Abstract
High levels of fatty acid synthase (FAS) expression have been reported in hormone receptor-positive tumors, including prostate, breast, and ovarian cancers, and its inhibition reduces tumor growth in vitro and in vivo. Similar to other hormone receptor-positive tumor types, meningiomas are progesterone receptor- and estrogen receptor-immunoreactive brain tumors. To define the role of FAS in human meningioma growth control, we first analyzed the FAS expression using a tissue microarray containing 38 meningiomas and showed increased FAS expression in 70% of atypical WHO grade II and anaplastic WHO grade III meningiomas compared with 10% of benign WHO grade I tumors. We next confirmed this finding by real-time PCR and Western blotting. Second, we demonstrated that treatment with the FAS inhibitor, cerulenin (Cer), significantly decreased meningioma cell survival in vitro. Third, we showed that Cer treatment reduced FAS expression by modulating Akt phosphorylation (activation). Fourth, we demonstrated that Cer treatment of mice bearing meningioma xenografts resulted in significantly reduced tumor volumes associated with increased meningioma cell death. Collectively, our data suggest that the increased FAS expression in human meningiomas represents a novel therapeutic target for the treatment of unresectable or malignant meningioma.
Keywords: fatty acid synthase, meningioma, therapy
Meningiomas are among the most frequent tumors of the brain and spinal cord, accounting for 15%–20% of all central nervous system tumors.1 While the vast majority of meningiomas are benign World Health Organization (WHO) grade I tumors, atypical WHO grade II and anaplastic WHO grade III meningiomas with aggressive clinical behavior and substantially reduced survival rates occur in about 15%–20% and 1%–2%, respectively.1 Treatment options for WHO grade II and III meningiomas are limited, and few biologically based targets exist for these tumors.
Previous genetic studies have identified several genes associated with meningioma pathogenesis, such as losses of heterozygosity at chromosome 22q, 1p, 14q, 6q, and 10.2,3 Additionally, atypical or anaplastic meningiomas harbor changes involving the CDKN2A, p14, and CDKN2B tumor suppressor genes.4 While these genes provide important insights into meningioma formation and progression, they have not resulted in the discovery of new targets for meningioma therapy.
Meningiomas express female hormone receptors, including progesterone receptors (PR) and estrogen receptors (ER).5 There is also a clear female predilection in meningiomas, and an association with breast cancer has been reported.6 Previous studies have shown that PR expression in meningioma is inversely related to the malignancy grade.7 This finding has suggested that hormonal therapy might have a role in the treatment of these tumors; however, several studies have revealed antiestrogen therapy not to be effective.
Recently, fatty acid synthase (FAS) inhibitors have been employed for the treatment of hormone receptor-positive tumors (reviewed in ref. 8). FAS is a multifunctional enzyme that catalyzes the synthesis of long-chain fatty acids from malonyl-CoA, an intermediate derived from the carboxylation of acetyl-CoA. In humans, FAS is highly expressed in hormone-sensitive cells and is regulated both by oestradiol and progesterone.9 FAS has been shown to be upregulated in many human tumors (reviewed in ref. 10). Recently, FAS has been proposed to be effective against glioblastoma cells, opening the possibility to treat intracranial tumors by FAS inhibitors.11
To examine the role of FAS in meningioma growth, we demonstrate for the first time that human meningiomas express high levels of FAS. We also show that FAS expression is regulated by Akt pathway activity. Moreover, we demonstrate that the natural FAS inhibitor, cerulenin (Cer), effectively reduces the meningioma growth by inducing apoptosis in vitro and in vivo. These data suggest that FAS inhibition may represent an additional option for the treatment of unresectable or aggressive meningioma.
Materials and Methods
Tumor Material
Paraffin-embedded biopsy samples from a total of 38 meningiomas of different histological subtypes and grades of malignancy (20 WHO grade I including 10 tumors of meningothelial, 4 of fibrous, and 6 of transitional subtype, respectively; 11 atypical meningiomas WHO grade II, 7 anaplastic meningiomas WHO grade III including one rhabdoid meningioma) were used for the generation of the tissue microarray (TMA). All meningiomas were graded according to the current 2007 WHO classification of brain tumors.1 Additional snap-frozen tumor material stored at −80°C was available from 11 cases for PCR and Western blot studies.
Immunohistochemistry
Immunohistochemical detection of FAS in paraffin-embedded tumor tissue was performed using standard procedures as described previously in detail.12 FAS immunostaining was performed using an anti-FAS antibody from BD Biosciences (1:50), as previously reported.13 Negative controls comprised omission of the primary antibody and its substitution with an irrelevant mouse monoclonal antibody (data not shown). In order to assess the intensity of immunoexpression, FAS expression was graded semiquantitatively as follows: −, no expression; +, weak patchy expression; ++, focal strong expression; +++, strong expression in more than 50% of tumor tissue. Expression of cleaved-caspase 3 using an antibody suitable for detection of apoptosis using paraffin-embedded tissue (Cell Signaling, 1:200), as well as apoptosis detection by TUNEL staining as described recently12 was evaluated by counting 10 randomly selected high-power fields (×400) in vital tumor tissue of both Cer and C75-treated xenografts.
Cell Culture and Inhibitors
The cell lines HBL52 (Cell lines service) and Ben-Men-1 (essentially characterized in14) were both derived from benign meningiomas WHO grade I. The meningioma cell lines F5 and SF3061 (both characterized in15) were derived from malignant meningiomas, the same as for IOMM-Lee16 and KT21MG1.17 Cells were maintained in Dulbecco's modified Eagle medium (PAA, Linz) with 4.5 g/l glucose supplemented with 10% fetal calf serum, l-glutamine (2 µM), and penicillin (50 IU/mL) at 37°C in 5% CO2. Wortmannin, PD98059, and Cer were obtained from Sigma, C75 was from Alexis Biochemicals, LY294002 was from Alomone Labs, and insulin was from Aventis. Cer (in ethanol) and C75 (in DMSO) were added to the cultures 24 hours before cell harvesting to give a final concentration of 1, 5, and 10 µg/mL, respectively. For insulin stimulation, cells were grown serum-starved 24 hours before treatment. Insulin was then added to a final concentration of 100, 200, 400, and 800 nM. For Wortmannin, PD98059, and LY294002 treatment, cells were first grown serum-starved for 24 hours, then insulin was added to a final concentration of 400 nM for an additional 24 hours. Inhibitors were then added to a final concentration of 10, 20, and 30 µM, respectively.
Real-Time PCR Measurement of mRNA Levels
Total RNA from frozen tissue and from cell pellets was extracted using the RNeasy Mini Kit (QIAGEN) according to the manufacturer's instructions and eluted in 30 µl RNase-free water. RNA concentration was determined at A260 and adjusted to 1 µg/10 µl for Reverse Transcription (Reverse Transcription System, Promega). FAS expression levels were analyzed by real-time PCR on the realplex light cycler system (Eppendorf) using the DNA Master SYBR Green I Kit (Roche Applied Science) in at least 3 independent experiments. The sequences of the primers were as follows: FAS(sense) 5′-TGGCTGCCTACTACATCGACT-3′; FAS (antisense) 5′-GTGCTCCATGTCCGTGAACT-3′; ESR1 (sense) 5′-CAGACACTTTGATCCACCTGA-3′; ESR1 (antisense) 5′-CTCCAGCAGCAGGTCATAGA-3′; PR (sense) 5′-TTGAGGCAAAAAGGAGTTGT-3′; PR (antisense) 5′-GAAGGGGTTTCACCATCC-3′. The Ct values were normalized to the house keeping gene beta-actin (sense 5′-ACCACCCCAGCCATGTACG-3′; antisense 5′-ATGTCACGCACGATTTCCC-3′). Cycling conditions were as follows: 10 minutes initializing denaturation, followed by 40 cycles of 95°C for 20 seconds, 55°C for 20 seconds (58°C for ESR1 and PR), and 72°C for 30 seconds. The specificity of the PCR products was controlled by melting-curve analysis.
Western Blot Analyses
Fresh samples from surgically removed meningiomas were snap-frozen in liquid nitrogen and stored at −80°C. The tissue was homogenized in 300 µl lysis buffer (20 mM Tris, 150 mM NaCl, 1 mM EGTA, 1 mM EDTA, 2.5 mM sodium-pyrophosphate, 1% Triton-X100, 1 mM sodium-orthovanadate, 1 mM PMSF, 1 µg/mL leupeptin, and 10 µg/mL aprotinin), incubated on ice for 30 minutes and centrifuged (4°C, 14000 rpm) for 15 minutes. The supernatant was collected and total protein content measured at 595 nm using the Bradford protein assay (BioRad). Cells were harvested and resuspended in 50 µl lysis buffer (see above). For protein level detection, 20 µg protein per sample were loaded on a 8% SDS polyacrylamide gel for electrophoresis. Proteins were transferred to a nitrocellulose membrane at 500 mA for 1 hour. Western blot analysis was done after blocking the membrane with 5% low fat milk in TBS + 0.1% Tween20 for 1 hour. The membrane was incubated with the specific antibody at 4°C overnight. The following antibodies were used: monoclonal antibody against human FAS (BD Biosciences), polyclonal antibodies against caspase-3, PARP, Bax, and total-Akt (Cell Signaling Technology), and polyclonal antibodies against Bcl-2, Bcl-xL, and phospho-Akt (Santa Cruz Biotechnology). Secondary antibody detection was done using HRP-linked antimouse and antirabbit IgG, respectively (GE Healthcare). After a 1 hour incubation, HRP activity was visualized by applying the enhanced chemiluminescent substrate (GE Healthcare) followed by exposuring the membrane to the LAS 3000 Imager (Fuji, Stamford). Equal protein loading was confirmed by reprobing the membranes with antiactin antibody (Sigma) following membrane stripping using Restore Stripping Buffer (Pierce).
Assessment of Serum FAS Levels by ELISA
FAS serum levels of 24 patients suffering from intracranial meningiomas, malignant gliomas, or lumbar spine disc prolaps were determined by direct ELISA. Diluted serum was applied on 96-well microtiter plates in triplicate and incubated overnight at 4°C. After a washing step, the wells were blocked with 5% skim milk in PBST for 1 hour and monoclonal antihuman FAS antibody was applied for 2 hours at room temperature. Secondary antibody incubation was done using an alkaline phosphatase-linked antibody and signals were detected by adding PNPP substrate (BioRad) for color development and measuring the absorbance at 450 nm after 24 hours using a KC4 Lambda ELISA reader (MWG Biotech). For controls, every third well per serum was incubated without primary antibody and the absorbance was subtracted as background.
Nuclear Fragmentation Assay
To visualize the nuclei of Cer- and C75-treated cells, we used the fluorescence dye Hoechst 33258 (Sigma), which specifically binds to chromatin, as previously reported.18 In brief, after incubation with 10 µg/mL Cer and C75 or ethanol and DMSO alone for 24 hours, cells were fixed with 4% PFA for 20 minutes. Hoechst 33258 was added in a final concentration of 2 µg/mL (in PBS) and incubated for a further 20 minutes. Cells were examined by fluorescence microscopy using an Axiovert 25 (Zeiss) at 20× and 40× magnification and an excitation and emission of 352 nm and 461 nm, respectively. All experiments were done in triplicate.
Viability Assays
Two assays (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrasodium bromide (MTT) and BrdU incorporation assays) were performed. First, the MTT assay was performed following the plating of 5 × 103 Ben-Men-1 or IOMM-Lee cells per well in 96-well plates. Following overnight culture, Cer and C75 were added to a final concentration of 1.5 and 10 µg/ml, respectively. Ethanol and DMSO controls were run simultaneously. Each condition was run in triplicate and in 3 independent experiments. After 24 hours of incubation, MTT was added for a further 4 hours incubation step to allow its reduction to a blue formazane dye. The cells were then lysed by adding 100 µl color development solution (isopropanol with 0.04 N HCl) per well, and after 30 minutes incubation at 37°C, absorbance at 570 nm (reference wavelength 630 nm) was measured using a FluoroStar microplate reader (BMG).
Second, cell proliferation was also studied by BrdU incorporation. Ben-Men-1 and IOMM-Lee cells were cultured and treated as described above. After 24 hours, cells were labeled with the pyrimidine analogue BrdU for 6 hours according to the manufacturer's instructions (BrdU Cell Proliferation ELISA, Roche Applied Science). Cellular proliferation was determined by measuring luminescence using a LUMIstar galaxy microplate luminometer (BMG).
Animal Studies
Fifteen female SCID mice were purchased from Jackson Laboratories. The body weight of each mouse was monitored weakly. IOMM-Lee cells (1 × 10−6) in 100 µl matrigel (BD Biosciences) were injected subcutaneously as previously reported.16 After 2 weeks, mice were randomly divided into 3 treatment groups: Cer-treated (N = 5), C75-treated (N = 5), and control mice, respectively. Cer was injected daily over 7 days at a dosis of 80 mg/kg intraperitoneal (i.p.) in DMSO as described in ref. 19. C75 was applied at an initial dose of 40 mg/kg followed by a weekly dose of 30 mg/kg in RPMI medium as described in ref. 20. Control animals received the appropriate volume of DMSO or RPMI. After a total of 4 weeks of tumor growth including 2 weeks of treatment, surviving mice were euthanized, individual tumors were weighted and then partly formalin fixed for histological and immunohistochemical studies, or snap-frozen in liquid nitrogen for subsequent Western blot studies. Organs from all animals including brain were subjected to histological investigation following formalin fixation and paraffin embedding in order to determine the side effects of treatment, as well as the presence of tumor metastases. All animal studies were performed according to animal care guidelines of the University of Jena and the Land Thueringen.
Statistical Analyses
Differences between meningioma tumor groups and treated cells were evaluated using Mann–Whitney and student's t-test, respectively. Correlation of FAS, PR, and ER mRNA levels were studied by determining Spearman's correlation coefficient. A chi-square test was applied to evaluate the expression differences in the TMA. P < 0.05 was considered statistically significant. All statistical evaluations were performed with the SPSS 13.0 software package.
Results
FAS is Highly Expressed in Aggressive Human Meningiomas
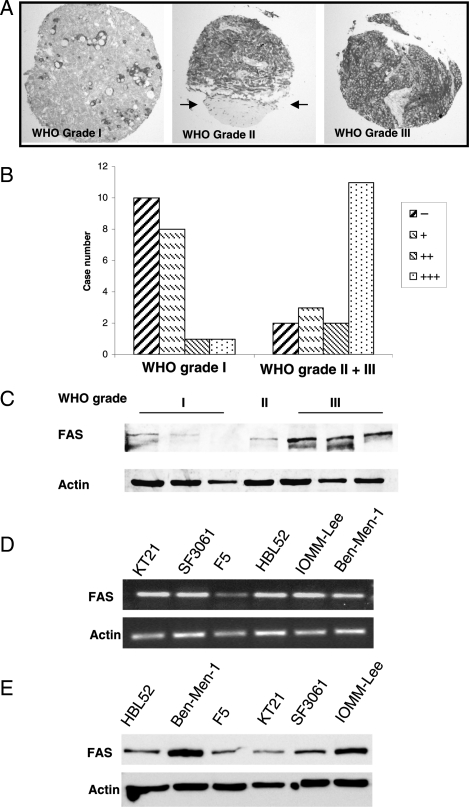
First, we determined the frequency of FAS immunoexpression using a TMA comprising a total of 38 meningiomas WHO grade I, II, and III, respectively. As shown in Fig. 1A (left), FAS expression was seen cytoplasmically in immunopositive tumors. Adjacent normal dural tissue did not show FAS immunopositivity (Fig. 1A, middle). While in benign tumors FAS expression was generally weak to moderate with a patchy distribution of immunostaining (Fig. 1A, left), strong and diffuse FAS expression was observed in aggressive meningiomas (Fig. 1A, right). Moreover, semiquantitative assessment of FAS staining revealed a clearly increased frequency of FAS-immunopositive tumors among aggressive meningiomas of WHO grade II or III compared with benign WHO grade I tumors (Fig. 1B), demonstrating increased FAS expression in aggressive meningiomas (chi-square test: P < 0.01). This finding was confirmed by real-time PCR (Fig. S1) and by Western blotting (Fig. 1C).
Fig. 1.
Expression of fatty acid synthase (FAS) in human meningioma samples. (A) Immunodetection of FAS in biopsy samples using a tissue microarray (TMA) comprising 38 meningioma specimens of different grades of malignancy. Left: In benign meningiomas WHO grade I, FAS expression is focally concentrated around pseudopsammoma bodies. Middle: FAS immunoexpression is increased in an atypical grade II meningioma. The upper part shows the tumor tissue with strong FAS immunopositivity, while the adjacent normal meningeal tissue (arrows) does completely lack FAS expression. Right: Strong diffuse FAS expression in an anaplastic meningioma WHO grade III. (B) Semiquantitative analysis of FAS immunoexpression in the 38 TMA samples reveales a significantly increased number (Chi-square test: P < 0.01) of meningiomas with moderate (++) or strong (++ +) FAS positivity among aggressive WHO grade II and III meningiomas, while in 18 of 20 benign meningiomas, FAS immunoexpression is absent (−) or weak (+). (C) Western blot analyses of FAS protein expression show an increase of FAS amount in atypical (GII) and anaplastic (GIII) meningiomas compared to benign GI tumors. (D) Characterization of FAS expression and function in different benign and malignant meningioma cell lines by RT-PCR and Western blot analysis (E), revealing strong FAS protein expression especially in Ben-Men-1 and IOMM-Lee cells.
FAS Expression in Meningiomas is Not Related to ER or PR Expression, or to FAS Serum Levels
In order to test whether FAS expression is associated with tumor levels of estrogen receptor (ER) or PR levels, mRNA levels of ER and PR from a total of 13 meningiomas were correlated with FAS mRNA levels. However, no association between FAS and ER (Pearson's r = −0.02) or PR (r = −0.34) levels was observed. In addition, immunohistochemical staining of TMA slides with antibodies against PR and ER did not reveal any association with FAS expression (not shown). Among the group of 9 patients who underwent neurosurgical meningioma resection, we measured the FAS serum levels preoperatively and compared the values to preoperative serum values from 5 patients with malignant gliomas and 10 patients undergoing lumbar discectomy. We found no significant differences between the groups, with the highest values in the glioma group (0.40 ± 0.15), followed by the discectomy (0.33 ± 0.04) and the meningioma group (0.26 ± 0.06).
FAS Inhibition by Cer and C75 in Meningioma Cells
First, we analyzed the FAS expression in 6 different human meningioma lines, including 2 lines (HBL52 and Ben-Men-1) derived from benign WHO grade I tumors, which showed the presence of FAS mRNA (Fig. 1D) and protein (Fig. 1E) in all cell lines. However, the malignant meningioma cell lines F5 and KT21 had only low FAS contents, while the Ben-Men-1 cells had surprisingly high FAS levels. This might be caused by inter-tumoral heterogeneity or by cell line senescence, for instance. However, for further functional studies and for the intention to test FAS inhibitors, we selected Ben-Men-1 and IOMM-Lee cells showing the highest FAS protein expression.
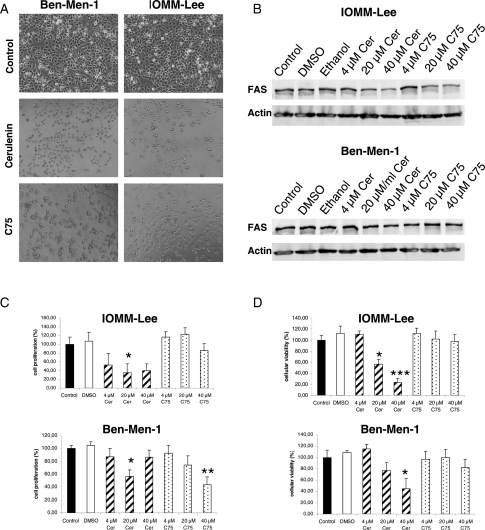
In order to explore a potential therapeutic use of FAS inhibitors for human meningioma, we next treated Ben-Men-1 and IOMM-Lee cells with 2 known FAS inhibitors, Cer and C75. As shown in Fig. 2A, both inhibitors had substantially reduced cell survival in IOMM-Lee cells, whereas Ben-Men-1 cells were less dramatically affected. This might be caused by the inherent biology of Ben-Men-1 cells derived from a benign meningioma with low proliferation activity.14 Western blot analysis of FAS levels following C75 or Cer treatment confirmed that FAS protein expression was strongly reduced by Cer, whereas, it was less affected by C75 treatment (Fig. 2B, upper panel). Similarly, FAS levels in Ben-Men-1 cells were less strongly reduced by Cer treatment, but no clear response was seen following C75 treatment (Fig. 2B, lower panel).
Fig. 2.
FAS inhibition by Cer and C75 in human meningioma cells. (A) Representative pictures of Ben-Men-1 (left panel) and IOMM-Lee (right panel) cells following treatment with 40 µM Cer (middle figures) or 40 µM C75 (lower figures) compared with untreated cells. It is clearly seen that the density of surviving cells is reduced in cells treated with both inhibitors, while effects appear to be stronger in IOMM-Lee cells and by Cer compared with C75 treatment, respectively. (B) FAS protein levels in IOMM-Lee cells are significantly reduced by Cer and C75 treatment (upper figure), while Ben-Men-1 cells do not show reduced FAS levels following treatment with both inhibitors (lower figure). FAS levels of both cell lines are not affected by the solvent DMSO or ethanol. (C) Effect of Cer and C75 on meningioma cell proliferation. While in Ben-Men-1 cells only moderate effects are seen following treatment with C75 or Cer (lower figure), Cer but not C75 does significantly reduce cell proliferation in malignant IOMM-Lee cells (upper figure)(*P < 0.05; **P < 0.01). (D) Survival of meningioma cells is significantly reduced by Cer treatment in both IOMM-Lee (upper figure) and Ben-Men-1 (lower figure) cells, while C75 is not effective (*P < 0.05; ***P < 0.001). Data are derived from 3 independent experiments.
Next, we demonstrated that IOMM-Lee cell proliferation was significantly reduced following Cer treatment (Fig. 2C, upper panel), while the same treatment had only limited effects in Ben-Men-1 cells (Fig. 2C, lower panel). In addition, compared with Cer treatment, C75 was clearly less effective at reducing meningioma cell growth. Comparable data were obtained using MTT-based assays, strengthening the finding that Cer treatment is more effective than C75 treatment in meningioma cells, and that IOMM-lee cells are more responsive to FAS inhibition (Fig. 2D, upper panel) than Ben-Men-1 cells (Fig. 2D, lower panel).
FAS Expression is Regulated by Akt Signaling
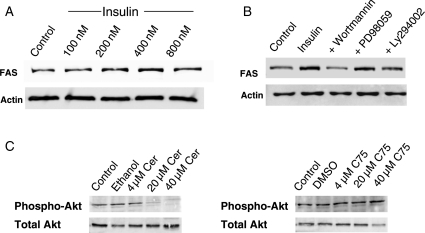
Since human meningiomas have been shown to proliferate in response to insulin stimulation, we next studied the effect of insulin treatment on FAS expression in meningioma. Stimulation with insulin resulted in an induction of FAS expression (Ben-Men-1 data presented in Fig. 3A; comparable results were achieved with IOMM-Lee cells). Previous studies have shown that insulin can lead to increased PI3 kinase (PI3-K) pathway activation.21,22 To determine whether FAS levels are regulated by PI3-K signaling, we employed 2 known inhibitors of PI3-K, wortmannin and LY294002, and showed that these inhibitors reduced the FAS expression induced by insulin in both Ben-Men-1 and IOMM-Lee cells (IOMM-Lee data presented in Fig. 3B). No effect of Erk1/2 inhibition (PD98059) on FAS expression was observed.
Fig. 3.
FAS expression can be induced in serum-starved IOMM-Lee cells by insulin stimulation (A). Inhibition of PI3K signaling by wortmannin or Ly294002 effectively downregulates the FAS levels in serum-starved IOMM-Lee cells stimulated with insulin, while inhibition of MAPK/ERK signaling by PD98059 has no effect on FAS protein expression (B). Comparable data were seen in Ben-Men-1 cells (not shown). (C) Effects of the FAS inhibitors Cer and C75 on PI3 kinase/Akt phosphorylation levels. While IOMM-Lee cells show cleary reduced phospho-Akt levels following Cer treatment (left figures), C75 is not effective (right figures). Comparable data were achieved for Ben-Men-1 cells (not shown).
Based on studies demonstrating that Cer inhibits PI3-K signaling in various cell types, we next sought to determine whether the Cer-induced reduction in meningioma cell growth reflected PI3-K-mediated inhibition of FAS expression. As shown in Fig. 3C, Cer treatment resulted in a decrease of phospho-Akt protein levels, whereas the less effective C75 inhibitor did not substantially affect phospho-Akt (activated Akt) levels. These data suggest that FAS is regulated by PI3-K signaling in meningioma cells.
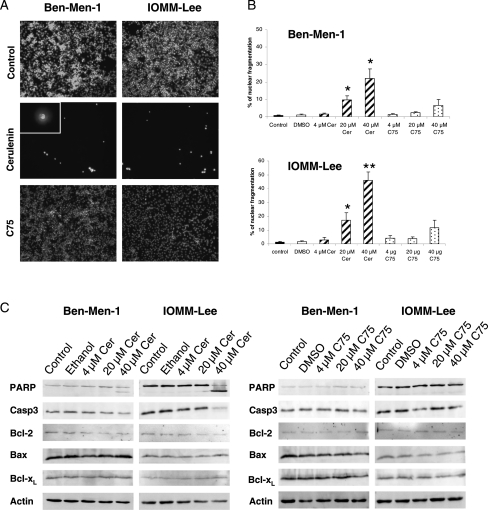
Cer but Not C75 Induces Apoptosis in Meningioma Cells
To further delineate the cellular effects of FAS inhibition in meningioma cells, we examined apoptosis following the administration of FAS inhibitors. As shown in Fig. 4, assessment of nuclear fragmentation using HOECHST 33258 staining as a marker for apoptotic cell death revealed significantly increased nuclear fragmentation after Cer treatment in Ben-Men-1 and IOMM-Lee cells. Western blot studies following Cer treatment resulted in apoptosis as demonstrated by reduced uncleaved PARP (116 kDa), the presence of PARP cleavage products (89 kDa), and a reduction in the expression of the antiapoptotic bcl-2 protein in Ben-Men-1 and IOMM-Lee cells (Fig. 4C). While we did not detect caspase-3 cleavage products (17 kDa), the reduction of uncleaved caspase-3 and Bcl-2 following Cer treatment indicates an induction of tumor cell apoptosis. In contrast, levels of Bcl-xL and Bax appeared to be unchanged.
Fig. 4.
FAS inhibitors induce apoptotic cell death in meningioma cells. (A) Representative nuclear fragmentation pictures of Ben-Men-1 and IOMM-Lee cells following treatment with 40 Cer or 40 µM C75 compared with untreated cells. A strong reduction in number of nuclei and a broad nuclear fragmentation is seen in case of Cer-treated (middle figures) cells. The inset shows magnification of nuclear fragmentation. (B) Quantification of nuclear fragmentation using HOECHST 33258 staining following application of FAS inhibitors shows a significant increase of apoptotic nuclear figures especially in malignant IOMM-Lee cells (lower figure; *P < 0.05; **P < 0.01) and less pronounced in Ben-Men-1 cells (upper figure; *P < 0.05) following Cer treatment. In contrast, C75 causes only minor increases in the rate of meningioma cell nuclei fragmentation. Data are derived from 3 independent experiments. (C) Levels of the apoptosis-related proteins PARP, caspase-3, and Bcl-2 are dose dependently reduced by Cer in IOMM-Lee cells but not in Ben-Men-1 cells (left figures), while Bcl-xL and Bax levels are not affected. In contrast, C75 does not change the protein levels of apoptosis-related proteins (right figures).
Cer Treatment Reduces Meningioma Tumor Growth In Vivo
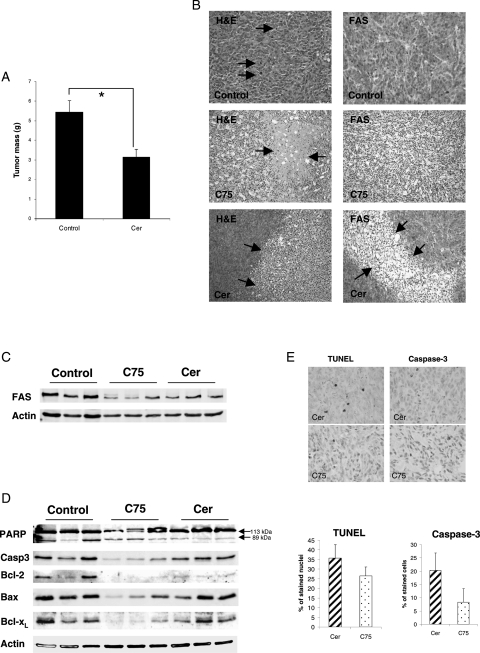
In order to determine the potential in vivo efficacy of Cer and C75 for meningioma treatment, we injected IOMM-Lee cells subcutaneously into SCID mice.16 Two weeks after tumor cell inoculation, palpable tumor masses had developed, and C75 or Cer treatment was started by intraperitoneal administration.19,20 Control animals received either DMSO or RPMI. In 1 animal from the C75 and Cer group, respectively, tumor formation did not occur. After a total of 4 weeks of tumor growth including 2 weeks treatment, control animals were euthanized. While all Cer-treated animals survived this 4-week period, all C75-treated animals died within the first week after onset of treatment, possibly due to the toxic side effects of the substance. Tumor preparations revealed significantly less tumor mass formation in Cer-treated animals compared with controls (Fig. 5A). Histological analyses of the tumors showed widespread necrosis especially in Cer-treated tumors, while control tumors mostly showed a solid tumor growth with abundant mitotic activity (Fig. 5B). TUNEL staining revealed no specific detection of apoptotic nuclei within these regions, while non-necrotic surrounding tumor tissue showed clear evidence of apoptosis by TUNEL-positive nuclei (Supplementary Material, Fig. S2). Immunohistochemical analyses of FAS expression in the tumors revealed that FAS levels were reduced in Cer- and C75-treated animals, but the reduction was more pronounced in C75-treated animals (Fig. 5B, right column). This was confirmed by Western blot analysis (Fig. 5C). Detection of apoptosis-related proteins in the tumor xenografts showed apoptosis induction in the Cer- and C75-treated animals compared with the control group, as indicated by reduced levels of uncleaved caspase-3, Bcl-2, and Bcl-xL (Fig. 5D). Quantification of TUNEL-positive nuclei and cells showing cytoplasmic and perinuclear active caspase-3 staining revealed higher percentages in paraffin sections from tumor xenografts treated with Cer than tumors treated with C75, respectively (Fig. 5E).
Fig. 5.
Inhibition of FAS affects meningioma tumor growth in vivo. (A) After a 4-week treatment period, Cer-treated animals show significantly lower tumor mass compared with control animals (*P < 0.05). (B) Histological (left panel) and immunohistochemical (right panel) analyses of mouse xenografts show reduced cell density and large necroses in Cer- or C75-treated animals, respectively. Control tumors show a high rate of proliferation as indicated by frequent mitoses (arrows). Immunohistochemistry shows that FAS protein levels are reduced especially in C75-treated animals. (C) Both Cer and C75 treatment reduce intratumoral FAS levels as determined by Western blotting. (D) Determination of apoptosis-related factors in tumors from treated animals and controls. C75-treated animals show clear apoptosis induction by changed protein levels of PARP, caspase-3, Bcl-2, Bax, and Bcl-xL. Cer-treated animals show apoptosis induction to a lesser extent compared with controls. (E) Quantification of apoptosis in Cer- and C75-treated xenografts by TUNEL assays and staining of active caspase-3. Representative immunostainings are shown in the upper figure. Lower figures show the percentage of stained tumor cells in both groups (differences are not statistically significant).
Discussion
Previous reports have shown that inhibition of FAS has substantial effects on tumor growth in various types of cancers, especially tumors known to express male and female hormone receptors. Here we demonstrate that FAS expression is increased in human meningiomas, and that FAS inhibition reduces cell growth and induces apoptosis in human meningioma cells in vitro and in vivo. These data suggest that FAS inhibitors might be a treatment option for unresectable or recurrent aggressive meningiomas.
Recently there has been renewed interest in the role of lipid metabolism in human cancer. While FAS activity is very low in normal cells (liver and adipose tissue), it is well known that increased de novo fatty acid synthesis by FAS is one of the major metabolic hallmarks of cancer cells. In addition, fatty acids generated by FAS in normal tissue are derived from excessive carbohydrates and are converted to triglycerides for energy storage. In tumor cells, however, the newly synthesized fatty acids are predominantly needed for the generation of phospholipids.23 Therefore, inhibition of this tumor-specific fatty acid synthesis may provide a reasonable approach to target human cancer. In this regard, inhibitors of FAS have been shown to significantly reduce tumor growth in human breast, colon, and prostate cancer cells.9,19,20,24
AntiTumor Effects of Cer in Meningiomas
Cerulenin is a natural antibiotic product of the fungus Cephalosporium ceruleans. Cer binds covalently to FAS in the β-ketoacyl-synthase domain,25 resulting in irreversible FAS inhibition. Intraperitoneal Cer treatment of nude mice with xenografts of ovarian cancer cells,19 with the same dosing regimen used in the present study, resulted in increased animal survival. Our studies showed that Cer has both antiproliferative and cytotoxic effects on meningioma cells, as previously reported in other cell types.24,26–29 We also found an induction of apoptosis by Cer administration, as evidenced by PARP cleavage and depletion of Bcl-xL.28 In our xenograft studies, we also observed an antitumor effect of Cer treatment. Western blotting analyses in cell culture systems and xenograft tumors revealed a reduction of the antiapoptotic protein Bcl2, together with PARP cleavage and reduced caspase-3.
AntiTumor Effects of C75 in Meningiomas
The second FAS inhibitor we tested was the synthetic drug C75. C75 is a more stable, slow-binding inhibitor of FAS, which shows higher in vitro growth inhibition in breast cancer cells compared with Cer.30 C75 has been used in subsequent studies including xenograft studies, demonstrating antitumor effects without systemic toxicity except reversible body weight loss.20,31,32 Alli et al.31 also demonstrated that mammary cancer in neu-N transgenic mice treated with C75 had reduced intratumoral FAS levels. We also observed that C75 is highly effective in downregulating FAS expression in meningioma xenografts by both Western blotting and immunohistochemistry. While other studies have revealed that C75 can reduce FAS expression in a mammary cancer model,31 the presence of lung metastasis in C75-, but not in Cer-treated animals suggests that Cer is more effective than C75 in meningioma tumors. This is also supported by the significant antiproliferative effects of Cer-treated compared with C75-treated meningioma cells.
FAS has been shown to be highly active during embryogenesis and in fetal lungs, being essential for the production of lung surfactant.33 In adult tissues, FAS expression is regulated by progesterone and estradiol.34 While meningiomas express both PR and ER, we did not find a correlation between PR/ER expression and FAS levels. However, while clinical trials using antigestational agents have been disappointing so far, combined therapy with FAS inhibitors might offer additional options, especially after dissection of PR-negative/positive tumors. Moreover, a detailed correlation analysis of FAS levels with PR expression and NF2 deletion status could help to distinguish tumors sensitive to combined FAS/PR inhibition, because PR status has been shown to represent a clinical marker for genetic subgroups of meningioma especially regarding genes at the NF2 gene locus.35 A loss of PR expression has been related to increased apoptosis and early recurrence.36
Intratumoral FAS levels are strongly dependent on PI3K/Akt signaling. Sterol regulatory element binding protein-1C (SREB1C), which is the major regulator for FAS expression in normal and tumor cells, is downregulated by both PI3K/Akt and MAPK signaling.37 In ovarian carcinoma cells, a positive feedback between AKT activation and FAS expression has been reported.32 Furthermore, there is a positive relationship between FAS and pAkt expression in prostate tumor samples.38 FAS inhibition by C75 or Cer leads to downregulation of phospho-Akt in SKOV3 cells,32 which was also observed in both meningioma cells tested. Insulin-dependent activation of the PI3K/Akt pathway is associated with tumorigenesis and apoptosis resistance. In neuroblastoma, IGF-1 mediated Akt activation correlates with poor prognosis and apoptosis resistance.39 Together with the previous findings that aggressive meningiomas are characterized by activation of the PI3K/Akt pathway,12 our findings of increased FAS levels in aggressive meningiomas mediated by PI3K signaling expand the knowledge about deregulated lipid metabolism in meningiomas and its role for meningioma cell proliferation and apoptosis. Moreover, the substantial antitumor activity of FAS inhibitors suggests a new treatment option in unresectable and malignant meningiomas.
Supplementary Material
Supplementary Material is available at Neuro-Oncology online.
Funding
The study was supported in part by a grant from the Deutsche Forschungsgemeinschaft (SFB604 A1) to C.M.
Acknowledgements
We highly appreciate the technical assistance of C. Doering, C. Ranke, I. Meyer, and D. Weber for histological and immunohistochemical analyses. We also thank K. Schorr for help with the animal studies.
Conflict of interest statement. None declared.
References
- 1.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114:97–109. doi: 10.1007/s00401-007-0243-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gutmann DH, Giordano MJ, Fishback AS, Guha A. Loss of merlin expression in sporadic meningiomas, ependymomas and schwannomas. Neurology. 1997;49:267–270. doi: 10.1212/wnl.49.1.267. [DOI] [PubMed] [Google Scholar]
- 3.Weber RG, Bostrom J, Wolter M, et al. Analysis of genomic alterations in benign, atypical, and anaplastic meningiomas: toward a genetic model of meningioma progression. Proc Natl Acad Sci USA. 1997;94:14719–14724. doi: 10.1073/pnas.94.26.14719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bostrom J, Meyer-Puttlitz B, Wolter M, et al. Alterations of the tumor suppressor genes CDKN2A (p16(INK4a)), p14(ARF), CDKN2B (p15(INK4b)), and CDKN2C (p18(INK4c)) in atypical and anaplastic meningiomas. Am J Pathol. 2001;159:661–669. doi: 10.1016/S0002-9440(10)61737-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perry A, Gutmann DH, Reifenberger G. Molecular pathogenesis of meningiomas. J Neurooncol. 2004;70:183–202. doi: 10.1007/s11060-004-2749-0. [DOI] [PubMed] [Google Scholar]
- 6.Custer BS, Koepsell TD, Mueller BA. The association between breast carcinoma and meningioma in women. Cancer. 2002;94:1626–1635. doi: 10.1002/cncr.10410. [DOI] [PubMed] [Google Scholar]
- 7.Perry A, Cai DX, Scheithauer BW, et al. Merlin, DAL-1, and progesterone receptor expression in clinicopathologic subsets of meningioma: a correlative immunohistochemical study of 175 cases. J Neuropathol Exp Neurol. 2000;59:872–879. doi: 10.1093/jnen/59.10.872. [DOI] [PubMed] [Google Scholar]
- 8.Kuhajda FP. Fatty acid synthase and cancer: new application of an old pathway. Cancer Res. 2006;66:5977–5980. doi: 10.1158/0008-5472.CAN-05-4673. [DOI] [PubMed] [Google Scholar]
- 9.Pizer ES, Kurman RJ, Pasternack GR, Kuhajda FP. Expression of fatty acid synthase is closely linked to proliferation and stromal decidualization in cycling endometrium. Int J Gynecol Pathol. 1997;16:45–51. doi: 10.1097/00004347-199701000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7:763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]
- 11.Zhao W, Kridel S, Thorburn A, et al. Fatty acid synthase: a novel target for antiglioma therapy. Br J Cancer. 2006;95:869–878. doi: 10.1038/sj.bjc.6603350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mawrin C, Sasse T, Kirches E, et al. Different activation of mitogen-activated protein kinase and Akt signaling is associated with aggressive phenotype of human meningiomas. Clin Cancer Res. 2005;11:4074–4082. doi: 10.1158/1078-0432.CCR-04-2550. [DOI] [PubMed] [Google Scholar]
- 13.Evert M, Schneider-Stock R, Dombrowski F. Overexpression of fatty acid synthase in chemically and hormonally induced hepatocarcinogenesis of the rat. Lab Invest. 2005;85:99–108. doi: 10.1038/labinvest.3700206. [DOI] [PubMed] [Google Scholar]
- 14.Puttmann S, Senner V, Braune S, et al. Establishment of a benign meningioma cell line by hTERT-mediated immortalization. Lab Invest. 2005;85:1163–1171. doi: 10.1038/labinvest.3700307. [DOI] [PubMed] [Google Scholar]
- 15.Cuevas IC, Slocum AL, Jun P, et al. Meningioma transcript profiles reveal deregulated Notch signaling pathway. Cancer Res. 2005;65:5070–5075. doi: 10.1158/0008-5472.CAN-05-0240. [DOI] [PubMed] [Google Scholar]
- 16.Surace EI, Lusis E, Haipek CA, Gutmann DH. Functional significance of S6K overexpression in meningioma progression. Ann Neurol. 2004;56:295–298. doi: 10.1002/ana.20201. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka K, Sato C, Maeda Y, et al. Establishment of a human malignant meningioma cell line with amplified c-myc oncogene. Cancer. 1989;64:2243–2249. doi: 10.1002/1097-0142(19891201)64:11<2243::aid-cncr2820641110>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 18.Braeuninger S, Chamaon K, Kropf S, et al. Short incubation with 2-methoxyestradiol kills malignant glioma cells independent of death receptor 5 upregulation. Clin Neuropathol. 2005;24:175–183. [PubMed] [Google Scholar]
- 19.Pizer ES, Wood FD, Heine HS, et al. Inhibition of fatty acid synthesis delays disease progression in a xenograft model of ovarian cancer. Cancer Res. 1996;56:1189–1193. [PubMed] [Google Scholar]
- 20.Gabrielson EW, Pinn ML, Testa JR, Kuhajda FP. Increased fatty acid synthase is a therapeutic target in mesothelioma. Clin Cancer Res. 2001;7:153–157. [PubMed] [Google Scholar]
- 21.Powell DJ, Hajduch E, Kular G, Hundal HS. Ceramide disables 3-phosphoinositide binding to the pleckstrin homology domain of protein kinase B (PKB)/Akt by a PKCzeta-dependent mechanism. Mol Cell Biol. 2003;23:7794–7808. doi: 10.1128/MCB.23.21.7794-7808.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stratford S, Hoehn KL, Liu F, Summers SA. Regulation of insulin action by ceramide: dual mechanisms linking ceramide accumulation to the inhibition of Akt/protein kinase B. J Biol Chem. 2004;279:36608–36615. doi: 10.1074/jbc.M406499200. [DOI] [PubMed] [Google Scholar]
- 23.Kuhajda FP, Jenner K, Wood FD, et al. Fatty acid synthesis: a potential selective target for antineoplastic therapy. Proc Natl Acad Sci USA. 1994;91:6379–6383. doi: 10.1073/pnas.91.14.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Furuya Y, Akimoto S, Yasuda K, Ito H. Apoptosis of androgen-independent prostate cell line induced by inhibition of fatty acid synthesis. Anticancer Res. 1997;17:4589–4593. [PubMed] [Google Scholar]
- 25.Funabashi H, Kawaguchi A, Tomoda H, et al. Binding site of cerulenin in fatty acid synthetase. J Biochem. 1989;105:751–755. doi: 10.1093/oxfordjournals.jbchem.a122739. [DOI] [PubMed] [Google Scholar]
- 26.Li JN, Gorospe M, Chrest FJ, et al. Pharmacological inhibition of fatty acid synthase activity produces both cytostatic and cytotoxic effects modulated by p53. Cancer Res. 2001;61:1493–1499. [PubMed] [Google Scholar]
- 27.Zhou W, Simpson PJ, McFadden JM, et al. Fatty acid synthase inhibition triggers apoptosis during S phase in human cancer cells. Cancer Res. 2003;63:7330–7337. [PubMed] [Google Scholar]
- 28.Ho TS, Ho YP, Wong WY, et al. Fatty acid synthase inhibitors cerulenin and C75 retard growth and induce caspase-dependent apoptosis in human melanoma A-375 cells. Biomed Pharmacother. 2007;61:578–587. doi: 10.1016/j.biopha.2007.08.020. [DOI] [PubMed] [Google Scholar]
- 29.Menendez JA, Colomer R, Lupu R. Inhibition of tumor-associated fatty acid synthase activity enhances vinorelbine (Navelbine)-induced cytotoxicity and apoptotic cell death in human breast cancer cells. Oncol Rep. 2004;12:411–422. [PubMed] [Google Scholar]
- 30.Kuhajda FP, Pizer ES, Li JN, et al. Synthesis and antitumor activity of an inhibitor of fatty acid synthase. Proc Natl Acad Sci USA. 2000;97:3450–3454. doi: 10.1073/pnas.050582897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alli PM, Pinn ML, Jaffee EM, McFadden JM, Kuhajda FP. Fatty acid synthase inhibitors are chemopreventive for mammary cancer in neu-N transgenic mice. Oncogene. 2005;24:39–46. doi: 10.1038/sj.onc.1208174. [DOI] [PubMed] [Google Scholar]
- 32.Wang HQ, Altomare DA, Skele KL, et al. Positive feedback regulation between AKT activation and fatty acid synthase expression in ovarian carcinoma cells. Oncogene. 2005;24:3574–3582. doi: 10.1038/sj.onc.1208463. [DOI] [PubMed] [Google Scholar]
- 33.Wagle S, Bui A, Ballard PL, et al. Hormonal regulation and cellular localization of fatty acid synthase in human fetal lung. Am J Physiol. 1999;277:L381–L390. doi: 10.1152/ajplung.1999.277.2.L381. [DOI] [PubMed] [Google Scholar]
- 34.Kusakabe T, Maeda M, Hoshi N, et al. Fatty acid synthase is expressed mainly in adult hormone-sensitive cells or cells with high lipid metabolism and in proliferating fetal cells. J Histochem Cytochem. 2000;48:613–622. doi: 10.1177/002215540004800505. [DOI] [PubMed] [Google Scholar]
- 35.Claus EB, Park PJ, Carroll R, Chan J, Black PM. Specific genes expressed in association with progesterone receptors in meningioma. Cancer Res. 2008;68:314–322. doi: 10.1158/0008-5472.CAN-07-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Konstantinidou AE, Korkolopoulou P, Mahera H, et al. Hormone receptors in non-malignant meningiomas correlate with apoptosis, cell proliferation and recurrence-free survival. Histopathology. 2003;43:280–290. doi: 10.1046/j.1365-2559.2003.01712.x. [DOI] [PubMed] [Google Scholar]
- 37.Yang YA, Morin PJ, Han WF, et al. Regulation of fatty acid synthase expression in breast cancer by sterol regulatory element binding protein-1c. Exp Cell Res. 2003;282:132–137. doi: 10.1016/s0014-4827(02)00023-x. [DOI] [PubMed] [Google Scholar]
- 38.Van de ST, Roskams T, Lerut E, et al. High-level expression of fatty acid synthase in human prostate cancer tissues is linked to activation and nuclear localization of Akt/PKB. J Pathol. 2005;206:214–219. doi: 10.1002/path.1760. [DOI] [PubMed] [Google Scholar]
- 39.Opel D, Poremba C, Simon T, Debatin KM, Fulda S. Activation of Akt predicts poor outcome in neuroblastoma. Cancer Res. 2007;67:735–745. doi: 10.1158/0008-5472.CAN-06-2201. [DOI] [PubMed] [Google Scholar]