian RP, Furniss CS, Lamborn KR, et al. Dysregulation of PTEN and protein kinase B is associated with glioma histology and patient survival. Clin Cancer Res. 2002;8:1100–1106. [PubMed] [Google Scholar]
- 17.McGillicuddy LT, Fromm JA, Hollstein PE, et al. Proteasomal and genetic inactivation of the NF1 tumor suppressor in gliomagenesis. Cancer Cell. 2009;16:44–54. doi: 10.1016/j.ccr.2009.05.009. doi:10.1016/j.ccr.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Downward J. Targeting RAS signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3:11–22. doi: 10.1038/nrc969. doi:10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 19.Cheng CK, Fan QW, Weiss WA. PI3K signaling in glioma–animal models and therapeutic challenges. Brain Pathol. 2009;19:112–120. doi: 10.1111/j.1750-3639.2008.00233.x. doi:10.1111/j.1750-3639.2008.00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holland EC. Gliomagenesis: genetic alterations and mouse models. Nat Rev Genet. 2001;2:120–129. doi: 10.1038/35052535. doi:10.1038/35052535. [DOI] [PubMed] [Google Scholar]
- 21.Sonoda Y, Ozawa T, Aldape KD, Deen DF, Berger MS, Pieper RO. Akt pathway activation converts anaplastic astrocytoma to glioblastoma multiforme in a human astrocyte model of glioma. Cancer Res. 2001;61:6674–6678. [PubMed] [Google Scholar]
- 22.Marumoto T, Tashiro A, Friedmann-Morvinski D, et al. Development of a novel mouse glioma model using lentiviral vectors. Nat Med. 2009;15:110–116. doi: 10.1038/nm.1863. doi:10.1038/nm.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bachoo RM, Maher EA, Ligon KL, et al. Epidermal growth factor receptor and Ink4a/Arf: convergent mechanisms governing terminal differentiation and transformation along the neural stem cell to astrocyte axis. Cancer Cell. 2002;1:269–277. doi: 10.1016/s1535-6108(02)00046-6. doi:10.1016/S1535-6108(02)00046-6. [DOI] [PubMed] [Google Scholar]
- 24.Xiao A, Wu H, Pandolfi PP, Louis DN, Van Dyke T. Astrocyte inactivation of the pRb pathway predisposes mice to malignant astrocytoma development that is accelerated by PTEN mutation. Cancer Cell. 2002;1:157–168. doi: 10.1016/s1535-6108(02)00029-6. doi:10.1016/S1535-6108(02)00029-6. [DOI] [PubMed] [Google Scholar]
- 25.Xiao A, Yin C, Yang C, Di Cristofano A, Pandolfi PP, Van Dyke T. Somatic induction of Pten loss in a preclinical astrocytoma model reveals major roles in disease progression and avenues for target discovery and validation. Cancer Res. 2005;65:5172–5180. doi: 10.1158/0008-5472.CAN-04-3902. doi:10.1158/0008-5472.CAN-04-3902. [DOI] [PubMed] [Google Scholar]
- 26.Guertin DA, Sabatini DM. The pharmacology of mTOR inhibition. Sci Signal. 2009;2:e24. doi: 10.1126/scisignal.267pe24. [DOI] [PubMed] [Google Scholar]
- 27.Peterson TR, Laplante M, Thoreen CC, et al. DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell. 2009;137:873–886. doi: 10.1016/j.cell.2009.03.046. doi:10.1016/j.cell.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alessi DR, James SR, Downes CP, et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. doi:10.1016/S0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 29.Calleja V, Laguerre M, Parker PJ, Larijani B. Role of a novel PH-kinase domain interface in PKB/Akt regulation: structural mechanism for allosteric inhibition. PLoS Biol. 2009;7:e17. doi: 10.1371/journal.pbio.1000017. doi:10.1371/journal.pbio.1000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calleja V, Alcor D, Laguerre M, et al. Intramolecular and intermolecular interactions of protein kinase B define its activation in vivo. PLoS Biol. 2007;5:e95. doi: 10.1371/journal.pbio.0050095. doi:10.1371/journal.pbio.0050095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Easton JB, Houghton PJ. mTOR and cancer therapy. Oncogene. 2006;25:6436–6446. doi: 10.1038/sj.onc.1209886. doi:10.1038/sj.onc.1209886. [DOI] [PubMed] [Google Scholar]
- 32.Fan QW, Cheng C, Knight ZA, et al. EGFR signals to mTOR through PKC and independently of Akt in glioma. Sci Signal. 2009;2:ra4. doi: 10.1126/scisignal.2000014. doi:10.1126/scisignal.2000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakamura JL, Garcia E, Pieper RO. S6K1 plays a key role in glial transformation. Cancer Res. 2008;68:6516–6523. doi: 10.1158/0008-5472.CAN-07-6188. doi:10.1158/0008-5472.CAN-07-6188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu X, Pandolfi PP, Li Y, Koutcher JA, Rosenblum M, Holland EC. mTOR promotes survival and astrocytic characteristics induced by Pten/AKT signaling in glioblastoma. Neoplasia. 2005;7:356–368. doi: 10.1593/neo.04595. doi:10.1593/neo.04595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neshat MS, Mellinghoff IK, Tran C, et al. Enhanced sensitivity of PTEN-deficient tumors to inhibition of FRAP/mTOR. Proc Natl Acad Sci USA. 2001;98:10314–10319. doi: 10.1073/pnas.171076798. doi:10.1073/pnas.171076798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eshleman JS, Carlson BL, Mladek AC, Kastner BD, Shide KL, Sarkaria JN. Inhibition of the mammalian target of rapamycin sensitizes U87 xenografts to fractionated radiation therapy. Cancer Res. 2002;62:7291–7297. [PubMed] [Google Scholar]
- 37.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. doi:10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 38.Sabatini DM. mTOR and cancer: insights into a complex relationship. Nat Rev Cancer. 2006;6:729–734. doi: 10.1038/nrc1974. doi:10.1038/nrc1974. [DOI] [PubMed] [Google Scholar]
- 39.Zhang H, Bajraszewski N, Wu E, et al. PDGFRs are critical for PI3K/Akt activation and negatively regulated by mTOR. J Clin Invest. 2007;117:730–738. doi: 10.1172/JCI28984. doi:10.1172/JCI28984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo D, Hildebrandt IJ, Prins RM, et al. The AMPK agonist AICAR inhibits the growth of EGFRvIII-expressing glioblastomas by inhibiting lipogenesis. Proc Natl Acad Sci USA. 2009;106:12932–12937. doi: 10.1073/pnas.0906606106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cloughesy TF, Yoshimoto K, Nghiemphu P, et al. Antitumor activity of rapamycin in a Phase I trial for patients with recurrent PTEN-deficient glioblastoma. PLoS Med. 2008;5:e8. doi: 10.1371/journal.pmed.0050008. doi:10.1371/journal.pmed.0050008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thoreen CC, Kang SA, Chang JW, et al. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J Biol Chem. 2009;284:8023–8032. doi: 10.1074/jbc.M900301200. doi:10.1074/jbc.M900301200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Masri J, Bernath A, Martin J, et al. mTORC2 activity is elevated in gliomas and promotes growth and cell motility via overexpression of rictor. Cancer Res. 2007;67:11712–11720. doi: 10.1158/0008-5472.CAN-07-2223. doi:10.1158/0008-5472.CAN-07-2223. [DOI] [PubMed] [Google Scholar]
- 44.Read RD, Cavenee WK, Furnari FB, Thomas JB. A drosophila model for EGFR- Ras and PI3K-dependent human glioma. PLoS Genet. 2009;5:e1000374. doi: 10.1371/journal.pgen.1000374. doi:10.1371/journal.pgen.1000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mellinghoff IK, Wang MY, Vivanco I, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med. 2005;353:2012–2024. doi: 10.1056/NEJMoa051918. doi:10.1056/NEJMoa051918. [DOI] [PubMed] [Google Scholar]
- 46.Haas-Kogan DA, Prados MD, Tihan T, et al. Epidermal growth factor receptor, protein kinase B/Akt, and glioma response to erlotinib. J Natl Cancer Inst. 2005;97:880–887. doi: 10.1093/jnci/dji161. [DOI] [PubMed] [Google Scholar]
- 47.Reardon DA, Quinn JA, Vredenburgh JJ, et al. Phase 1 trial of gefitinib plus sirolimus in adults with recurrent malignant glioma. Clin Cancer Res. 2006;12:860–868. doi: 10.1158/1078-0432.CCR-05-2215. doi:10.1158/1078-0432.CCR-05-2215. [DOI] [PubMed] [Google Scholar]
- 48.Kreisl TN, Lassman AB, Mischel PS, et al. A pilot study of everolimus and gefitinib in the treatment of recurrent glioblastoma (GBM) J Neurooncol. 2009;92:99–105. doi: 10.1007/s11060-008-9741-z. doi:10.1007/s11060-008-9741-z. [DOI] [PubMed] [Google Scholar]
- 49.Doherty L, Gigas DC, Kesari S, et al. Pilot study of the combination of EGFR and mTOR inhibitors in recurrent malignant gliomas. Neurology. 2006;67:156–158. doi: 10.1212/01.wnl.0000223844.77636.29. doi:10.1212/01.wnl.0000223844.77636.29. [DOI] [PubMed] [Google Scholar]
- 50.Sarkaria JN, Yang L, Grogan PT, et al. Identification of molecular characteristics correlated with glioblastoma sensitivity to EGFR kinase inhibition through use of an intracranial xenograft test panel. Mol Cancer Ther. 2007;6:1167–1174. doi: 10.1158/1535-7163.MCT-06-0691. doi:10.1158/1535-7163.MCT-06-0691. [DOI] [PubMed] [Google Scholar]
- 51.Sos ML, Koker M, Weir BA, et al. PTEN loss contributes to erlotinib resistance in EGFR-mutant lung cancer by activation of Akt and EGFR. Cancer Res. 2009;69:3256–3261. doi: 10.1158/0008-5472.CAN-08-4055. doi:10.1158/0008-5472.CAN-08-4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang MY, Lu KV, Zhu S, et al. Mammalian target of rapamycin inhibition promotes response to epidermal growth factor receptor kinase inhibitors in PTEN- deficient and PTEN-intact glioblastoma cells. Cancer Res. 2006;66:7864–7869. doi: 10.1158/0008-5472.CAN-04-4392. doi:10.1158/0008-5472.CAN-04-4392. [DOI] [PubMed] [Google Scholar]
- 53.Fan QW, Knight ZA, Goldenberg DD, et al. A dual PI3 kinase/mTOR inhibitor reveals emergent efficacy in glioma. Cancer Cell. 2006;9:341–349. doi: 10.1016/j.ccr.2006.03.029. doi:10.1016/j.ccr.2006.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fan QW, Cheng CK, Nicolaides TP, et al. A dual phosphoinositide-3-kinase alpha/mTOR inhibitor cooperates with blockade of epidermal growth factor receptor in PTEN-mutant glioma. Cancer Res. 2007;67:7960–7965. doi: 10.1158/0008-5472.CAN-07-2154. doi:10.1158/0008-5472.CAN-07-2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reardon DA, Desjardins A, Vredenburgh JJ, et al. Phase 2 trial of erlotinib plus sirolimus in adults with recurrent glioblastoma. JNeurooncol. 2010;96:219–230. doi: 10.1007/s11060-009-9950-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chang SM, Wen P, Cloughesy T, et al. Phase II study of CCI-779 in patients with recurrent glioblastoma multiforme. Invest New Drugs. 2005;23:357–361. doi: 10.1007/s10637-005-1444-0. doi:10.1007/s10637-005-1444-0. [DOI] [PubMed] [Google Scholar]
- 57.Galanis E, Buckner JC, Maurer MJ, et al. Phase II trial of temsirolimus (CCI-779) in recurrent glioblastoma multiforme: a North Central Cancer Treatment Group study. J Clin Oncol. 2005;23:5294–5304. doi: 10.1200/JCO.2005.23.622. doi:10.1200/JCO.2005.23.622. [DOI] [PubMed] [Google Scholar]
- 58.Podsypanina K, Lee RT, Politis C, et al. An inhibitor of mTOR reduces neoplasia and normalizes p70/S6 kinase activity in Pten + /− mice. Proc Natl Acad Sci USA. 2001;98:10320–10325. doi: 10.1073/pnas.171060098. doi:10.1073/pnas.171060098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu K, Toral-Barza L, Discafani C, et al. mTOR, a novel target in breast cancer: the effect of CCI-779, an mTOR inhibitor, in preclinical models of breast cancer. Endocr Relat Cancer. 2001;8:249–258. doi: 10.1677/erc.0.0080249. doi:10.1677/erc.0.0080249. [DOI] [PubMed] [Google Scholar]
- 60.Shi Y, Gera J, Hu L, et al. Enhanced sensitivity of multiple myeloma cells containing PTEN mutations to CCI-779. Cancer Res. 2002;62:5027–5034. [PubMed] [Google Scholar]
- 61.Majumder PK, Febbo PG, Bikoff R, et al. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med. 2004;10:594–601. doi: 10.1038/nm1052. doi:10.1038/nm1052. [DOI] [PubMed] [Google Scholar]
- 62.Wendel HG, De Stanchina E, Fridman JS, et al. Survival signalling by Akt and eIF4E in oncogenesis and cancer therapy. Nature. 2004;428:332–337. doi: 10.1038/nature02369. doi:10.1038/nature02369. [DOI] [PubMed] [Google Scholar]
- 63.Yilmaz OH, Valdez R, Theisen BK, et al. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–482. doi: 10.1038/nature04703. doi:10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- 64.O'Reilly KE, Rojo F, She QB, et al. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500–1508. doi: 10.1158/0008-5472.CAN-05-2925. doi:10.1158/0008-5472.CAN-05-2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Breuleux M, Klopfenstein M, Stephan C, et al. Increased AKT S473 phosphorylation after mTORC1 inhibition is rictor dependent and does not predict tumor cell response to PI3K/mTOR inhibition. Mol Cancer Ther. 2009;8:742–753. doi: 10.1158/1535-7163.MCT-08-0668. doi:10.1158/1535-7163.MCT-08-0668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maira SM, Stauffer F, Brueggen J, et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol Cancer Ther. 2008;7:1851–1863. doi: 10.1158/1535-7163.MCT-08-0017. doi:10.1158/1535-7163.MCT-08-0017. [DOI] [PubMed] [Google Scholar]
- 67.Gulati P, Gaspers LD, Dann SG, et al. Amino acids activate mTOR complex 1 via Ca2+/CaM signaling to hVps34. Cell Metab. 2008;7:456–465. doi: 10.1016/j.cmet.2008.03.002. doi:10.1016/j.cmet.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Shackelford DB, Shaw RJ. The LKB1-AMPK pathway: metabolism and growth control in tumour suppression. Nat Rev Cancer. 2009;9:563–575. doi: 10.1038/nrc2676. doi:10.1038/nrc2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guo D, Chien S, Shyy JY. Regulation of endothelial cell cycle by laminar versus oscillatory flow: distinct modes of interactions of AMP-activated protein kinase and Akt pathways. Circ Res. 2007;100:564–571. doi: 10.1161/01.RES.0000259561.23876.c5. doi:10.1161/01.RES.0000259561.23876.c5. [DOI] [PubMed] [Google Scholar]
- 70.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. doi:10.1016/S0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 71.Gwinn DM, Shackelford DB, Egan DF, et al. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. doi:10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mahoney CL, Choudhury B, Davies H, et al. LKB1/KRAS mutant lung cancers constitute a genetic subset of NSCLC with increased sensitivity to MAPK and mTOR signalling inhibition. Br J Cancer. 2009;100:370–375. doi: 10.1038/sj.bjc.6604886. doi:10.1038/sj.bjc.6604886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ikenoue T, Hikiba Y, Kanai F, et al. Functional analysis of mutations within the kinase activation segment of B-Raf in human colorectal tumors. Cancer Res. 2003;63:8132–8137. [PubMed] [Google Scholar]
- 74.Dhomen N, Marais R. New insight into BRAF mutations in cancer. Curr Opin Genet Dev. 2007;17:31–39. doi: 10.1016/j.gde.2006.12.005. doi:10.1016/j.gde.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 75.Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–954. doi: 10.1038/nature00766. doi:10.1038/nature00766. [DOI] [PubMed] [Google Scholar]
- 76.Zheng B, Jeong JH, Asara JM, et al. Oncogenic B-RAF negatively regulates the tumor suppressor LKB1 to promote melanoma cell proliferation. Mol Cell. 2009;33:237–247. doi: 10.1016/j.molcel.2008.12.026. doi:10.1016/j.molcel.2008.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Guo D, Cloughesy TF, Radu CG, Mischel PS. AMPK: a metabolic checkpoint that regulates the growth of EGFR activated glioblastomas. Cell Cycle. 2010;9:4–5. doi: 10.4161/cc.9.2.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ding H, Shannon P, Lau N, et al. Oligodendrogliomas result from the expression of an activated mutant epidermal growth factor receptor in a RAS transgenic mouse astrocytoma model. Cancer Res. 2003;63:1106–1113. [PubMed] [Google Scholar]
- 79.Weiss WA, Burns MJ, Hackett C, et al. Genetic determinants of malignancy in a mouse model for oligodendroglioma. Cancer Res. 2003;63:1589–1595. [PubMed] [Google Scholar]
- 80.Guo D, Prins RM, Dang J, et al. EGFR signaling through an Akt-SREBP-1-dependent, rapamycin-resistant pathway sensitizes glioblastomas to antilipogenic therapy. Sci Signal. 2009;2:Ra82. doi: 10.1126/scisignal.2000446. doi:10.1126/scisignal.2000446. [DOI] [PMC free article] [PubMed] [Google Scholar]