Abstract
Pilocytic astrocytoma is commonly viewed as a benign lesion. However, disease onset is most prevalent in the first two decades of life, and children are often left with residual or recurrent disease and significant morbidity. The Hedgehog (Hh) pathway regulates the growth of higher WHO grade gliomas, and in this study, we have evaluated the activation and operational status of this regulatory pathway in pilocytic astrocytomas. Expression levels of the Hh pathway transcriptional target PTCH were elevated in 45% of tumor specimens analyzed (ages 1–22 years) and correlated inversely with patient age. Evaluation of a tissue array revealed oligodendroglioma-like features, pilomyxoid features, infiltration, and necrosis more commonly in specimens from younger patients (below the median patient age of 10 years). Immunohistochemical staining for the Hh pathway components PTCH and GLI1 and the proliferation marker Ki67 demonstrated that patients diagnosed before the age of 10 had higher staining indices than those diagnosed after the age of 10. A significant correlation between Ki67 and PTCH and GLI1 staining indices was measured, and 86% of Ki67-positive cells also expressed PTCH. The operational status of the Hh pathway was confirmed in primary cell culture and could be modulated in a manner consistent with a ligand-dependent mechanism. Taken together, these findings suggest that Hh pathway activation is common in pediatric pilocytic astrocytomas and may be associated with younger age at diagnosis and tumor growth.
Keywords: Hedgehog, pediatric brain tumor, pilocytic astrocytoma
Pilocytic astrocytoma is the most common pediatric brain tumor and commonly viewed as a benign lesion with excellent prognosis.1–4 Estimated 10-year survival rates range from 90% to 96.8%.3,5 However, the 20-year survival and progression-free survival rates of approximately 70% and 40%, respectively,6–8 reflect the indolent nature of this disease. Furthermore, survivors of pilocytic astrocytomas commonly suffer long-term sequelae related to disease and treatment.9–13 Thus, a better understanding of pilocytic astrocytoma biology is needed to guide the development of new therapies to decrease morbidity and improve survival.
Hedgehog (Hh) signaling is one mechanism implicated in the growth of two other types of primary brain tumors, medulloblastoma and malignant glioma.14,15 Additionally, recent limited clinical data indicate that targeted inhibition of the Hh pathway may be therapeutically beneficial for certain patients with medulloblastoma.16 In a prior survey of WHO grade I–IV glioma specimens that included a small collection of pilocytic astrocytomas from adult patients, protein and transcript expression profiles for the Hh receptor Patched (PTCH) suggested that the Hh pathway might be activated to a small extent in this astrocytoma entity.17 Therefore, in this study, we have investigated the Hh pathway in a larger panel of pilocytic astrocytoma specimens from pediatric patients. We demonstrate that the Hh pathway is operational in sporadic pilocytic astrocytomas and activated to a greater extent in tumors from younger patients. Furthermore, expression of the Hh pathway signal transduction components correlates with expression of the cellular proliferation marker Ki67.
Materials and Methods
Tissue Procurement
Brain tumor and epilepsy specimens from patients treated at the Vanderbilt Medical Center from 2003 to 2008 were obtained in accordance with the Institutional Review Board's approval. Primary brain tumors were phenotyped and graded using the World Health Organization criteria,18,19 and 20 specimens classified as pilocytic astrocytoma were used for these studies (Table 1). Pilocytic astrocytoma patient ages ranged from 1 to 22 years, and patients with a diagnosis of neurofibromatosis type 1 and pilomyxoid astrocytoma were not included. The epilepsy control specimens were obtained from patients who were 37–57 years old. The locations of the pilocytic astrocytomas varied: 14 in the cerebellum, 2 in the midbrain, 1 in the temporal lobe, 1 in the frontal lobe, 1 in the thalamus, and 1 in the suprasellar region (Table 1). RNA was extracted for quantitative real-time PCR (qRT-PCR) studies from 18 of the tumor specimens for which excess tissue was available for banking in a research tissue repository. Tissue cores were removed for tissue microarray construction from 17 of the tumor specimens that contained sufficient material in paraffin blocks. Primary cell cultures were generated from 4 of the tumor specimens for which adequate excess tissue was available.
Table 1.
Clinical and pathological features and the molecular analyses of pilocytic astrocytomas
| Sample | Age (y) | Primary/recurrent | Location | Pathology | PTCH RNA | PTCH IHC | GLI1 IHC | Ki67 IHC |
|---|---|---|---|---|---|---|---|---|
| 1 | 1.8 | P | Cerebellum | Pilomyxoid features | 2.30 | 9.01 | 0.29 | 0.15 |
| 2 | 2.1 | P | Cerebellum | Infiltration and oligodendroglioma-like | 5.72 | 4.67 | 0.79 | 0.35 |
| 3 | 2.5 | P | Cerebellum | Infiltration/pilomyxoid features | 11.5 | 15.15 | 6.90 | 0.10 |
| 4 | 2.6 | P | Cerebellum | Classic architecture | 3.56 | 6.29 | 2.38 | 1.08 |
| 5 | 3.4 | P | Cerebellum | Infiltration | 10.6 | 12.85 | 1.41 | 0.12 |
| 6 | 4.1 | P | Suprasellar | Pilomyxoid features and necrosis | 1.25 | 32.17 | 13.91 | 0.55 |
| 7 | 4.7 | P | Midbrain, exophytic | Pilomyxoid features | NA | 6.54 | 2.73 | 0.06 |
| 8 | 4.7 | P | Cerebellum | Infiltration | 5.01 | 28.26 | 19.68 | 0.93 |
| 9 | 5.3 | R | Midbrain, exophytic | Pilomyxoid and oligodendroglioma-like features | 7.42 | 24.05 | 18.18 | 1.85 |
| 10 | 6.7 | P | Cerebellum and midbrain | Pilomyxoid features and increased proliferation | 1.72 | 36.57 | 27.20 | 2.77 |
| 11 | 13.7 | P | Cerebellum | Classic architecture | 1.93 | 6.81 | 0.00 | 0.00 |
| 12 | 14 | R | Cerebellum | Classic architecture | NA | 4.90 | 0.37 | 0.18 |
| 13 | 14.3 | P | Cerebellum | Oligodendroglioma-like features | 3.80 | NA | NA | NA |
| 14 | 14.8 | P | Cerebellum | Classic architecture | 0.67 | 13.39 | 4.17 | 0.06 |
| 15 | 16.7 | P | Temporal lobe | Classic architecture | 1.03 | NA | NA | NA |
| 16 | 18.3 | P | Cerebellum | Classic architecture | 3.81 | NA | NA | NA |
| 17 | 18.8 | P | Cerebellum | Classic architecture | 0.820 | NA | NA | NA |
| 18 | 20.1 | P | Frontal lobe | Classic architecture | 1.23 | 9.70 | 0.74 | 0.03 |
| 19 | 21 | R | Cerebellum and suprasellar | Classic architecture | 1.41 | NA | NA | NA |
| 20 | 22 | P | Thalamus | Classic architecture | 1.86 | 3.49 | 2.68 | 0.09 |
Abbreviations: P, primary tumor; R, recurrent tumor; PTCH, patched; IHC, immunohistochemistry; Ki67-LE, Ki67 labeling index.
RNA Extraction, cDNA Synthesis, and qRT-PCR
Total RNA was extracted from brain tissue or primary cell cultures with the RNeasy Mini Kit (QIAGEN). Genomic DNA was removed (RNase-Free DNase Set, QIAGEN) and purified RNA was quantified (RiboGreen RNA Quantitation Kit, Molecular Probes). Single-stranded cDNA was synthesized with oligo(dT) and random hexamer primers (iScript cDNA Synthesis Kit, Bio-Rad). For negative controls, reverse transcriptase was omitted from the synthesis reaction (−RT). For all measurements, qRT-PCR was performed in triplicate for each sample and on the corresponding −RT control. For primary brain tumor and epilepsy specimens, qRT-PCR reactions were performed with SYBR Green Supermix (Bio-Rad), cDNA template, and 200 nM primers for PTCH and glyceraldehyde 3-phosphate dehydrogenase (GAPDH).17 Additionally, measurements in primary brain tumors were corroborated using the TaqMan primers for PTCH (Hs00970979_m1) and GAPDH (Hs99999905_m1). For primary cell cultures, qRT-PCR reactions were performed with the TaqMan Fast Universal PCR Master Mix (Applied Biosystems), cDNA template, and primers (TaqMan Gene Expression Assay, Applied Biosystems) for hPTCH (Hs00970979_m1), hGLI1 (Hs00171790_m1), and hGAPDH (Hs99999905_m1), respectively.15 For standard curves, qRT-PCR was performed on serial dilutions of a human cDNA mixture.17 For each amplicon, quantities were determined according to the standard curve method (User Bulletin #2, PE Applied Biosystems).
Tissue Microarray Construction and Immunohistochemical Analysis
Surgical pathology slides were reviewed (by T.W.A.) to identify tumor regions of interest and any normal brain structures. The slides were marked and delivered to the Vanderbilt Human Tissue Acquisition and Pathology Core to identify the corresponding regions on the formalin-fixed, paraffin-embedded donor blocks and construct a tissue microarray. Four tissue cores (1.0 mm diameter) were removed from each donor block, assigned a coded identifier, and manually placed in nonadjacent positions of the recipient microarray block. Twenty consecutive sections (5 µm) from the tissue microarray were mounted on silanized slides. Sections 1 and 20 were stained with hematoxylin and eosin to confirm sample integrity, and sections 2–19 were used for the immunohistochemical analysis.
Immunohistochemistry was performed with the following antibodies: PTCH-1 (1:100; Santa Cruz Biotechnologies), Ki67 (1:50; DAKO), Bmi-1 (1:100; Upstate), and Gli-1 (1:50; Cell Signaling). The slides were deparaffinized and microwaved in a citrate buffer (pH 6.0) for antigen retrieval. They were treated with 3% hydrogen peroxide solution, incubated in blocking serum, and incubated in primary antibody overnight at 4°C. Biotinylated secondary antibody was incubated overnight at 4°C, and immunodetection with 3,3′-diaminobenzidine or 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium (BCIP/NBT) substrate was performed using the Vectastain Elite ABC kit (Vector Laboratories).
Staining indices for each sample on the microarray were determined by dividing the average number of cells that stained positively for PTCH, GLI1, or Ki67 per high-power microscopic fields (hpf) by the total number of cells per hpf. Cells staining for PTCH, GLI1, and Ki67 were counted in 9 hpf for each sample on the single-antibody–labeled slides. The total numbers of cells were counted in 4 hpf for each sample on a slide stained with hematoxylin. Depending on tissue integrity, values for each tumor were obtained from at least 2 samples on every microarray slide. Tumor identity was determined only after the staining was scored.
Cell Culture and Hh Signaling Assays
Tumor samples were dissociated (Papain, Worthington Biochemical Corporation) and plated in DMEM/F12, 10% FBS, and 1× penicillin–streptomycin (Invitrogen). After primary cell cultures reached confluency, they were plated for 7 days in nontreated polystyrene flasks (BD-Falcon) in NeuroCult medium with supplements (NeuroCult NS-A Proliferation Kit; Stem Cell Technologies), 2 µg/mL heparin (Sigma), 20 ng/mL EGF (ProSpec-Tany TechnoGene), 10 ng/mL bFGF (ProSpec-Tany TechnoGene), and 1× penicillin-streptomycin (Invitrogen). The resulting spheres of cells were transferred to nontreated polystyrene multiwell plates (BD-Falcon) and cultured in triplicate for 40 hours either alone, with 50 nM smoothened agonist (SAG), with 500 nM SAG, or with 200 nM smoothened antagonist (SANT1). For assays of Hh pathway inhibition, confluent cultures were plated in nontreated polystyrene flasks (BD-Falcon) in NeuroCult medium with supplements (NeuroCult NS-A Proliferation Kit; Stem Cell Technologies), 2 µg/mL heparin (Sigma), 1× penicillin–streptomycin (Invitrogen), and 50 nM SAG. The resulting spheres of cells were transferred to multiwell plates and cultured in triplicate for 48 hours either with 50 nM SAG or with 200 nM SANT1. GLI1 and GAPDH levels were measured by qRT-PCR as described above.
Statistical Analysis
To measure the correlation between two variables, the Pearson product–moment correlation coefficient was calculated with a 99% confidence interval (GraphPad Prism). Student's t-test was used to compare the mean values from the 2 groups.
Results
PTCH mRNA Expression Levels in Pilocytic Astrocytomas Correlate Inversely with Age
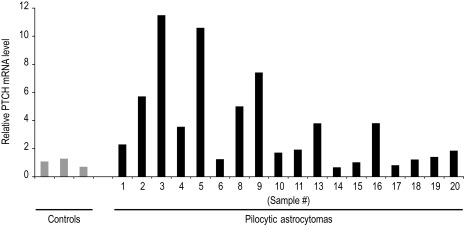
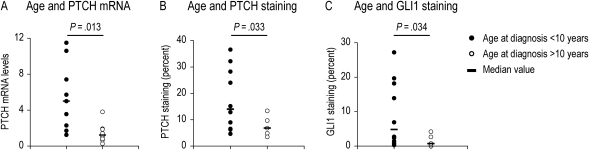
To evaluate the Hh pathway activity, PTCH mRNA levels were measured by qRT-PCR in 18 of the pilocytic astrocytomas evaluated in this study (Table 1) and in 3 specimens from patients with epilepsy (Fig. 1). PTCH is a signal transduction component and direct gene target of Hh signaling, whose expression level can serve as a metric for pathway activity.20,21 For all samples, PTCH levels were normalized to endogenous GAPDH levels and expressed as the fold difference relative to control samples resected from patients with epilepsy. Compared with control specimens, PTCH RNA expression was elevated in 45% of the pilocytic astrocytoma specimens (Fig. 1). A significant inverse correlation between PTCH expression levels and patient age (Pearson's test, r = −.59, P = .0097) suggests that the Hh pathway may be activated in pilocytic astrocytomas from younger patients. When grouped according to diagnosis before and after the median patient age of 10, median PTCH levels were 5.01 and 1.23, respectively (Student's t-test, P = .013; Fig. 2A).
Fig. 1.
PTCH mRNA expression in pilocytic astrocytomas. Graphic illustration of the relative PTCH expression levels from samples listed in Table 1 revealed a significant inverse correlation with patient age (Pearson's test, r = −.59, P = .0097).
Fig. 2.
Comparison of age at diagnosis and molecular analyses of Hedgehog pathway activation. (A) When grouped according to diagnosis before and after the age of 10 years, median PTCH levels are 5.01 and 1.23, respectively (Student's t-test, P = .013). (B and C) Higher staining indices for PTCH and GLI1 are measured in tumors from younger patients. In specimens from patients diagnosed before and after the age of 10, the median values for PTCH are 14.00 and 6.81, respectively (Student's t-test, P = .033; B) and for GLI1 they are 4.82 and 0.74 (Student's t-test, P = .034; C).
PTCH and GLI1 Protein Staining Indices in Pilocytic Astrocytomas Correlate with that of a Cellular Marker for Proliferation
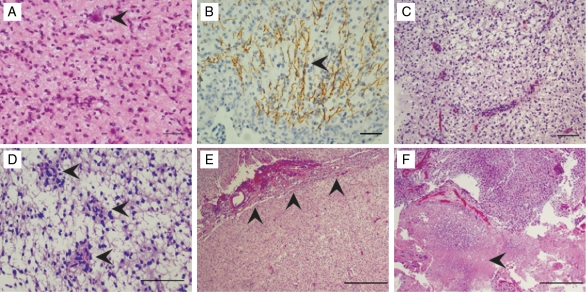
To corroborate these findings, pathological features of the 20 pilocytic astrocytoma specimens (Table 1) were reviewed for tissue microarray construction. Compact growth patterns and biphasic architectural organizational patterns predominated in all specimens. However, 9 of the patient samples demonstrated at least one additional histological feature that included oligodendroglioma-like qualities, pilomyxoid features, leptomeningeal spread, focal areas of increased proliferation, necrosis, or focal infiltrative growth (Fig. 3). These pathological features were more prevalent in patients diagnosed with pilocytic astrocytoma before the age of 10 (Table 1), and several have been associated with poorer outcome.4,22–24 Four tissue cores from 17 of the donor paraffin blocks that contained sufficient material were included in a recipient microarray block. Immunohistochemistry was performed for the Hh receptor PTCH and the Hh transcription factor GLI1, and staining was scored in an investigator-blinded fashion. Adequate tissue for the immunohistochemical analysis was available for 15 of the 17 tumors on the tissue microarray sections.
Fig. 3.
Putative aggressive pathological features in pilocytic astrocytoma specimens. (A–F) Putative aggressive features noted on review of 20 anonymized pilocytic astrocytoma specimens. Tumor infiltration demonstrated by entrapped neuronal cell bodies (arrowhead in A) and axons coursing through the neoplasm (arrowhead in B, neurofilament stain), oligodendroglioma-like features (C), pilomyxoid features (arrowheads in D), leptomeningeal spread (arrowheads in E), and necrosis (arrowhead in F) were noted in 9 of the patient samples. These pathological features were more prevalent in patients diagnosed prior to the age of 10 (Table 1). Scale bar indicates 50 µm in A and B, 100 µm in C and D, and 500 µm in E and F.
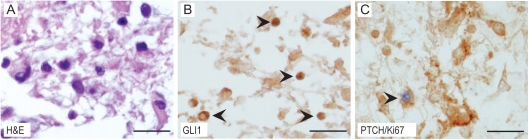
Single-label staining for PTCH or GLI1 produced concordant results for each donor tissue core, with greater numbers of cells labeled for PTCH than GLI1 in all instances (Fig. 4 and Supplementary Material, Fig. S1). A notable feature of this type of analysis was variability in PTCH or GLI1 staining indices among some of the tissue cores sampled from the same tumor (Supplementary Material, Table S1 and Fig. S2). This suggests that pilocytic astrocytomas are heterogeneous with respect to Hh pathway component expression. Consistent with this interpretation, the PTCH or GLI1 staining indices from one portion of a tumor did not always correspond with the PTCH mRNA expression level measured in another portion of the same tumor (Table 1). However, as for PTCH mRNA measurements, higher staining indices for PTCH and GLI1 were measured in tumors from younger patients (Fig. 2). In specimens from patients diagnosed before and after the age of 10, the median values for PTCH were 14.00 and 6.81, respectively (Student's t-test, P = .033; Fig. 2B), and for GLI1 they were 4.82 and 0.74 (Student's t-test, P = .034; Fig. 2C).
Fig. 4.
Hedgehog pathway component expression in a pilocytic astrocytoma specimens. (A–C) Representative image of a pilocytic astrocytoma from a tissue microarray stained with hematoxylin and eosin (A) and for GLI1 (arrowheads in B), and PTCH and Ki67 (C). Arrowhead in C demonstrates a PTCH+/Ki67+ cells. Scale bar = 50 µm.
To evaluate cellular proliferation, Ki67 immunohistochemistry was performed with the pilocytic astrocytoma tissue microarray. Consistent with other reports, Ki67 labeling indices ranged from 0% to 2.77% and were higher in younger patients (Table 1).25,26 In addition, a significant correlation between Ki67 and PTCH (Pearson's test, r = .68, P = .0058) and between Ki67 and GLI1 (Pearson's test, r = .82, P = .0002) staining indices was measured. To corroborate these observations, double-labeling experiments were performed and revealed that 86% of Ki67-positive cells expressed PTCH across all tumors (Fig. 4C and data not shown).
Hh Pathway Modulation Can Be Measured in Primary Cells Cultured from Pilocytic Astrocytomas
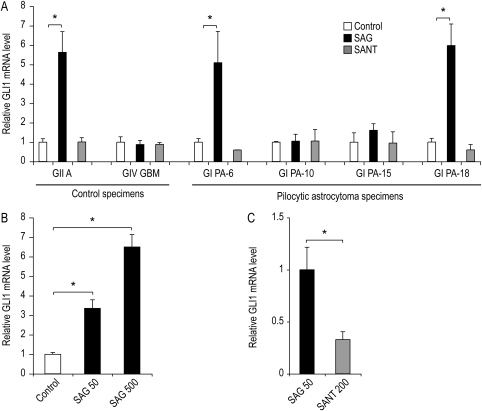
In order to assess the operational status of the Hh pathway, primary cell cultures were generated from 4 pilocytic astrocytomas (sample numbers 6, 10, 15, and 18; Table 1) and 2 higher-grade adult astrocytomas that served as controls. Under culture conditions that are required for eliciting an Hh pathway response,17 the addition of an SAG (50 nM) induced a significant increase in GLI1 mRNA expression in 2 of the 4 pilocytic astrocytoma cultures (sample numbers 6 and 18; P < .05, Student's t-test; Fig. 5A). The basal expression levels of GLI1 transcript were not reduced by treatment with a SANT1 (200 nM)27 in any of the astrocytoma cultures (Fig. 5A). The levels of GLI1 induction in the pilocytic astrocytoma cultures were comparable to those observed in a Hh-responsive WHO grade II astrocytoma control culture (Fig. 5A). Consistent with prior studies, GLI1 expression levels were not induced by the SAG treatment of a primary GBM culture that served as a negative control in the analysis.15,17 The findings from this survey of 4 tumors provide evidence that the Hh pathway is operationally intact within a subset of pilocytic astrocytomas.
Fig. 5.
Characterization of Hedgehog pathway responsiveness in primary cell cultures from pilocytic astrocytomas. (A) Astrocytic tumors were grown as spheres under stem cell culture conditions. Cells from a Hh-responsive astrocytoma (GII A), a Hh-unresponsive GBM (GIV GBM), and 4 pilocytic astrocytomas (GI PA-6, GI PA-10, GI PA-15, and GI PA-18) were cultured for 36 hours either alone (control), with 50 nM SAG, or with 200 nM SANT1. In triplicate wells for each cell line and culture condition, the GLI1 levels were normalized to GAPDH and expressed relative to the untreated control cultures. (B) To confirm a dose-dependent response to Hh pathway stimulation, the GI PA-18 cells were cultured in triplicate wells either alone (control), with 50 nM SAG, or with 200 nM SAG. After 40 hours, the GLI1 levels were normalized to GAPDH and expressed relative to the untreated control cultures. (C) To determine whether Hh signaling could be inhibited following pathway activation, the GI PA-18 cells were cultured in the presence of SAG (50 nM) for 7 days and then re-plated in triplicate wells containing either 50 nM SAG or 200 nM SANT1. After an additional 48 hours in culture, the GLI1 levels were normalized to GAPDH and expressed relative to the SAG-treated cultures. GI, WHO grade I; GII, WHO grade II; GIV, WHO grade IV; A, astrocytoma; AA, anaplastic astrocytoma; GBM, glioblastoma multiforme; PA, pilocytic astrocytoma; SAG, smoothened agonist; SANT, smoothened antagonist; *P ≤ .05.
To confirm the modulatory status of the Hh pathway, dose-dependent induction of GLI1 mRNA with SAG was assayed (Fig. 5B). To determine whether once activated the pathway could then be inhibited, a culture was generated in the presence of SAG. After 7 days, a portion of the spheres from the parent culture was re-plated in the presence of SANT1. The GLI1 levels were elevated in the parent culture derived in the presence of SAG and could be reduced to basal levels by SANT1 treatment (Fig. 5C). These data demonstrate that Hh signaling in a pilocytic astrocytoma primary culture can be modulated by either activation or inhibition, thereby confirming the operational status of the pathway.
Discussion
A role for aberrant Hh signaling in tumorigenesis was first appreciated with the discovery that the autosomal dominant disorder, Gorlin's syndrome, is associated with mutations in the PTCH gene.28,29 Patients with Gorlin's syndrome (also called basal cell nevus syndrome) display an increased incidence of basal cell carcinoma and medulloblastoma. Since that time, mutations in PTCH,30–32 Smoothened (SMOH),33,34 or Suppressor-of-Fused (SUFU)35 have been identified in approximately 30% of sporadic medulloblastoma specimens. Mutations in these 3 Hh components confer “ligand-independent” activation of the pathway. In contrast, “ligand-dependent” activation of the Hh pathway has been identified in a broader array of malignancies36 including a subset of malignant gliomas.17,37–39 Here, we show ligand-dependent activation of the Hh pathway in a subset of pilocytic astrocytomas, low-grade gliomas that are clinically and genetically distinct from malignant gliomas.
Hh signaling in primary brain tumors and the divergent mechanisms by which the pathway is activated illustrate several features that are pertinent to the findings in this study. First, the incidence of Hh pathway activation in sporadic medulloblastomas can be defined with greater precision by mutational analysis. In the case of gliomas, the proportion of tumors in which the pathway is activated is less well defined in the absence of clear thresholds for PTCH and GLI1 expression levels to define an “on” or “off” state. Second, in contrast to medulloblastomas, in which clonal deregulation of Hh signaling is driven by mutations in the pathway, marked cellular heterogeneity in the expression of Hh pathway components suggests that the Hh pathway is activated in only a portion of tumor cells in malignancies with a ligand-dependent mechanism of activation. Lastly, a requirement for the Hh pathway activity in the growth of medulloblastomas and malignant gliomas has been demonstrated in animal transplantation models.14,15
In these studies, we demonstrate that the Hh pathway is activated in a subset of pilocytic astrocytomas. Complementary methods to assess the expression of the Hh pathway components and gene targets PTCH and GLI1 were utilized, and multiple regions of the same tumor were sampled. Pilocytic astrocytomas are characterized by marked heterogeneity in cytoarchitecture and patterns of cellular organization. With regard to PTCH and GLI1 expression, we find that levels vary with respect to the portion of tumor sampled. Signaling assays with primary cell cultures confirm that the Hh pathway is operational in pilocytic astrocytomas. The Hh pathway could be modulated in only a subset of primary cell cultures, consistent with heterogeneity in either the operational status of the pathway or the portion of tumor selected for culture. The ability to significantly induce GLI1 mRNA with SAG combined with the inability to inhibit the basal GLI1 levels with an antagonist suggests that Hh signaling is not constitutively activated.27,40 Therefore, as in WHO grade II and III gliomas, the Hh pathway appears to be activated in a ligand-dependent manner in pilocytic astrocytomas.15,17 A role for Hh signaling in pilocytic astrocytomas cannot be determined in the absence of animal models for sporadic disease. However, a notable feature of our studies is the demonstration of a significant correlation between Ki67 and PTCH and GLI1 staining. This finding suggests that, as for other primary brain tumor types,14,15 Hh signaling may regulate the growth of pilocytic astrocytomas.
Combined with the prior measurements of low PTCH expression in adult pilocytic astrocytomas,17 the inverse correlation between PTCH mRNA and patient age in this study suggests that the Hh pathway may be activated to a greater extent in younger patients. Although some studies suggest that younger age at diagnosis portends shorter progression-free survival,41,42 this has not been validated by other series.1,4,43 Additionally, we observed that potentially aggressive histological features were more commonly associated with tumors from younger patients. In particular, some series suggest that necrosis, oligodendroglioma-like features, and infiltrative growth are associated with decreased event-free survival.4,24 Evaluation of a larger series of tumor samples will be required to determine if the Hh pathway is activated to a greater extent in pilocytic astrocytomas from younger patients and whether this correlates with specific histopathologic features and tumor progression.
Recent findings implicate aberrant activation of the MAPK pathway, due to BRAF gene rearrangements or mutations, in 66%–85% of sporadic pilocytic astrocytomas.44–47 Integration of MAPK and Hh signaling, by ERK-mediated control of the GLI function, has been implicated in the regulation of cellular proliferation in basal cell and gastric carcinomas.48–50 Whether the MAPK and the Hh pathways function synergistically to regulate the growth of pilocytic astrocytomas warrants further study. In this respect, there remains an important need for animal models of sporadic pilocytic astrocytomas.
Supplementary Material
Supplementary material is available at Neuro-Oncology online.
Conflict of interest statement. None declared.
Funding
This work was supported by grants to M.K.C. from the Childhood Brain Tumor Foundation, National Brain Tumor Society, National Institutes of Health (K02 NS053614), and the Doris Duke Charitable Foundation.
Acknowledgments
We thank Vandana Grover for assistance with statistical analysis, and Nicholas K. Foreman for critical reading of this manuscript. Histological services were performed in part by the Vanderbilt Medical Center (VMC) Human Tissue Acquisition and Pathology Shared Resource (supported by the Vanderbilt Ingram Cancer Center, P30 CA68485).
References
- 1.Pencalet P, Maixner W, Sainte-Rose C, Lellouch-Tubiana A, Cinalli G, Zerah M, et al. Benign cerebellar astrocytomas in children. J Neurosurg. 1999;90:265–273. doi: 10.3171/jns.1999.90.2.0265. [DOI] [PubMed] [Google Scholar]
- 2.Fisher PG, Breiter SN, Carson BS, Wharam MD, Williams JA, Weingart JD, et al. A clinicopathologic reappraisal of brain stem tumor classification. Identification of pilocystic astrocytoma and fibrillary astrocytoma as distinct entities. Cancer. 2000;89:1569–1576. doi: 10.1002/1097-0142(20001001)89:7<1569::aid-cncr22>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 3.Burkhard C, Di Patre PL, Schuler D, Schuler G, Yasargil MG, Yonekawa Y, et al. A population-based study of the incidence and survival rates in patients with pilocytic astrocytoma. J Neurosurg. 2003;98:1170–1174. doi: 10.3171/jns.2003.98.6.1170. [DOI] [PubMed] [Google Scholar]
- 4.Fernandez C, Figarella-Branger D, Girard N, Bouvier-Labit C, Gouvernet J, Paz Paredes A, et al. Pilocytic astrocytomas in children: prognostic factors—a retrospective study of 80 cases. Neurosurgery. 2003;53:544–553. doi: 10.1227/01.neu.0000079330.01541.6e. discussion 554–555. [DOI] [PubMed] [Google Scholar]
- 5.2005. CBTRUS: Central Brain Tumor Registry of the United States. www.cbtrus.org . [Google Scholar]
- 6.Austin EJ, Alvord EC., Jr Recurrences of cerebellar astrocytomas: a violation of Collins' law. J Neurosurg. 1988;68:41–47. doi: 10.3171/jns.1988.68.1.0041. [DOI] [PubMed] [Google Scholar]
- 7.Wallner KE, Gonzales MF, Edwards MS, Wara WM, Sheline GE. Treatment results of juvenile pilocytic astrocytoma. J Neurosurg. 1988;69:171–176. doi: 10.3171/jns.1988.69.2.0171. [DOI] [PubMed] [Google Scholar]
- 8.Fisher PG, Tihan T, Goldthwaite PT, Wharam MD, Carson BS, Weingart JD, et al. Outcome analysis of childhood low-grade astrocytomas. Pediatr Blood Cancer. 2008;51:245–250. doi: 10.1002/pbc.21563. [DOI] [PubMed] [Google Scholar]
- 9.Cohen KJ, Broniscer A, Glod J. Pediatric glial tumors. Curr Treat Options Oncol. 2001;2:529–536. doi: 10.1007/s11864-001-0074-9. [DOI] [PubMed] [Google Scholar]
- 10.Aarsen FK, Van Dongen HR, Paquier PF, Van Mourik M, Catsman-Berrevoets CE. Long-term sequelae in children after cerebellar astrocytoma surgery. Neurology. 2004;62:1311–1316. doi: 10.1212/01.wnl.0000120549.77188.36. [DOI] [PubMed] [Google Scholar]
- 11.Beebe DW, Ris MD, Armstrong FD, Fontanesi J, Mulhern R, Holmes E, et al. Cognitive and adaptive outcome in low-grade pediatric cerebellar astrocytomas: evidence of diminished cognitive and adaptive functioning in National Collaborative Research Studies (CCG 9891/POG 9130) J Clin Oncol. 2005;23:5198–5204. doi: 10.1200/JCO.2005.06.117. [DOI] [PubMed] [Google Scholar]
- 12.Merchant TE, Conklin HM, Wu S, Lustig RH, Xiong X. Late effects of conformal radiation therapy for pediatric patients with low-grade glioma: prospective evaluation of cognitive, endocrine, and hearing deficits. J Clin Oncol. 2009;27:3691–3697. doi: 10.1200/JCO.2008.21.2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Turner CD, Chordas CA, Liptak CC, Rey-Casserly C, Delaney BL, Ullrich NJ, et al. Medical, psychological, cognitive and educational late-effects in pediatric low-grade glioma survivors treated with surgery only. Pediatr Blood Cancer. 2009;53:417–423. doi: 10.1002/pbc.22081. [DOI] [PubMed] [Google Scholar]
- 14.Berman DM, Karhadkar SS, Hallahan AR, Pritchard JI, Eberhart CG, Watkins DN, et al. Medulloblastoma growth inhibition by hedgehog pathway blockade. Science. 2002;297:1559–1561. doi: 10.1126/science.1073733. [DOI] [PubMed] [Google Scholar]
- 15.Sarangi A, Valadez JG, Rush S, Abel TW, Thompson RC, Cooper MK. Targeted inhibition of the hedgehog pathway in established malignant glioma xenografts enhances survival. Oncogene. 2009;28:3468–3476. doi: 10.1038/onc.2009.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rudin CM, Hann CL, Laterra J, Yauch RL, Callahan CA, Fu L, et al. Treatment of medulloblastoma with hedgehog pathway inhibitor GDC-0449. N Engl J Med. 2009;361:1173–1178. doi: 10.1056/NEJMoa0902903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ehtesham M, Sarangi A, Valadez JG, Chanthaphaychith S, Becher MW, Abel TW, et al. Ligand-dependent activation of the hedgehog pathway in glioma progenitor cells. Oncogene. 2007;26:5752–5761. doi: 10.1038/sj.onc.1210359. [DOI] [PubMed] [Google Scholar]
- 18.Kleihues P, Louis DN, Scheithauer BW, Rorke LB, Reifenberger G, Burger PC, et al. The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol. 2002;61:215–225. doi: 10.1093/jnen/61.3.215. discussion 226–229. [DOI] [PubMed] [Google Scholar]
- 19.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK. WHO Classification of Tumours of the Central Nervous System. 4th ed. Lyon, France: IARC Press; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berman DM, Karhadkar SS, Maitra A, Montes De Oca R, Gerstenblith MR, Briggs K, et al. Widespread requirement for hedgehog ligand stimulation in growth of digestive tract tumours. Nature. 2003;425:846–851. doi: 10.1038/nature01972. [DOI] [PubMed] [Google Scholar]
- 21.Karhadkar SS, Bova GS, Abdallah N, Dhara S, Gardner D, Maitra A, et al. Hedgehog signalling in prostate regeneration, neoplasia and metastasis. Nature. 2004;431:707–712. doi: 10.1038/nature02962. [DOI] [PubMed] [Google Scholar]
- 22.Dirven CM, Koudstaal J, Mooij JJ, Molenaar WM. The proliferative potential of the pilocytic astrocytoma: the relation between MIB-1 labeling and clinical and neuro-radiological follow-up. J Neurooncol. 1998;37:9–16. doi: 10.1023/a:1005905009449. [DOI] [PubMed] [Google Scholar]
- 23.Bowers DC, Gargan L, Kapur P, Reisch JS, Mulne AF, Shapiro KN, et al. Study of the MIB-1 labeling index as a predictor of tumor progression in pilocytic astrocytomas in children and adolescents. J Clin Oncol. 2003;21:2968–2973. doi: 10.1200/JCO.2003.01.017. [DOI] [PubMed] [Google Scholar]
- 24.Tibbetts KM, Emnett RJ, Gao F, Perry A, Gutmann DH, Leonard JR. Histopathologic predictors of pilocytic astrocytoma event-free survival. Acta Neuropathol. 2009;117:657–665. doi: 10.1007/s00401-009-0506-3. [DOI] [PubMed] [Google Scholar]
- 25.Ito S, Hoshino T, Shibuya M, Prados MD, Edwards MS, Davis RL. Proliferative characteristics of juvenile pilocytic astrocytomas determined by bromodeoxyuridine labeling. Neurosurgery. 1992;31:413–418. doi: 10.1227/00006123-199209000-00005. discussion 419. [DOI] [PubMed] [Google Scholar]
- 26.Haapasalo H, Sallinen S, Sallinen P, Helen P, Jaaskelainen J, Salmi TT, et al. Clinicopathological correlation of cell proliferation, apoptosis and p53 in cerebellar pilocytic astrocytomas. Neuropathol Appl Neurobiol. 1999;25:134–142. doi: 10.1046/j.1365-2990.1999.00157.x. [DOI] [PubMed] [Google Scholar]
- 27.Chen JK, Taipale J, Young KE, Maiti T, Beachy PA. Small molecule modulation of smoothened activity. Proc Natl Acad Sci USA. 2002;99:14071–14076. doi: 10.1073/pnas.182542899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hahn H, Wicking C, Zaphiropoulous PG, Gailani MR, Shanley S, Chidambaram A, et al. Mutations of the human homolog of Drosophila patched in the nevoid basal cell carcinoma syndrome. Cell. 1996;85:841–851. doi: 10.1016/s0092-8674(00)81268-4. [DOI] [PubMed] [Google Scholar]
- 29.Johnson RL, Rothman AL, Xie J, Goodrich LV, Bare JW, Bonifas JM, et al. Human homolog of patched, a candidate gene for the basal cell nevus syndrome. Science. 1996;272:1668–1671. doi: 10.1126/science.272.5268.1668. [DOI] [PubMed] [Google Scholar]
- 30.Pietsch T, Waha A, Koch A, Kraus J, Albrecht S, Tonn J, et al. Medulloblastomas of the desmoplastic variant carry mutations of the human homologue of Drosophila patched. Cancer Res. 1997;57:2085–2088. [PubMed] [Google Scholar]
- 31.Raffel C, Jenkins RB, Frederick L, Hebrink D, Alderete B, Fults DW, et al. Sporadic medulloblastomas contain PTCH mutations. Cancer Res. 1997;57:842–845. [PubMed] [Google Scholar]
- 32.Wolter M, Reifenberger J, Sommer C, Ruzicka T, Reifenberger G. Mutations in the human homologue of the Drosophila segment polarity gene patched (PTCH) in sporadic basal cell carcinomas of the skin and primitive neuroectodermal tumors of the central nervous system. Cancer Res. 1997;57:2581–2585. [PubMed] [Google Scholar]
- 33.Lam CW, Xie J, To KF, Ng HK, Lee KC, Yuen NW, et al. A frequent activated smoothened mutation in sporadic basal cell carcinomas. Oncogene. 1999;18:833–836. doi: 10.1038/sj.onc.1202360. [DOI] [PubMed] [Google Scholar]
- 34.Zurawel RH, Allen C, Chiappa S, Cato W, Biegel J, Cogen P, et al. Analysis of PTCH/SMO/SHH pathway genes in medulloblastoma. Genes Chromosomes Cancer. 2000;27:44–51. doi: 10.1002/(sici)1098-2264(200001)27:1<44::aid-gcc6>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 35.Taylor MD, Liu L, Raffel C, Hui CC, Mainprize TG, Zhang X, et al. Mutations in SUFU predispose to medulloblastoma. Nat Genet. 2002;31:306–310. doi: 10.1038/ng916. [DOI] [PubMed] [Google Scholar]
- 36.Scales SJ, de Sauvage FJ. Mechanisms of hedgehog pathway activation in cancer and implications for therapy. Trends Pharmacol Sci. 2009;30:303–312. doi: 10.1016/j.tips.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 37.Katayam M, Yoshida K, Ishimori H, Katayama M, Kawase T, Motoyama J, et al. Patched and smoothened mRNA expression in human astrocytic tumors inversely correlates with histological malignancy. J Neurooncol. 2002;59:107–115. doi: 10.1023/a:1019660421216. [DOI] [PubMed] [Google Scholar]
- 38.Bar EE, Chaudhry A, Lin A, Fan X, Schreck K, Matsui W, et al. Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like cancer cells in glioblastoma. Stem Cells. 2007;25:2524–2533. doi: 10.1634/stemcells.2007-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clement V, Sanchez P, de Tribolet N, Radovanovic I, Ruiz i Altaba A. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr Biol. 2007;17:165–172. doi: 10.1016/j.cub.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Svard J, Heby-Henricson K, Persson-Lek M, Rozell B, Lauth M, Bergstrom A, et al. Genetic elimination of Suppressor of fused reveals an essential repressor function in the mammalian hedgehog signaling pathway. Dev Cell. 2006;10:187–197. doi: 10.1016/j.devcel.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 41.Gajjar A, Sanford RA, Heideman R, Jenkins JJ, Walter A, Li Y, et al. Low-grade astrocytoma: a decade of experience at St. Jude Children's Research Hospital. J Clin Oncol. 1997;15:2792–2799. doi: 10.1200/JCO.1997.15.8.2792. [DOI] [PubMed] [Google Scholar]
- 42.Rivera-Luna R, Zapata-Tarres M, Medina-Sanson A, Lopez-Aguilar E, Niembro-Zuniga A, Amador Zarco J, et al. Long-term survival in children under 3 years of age with low-grade astrocytoma. Childs Nerv Syst. 2007;23:543–547. doi: 10.1007/s00381-006-0287-0. [DOI] [PubMed] [Google Scholar]
- 43.Desai KI, Nadkarni TD, Muzumdar DP, Goel A. Prognostic factors for cerebellar astrocytomas in children: a study of 102 cases. Pediatr Neurosurg. 2001;35:311–317. doi: 10.1159/000050443. [DOI] [PubMed] [Google Scholar]
- 44.Bar EE, Lin A, Tihan T, Burger PC, Eberhart CG. Frequent gains at chromosome 7q34 involving BRAF in pilocytic astrocytoma. J Neuropathol Exp Neurol. 2008;67:878–887. doi: 10.1097/NEN.0b013e3181845622. [DOI] [PubMed] [Google Scholar]
- 45.Jones DT, Kocialkowski S, Liu L, Pearson DM, Backlund LM, Ichimura K, et al. Tandem duplication producing a novel oncogenic BRAF fusion gene defines the majority of pilocytic astrocytomas. Cancer Res. 2008;68:8673–8677. doi: 10.1158/0008-5472.CAN-08-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pfister S, Janzarik WG, Remke M, Ernst A, Werft W, Becker N, et al. BRAF gene duplication constitutes a mechanism of MAPK pathway activation in low-grade astrocytomas. J Clin Invest. 2008;118:1739–1749. doi: 10.1172/JCI33656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jones DT, Kocialkowski S, Liu L, Pearson DM, Ichimura K, Collins VP. Oncogenic RAF1 rearrangement and a novel BRAF mutation as alternatives to KIAA1549:BRAF fusion in activating the MAPK pathway in pilocytic astrocytoma. Oncogene. 2009;28:2119–2123. doi: 10.1038/onc.2009.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Riobo NA, Haines GM, Emerson CP., Jr Protein kinase C-delta and mitogen-activated protein/extracellular signal-regulated kinase-1 control GLI activation in hedgehog signaling. Cancer Res. 2006;66:839–845. doi: 10.1158/0008-5472.CAN-05-2539. [DOI] [PubMed] [Google Scholar]
- 49.Schnidar H, Eberl M, Klingler S, Mangelberger D, Kasper M, Hauser-Kronberger C, et al. Epidermal growth factor receptor signaling synergizes with hedgehog/GLI in oncogenic transformation via activation of the MEK/ERK/JUN pathway. Cancer Res. 2009;69:1284–1292. doi: 10.1158/0008-5472.CAN-08-2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Seto M, Ohta M, Asaoka Y, Ikenoue T, Tada M, Miyabayashi K, et al. Regulation of the hedgehog signaling by the mitogen-activated protein kinase cascade in gastric cancer. Mol Carcinog. 2009;48:703–712. doi: 10.1002/mc.20516. [DOI] [PubMed] [Google Scholar]