Abstract
Antiepileptic drugs (AEDs) are frequently used to treat seizures in glioma patients. AEDs may have an unrecognized impact in modulating O6-methylguanine-DNA methyltransferase (MGMT), a DNA repair protein that has an important role in tumor cell resistance to alkylating agents. We report that levetiracetam (LEV) is the most potent MGMT inhibitor among several AEDs with diverse MGMT regulatory actions. In vitro, when used at concentrations within the human therapeutic range for seizure prophylaxis, LEV decreases MGMT protein and mRNA expression levels. Chromatin immunoprecipitation analysis reveals that LEV enhances p53 binding on the MGMT promoter by recruiting the mSin3A/histone deacetylase 1 (HDAC1) corepressor complex. However, LEV does not exert any MGMT inhibitory activity when the expression of either p53, mSin3A, or HDAC1 is abrogated. LEV inhibits malignant glioma cell proliferation and increases glioma cell sensitivity to the monofunctional alkylating agent temozolomide. In 4 newly diagnosed patients who had 2 craniotomies 7–14 days apart, prior to the initiation of any tumor-specific treatment, samples obtained before and after LEV treatment showed the inhibition of MGMT expression. Our results suggest that the choice of AED in patients with malignant gliomas may have an unrecognized impact in clinical practice and research trial design.
Keywords: gliomas, levetiracetam, MGMT, mSin3A/HDAC1, p53, temozolomide
Monofunctional methylating agents, like temozolomide (TMZ), and DNA cross-linking chlorethylating agents, such as carmustine (BCNU) and lomustine (CCNU), are widely used in patients with malignant gliomas.1,2 Their clinical effectiveness is attributed, in part, to their relatively high yields of cytotoxic O6-alkylguanine DNA adducts, which inhibit cellular division and lead to apoptosis.2–4 The unsatisfactory results of chemotherapy are chiefly attributed to chemoresistance, which in the case of alkylating agents relies heavily on O6-methylguanine-DNA methyltransferase (MGMT).
In normal cells, MGMT is a ubiquitous DNA repair protein, which is involved in protecting the genome from the mutagenic actions of endogenous and environmental carcinogens.5 MGMT is highly expressed in human cancers and its expression directly correlates with experimental and clinical resistance to alkylating agents.6–10 MGMT functions by a stoichiometric and suicidal reaction mechanism in which alkyl groups bound to the O6-position of guanine are transferred to a cysteine in its active site, resulting in the direct restoration of the normal base and self-inactivation of MGMT.11 O6-alkylguanine lesions induced in DNA by methylating and chloroethylating agents are excellent substrates for MGMT11,12 making MGMT one of the most important DNA repair mechanisms of alkylating drug-induced DNA damage. Previous studies13–16 have shown that cellular accumulation of p53 protein downregulates MGMT expression. MGMT promoter hypermethylation is a common event in primary human neoplasms, resulting in MGMT silencing.14,17 MGMT promoter hypermethylation in malignant gliomas is a useful predictor of tumor responsiveness to alkylating agents.18,19 For gliomas that retain the ability to express MGMT, studying potential drug interactions that modulate MGMT could help optimize chemotherapy with alkylating agents.
Convulsions are common in brain tumor patients, and antiepileptic drugs (AEDs) are widely used for seizure treatment. In a brain tumor patient, the ideal AED would effectively suppress seizures without causing any unwanted side effects, potentially enhance chemotherapy at multiple levels by pharmacokinetic, pharmacodynamic, and ultimately pharmacoepigenetic interactions, and protect the surrounding normal brain from the unwanted effects of chemotherapy. Given the accumulated evidence regarding the transcriptional regulatory activity of AEDs via histone deacetylase (HDAC) modulation, we assessed the potential impact of AEDs (levetiracetam [LEV], valproic acid, phenytoin, and phenobarbital) on MGMT expression in gliomas. We compared several AEDs to identify an MGMT inhibitor that could effectively enhance the cytotoxic activity of alkylating agents. We also assessed the role of p53 and one of the most common p53 corepressor systems, mSin3A/HDAC1,20 whose impaired function has been reported in other human cancers.21 Additionally, we compared the effect of LEV on MGMT expression levels in normal astrocytes and glioblastoma cells and investigated the subsequent impact of MGMT inhibition on proliferation of the glioblastoma cells when using LEV and alkylating agents together, and the effect of LEV on MGMT expression in glioblastoma patients.
Materials and Methods
Cell Culture
Hs683, SW1088, SW1783, U87, A-172, LN18, T98G, and U138 human glioma cells were obtained from the American Type Culture Collection (ATCC). Normal human astrocytes were purchased from Lonza Inc. Hs683, SW1088, SW1783, A-172, and LN18 cells were grown in a Dulbecco's modified Eagle medium (Invitrogen), U87 and U138 cells were grown in a minimum essential medium (Invitrogen), and T98G cells were grown in Eagle's minimal essential medium (ATCC) supplemented with 10% fetal bovine serum (GIBCO, Invitrogen Corporation) and 100 µg/mL of penicillin–streptomycin at 37°C in 5% CO2. Normal astrocytes were grown in an astrocyte basal medium containing AGM SingleQuots from Lonza Inc.
Antibodies and Drugs
p53 (sc-126), normal rabbit (sc-2027), and mouse (sc-2025) serum antibodies were purchased from Santa Cruz Biotechnology. MGMT (MAB16200) and mSin3A (06-913) antibodies were purchased from Millipore. The HDAC1 antibody (2062) was purchased from Cell Signaling Technology, and the β-actin (A2066) antibody was purchased from Sigma-Aldrich. LEV (UCB, Inc.), valproic acid (Teva Pharmaceuticals), phenytoin (Alpharma USPD Inc.), and phenobarbital (Qualitest Pharmaceuticals, Inc.) are all available in liquid form. TMZ (Schering Plough Inc.) and BCNU (Bristol-Myers Squibb Co.) were used in tissue culture using a previously described methodology.22,23 Control ethanol concentrations were matched with the corresponding ethanol concentrations for the liquid AED plus medium formulations used and were <0.0005%.
RNA Interference–Mediated Transfections
U138 cells were plated (2 × 105) for 24 hours and then treated daily with LEV (40 µg/mL) for another 72 hours. Cells were subsequently transfected with control siRNA (nonspecific; 100 nmol/L), mSin3A siRNA (100 nmol/L), and HDAC1 siRNA (100 nmol/L), alone or in combination, with or without LEV. Cells were harvested 24 hours post-transfection, and total RNA was isolated for real-time PCR. In another experiment, U138 cells were plated (2 × 105) and 24 hours later transfected with MGMT siRNA (100 nmol/L) and control siRNA (100 nmol/L). Cells were harvested at 24, 48, 72, and 96 hours for Western blot analysis. In another experiment, U138 cells were plated (2 × 105) for 24 hours, subsequently treated daily with LEV (40 µg/mL) for 72 hours, and further transfected with p53 siRNA (20 nmol/L) for another 24 hours, and then cells were harvested and total RNA was isolated for real-time PCR. p53, MGMT, mSin3A, and HDAC1 siRNAs were purchased from Dharmacon.
Methylthiazol Tetrazolium Assays
LN18, T98G, and U138 cells were plated (2 × 104) in a 24-well plate for 24 hours, treated daily with 2 concentrations of LEV (20 or 40 µg/mL) and harvested at 24, 48, 72, and 96 hours for methylthiazol tetrazolium (MTT) assays. In another experiment, normal human astrocytes (2 × 104) were plated for 24 hours, treated daily with different concentrations of LEV (10, 20, 30, or 40 µg/mL) for 96 hours, and then cells were harvested and MTT assays were performed. In another experiment, glioma cells and normal human astrocytes were treated daily with LEV (40 µg/mL) for 72 hours and then treated with TMZ (150 µg/mL) or BCNU (50 µg/mL) for another 48 hours before MTT assays were performed. MTT assay kits were purchased from ATCC. MTT assays were performed following the manufacturer's instructions.
Reporter Assays
For reporter assays, U138 cells (5 × 104) and normal human astrocytes (5 × 104) were seeded in 12-well plates and 24 hours later transfected with p21-luc24 in the presence or the absence of LEV (20 and 40 µg/mL) using lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. The amount of total transfected DNA was kept constant by adding empty plasmid. Twenty-four hours post-transfection, cells were harvested and lysed, and luciferase activity was measured using a dual luciferase reporter assay system (Promega Corporation).
Western Blotting
Human glioma cells (Hs683, SW1088, SW1783, A-172, U87, and U138) were plated (1 million cells) in 10-cm2 petri dishes, and 24 hours later, cell lysates were collected. In another experiment, normal human astrocytes (2 × 105), U138, LN18, and T98G (2 × 105) cells were grown in 10-cm2 plates for 24 hours. They were further treated daily with different concentrations of LEV (10, 20, 30, or 40 µg/mL) for another 96 hours when cell lysates were collected. In another experiment, U138 cells were treated similarly daily for 96 hours with different concentrations of valproic acid (10, 25, 50, 100, or 150 µg/mL), phenytoin (2.5, 5, 10, 15, or 20 µg/mL), and phenobarbital (10, 20, 30, or 40 µg/mL), and cell lysates were collected for western blot analysis. Proteins were separated on 10% SDS–PAGE and transferred to nitrocellulose membranes. Membranes were blocked with 5% dry milk and probed with p53, MGMT, and actin antibodies. After incubation with primary antibody for 2 hours, membranes were washed with tris-buffered saline containing 0.5% Tween 20, and subsequently incubated for 1 hour with horseradish peroxidase (HRP)-conjugated secondary antibody (goat anti-rabbit IgG-HRP [sc-2004] and goat anti-mouse IgG-HRP [sc-2005]; Santa Cruz Biotechnology). Specific proteins were detected by enhanced chemiluminescence from GE Healthcare Bio-Sciences Corp. and exposed to X-ray film (Kodak). To determine whether the total amounts of protein loaded in each lane are comparable, the membranes were probed with β-actin. Similarly, Western blot analysis was performed using siRNA-transfected samples.
Quantitative Real-Time PCR
For quantitative real-time PCR (qRT-PCR), U138, LN18, and T98G cells and normal human astrocytes (2 × 105) were plated in 10-cm2 petri dishes for 24 hours and subsequently LEV was added daily at different concentrations (10, 20, 30, or 40 µg/mL) for 96 hours, and then cells were collected and washed twice with phosphate-buffered saline and lysed in Trizole reagent (Invitrogen). Total RNA was isolated using the Qiagen columns purchased from Qiagen and reverse transcribed using a SuperScript™ First-Strand Synthesis System (Invitrogen) for RT–PCR (Cat. No. 12371-019). Real-time PCR was performed using ABI 7300 series sequence detection system (Applied Biosystems). Total RNA up to 1 µg from individual samples was reverse transcribed in 20 µL of a reaction mixture using a cDNA synthesis kit (Invitrogen). Fifty nanograms of the resulting cDNA were used in a total PCR volume of 20 µL. Real-time PCR was performed using the TaqMan gene expression assay primers (Applied Biosystems) for MGMT (Hs00172470_m1), p53 (Hs00153349_m1), mSin3A (Hs00411592_m1), HDAC1 (Hs00606262-g1), and actin (4333762F). As mentioned above, RNA interference–mediated transfected samples were also processed. The quantitative relative mRNA levels of p53 and MGMT, and mSin3A and HDAC1 were calculated using the ΔΔCt method with β-actin mRNA as an endogenous control. For regular semi-quantitative RT–PCR, RNA was isolated from U138, LN18, and T98G cells treated daily with LEV (40 µg/mL) for 96 hours and cDNA was prepared as described earlier. Primers were designed using the Vector NTI program (Invitrogen), and PCRs were performed by using a Veriti 96-well Thermal Cycler (Applied Biosystems). The gene products were amplified with Platinum PCR Super Mix (Cat. No. 11306-016; Invitrogen) and 30–35 cycles were performed (95°C, 30 seconds; 60°C, 30 seconds; 72°C, 30 seconds). Electrophoresis was performed at 100 V in 1× TAE buffer for 1 hour, and 10 µL of each sample was loaded on a 2% agarose gel containing ethidium bromide. The gel was imaged using a UV transilluminator. Glyceraldehydephosphate dehydrogenase (GAPDH) was used as an internal control.
Primer sequences used in this study were as follows:
- MGMT mRNA primers:
- forward: 5′ ACCGTTTGCGACTTGGTACTTG 3′
- reverse: 5′ GTCGCTCAAACATCCATCCTAC 3′
- GAPDH mRNA primers
- forward: 5′ CGAGCCACATCGCTCAGACAC 3′
- reverse: 5′ CCAGTGGACTCCACGACGTAC 3′.
Chromatin Immunoprecipitation and Re-chromatin Immunoprecipitation Assays
Chromatin immunoprecipitation (ChIP) assays were performed using the manufacturer's instructions (Upstate) with minor modifications.25,26 U138 cells (2 × 105) were plated and 24 hours later LEV (40 µg/mL) was added daily for 96 hours, and cell lysates were prepared. In another experiment, LEV was added daily for 72 hours, cells were then transfected with control (nonspecific; 20 nmol/L) and p53 siRNAs (20 nmol/L) and 24 hours post-transfection cell lysates were collected. Direct ChIP was also performed in the absence of LEV to assess the recruitment of p53, mSin3A, and HDAC1 to the MGMT promoter. Chromatin was cross-linked using 1.5% formaldehyde for 10 minutes at 37°C. Cells were collected after 2 washings with PBS containing a protease inhibitor cocktail (Complete Protease Inhibitor Cocktail—sc-29130—Santa Cruz Biotechnology). Cells were lysed with SDS lysis buffer (1% SDS, 10 mM EDTA, 50 mM Tris–HCl, pH 8.1). The cell suspension was then incubated on ice for 10–15 minutes and centrifuged at 1000 × g. The cell lysates (400 µL) were sonicated 25 times and each time a 10-second pulse was administered with a 20-second gap (Mesonix Inc.). After centrifugation, 50 µL of the supernatant was used to check DNA fragmentation as well as input and the remaining 350 µL was used for ChIP. For re-ChIP assays, lysates were initially incubated with p53 antibody and then the immunocomplexes were eluted at 37°C for 30 min in re-ChIP buffer (1% Triton X-100, 2 mM EDTA, 150 mM NaCl, 20 mM Tris–HCl, pH 8.1). The primary immunocomplexes were incubated with mSin3A and HDAC1 antibodies, and final immunocomplexes were eluted in 500 µL of 1% SDS, 0.1 M NaHCO3 buffer, and processed as described earlier.
The sequence of the primers used in this study:
MGMT forward: 5′ GCTCCAGGGAAGAGTGTCCTCTGCTCCCT 3′
MGMT reverse: 5′ GGCCTGTGGTGGGCGATGCCGTCCAG 3′.
Histological Studies
Glioblastoma samples were obtained from 4 patients who had a second surgical resection within 7–14 days from their initial diagnostic biopsy or resection and who were not on any AED prior to the first operation and were placed on LEV for the 7 to 14 days between the first and the second operations. For immunohistochemistry and histological staining, paraffin-embedded tissues were used to identify MGMT. Sections (4–6 µm thick) were mounted on positively charged superfrost slides (Fischer Scientific, Co.) and dried overnight. Sections were deparaffinized in xylene, treated with a graded series of alcohol (100%, 95%, and 80% ethanol [vol/vol] in deionized H2O), and rehydrated in deionized water and PBS (pH 7.5). Antigen retrieval was achieved by placing slides in 97°C 1 M citrate buffer (pH 6.0) for 10 minutes. Slides were then washed with PBS that contained 0.1% triton and 0.1% BSA. Endogenous peroxidase was blocked with 3% hydrogen peroxide in PBS, whereas nonspecific binding was blocked with 10% normal goat serum and 2% BSA in PBS. The slides were then incubated at 4°C overnight in a moist chamber with a monoclonal mouse anti-MGMT antibody (Chemicon-Millipore; #MAB16200, 1:100 dilution) and then washed with PBS containing 0.1% Triton and 0.1% BSA in PBS. After 1-hour incubation at room temperature with a peroxidase-conjugated mouse IgG secondary antibody (Santa Cruz Biotechnology, 1:500 dilution), a positive reaction was visualized by incubating the slides with stable 3,3′-diaminobenzidine (Invitrogen) for 8–10 min. Counterstain was achieved by rinsing the sections with 2 changes of tap water, placing them in Gill's filtered hematoxylin (EMD Chemicals) for 5 minutes, then successive dips into tap water, acid alcohol (EMD Chemicals), tap water, lithium carbonate (EMD Chemicals), and tap water. Slides were dehydrated and mounted with Crystal Mount (Fischer Scientific, Co.).
Statistical Analysis
Each experiment was repeated 3 times. Data are presented as the mean ± SD. Statistical analysis was done using the Student's t-test, assuming equal variance, and P-values were calculated based on the 2-tailed test. A P-value of <.05 was considered statistically significant.
Results
LEV Inhibited MGMT Protein in Glioblastoma Cell Lines
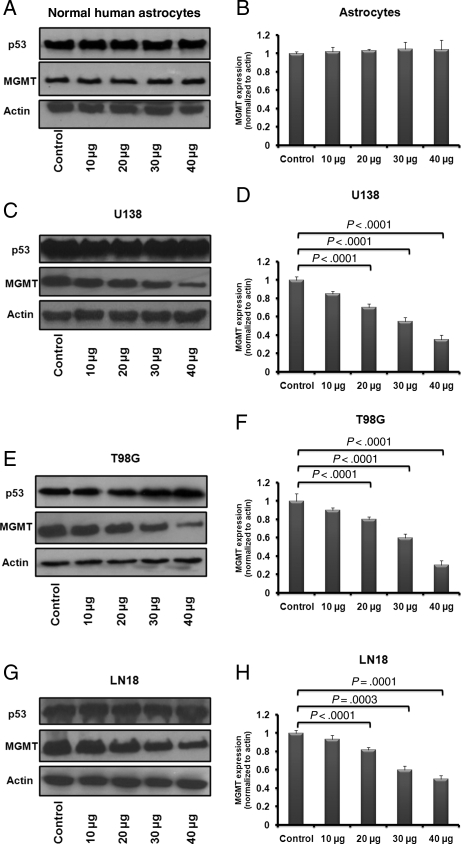
LEV concentrations used in tissue culture reflected the clinical serum therapeutic range (10, 20, 30, and 40 µg/mL) achieved in patients at oral doses of 500–1000 mg twice daily. Our results should be cautiously interpreted given that serum concentrations may not directly correlate with concentrations achieved in the extracellular intratumor space where, due to tumor heterogeneity, interstitial concentration of AED may be highly variable. In our study, in vitro, LEV did not inhibit MGMT protein in normal human astrocytes but did have a significant MGMT inhibitory effect in each of the 3 glioblastoma cell lines tested (U138, LN18, and T98G; Fig. 1A–H; P < .003). These glioblastoma cell lines (U138, LN18, and T98G) were the focus of our study due to their phenotype, as they are known to express MGMT and known to have mutated p53. Several other glioma cell lines were screened for their p53 and MGMT expression status to include low- (Hs683), medium- (SW1088 and SW1783), and high-grade gliomas (A172, U87, and U138). In the cell lines tested, for those expressing MGMT (unmethylated promoter and/or mutated p53), the level of MGMT expression directly correlated with the glioma grade (Supplementary Material, Fig. S1). Significant inhibition of MGMT was observed starting at 72–96 hours after initiation of daily treatment with LEV as described in the “Materials and Methods” section. Earlier time points (24 and 48 hours) along with 72 and 96 hours were screened using U138 glioma cells, and both LEV (40 µg/mL) and siRNA MGMT knock-downtime-course experiments were performed (Supplementary Material, Fig. S2A–D), showing the earliest significant suppression of MGMT at 72 hours. Other AEDs (valproic acid, phenytoin, and phenobarbital) were screened. At 96 hours, valproic acid increased MGMT expression, phenytoin had no significant effect, and phenobarbital had a minimal inhibitory effect (Supplementary Material, Fig. S3). At 96 hours, the MGMT protein levels in U138 cells were 30% (P < .0001), 45% (P < .0001), and 65% (P < .0001) lower after daily treatment with LEV 20, 30, or 40 µg/mL (Fig. 1D). For T98G cells, at 96 hours, MGMT expression was 20% (P < .0001), 40% (P < .0001), and 70% (P < .0001) lower after daily LEV treatment with 20, 30 or 40 µg/mL (Fig. 1F). The results were similar in LN18 cells at 96 hours with MGMT suppression of 29% (P < .0001), 40% (P = .0003), and 50% (P = .0001) after daily treatment with 20, 30, or 40 µg/mL of LEV (Fig. 1H). When treated daily with LEV (10 µg/mL), all the glioblastoma cell lines tested showed a small (10%–15%) but not significant decrease in the MGMT protein levels.
Fig. 1.
The effect of LEV on MGMT protein expression in normal human astrocytes and glioblastoma cells. The effect of LEV on MGMT and p53 protein expression in normal human astrocytes and 3 MGMT-expressing glioblastoma cell lines (U138, LN18, and T98G) was analyzed by Western blotting. Cells were treated daily with LEV-enriched specific medium for 96 hours at concentrations covering the serum therapeutic range for seizures of 10, 20, 30, or 40 µg/mL.
LEV Inhibited MGMT Transcription in Brain Tumor Cells and Had a Protective Effect on Normal Astrocytes
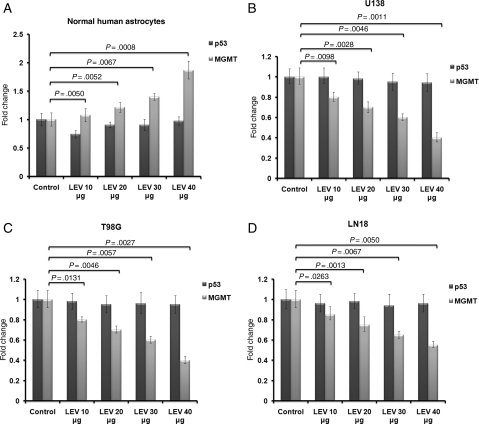
LEV significantly inhibited MGMT transcription in all 3 glioblastoma cell lines tested (U138, LN18, and T98G; all known to express MGMT and known to have mutated p53; Fig. 2 and Supplementary Material, Fig. S4). MGMT transcription at 72 hours was between 30% and 60% lower in T98G cells after daily treatment with either 20 (P < .005), 30 (P < .006), or 40 µg/mL (P < .003) of LEV (Fig. 2C). Similarly, MGMT transcription at 72 hours was between 30% and 60% lower in U138 cells after daily treatment with either 20 (P < .003), 30 (P < .005), or 40 µg/mL of LEV (P < .002; Fig. 2B), and under similar conditions, MGMT transcription was lower with 25%–45% (P < .006) for LN18 (Fig. 2D). After daily treatment with LEV, at 72 hours, MGMT transcription increased in normal astrocytes from 22% to 87% when treated with 20, 30, or 40 µg/mL (P < .006), suggesting a protective role in the normal brain (Fig. 2A). This is in keeping with the known neuroprotective effect for LEV, as already suggested by others via a different mechanism.27
Fig. 2.
The effect of LEV on MGMT transcription in normal human astrocytes and in glioblastoma cells. The effect of LEV on MGMT and p53 transcription in normal human astrocytes and 3 MGMT-expressing glioblastoma cell lines (U138, LN18, and T98G) was analyzed by qRT-PCR. Cells were treated daily with LEV-enriched specific medium for 96 hours at concentrations covering the serum therapeutic range for seizures of 10, 20, 30, or 40 µg/mL.
LEV Directly Inhibited Glioma Cell Proliferation
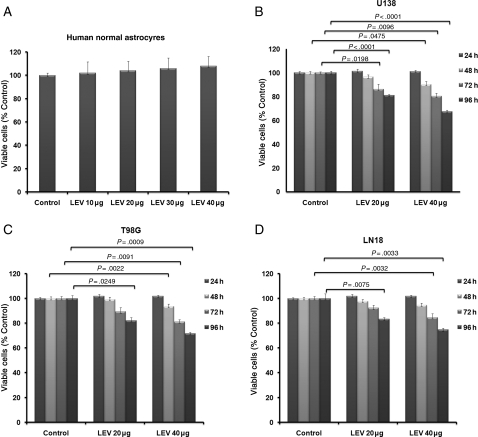
Daily treatment with LEV (either 20 or 40 µg/mL) exerted a time- and dose-dependent inhibitory effect on the growth of all glioblastoma cell lines tested (U138, T98G, and LN18; all known to express MGMT known to have mutated p53), whereas normal human astrocytes were not affected (Fig. 3A–D; P < .004). Furthermore, LEV decreased p53-mediated p21 activity in normal human astrocytes (P < .04) and increased in glioblastoma cells (U138) after 24 hours of treatment with LEV at 20 or 40 µg/mL (P < .04; Supplementary Material, Fig. S5).
Fig. 3.
The effect of LEV on glioma cell growth. Growth inhibition assays (MTT) were performed on normal human astrocytes and 3 MGMT-expressing glioblastoma cell lines (U138, LN18 and T98G) after daily treatment with 2 different concentrations (20 and 40 µg/mL) of LEV at 4 different time points (24, 48, 72, and 96 hours).
LEV Inhibited MGMT Expression by Increasing p53 Binding on the MGMT Promoter via Recruitment of the Transcriptional Corepressors mSin3A and HDAC1
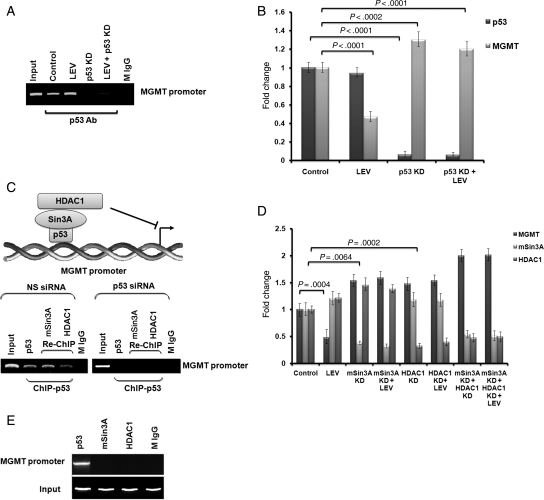
LEV increased p53 binding on the MGMT promoter in U138 cells treated daily for 96 hours with 40 µg/mL of LEV (Fig. 4A). siRNA p53 knock-down U138 cells showed decreased p53 binding to the MGMT promoter despite the presence of LEV (Fig. 4A). Similar to findings suggested by direct ChIP assay (Fig. 4A), siRNA p53 knock-down U138 cells showed increased MGMT transcription and the LEV MGMT inhibitory effect was lost (Fig. 4B; P < .0002). These results show that LEV MGMT inhibitory effect relies on the presence of the residual functional activity of mutated p53 (Fig. 4B). There is no mSin3A and HDAC1 recruitment on the MGMT promoter in p53 knock-down U138 cells (Fig. 4C). mSin3A siRNA knock-down or HDAC1 siRNA knock-down either alone or in combination translate into high levels of MGMT despite the presence of LEV as its MGMT inhibitory effect is completely lost in these knock-down states (Fig. 4D; P < .006). LEV increases mSin3A and HDAC1 transcription by 21% in U138 cells treated daily with 40 µg/mL of LEV for 96 hours (P < .003; Fig. 4D). A direct ChIP assay revealed no spontaneous mSin3A or HDAC1 recruitment to the MGMT promoter despite the presence of p53 (Fig. 4E).
Fig. 4.
The effect of LEV on p53 binding on the MGMT promoter and on the role of the mSinA/HDAC1 transcriptional corepressor system. U138 cells were treated daily with LEV (40 µg/mL)-enriched specific medium for 96 hours. ChIP assays were performed in the presence or the absence of knock-down p53 (A). p53 and MGMT transcription was measured by qRT-PCR in knock-down p53 U138 cells in the presence or the absence of LEV (B). Re-Chip assays were performed in the presence or absence of knock-down p53 (C). MGMT, mSin3A, and HDAC1 transcription were measured by qRT-PCR in knock-down mSin3A or/and knock-down HDAC1 U138 cells in the presence or the absence of LEV (D). Direct ChIP analysis was performed using U138 cell lysates to investigate the recruitment of p53, mSin3A, and HDAC1 on MGMT promoter (E).
LEV Sensitized Glioblastoma Cells to TMZ
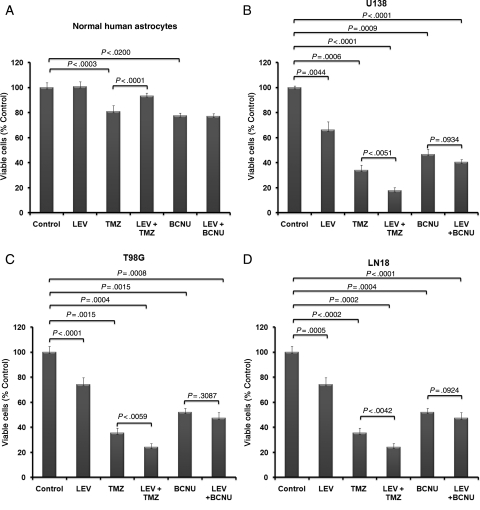
LEV had an independent significant inhibitory effect on glioblastoma cells. When treated daily with LEV (40 µg/mL) for 72 hours, cell growth inhibition was 29% in T98G cells (P < .002), 34% in U138 cells (P < .001), and 25% in LN18 (P < .0001). LEV significantly enhanced the cytotoxic activity of TMZ; after daily treatment with LEV (40 µg/mL) for 72 hours followed by treatment with TMZ (150 µg/mL) for another 48 hours, cell growth inhibition was enhanced by 39% in T98G glioma cells (P < .006), 48% in U138 glioma cells (P < .001), and 32% in LN18 (P < .005). LEV did not significantly enhance BCNU cytotoxicity in any of the glioblastoma cell lines tested. In contrast, LEV significantly protected (P < .0001) against TMZ-mediated cytotoxicity on normal human astrocytes (Fig. 5A–D).
Fig. 5.
LEV sensitizes glioma cells to TMZ. MGMT-expressing glioblastoma cell lines T98G, U138, and LN18 and normal human astrocytes were treated daily with LEV (40 µg/mL) for 72 hours and then treated with TMZ (150 µg/mL) or BCNU (50 µg/mL) for another 48 hours before MTT assays were performed.
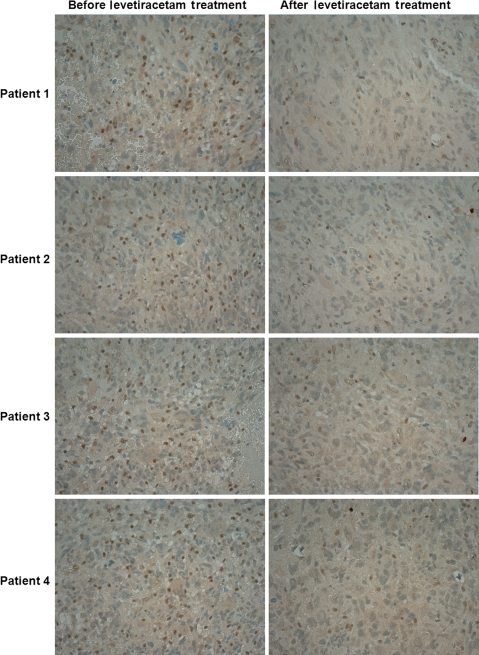
LEV Decreased MGMT Expression in Brain Tumor Samples
MGMT immunohistochemistry was performed on glioblastoma samples from 4 patients who required a second surgery within 7–14 days of their initial diagnoses and resections. These patients were maintained on LEV for seizure prophylaxis and have not received any tumor-specific treatment (chemoradiation—TMZ and IMRT). LEV was used for seizure prophylaxis at a dose of 500–1000 mg twice a day, which correlated with serum levels of 20–40 µg/mL. All LEV serum levels were drawn after at least 4 days of LEV twice daily treatment to ensure that steady state was reached. Immunohistochemistry results demonstrated reduced MGMT expression in patient brain tumor samples after treatment with LEV (Fig. 6).
Fig. 6.
LEV effect on the MGMT expression in glioblastoma samples. MGMT immunohistochemistry was performed on glioblastoma samples from 4 newly diagnosed patients who had 2 craniotomies 7–14 days apart, prior to initiation of any tumor-specific treatment, samples were obtained before and after LEV treatment. LEV was used at a dose of 500–1000 mg twice daily, which correlated with serum levels of 20–40 µg/mL.
Discussion
LEV has been increasingly used as an alternative AED in glioma patients because of its high therapeutic index, pharmacokinetics, and favorable side effect profile. A semi-quantitative pharmacokinetic rating system focusing on AED use in epilepsy patients, using different pharmacokinetic characteristics allowing for direct comparison among AEDs rated LEV as the most optimal among topiramate, lamotrigine, zonisamide, ethosuximide, and clonazepam, with valproic acid being the least optimal.28 LEV crosses the blood–brain barrier rapidly, and its concentration in the cerebrospinal fluid rises linearly in a dose-dependent manner.29 LEV is undergoing limited metabolization (25%–30% by extrahepatic hydrolysis in blood), is excreted unchanged in urine, is not bound to plasma proteins, and does not induce or inhibit drug-metabolizing enzymes (ie, cytochrome P450 system), and drug interactions are limited and mostly significant when patients are comedicated with other AEDs. Enzyme inducing AEDs significantly increase LEV clearance by 24%–37%.30 Age has a significant impact in LEV clearance, as older adults have lower clearance and require a mean 40% lower dose of LEV to achieve the same serum level as young adults.30 In human body tissues, LEV has 100% bioavilability, is rapidly absorbed, and reaches peak concentration within 1 hour.
The standard of care for patients with glioblastoma is maximal surgical resection followed by combining TMZ with external beam radiotherapy (chemoradiation) followed by first-line TMZ as a single agent for 6 months or until recurrence.1 AEDs are routinely used for the treatment of seizures in brain tumor patients. A growing body of evidence, including the results of our study, suggests that select AEDs could lead to significant pharmacoepigenetic interactions. Valproic acid, for example, has been shown in vitro to have a significant HDAC inhibitory activity with subsequent modulatory activity of target genes involved in various diseases. In contrast, AEDs such as LEV, phenytoin, carbamazepine, and phenobarbital have been previously reported in vitro to have no HDAC inhibitory activity.31 Our study does not provide direct evidence that LEV effect is related to direct modulation of HDAC enzymatic activity. Our study suggests that LEV activity may be strictly related to increasing transcription of HDAC1 and mSin3A and to recruiting of the HDAC1/mSin3A corepressor complex (Fig. 4C and D). This underlines the complexity of both epigenomic modulations through HDACs and the pharmacoepigenomic interactions potentially triggered by small molecules like AEDs.
The present study confirms, as have previous studies,13–16 that p53 protein downregulates MGMT expression. The amount of time, p53 protein levels, and functional quality required for this downregulation are unclear. It appears that p53 gene transfer, by transfection,13,32 regulation of expression,33 or infection with adenoviral constructs33 that result in modest to high levels of p53 expression leads to an inhibition of MGMT expression. Here, we show that p53 binds directly to the MGMT promoter and inhibits MGMT expression (Fig. 4A and B). We also confirm in the cell lines tested for the MGMT expressing gliomas—HS683 (LG), SW1783 (MG), and U138 (HG) (with mutated p53 and unmethylated MGMT promoter)—that the MGMT expression levels directly correlate with glioma grade (Supplementary Material, Fig. S1).
The LEV concentrations used in tissue culture in our study reflect the clinical serum therapeutic range (10, 20, 30, and 40 µg/mL) usually achieved at oral doses of 500–1000 mg twice daily. Caution should be exercised in interpreting our results. Even if serum concentrations may directly correlate with concentrations in the cerebrospinal fluid, AED concentrations achieved in the interstitial intratumor space, given tumor heterogeneity, may be highly variable. We showed that LEV enhances the MGMT transcriptional inhibitory activity of mutated p53 by facilitating the recruitment of one of the pivotal p53 regulatory corepressor complexes mSin3A/HDAC1 (Fig. 4C). We demonstrate that p53-induced MGMT transcription inhibition requires simultaneous participation of both elements of the mSin3A/HDAC1 corepressor complex, which, in turn, is recruited by LEV (Fig. 4D). We also show that LEV MGMT inhibition uniquely relies on each of the elements of p53, mSin3A/HDAC1 corepressor system activity as the inhibitory activity is lost when either p53 or mSin3A or HDAC1 is abrogated (Fig. 4B and D). To our knowledge, this is the first evidence of an AED's ability, LEV, to reestablish a more differentiated phenotype feature by restoring the MGMT inhibitory activity of mutated p53 through recruitment of the mSin3A/HDAC1 corepressor system. Decreased mSin3A expression has been reported in other human cancers.21 mSin3A/HDAC1 is a widespread corepressor system, suggesting that LEV may have far more reaching gene regulatory effects in many other cancers that will have to be elucidated.
In our study, LEV was the only AED capable of significant MGMT inhibition (Supplementary Material, Fig. S3A and B), whereas valproic acid significantly increased MGMT protein levels in a direct dose-dependent manner (Supplementary Material, Fig. S3C and D). The opposing effects of LEV and valproic acid on the MGMT expression do not translate in opposing cytotoxic effects. Our further studies focusing on the glioblastoma cell lines chosen (U138, T98G, and LN18) reveal that, despite the MGMT stimulatory effect, valproic acids maintains an overall cytotoxic effect (data not shown) in keeping with prior data, suggesting valproic acid cytotoxic role. Phenytoin has no MGMT modulatory activity, possibly consistent with reports demonstrating no HDAC regulatory activity31 (Supplementary Material, Fig. S3E and F). We further report that LEV significantly enhances the cytotoxic effect of TMZ but not BCNU (Fig. 5B–D), underscoring the differential role of p53, MGMT, and other DNA repair mechanisms involved in counteracting the DNA damage induced by a methylating monofunctional alkylator like TMZ vs a chlorethylating DNA cross-linking alkylator like BCNU. We also show that glioblastoma samples in patients exposed to LEV who have not received any other significant therapeutic intervention exhibit MGMT suppression (Fig. 6). In our study, LEV exerts a protective role on normal astrocytes when exposed to TMZ potentially expanding the racetam beneficial effects (Fig. 5A). Given the LEV MGMT inhibitory effect noted in the glioblastoma cell lines tested (U138, T98G, and LN18; all expressing MGMT/mutated p53), LEV could potentially modulate the MGMT expression profile of glioma cells that do not usually express MGMT, either by demethylating the MGMT promoter site in cells where MGMT expression was silenced by methylation of the promoter or by impairing wt-p53 MGMT suppressive function in wt-p53 glioma cells. LEV did not change the MGMT-negative expression status of MGMT-negative/wt-p53 glioblastoma cells (U87; data not shown). Beyond pharmacodynamic and pharmacokinetic interactions, our findings underscore the potential role of the pharmacoepigenomic effects of AEDs. Our results are to be considered preliminary given the limited number of cell lines used and selected number of experiments. Further studies will be needed to assess AEDs and correlate MGMT levels in glioma translational models and glioma patients. If confirmed, more studies will be required in clinical practice in the context of a specific therapeutic intervention for seizure treatment in alkylating agent based clinical trials, which may require stratification of patients by both MGMT expression and specific AED use.
Supplementary Material
Supplementary material is available at Neuro-Oncology online.
Acknowledgments
We thank Beth Isley for the assistance with the immunohistochemistry experiments.
Conflict of interest statement. None declared.
References
- 1.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. doi:10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Burton EC, Prados M. Malignant gliomas. Curr Treat Options Oncol. 2000;1(5):459–468. doi: 10.1007/s11864-000-0073-2. [DOI] [PubMed] [Google Scholar]
- 3.Erickson LC, Bradley MO, Ducore JM, Ewig RA, Kohn KW. DNA crosslinking and cytotoxicity in normal and transformed human cells treated with antitumor nitrosoureas. Proc Natl Acad Sci USA. 1980;77(1):467–471. doi: 10.1073/pnas.77.1.467. doi:10.1073/pnas.77.1.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erickson LC, Laurent G, Sharkey NA, Kohn KW. DNA cross-linking and monoadduct repair in nitrosourea-treated human tumour cells. Nature. 1980;288(5792):727–729. doi: 10.1038/288727a0. doi:10.1038/288727a0. [DOI] [PubMed] [Google Scholar]
- 5.Pegg AE. Repair of O(6)-alkylguanine by alkyltransferases. Mutat Res. 2000;462(2–3):83–100. doi: 10.1016/s1383-5742(00)00017-x. [DOI] [PubMed] [Google Scholar]
- 6.Bobola MS, Berger MS, Silber JR. Contribution of O6-methylguanine-DNA methyltransferase to resistance to 1,3-(2-chloroethyl)-1-nitrosourea in human brain tumor-derived cell lines. Mol Carcinog. 1995;13(2):81–88. doi: 10.1002/mc.2940130204. doi:10.1002/mc.2940130204. [DOI] [PubMed] [Google Scholar]
- 7.Bobola MS, Blank A, Berger MS, Silber JR. Contribution of O6-methylguanine-DNA methyltransferase to monofunctional alkylating-agent resistance in human brain tumor-derived cell lines. Mol Carcinog. 1995;13(2):70–80. doi: 10.1002/mc.2940130203. doi:10.1002/mc.2940130203. [DOI] [PubMed] [Google Scholar]
- 8.Belanich M, Pastor M, Randall T, et al. Retrospective study of the correlation between the DNA repair protein alkyltransferase and survival of brain tumor patients treated with carmustine. Cancer Res. 1996;56(4):783–788. [PubMed] [Google Scholar]
- 9.Silber JR, Blank A, Bobola MS, et al. O6-methylguanine-DNA methyltransferase-deficient phenotype in human gliomas: frequency and time to tumor progression after alkylating agent-based chemotherapy. Clin Cancer Res. 1999;5(4):807–814. [PubMed] [Google Scholar]
- 10.Tanaka S, Kobayashi I, Utsuki S, et al. O6-methylguanine-DNA methyltranspherase gene expression in gliomas by means of real-time quantitative RT-PCR and clinical response to nitrosoureas. Int J Cancer. 2003;103(1):67–72. doi: 10.1002/ijc.10757. doi:10.1002/ijc.10757. [DOI] [PubMed] [Google Scholar]
- 11.Pegg AE, Dolan ME, Moschel RC. Structure, function, and inhibition of O6-alkylguanine-DNA alkyltransferase. Prog Nucleic Acid Res Mol Biol. 1995;51:167–223. doi: 10.1016/s0079-6603(08)60879-x. doi:10.1016/S0079-6603(08)60879-X. [DOI] [PubMed] [Google Scholar]
- 12.Gerson SL, Willson JK. O6-alkylguanine-DNA alkyltransferase. A target for the modulation of drug resistance. Hematol Oncol Clin North Am. 1995;9(2):431–450. [PubMed] [Google Scholar]
- 13.Grombacher T, Eichhorn U, Kaina B. p53 is involved in regulation of the DNA repair gene O6-methylguanine-DNA methyltransferase (MGMT) by DNA damaging agents. Oncogene. 1998;17(7):845–851. doi: 10.1038/sj.onc.1202000. doi:10.1038/sj.onc.1202000. [DOI] [PubMed] [Google Scholar]
- 14.Harris LC, Remack JS, Houghton PJ, Brent TP. Wild-type p53 suppresses transcription of the human O6-methylguanine-DNA methyltransferase gene. Cancer Res. 1996;56(9):2029–2032. [PubMed] [Google Scholar]
- 15.Guo W, Liu X, Lee S, Park NH. High O6-methylguanine methyl transferase activity is frequently found in human oral cancer cells with p53 inactivation. Int J Oncol. 1999;15(4):817–821. doi: 10.3892/ijo.15.4.817. [DOI] [PubMed] [Google Scholar]
- 16.Natsume A, Ishii D, Wakabayashi T, et al. IFN-beta down-regulates the expression of DNA repair gene MGMT and sensitizes resistant glioma cells to temozolomide. Cancer Res. 2005;65(17):7573–7579. doi: 10.1158/0008-5472.CAN-05-0036. [DOI] [PubMed] [Google Scholar]
- 17.Silber, Blank A, Bobola MS, et al. O6-methylguanine-DNA methyltransferase-deficient phenotype in human gliomas: frequency and time to tumor progression after alkylating agent-based chemotherapy. Clin Cancer Res. 1999;5(4):807–814. [PubMed] [Google Scholar]
- 18.Esteller M, Garcia-Foncillas J, Andion E, et al. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med. 2000;343(19):1350–1354. doi: 10.1056/NEJM200011093431901. doi:10.1056/NEJM200011093431901. [DOI] [PubMed] [Google Scholar]
- 19.Esteller M, Toyota M, Sanchez-Cespedes M, et al. Inactivation of the DNA repair gene O6-methylguanine-DNA methyltransferase by promoter hypermethylation is associated with G to A mutations in K-ras in colorectal tumorigenesis. Cancer Res. 2000;60(9):2368–2371. [PubMed] [Google Scholar]
- 20.Murphy M, Ahn J, Walker KK, et al. Transcriptional repression by wild-type p53 utilizes histone deacetylases, mediated by interaction with mSin3a. Genes Dev. 1999;13(19):2490–2501. doi: 10.1101/gad.13.19.2490. doi:10.1101/gad.13.19.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suzuki H, Ouchida M, Yamamoto H, et al. Decreased expression of the SIN3A gene, a candidate tumor suppressor located at the prevalent allelic loss region 15q23 in non-small cell lung cancer. Lung Cancer. 2008;59(1):24–31. doi: 10.1016/j.lungcan.2007.08.002. doi:10.1016/j.lungcan.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Pyrko P, Schonthal AH, Hofman FM, Chen TC, Lee AS. The unfolded protein response regulator GRP78/BiP as a novel target for increasing chemosensitivity in malignant gliomas. Cancer Res. 2007;67(20):9809–9816. doi: 10.1158/0008-5472.CAN-07-0625. doi:10.1158/0008-5472.CAN-07-0625. [DOI] [PubMed] [Google Scholar]
- 23.Biroccio A, Bufalo DD, Ricca A, et al. Increase of BCNU sensitivity by wt-p53 gene therapy in glioblastoma lines depends on the administration schedule. Gene Ther. 1999;6(6):1064–1072. doi: 10.1038/sj.gt.3300935. doi:10.1038/sj.gt.3300935. [DOI] [PubMed] [Google Scholar]
- 24.Gong J, Ammanamanchi S, Ko TC, Brattain MG. Transforming growth factor beta 1 increases the stability of p21/WAF1/CIP1 protein and inhibits CDK2 kinase activity in human colon carcinoma FET cells. Cancer Res. 2003;63(12):3340–3346. [PubMed] [Google Scholar]
- 25.Liu W, Konduri SD, Bansal S, et al. Estrogen receptor-alpha binds p53 tumor suppressor protein directly and represses its function. J Biol Chem. 2006;281(15):9837–9840. doi: 10.1074/jbc.C600001200. doi:10.1074/jbc.C600001200. [DOI] [PubMed] [Google Scholar]
- 26.Sayeed A, Konduri SD, Liu W, et al. Estrogen receptor alpha inhibits p53-mediated transcriptional repression: implications for the regulation of apoptosis. Cancer Res. 2007;67(16):7746–7755. doi: 10.1158/0008-5472.CAN-06-3724. doi:10.1158/0008-5472.CAN-06-3724. [DOI] [PubMed] [Google Scholar]
- 27.Ueda Y, Doi T, Takaki M, et al. Levetiracetam enhances endogenous antioxidant in the hippocampus of rats: in vivo evaluation by brain microdialysis combined with ESR spectroscopy. Brain Res. 2009;1266:1–7. doi: 10.1016/j.brainres.2009.02.040. [DOI] [PubMed] [Google Scholar]
- 28.Patsalos PN. Properties of antiepileptic drugs in the treatment of idiopathic generalized epilepsies. Epilepsia. 2005;46(suppl 9):140–148. doi: 10.1111/j.1528-1167.2005.00326.x. [DOI] [PubMed] [Google Scholar]
- 29.Doheny HCRN, Whittington MA, Jefferys JG, Patsalos PN. Blood and cerebrospinal fluid pharmacokinetics of the novel anticonvulsant levetiracetam (ucb L059) in the rat. Epilepsy Res. 1999;34(2–3):161–168. doi: 10.1016/s0920-1211(98)00104-1. doi:10.1016/S0920-1211(98)00104-1. [DOI] [PubMed] [Google Scholar]
- 30.Hirsch LJ, Arif H, Buchsbaum R, et al. Effect of age and comedication on levetiracetam pharmacokinetics and tolerability. Epilepsia. 2007;48(7):1351–1359. doi: 10.1111/j.1528-1167.2007.01043.x. doi:10.1111/j.1528-1167.2007.01043.x. [DOI] [PubMed] [Google Scholar]
- 31.Eyal S, Yagen B, Sobol E, et al. The activity of antiepileptic drugs as histone deacetylase inhibitors. Epilepsia. 2004;45(7):737–744. doi: 10.1111/j.0013-9580.2004.00104.x. doi:10.1111/j.0013-9580.2004.00104.x. [DOI] [PubMed] [Google Scholar]
- 32.Nutt CL, Loktionova NA, Pegg AE, Chambers AF, Cairncross JG. O(6)-methylguanine-DNA methyltransferase activity, p53 gene status and BCNU resistance in mouse astrocytes. Carcinogenesis. 1999;20(12):2361–2365. doi: 10.1093/carcin/20.12.2361. doi:10.1093/carcin/20.12.2361. [DOI] [PubMed] [Google Scholar]
- 33.Srivenugopal KS, Shou J, Mullapudi SR, et al. Enforced expression of wild-type p53 curtails the transcription of the O(6)-methylguanine-DNA methyltransferase gene in human tumor cells and enhances their sensitivity to alkylating agents. Clin Cancer Res. 2001;7(5):1398–1409. [PubMed] [Google Scholar]