Abstract
Bevacizumab, a monoclonal antibody against vascular endothelial growth factor, has shown promise in treating recurrent adult high-grade glioma (HGG). However, there is very little data on recurrent or progressive pediatric HGG treated with bevacizumab. We report the results of a single institution experience using bevacizumab and irinotecan in children who relapsed or progressed following standard therapy. Twelve pediatric patients with recurrent or progressive HGG received bevacizumab at 10 mg/kg every 2 weeks with irinotecan at 125 mg/m2. Magnetic resonance imaging (MRI) was performed prior to therapy and every 8 weeks subsequently. Ten patients had supratentorial HGG; 2 had DIPG. Radiological responses were defined according to MacDonald's criteria. Progression-free survival (PFS), overall survival (OS), and toxicities were analyzed. Ten (83.3%) patients tolerated bevacizumab without serious toxicity. Therapy was discontinued in 1 patient because of anaphylaxis. Another patient developed grade III delayed wound healing and deep vein thrombosis. Two patients (16.7%) experienced a partial response after the first MRI. No complete radiographic responses were seen. Stable disease was noted in 4 (33.3%) patients. The median PFS and OS were 2.25 and 6.25 months, respectively. A diffuse invasive recurrence pattern was noted in 5 (45.5%) patients. Treatment tolerance, toxicity, and recurrence profiles were comparable to adult HGG patients treated with bevacizumab. However, the radiological response rate, response duration, and survival appeared inferior in pediatric patients. Genetic differences in pediatric gliomas might account for this difference.
Keywords: antiangiogenic therapy, bevacizumab, high-grade glioma
Pediatric high-grade gliomas (HGG) are relatively rare in the context of other pediatric primary CNS neoplasms, constituting 6%–10% of all brain tumors.1 These tumors in children may consist of astrocytomas with histological documentation of either a WHO III or IV pathology, either of pure astroglial or mixed glial neuronal lineage, and radiographically diagnosed diffuse intrinsic pontine gliomas (DIPGs). Current treatment of these tumors involves maximal surgical resection for cortical-based lesions whenever possible followed by involved-field radiation and chemotherapy, with most patients succumbing to their disease within 12–18 months.2 Salvage treatment with chemotherapy has yielded only poor results, although drugs like irinotecan have showed some promise, presumably because of their ability to cross the blood–brain barrier.
Recent research efforts have been aimed at devising new molecular-targeted therapies. Vascular endothelial growth factor (VEGF) has been identified as a potential target in HGG, since VEGF is an important stimulus for angiogenesis and possibly tumor invasion. Bevacizumab (Avastin®; Genentech) was one of the first angiogenesis inhibitors to be used in clinical settings. It has been approved by the Food and Drug Administration for use in treating metastatic colorectal,3 breast,4 nonsmall-cell lung,5 and renal cell6 cancer and was recently approved for use in treating recurrent adult glioblastoma multiforme (GBM). In 2 small Phase II trials in recurrent adult GBMs, the combination of bevacizumab and irinotecan resulted in a radiological response of 47%–67% and a 6-month survival of 62%–77%,7–8 which was greater than the predicted 10%–15% for prior salvage chemotherapy.9 This is in agreement with data from our own institution, where we have shown a radiological response of 74% and a median survival of 9 months.10
Although the use of bevacizumab is growing among patients with recurrent adult HGG, there is a paucity of published literature on the use of bevacizumab in the treatment of recurrent pediatric HGG. This report serves to throw light on the early experience of using bevacizumab in conjunction with concurrent irinotecan at our institution.
Materials and Methods
Institutional review board approval was obtained for a retrospective analysis that was conducted from clinical records for 12 consecutive patients who were diagnosed with recurrent pediatric HGG between September, 2005 and July, 2008 at New York University Langone Medical Center, New York, NY. There were 10 patients with supratentorial HGG and 2 with DIPG. Both DIPG patients were found to have HGG at the time of autopsy and were hence included in the analysis. All patients underwent maximal surgical resection of the tumor when feasible at the time of initial diagnosis followed by radiation therapy and chemotherapy. Involved-field radiation was administered at 1.8 Gy per fraction to a dose of 54–59.4 Gy using a conformal technique. Temozolomide was the initial chemotherapy of choice and was used in 11 patients both during and following radiotherapy.
Salvage treatment with bevacizumab was started after confirmation of tumor recurrence by radiological (n = 9) or histopathological diagnosis (n = 3). Bevacizumab was administered intravenously at 10 mg/kg concurrently with irinotecan at 125 mg/m2 every 2 weeks until disease progression or the onset of dose-limiting toxicity. Patients were assessed with regular clinical examinations and contrast-enhanced magnetic resonance imaging (MRI) was performed every 8 weeks. Dynamic susceptibility-weighted contrast-enhanced perfusion imaging was obtained in addition to conventional MRI studies after 2006 in 6 patients. Relative cerebral blood volume and vascular permeability measurements were obtained from the perfusion data. Toxicities were assessed according to the National Cancer Institute Common Toxicity Criteria (Version 3.0).
MacDonald criteria, which use maximal cross-sectional T1 contrast images on MRI as well as Fluid Attenuated Inversion Recovery (FLAIR) sequences, were used to define the radiological response. Progression was defined as a 25% or greater increase in the size of a pre-existing enhancing lesion, appearance of a new lesion, or neurological deterioration that cannot be attributed to another cause. Progression-free survival (PFS) was measured from the time of the initial bevacizumab treatment to the date of first radiological/clinical progression, and overall survival (OS) was measured from the time of bevacizumab therapy to the time of death. Event-time distributions were estimated using the Kaplan–Meier method. Survival analysis was performed using the SPSS statistical software package (Version 17; SPSS, Inc.).
Results
Patient Characteristics
The patient characteristics are shown in Table 1. The median age at the time of initial diagnosis was 14.75 years (range 4–22). Ten of these patients had tumors located in the supratentorial region. Anaplastic glioma (WHO grade III) accounted for a majority of tumors at the time of initial diagnosis. One patient was diagnosed with a radiation therapy–induced HGG. Two patients with progressive DIPG were diagnosed clinically without pathology. The median Karnofsky performance status for the entire patient population at the start of chemotherapy was 90 (range 70–100). The median number of recurrences that occurred prior to receiving bevacizumab was 1 (range 1–4). The median time to first recurrence from the time of initial diagnosis was 7.6 months (range 1–18). Two of the patients had re-resection of the tumor prior to bevacizumab therapy, whereas a third underwent stereotactic biopsy.
Table 1.
Patient characteristics
| Characteristic | n (%) |
|---|---|
| Age | |
| Median | 14.75 |
| Range | 4–22 |
| Sex | |
| Male | 7 (58.3) |
| Female | 5 (41.7) |
| WHO tumor grade | |
| Grade III | 9 (75) |
| Grade IV | 3 (25) |
| KPS score | |
| 100 | 2 |
| 90 | 4 |
| 80 | 5 |
| 70 | 1 |
| Prior chemotherapy | |
| Temozolomide | 11 |
| Carboplatin | 2 |
| Vincristine | 2 |
| Carmustine | 1 |
| Lomustine | 1 |
| Etopside | 1 |
| Cyclophosphamide | 1 |
| Motaxefin gadolinium | 1 |
| O6-benzylguanine | 1 |
| Repeated resection | |
| None | 9 |
| Gross total resection | 2 |
| Subtotal resection | 0 |
| Stereotactic biopsy | 1 |
| Time to recurrence from diagnosis | |
| Median | 7.625 |
| Range | 1–17.5 |
| Number of recurrences prebevacizumab | |
| Median | 1 |
| Range | 1–4 |
| Number of bevacizumab infusions | |
| Median | 5.5 |
| Range | 2–18 |
Abbreviation: KPS, Karnofsky performance status.
Treatment Compliance and Toxicities
The median follow-up was 5.5 months (range 2.5–18.5). The median number of bevacizumab infusions received was 5.5 (range 2–18). Two patients experienced grade III/IV toxicities (Table 2). One patient experienced an acute anaphylactic reaction to bevacizumab soon after the first treatment, after which therapy had to be discontinued. The second patient presented with poor wound healing and subsequently, deep vein thrombosis. The other 10 patients tolerated the treatment well with grade I/II toxicities that included nausea, vomiting, diarrhea, and constipation. No cases of symptomatic intra-cranial hemorrhage were noted.
Table 2.
Toxicities
| Grade II (n = 2) |
Grade III (n = 2) |
Grade IV (n = 1) |
|||
|---|---|---|---|---|---|
| Skin rash | 1 | Deep vein thrombosis | 1 | Anaphylaxis | 1 |
| Intra-tumoral hemorrhage | 1 | Delayed wound healing | 1 | ||
Radiological Response
Using MacDonald's criteria, no patients demonstrated a complete response to bevacizumab therapy (Table 3). Two patients (16.7%) showed a partial radiological response (Fig. 1). A 25% reduction in the relative cerebral blood volume and vascular permeability was observed in both the patients who showed a radiological response. Four patients (2 with stable disease and 2 with progression on conventional MRI) showed no changes in perfusion imaging.
Table 3.
Radiological responses
| Radiological response | n = 12 (%) |
|---|---|
| Complete response | 0 (0) |
| Improvement | 2 (16.7) |
| Stable | 4 (33.3) |
| Progression | 6 (50) |
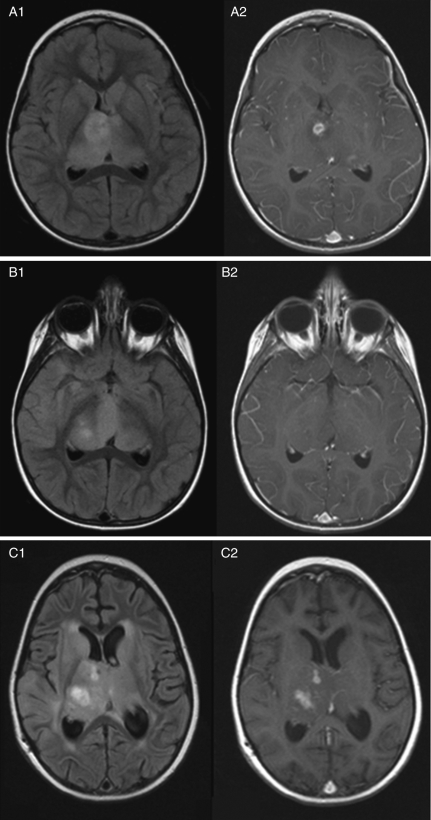
Fig. 1.
Four-year-old girl with recurrent anaplastic astrocytoma treated with bevacizumab and irinotecan. Image sets 1 and 2 represent T2/FLAIR and contrast-enhanced MRI sequences, respectively. Series A and B represent MRI images taken 2 weeks prior to treatment and 8 weeks after the start of treatment. Series C shows local tumor progression 5 months after the start of treatment.
Local Control and Survival
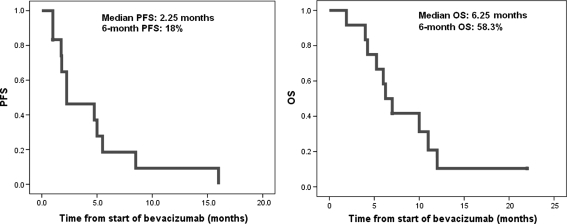
At the time of analysis, 11 patients have experienced relapse and 10 patients have succumbed to tumor progression. The median PFS and OS were 2.25 and 6.25 months, respectively (Fig. 2). The 6-month PFS and OS were 18% and 58.3%, respectively. Six (54.5%) of the patients had local recurrence, whereas 5 (45.5%) had diffuse patterns of recurrence. Three patients who failed as diffuse relapse also had radiological evidence of leptomeningeal disease. There were 2 long-time survivors in the study with a PFS of 8.5 and 16 months, respectively.
Fig. 2.
Progression-free survival (PFS) and overall survival (OS).
Discussion
There is a desperate need for new therapeutic options in both adults and children with recurrent HGG. The response rates with conventional chemotherapy are poor and the median survival following the documentation of clinical progression remains 4–6 months.11 In this context, the antiangiogenic approach represents the most promising treatment to date in the management of recurrent pediatric gliomas, given the success in the recurrent adult HGG.7–8,10 In a series of 61 patients with adult recurrent HGG treated with bevacizumab therapy, we had shown a radiological response of 74% (which included 13% complete responses) and a median survival of 9 months.10 A recent case report demonstrated that bevacizumab treatment in an adult patient diagnosed with a DIPG caused a dramatic clinical response, as well as significant reduction in contrast enhancement and FLAIR in the pons, making a compelling case for using bevacizumab in pediatric DIPG patients.12 The encouraging results from our own institution, as well as those of others on adult HGG, prompted us to incorporate the use of bevacizumab in recurrent pediatric HGG. However, the radiological response rate of 16.7% (with no complete responders) and a median PFS and OS of 2.5 and 5.5 months, respectively, in this series appear to be inferior compared with the adult HGG data from institutions including our own7,9,10 and no better than historical controls (Table 4). Tolerance to therapy and toxicities, though, appeared to be similar between the 2 groups.
Table 4.
Pediatric patients' survival compared with adult series
| Survival characteristics | Pediatric HGG (range) | Adult HGG10 | Adult HGG7 | Adult HGG9 |
|---|---|---|---|---|
| Median PFS (months) | 2.25 (1–16) | 5 | 5.75 | 5.6 |
| Median OS (months) | 6.25 (1.9–22) | 9 | 10 | 8.7 |
| Radiological response (%) | 16.7 | 73.6 | 63 | 37.8 |
Abbreviations: HGG, high-grade glioma; PFS, progression-free survival; OS, overall survival.
There is minimal published data on the use of bevacizumab therapy in pediatric gliomas. Packer et al.13 reported on the use of bevacizumab with irinotecan in multiply recurrent low-grade gliomas in 10 pediatric patients. Complete response was seen in 1 patient, and partial responses were noted in 3 patients. Two dose-limiting toxicities were seen that included transient leukoencephalopathy and proteinuria.
Recently, concerns have been raised about a possible change in the pattern of relapse in adult HGG patients treated with antiangiogenic therapy, as increased invasiveness has been noted in 30%–50% of these patients.14–15 It is not clear whether this phenomenon relates to a change in the growth pattern of an invasive tumor or better control of the primary tumor, permitting remote manifestations of the tumor to emerge. It is interesting to note that in our series, in spite of lack of radiological responses, the invasiveness of the tumors persisted, and almost half the patients had recurrence as diffuse disease with or without leptomeningeal disease. Similar patterns of distant noncontrast enhancing recurrences have been described in progressive pediatric HGG in another small study.16
A limitation of the present study is the small sample size and the retrospective nature of the study, which may bring the issue of possible selection bias. In fact, one can argue that these results can be considered speculative at best. However, recurrent pediatric HGG is a relatively rare disease compared with adults, and all patients seen during the interval between 2005 and 2008 at our institution were treated with this approach. The median number of recurrences prior to therapy, the median time to bevacizumab therapy from the time of initial diagnosis, and median bevacizumab infusions were similar to the recurrent adult HGG population from our own and other institutions.7–8,10 A study being conducted through the Pediatric Brain Tumor Consortium (PBTC) that investigated the use of bevacizumab and irinotecan in recurrent pediatric HGG found that among the 30 patients assessed, there were no sustained radiological responses and a median PFS of 9 weeks.17 Additionally, Parekh et al.16 at the Children's Hospital in Los Angeles observed only 1 complete response and 1 mixed response among 7 patients with pediatric HGG, with a median PFS of 10 weeks. Both of these studies are supportive of our results.
One may speculate that molecular differences, such as different escape mechanisms to anti-VEGF therapies, between HGG arising in adults and children may account for the observed variations in response and survival. One notable difference between pediatric and adult HGG is that particular chromosomal imbalances (+1q, +3q, +16q, −8q, and −17p) are more often seen in pediatric glioblastomas.18 Whereas epidermal growth factor receptor (EGFR) amplification occurs frequently in adult HGG, it is not generally present in pediatric HGG, although increased immunoreactivity for EGFR in pediatric HGG has been observed and suggests that another activation pathway apart from amplification may be responsible.19–20 This activation of EGFR can be, in part, the reason for the increase in the incidence of the invasive phenotype observed after therapy with bevacizumab. A recent study showed that VEGF-A expression in recurrent glioblastomas in young patients was less prevalent than in adults.21 Whereas gliomas displaying 1p19q loss of heterozygosity in adults correlates with a favorable outcome, this is not the case for children.22 It has also been shown that VEGF/VEGFR expression is not a predictor of response to antiangiogenic therapy.23 As a result, one should remain cautious about extrapolating results from adult to pediatric brain tumor populations in the use antiangiogenic therapy.
Conclusion
Tolerance to treatment and the toxicity profile of bevacizumab therapy in recurrent pediatric HGG were comparable to adult populations. However, the rate and duration of radiological responses as well as survival appeared inferior compared with adults. Increased invasiveness coupled with lack of radiological responses may reflect possible differences in the genetic make-up of pediatric gliomas and needs to be studied in future prospective trials.
Conflict of interest statement. None declared.
References
- 1.Halperin E, Constine L, Tarbell N, Kun L. Pediatric Radiation Oncology. 4th ed. Philadelphia: Lippincott Williams & Wilkins; 2004. [Google Scholar]
- 2.Mueller S, Chang S. Pediatric brain tumors: current treatment strategies and future therapeutic approaches. Neurotherapeutics. 2009;6(3):570–586. doi: 10.1016/j.nurt.2009.04.006. doi:10.1016/j.nurt.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–2342. doi: 10.1056/NEJMoa032691. doi:10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 4.Traina TA, Rugo HS, Dickler M. Bevacizumab for advanced breast cancer. Hematology/Oncology Clinics of North America. 2007;21(2):303–319. doi: 10.1016/j.hoc.2007.03.006. doi:10.1016/j.hoc.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Johnson DH, Fehrenbacher L, Novotny WF, et al. Randomized Phase II Trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2004;22(11):2184–2191. doi: 10.1200/JCO.2004.11.022. doi:10.1200/JCO.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 6.Escudier B, Cosaert J, Jethwa S. Targeted therapies in the management of renal cell carcinoma: role of bevacizumab. Biologics. 2008;2(3):517–530. doi: 10.2147/btt.s3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vredenburgh JJ, Desjardins A, Herndon JE, 2nd, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13(4):1253–1259. doi: 10.1158/1078-0432.CCR-06-2309. doi:10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 8.Chen W, Delaloye S, Silverman DHS, et al. Predicting treatment response of malignant gliomas to bevacizumab and irinotecan by imaging proliferation with [18F] fluorothymidine positron emission tomography: a Pilot Study. J Clin Oncol. 2007;25(30):4714–4721. doi: 10.1200/JCO.2006.10.5825. doi:10.1200/JCO.2006.10.5825. [DOI] [PubMed] [Google Scholar]
- 9.Friedman HS, Prados MD, Wen PY, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–4740. doi: 10.1200/JCO.2008.19.8721. doi:10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 10.Narayana A, Kelly P, Golfinos J, et al. Antiangiogenic therapy using bevacizumab in recurrent high-grade glioma: impact on local control and patient survival. J Neurosurg. 2009;110(1):173–180. doi: 10.3171/2008.4.17492. doi:10.3171/2008.4.17492. [DOI] [PubMed] [Google Scholar]
- 11.Gruber ML, Buster WP. Temozolomide in combination with irinotecan for treatment of recurrent malignant glioma. Am J Clin Oncol. 2004;27(1):33–38. doi: 10.1097/01.coc.0000045852.88461.80. doi:10.1097/01.coc.0000045852.88461.80. [DOI] [PubMed] [Google Scholar]
- 12.Torcuator R, Zuniga R, Loutfi R, Mikkelsen T. Bevacizumab and irinotecan treatment for progressive diffuse brainstem glioma: case report. J Neurooncol. 2009;93(3):409–412. doi: 10.1007/s11060-008-9782-3. doi:10.1007/s11060-008-9782-3. [DOI] [PubMed] [Google Scholar]
- 13.Packer RJ, Jakacki R, Horn M, et al. Objective response of multiply recurrent low-grade gliomas to bevacizumab and irinotecan. Pediatric Blood Cancer. 2009;52(7):791–795. doi: 10.1002/pbc.21935. doi:10.1002/pbc.21935. [DOI] [PubMed] [Google Scholar]
- 14.Norden AD, Young GS, Setayesh K, et al. Bevacizumab for recurrent malignant gliomas: Efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70(10):779–787. doi: 10.1212/01.wnl.0000304121.57857.38. doi:10.1212/01.wnl.0000304121.57857.38. [DOI] [PubMed] [Google Scholar]
- 15.Narayana A, Golfinos JG, Fischer I, et al. Feasibility of using bevacizumab with radiation therapy and temozolomide in newly diagnosed high-grade glioma. Int J Radiat Oncol Biol Phys. 2008;72(2):383–389. doi: 10.1016/j.ijrobp.2008.05.062. doi:10.1016/j.ijrobp.2008.05.062. [DOI] [PubMed] [Google Scholar]
- 16.Parekh C, Jubran R, Finlay JL, Dhall G. Treatment of children with recurrent or progressive high-grade gliomas with irinotecan, temozolomide, and bevacizumab [abstract]. In: Abstracts for the Thirteenth Annual Meeting of the Society for Neuro-Oncology. Neuro-Oncology. 2008;10(5):759–915. [Google Scholar]
- 17.Gururangan S, Chi S, Onar A, et al. Phase II study of bevacizumab plus irinotecan in children with recurrent malignant glioma and diffuse brainstem glioma—a Pediatric Brain Tumor Consortium study (PBTC-022) [abstract]. In: Abstracts for the Thirteenth Annual Meeting of the Society for Neuro-Oncology. Neurooncology. 2008;10(5):759–915. [Google Scholar]
- 18.Rickert CH, Strater R, Kaatsch P, et al. Pediatric High-grade astrocytomas show chromosomal imbalances distinct from adult cases. Am J Pathol. 2001;158(4):1525–1532. doi: 10.1016/S0002-9440(10)64103-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pfister S, Hartmann C, Korshunov A. Histology and molecular pathology of pediatric brain tumors. J Child Neurol. 2009;24(11):1375–1386. doi: 10.1177/0883073809339213. doi:10.1177/0883073809339213. [DOI] [PubMed] [Google Scholar]
- 20.Sung T, Miller DC, Hayes RL, Alonso M, Yee H, Newcomb EW. Preferential inactivation of the p53 tumor suppressor pathway and lack of EGFR amplification distinguish de novo high grade pediatric astrocytomas from de novo adult astrocytomas. Brain Pathol. 2000;10(2):249–259. doi: 10.1111/j.1750-3639.2000.tb00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nghiemphu PL, Liu W, Lee Y, et al. Bevacizumab and chemotherapy for recurrent glioblastoma: A single-institution experience. Neurology. 2009;72(14):1217–1222. doi: 10.1212/01.wnl.0000345668.03039.90. doi:10.1212/01.wnl.0000345668.03039.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pollack IF, Finkelstein SD, Burnham J, et al. Association between chromosome 1p and 19q loss and outcome in pediatric malignant gliomas: results from the CCG-945 cohort. Pediatr Neurosurg. 2003;39(3):114–121. doi: 10.1159/000071647. doi:10.1159/000071647. [DOI] [PubMed] [Google Scholar]
- 23.Bartels U, Hawkins C, Ma J, et al. Vascularity and angiogenesis as predictors of growth in optic pathway/hypothalamic gliomas. J Neurosurg: Pediatrics. 2006;104(5):314–320. doi: 10.3171/ped.2006.104.5.314. doi:10.3171/ped.2006.104.5.314. [DOI] [PubMed] [Google Scholar]