Abstract
We detected distinct plasma concentration profiles of S100B, neuropeptide Y, and secretagogin in 3 of 191 patients enrolled in a previous study investigating brain-tissue–related markers in the blood of patients with atrial fibrillation. Intriguingly, 2 of these 3 patients, both of whom were without neurological symptoms at the time of blood sampling, were diagnosed with malignant glioma (MG) approximately 1 year later. To our knowledge, this is the first report indicating that distinct blood biomarker profiles may be detected long before clinical manifestation of MG.
Keywords: biomarker, blood, glioma, neurooncology
Malignant gliomas (MGs), including glioblastoma and anaplastic astrocytoma, are the most common primary brain tumors of adults.1 So far, no blood biomarkers have been established for screening and early diagnosis, differential diagnosis, or follow-up of MG, although several potential markers have been investigated in brain tumor patients.2,3 To our knowledge, there are no previous reports on biochemical blood alterations preceding clinical manifestation of MG. Here, we report that we unexpectedly detected markedly elevated plasma concentrations of S100B, neuropeptide Y (NPY), and secretagogin (SCGN) in 3 (Patients 1–3) of 191 patients enrolled in a previous study investigating brain-tissue–related markers in the blood of patients with atrial fibrillation (AF).4 Intriguingly, 2 of these 3 patients (Patients 1 and 2) were diagnosed with MG approximately 1 year after blood sampling. Our observation indicates that S100B, NPY, and SCGN could be useful as blood biomarkers in brain tumor patients.
Patients and Methods
All 191 patients described in this report were enrolled in a previous study investigating the value of blood biomarkers (S100B, NPY, SCGN, matrix metalloprotease 9, brain natriuretic peptide, and brain fatty acid–binding protein) for the detection of ischemic brain lesions in patients with AF.4 Assessment by an experienced neurologist showed that all patients were free from major neurological deficits at the time of inclusion in the study. Exclusion criteria included history of stroke or neurodegenerative disease. In all patients enrolled in the study, we repeatedly sampled peripheral blood using EDTA-treated tubes beginning within 3 days after hospital admission.4
Measurement of concentrations of the individual makers was performed by Biosite and Inverness Medical Company using a fluorescence bioassay as described previously.4 Data were handled in a double-blind manner and the marker blood concentrations for all patients were disclosed to us only after the end of the enrollment period. At data disclosure, we noted marked elevations of S100B, NPY, and SCGN plasma concentrations in 3 of the 191 investigated patients.
Retrospective evaluation of the clinical history of these 3 patients revealed that 2 of them (Patients 1 and 2) had been diagnosed with MG 11 and 13 months after blood sampling, respectively, and had already succumbed to the disease. For the present report, the clinical history of all the 3 patients was reconstructed from hospital files. In addition, Patient 3 was interviewed about his current health status by telephone in September 2009.
To exclude the possibility that more than 2 of our patients were diagnosed with a brain tumor, a search for all patients of our study cohort was performed in the databases of the Austrian Brain Tumor Registry (ABTR) and the Austrian National Cancer Registry (ANCR) (date of search: November 2009).5
We calculated the expected incidence of MG in a cohort of 191 persons of the Austrian population based on the estimated overall Austrian brain tumor incidence from the ABTR. Standardized incidence ratios (SIRs) were calculated a the ratio of observed/expected cases and were expressed a percentage. Age-specific incidence rates for individual age cohorts were obtained from the ABTR. Annual population estimates were provided by the ANCR. The number of expected malignant brain tumor cases was determined by calculating the sum of age-specific incidence rates times the corresponding age-specific person time under risk.
Results
Patients
Patient 1
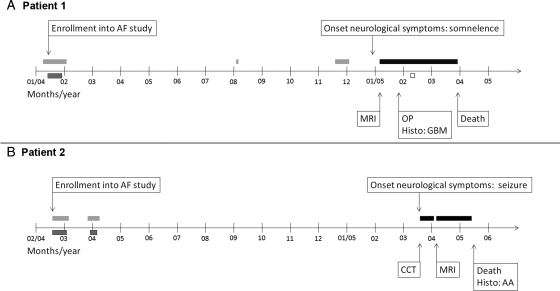
The clinical course of Patient 1 is depicted in Fig. 1A. Patient 1 was a 64-year-old man when he was enrolled in our study because of AF in January 2004. Beginning January 19, 2004, 7 blood samples for the measurement of blood biomarkers were taken over a period of 8 days. At this time, the patient displayed no neurological symptoms.
Fig. 1.
Clinical course of patients 1 and 2. (A) Time line showing the clinical course of Patient 1 from enrollment into our study until death. Gray bars without frame: patient admitted to hospital for cardiac reasons, no neurological symptoms; gray bar with black frame: sampling for analysis of blood biomarkers (n = 7, blood samples on different days); black bar: patient admitted to hospital due to neurological symptoms/brain tumor; white bar with black frame: temozolomide therapy (200mg/m2 for 5 days). (B) Time line showing the clinical course of Patient 2 from enrollment into our study until death. Gray bar without frame: patient admitted to hospital for cardiac reasons, no neurological symptoms; gray bar with black frame: blood sampling for analysis of blood biomarkers (n = 13, blood samples on different days); black bar: patient admitted to hospital due to neurological symptoms/brain tumor. AA, anaplastic astrocytoma; AF, atrial fibrillation; CCT, cranial computer tomography; Histo, histological diagnosis; OP, neurosurgical operation; MRI, brain magnetic resonance imaging.
The patient's prior clinical history included valvular aortic stenosis, renal artery stenosis, coronary artery disease, and myocardial infarction several years earlier and arterial hypertension, noninsulin-dependent diabetes mellitus type II, peripheral arterial disease stage IIa, erythrocytosis, and transient facial paresis 8 years earlier.
The patient was re-hospitalized twice (August 2004 and November 2004) in the course of the following months due to cardiac reasons. At both hospital stays, he did not exhibit any neurological symptoms.
On January 4, 2005, the patient was hospitalized due to progressive somnolence for 3 days. Neuroradiology showed a 5.5 × 2.5 cm large tumor in the right temporal lobe with marked perifocal edema and contrast enhancement. The tumor was subtotally resected on January 25, 2005. The histological diagnosis was glioblastoma multiforme (WHO grade IV).1 The patient received oral chemotherapy with temozolomide at a dosage of 200 mg/m2 for 1 cycle (5/28 days), which was well tolerated. In February 2005, the patient suffered deep venous thrombosis of the left leg and anticoagulant therapy was initiated. At the end of February 2005, the patient developed progressive confusion and neuroradiology showed a significant tumor progression. The patient died on March 26, 2005.
Patient 2
The clinical course of Patient 2 is depicted in Fig. 1B. Patient 2 was a 72-year-old woman when she was enrolled in our study because of AF in February 2004. Beginning February 16, 2004, 7 blood samples for the measurement of blood biomarkers were taken over a period of 15 days. At this time, the patient displayed no neurological symptoms.
The patient's prior clinical history included glaucoma, cataract, uterine myoma, parotid tumor of unknown dignity, and arterial hypertension.
The patient was re-hospitalized for 8 days in March and April 2004 for cardiac electroversion. At this hospital stay, 6 further blood samples for determination of blood biomarker levels were taken over a period of 6 days. During this period, the patient did not exhibit any neurological symptoms, and she was well when she was discharged.
On March 3, 2005, the patient suffered a grand mal seizure and was admitted to the hospital. Neuroradiology revealed a diffuse tumor in the thalamic, temporo-insular, and occipital regions, showing contrast enhancement. Ancillary neuroradiological investigations (positron emission tomography) were scheduled, and the patient was discharged but was readmitted a few days later because of increasing disorientation and repeated falls. The tumor was not biopsied or operated because of severe thrombocytopenia (probably heparin-induced). There was progressive worsening of the clinical condition with progressive neurological symptoms, and the patient died on May 18, 2005. At autopsy, the histological diagnosis was anaplastic astrocytoma (WHO grade III).1
Patient 3
Patient 3 was enrolled in our study at age 30 and was admitted to the Emergency Department of our institution in March 2004 because of exercise-associated syncope. At presentation to the hospital, the neurological status was normal. An ECG revealed AF and laboratory analysis showed hypokalemia. The patient reported that he had been previously healthy. From March 9–11, 3 blood samples were taken for biomarker analysis. The AF subsided after electrolyte substitution, and the patient was discharged. In July 2004, electric cardioversion was performed because of recurrent AF. As of October 2009, the patient still has intermittent AF but is otherwise healthy and well. He has no neurological symptoms and works full time in an academic profession. Brain imaging has never been performed in Patient 3.
Patients 4–191
Of these 188 patients, 119 were men and 69 were women. The patient age at enrollment in our study ranged from 17.02 to 98.12 years (median 68.5 years). At enrollment in the study, all of these 188 patients had AF and none displayed neurological symptoms. None of these patients was registered with a brain tumor diagnosis in the databases of the ABTR or the ANCR as of November 2009.
Blood Biomarkers
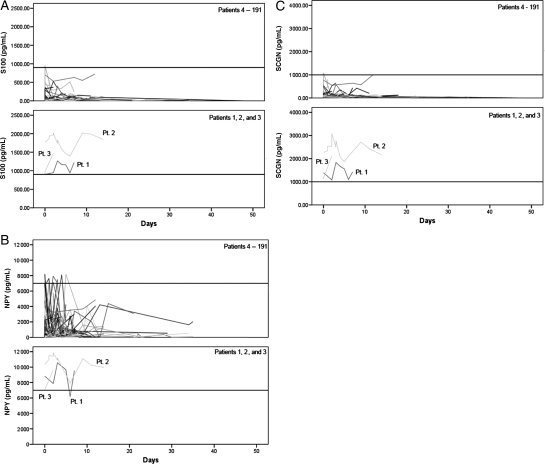
Plasma levels of S100B, NPY, and SCGN in all patients included in this report are summarized in Table 1 and Fig. 2. All 3 markers showed marked elevations in Patients 1–3 when compared with all other patients of our study cohort (Patients 4–191). None of the patients in our cohort had distinct profiles of BNP, MMP9, and BFABP blood levels (data not shown).
Table 1.
Plasma concentrations of S100B, NPY, and SCGN in all patients included in our study
| Brain tumor | Number of blood samples per patient | S100B (pg/mL) (median, range) | NPY (pg/mL) (median, range) | SCGN (pg/mL) (median, range) | |
|---|---|---|---|---|---|
| Patient 1 | Yes | 7 | 1160, 903–1266 | 9560, 6170–10 560 | 1424, 1076–1834 |
| Patient 2 | Yes | 13 | 1843, 1393–2023 | 10 890, 7930–11 860 | 2436, 1868–3078 |
| Patient 3 | No evidence | 3 | 977, 926–1477 | 7070, 6940–9619 | 1155, 1118–2124 |
| Patients 4–191 (n = 188) | No evidence | Range 1–16 (median 4) | <LOD, <LOD–947 | 20, <LOD–8200 | 48, <LOD–1076 |
Abbreviations: LOD, level of detection; NPY, neuropeptide Y; SCGN, secretagogin. All 3 markers are markedly elevated in Patients 1–3 when compared with all other patients (Patients 4–191).
Fig. 2.
Time course of blood biomarker concentrations. (A) Plasma concentrations of S100B in Patients 1–3 (lower panel) are markedly higher than that in all other patients of our study cohort (Patients 4–191; upper panel). (B) Plasma concentrations of NPY in Patients 1–3 (lower panel) are markedly higher than that in all other patients of our study cohort (Patients 4–191; upper panel). (C) Plasma concentrations of SCGN in Patients 1–3 (lower panel) are markedly higher than that in all other patients of our study cohort (Patients 4–191; upper panel). NPY, neuropeptide Y; Pt., patient; SCGN, secretagogin.
Estimation of Malignant Brain Tumor Incidence
The expected incidence of malignant brain tumors in our cohort of 191 persons was 0.139 leading to an estimated SIR of 14.39 (95% CI: 1.74–51.99).
Discussion
Here, we report markedly elevated plasma levels of S100B, NPY, and SCGN in the peripheral blood of 3 of the 191 AF patients, 2 of whom were diagnosed with MG 11 and 13 months after blood sampling. To our knowledge, this is the first report of distinct blood marker profiles preceding clinical manifestation of MG.
S100B, SCGN, and NPY are expressed in brain tissue and in some gliomas.6–9 S100B serum concentrations in patients diagnosed with glioma have been investigated in some studies,10–13 whereas there seem to be no data on NPY and SCGN blood concentrations in brain tumor patients.
Little is known about the growth kinetics of MG prior to diagnosis. However, clinical experience suggests that primary MG may develop within few months.14–16 Unfortunately, in neither of our 2 MG patients described in the present report was neuroimaging performed in the period of blood sampling. However, blood marker levels suggest that premalignant or early malignant disease might have been already present in both patients a long time (11 months in Patient 1 and 13 months in Patient 2) before clinical manifestation. It may be speculated that the disruption of the blood–brain barrier and/or the production of the proteins by tumor cells may have caused the elevated marker concentrations in our patients.
Patient 3, similar to the 2 patients, who were later diagnosed with MG, displayed markedly elevated blood levels of S100B, NPY, and SCGN when he presented to us with exercise-associated syncope and AF. It is unclear what has caused the marker elevations in this case. Perhaps brain ischemia related to the syncope-caused release of S100B, NPY, and SCGN into the patient's blood. It would be interesting to perform brain MRI to search for old ischemic lesions and to exclude the presence of a clinically silent brain neoplasia in this patient. However, due to ethical reasons, we decided not to burden the patient with potential brain disease based on our preliminary data. Anyway, the findings in Patient 3 indicate that plasma-level elevations of S100B, NPY, and SCGN may not be entirely specific for brain tumors. Indeed, elevated plasma concentrations of these proteins have been detected in some other diseases.4,6,17–19 However, most well-established and clinically useful tumor markers such as carcinoembryonic antigen, CA 19-9, or CA125 also lack perfect specificity.
Searches in the ABTR and ANCR databases, which record brain tumor cases nationwide in Austria,5 did not identify any other brain tumor patient in our cohort apart from Patients 1 and 2. Moreover, the expected number of malignant brain tumors in an Austrian population of 191 persons based on the estimated overall Austrian brain tumor incidence from the ABTR is 0.139. Thus, it is very unlikely that there are undetected MG cases among our study subjects. So, sensitivity for the detection of MG before onset of clinical symptoms by measurement of S100B, NPY, and SCGN plasma concentrations may be high.
To summarize, our observations suggest that S100B, NPY, and SCGN could be useful as blood biomarkers in brain tumor patients. Further studies that include the evaluation of sensitivity and specificity and the characterization of changes in blood concentrations during disease course and treatment are needed to establish the clinical usability of these markers in neuro-oncology. To this end, we have, at our center, initiated prospective studies including patients with other brain tumors, such as cancer metastasis and benign tumors, and sequential blood sampling during therapy.
Conflict of interest statement. None declared.
Funding
We acknowledge Biosite and Inverness Medical Company (Waltham, MA, USA) and Nadine Zielonke (Austrian National Cancer Registry) for their cooperation and support.
References
- 1.Louis DN, Ohgaki H, Wiestler OD, Cavanee WK. WHO Classification of Tumours of the Central Nervous System. Lyon: IARC Press; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jung CS, Foerch C, Schanzer A, Heck A, Plate KH, Seifert V, et al. Serum GFAP is a diagnostic marker for glioblastoma multiforme. Brain. 2007;130(pt 12):3336–3341. doi: 10.1093/brain/awm263. doi:10.1093/brain/awm263. [DOI] [PubMed] [Google Scholar]
- 3.Lin Y, Jiang T, Zhou K, Xu L, Chen B, Li G, et al. Plasma IGFBP-2 levels predict clinical outcomes of patients with high-grade gliomas. Neurooncology. 2009;11(5):468–476. doi: 10.1215/15228517-2008-114. doi:10.1215/15228517-2008-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gartner W, Zierhut B, Mineva I, Sodeck G, Leutmezer F, Domanovits H, et al. Brain natriuretic peptide correlates with the extent of atrial fibrillation-associated silent brain lesions. Clin Biochem. 2008;41(18):1434–1439. doi: 10.1016/j.clinbiochem.2008.09.096. doi:10.1016/j.clinbiochem.2008.09.096. [DOI] [PubMed] [Google Scholar]
- 5.Wohrer A, Waldhor T, Heinzl H, Hackl M, Feichtinger J, Gruber-Mosenbacher U, et al. The Austrian Brain Tumour Registry: a cooperative way to establish a population-based brain tumour registry. J Neurooncol. 2009;95(3):401–411. doi: 10.1007/s11060-009-9938-9. doi:10.1016/j.clinbiochem.2008.09.096. [DOI] [PubMed] [Google Scholar]
- 6.Gartner W, Lang W, Leutmetzer F, Domanovits H, Waldhausl W, Wagner L. Cerebral expression and serum detectability of secretagogin, a recently cloned EF-hand Ca(2+)-binding protein. Cereb Cortex. 2001;11(12):1161–1169. doi: 10.1093/cercor/11.12.1161. doi:10.1093/cercor/11.12.1161. [DOI] [PubMed] [Google Scholar]
- 7.Camby I, Nagy N, Lopes MB, Schafer BW, Maurage CA, Ruchoux MM, et al. Supratentorial pilocytic astrocytomas, astrocytomas, anaplastic astrocytomas and glioblastomas are characterized by a differential expression of S100 proteins. Brain Pathol. 1999;9(1):1–19. doi: 10.1111/j.1750-3639.1999.tb00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allen JM, Hoyle NR, Yeats JC, Ghatei MA, Thomas DG, Bloom SR. Neuropeptides in neurological tumours. J Neurooncol. 1985;3(3):197–202. doi: 10.1007/BF00165179. doi:10.1007/BF00165179. [DOI] [PubMed] [Google Scholar]
- 9.Pipp I, Wagner L, Rossler K, Budka H, Preusser M. Secretagogin expression in tumours of the human brain and its coverings. APMIS. 2007;115(4):319–326. doi: 10.1111/j.1600-0463.2007.apm_590.x. doi:10.1111/j.1600-0463.2007.apm_590.x. [DOI] [PubMed] [Google Scholar]
- 10.Song WS, Guo LB, Hong ZY, Li JJ, Wu J. Serum S100 protein and radiation-induced brain injury in astrocytoma patients. Di Yi Jun Yi Da Xue Xue Bao. 2005;25(6):723–725. [PubMed] [Google Scholar]
- 11.Rajendra A, Spinella PC, Drott HR, Dominguez TE, Sutton L, Helfaer M. S-100beta protein–serum levels in children with brain neoplasms and its potential as a tumor marker. J Neurooncol. 2004;67(3):345–349. doi: 10.1023/b:neon.0000024216.15923.77. doi:10.1023/B:NEON.0000024216.15923.77. [DOI] [PubMed] [Google Scholar]
- 12.Vos MJ, Postma TJ, Martens F, Uitdehaag BM, Blankenstein MA, Vandertop WP, et al. Serum levels of S-100B protein and neuron-specific enolase in glioma patients: a pilot study. Anticancer Res. 2004;24(4):2511–2514. [PubMed] [Google Scholar]
- 13.Ortiz-Munoz B, Menendez-Lopez A, Yaya-Tur R, Arribas-Alpuente L, Maiquez-Richart J, Bordes-Monmeneu M. S100 protein in tumours of the central nervous system. Rev Neurol. 2003;36(11):1011–1015. [PubMed] [Google Scholar]
- 14.Hofer S, Kollias S, Weller M. Evolution of glioblastoma. Acta Oncol. 2009;48(4):630–631. doi: 10.1080/02841860802637799. doi:10.1080/02841860802637799. [DOI] [PubMed] [Google Scholar]
- 15.Okamoto K, Ito J, Takahashi N, Ishikawa K, Furusawa T, Tokiguchi S, et al. MRI of high-grade astrocytic tumors: early appearance and evolution. Neuroradiology. 2002;44(5):395–402. doi: 10.1007/s00234-001-0725-3. doi:10.1007/s00234-001-0725-3. [DOI] [PubMed] [Google Scholar]
- 16.Landy HJ, Lee TT, Potter P, Feun L, Markoe A. Early MRI findings in high grade glioma. J Neurooncol. 2000;47(1):65–72. doi: 10.1023/a:1006494604527. doi:10.1023/A:1006494604527. [DOI] [PubMed] [Google Scholar]
- 17.Caglar K, Kutluk T, Varan A, Koray Z, Akyuz C, Yalcin B, et al. Leptin and neuropeptide Y plasma levels in children with cancer. J Pediatr Endocrinol Metab. 2005;18(5):485–489. doi: 10.1515/jpem.2005.18.5.485. [DOI] [PubMed] [Google Scholar]
- 18.Satoh C, Satoh F, Takahashi K, Murakami O, Sone M, Totsune K, et al. Elevated plasma immunoreactive neuropeptide Y concentrations and its increased urinary excretion in patients with advanced diabetic nephropathy. Endocr J. 1999;46(1):139–146. doi: 10.1507/endocrj.46.139. doi:10.1507/endocrj.46.139. [DOI] [PubMed] [Google Scholar]
- 19.Sedaghat F, Notopoulos A. S100 protein family and its application in clinical practice. Hippokratia. 2008;12(4):198–204. [PMC free article] [PubMed] [Google Scholar]