Abstract
Exposure to chronic and traumatic stress has been associated with the dysregulation of crucial stress response systems. Acculturation has been associated with unique forms of chronic psychosocial stress. The purpose of this study was to examine the effects of exposure to early traumatic stress and acculturation on dysregulation of the cortisol awakening response (CAR) in Mexican-American adults. Salivary cortisol samples were collected at awakening and 30, 45, and 60 minutes thereafter, on two consecutive weekdays from 59, healthy Mexican American adult males (26) and females (33), ages 18-38 years. Participants were assessed for level of acculturation and exposure to early trauma. Data were analyzed using a mixed effects regression model with repeated measures at four time points. Mixed effects regression results indicated a significant Early Trauma x Time interaction (p=.0029) and a significant Acculturation x Time interaction (p=.0015), after controlling for age and sex. Subsequent analyses of the interaction of Trauma x Acculturation x Time showed that more than minimal exposure to either risk factor was associated with attenuation of the awakening cortisol response (p=.0002). Higher levels of acculturation with greater Anglo-orientation were associated with attenuation of the CAR in Mexican-American adults. Both moderate and higher levels of exposure to early trauma were associated with an attenuated CAR. However, greater exposure to both risk factors was only incrementally worse than exposure to either one.
Keywords: Acculturation, Childhood Trauma, HPA axis, Cortisol, Mexican-Americans
Introduction
Childhood trauma is a significant problem in the United States and is associated with mental and physical health problems in adulthood (Heim et al., 2000a, 2001a; Gunnar and Vazquez, 2001, 2006; Dong et al., 2004; Nicolaidis et al., 2009), that lead to increased health care utilization and costs (Felitti, 1991; Felitti et al., 1998; Newman et al., 2000). Early exposure to trauma is a robust risk factor for the development of anxiety and depressive disorders in adulthood (Kessler and Magee, 1993; Kessler et al.,1997; Young et al., 1997; Kendler et al., 2001; Safren et al., 2002), and is associated with increased reports of emotional distress (Spertus et al., 2003), risk for suicidal behaviors ( McCauley et al., 1997), greater behavior problems (Messina and Grella 2006), greater psychiatric symptom severity (Yehuda et al., 2001), higher risk for substance use disorders (Epstein et al., 1998; Fleming et al., 1999), greater risk of ischemic heart disease (Dong et al., 2004), and more overall long-term physical health consequences (Lechner et al., 1993; Bendixen et al., 1994; McCauley et al., 1997; Moeller et al., 1993; Irish et al., 2009). Findings from the National Child Abuse and Neglect Data System (NCANDS; 2002) and the National Child Traumatic Stress Network (NCTSN; 2005), show that a significant percentage of children exposed to childhood trauma from maltreatment are Latino/Hispanic. When compared to Caucasian children, Latino/Hispanic children experience higher rates of domestic violence, community violence, and family-perpetrated abuse (Sanders-Phillips et al., 1995; Shaw et al., 2001). Latino/Hispanic children are also at greater risk for exposure to certain forms of early trauma including: general or complex traumas (72%), domestic violence (53%), caregiver impairment (47%), emotional abuse (42%), and traumatic loss (42%), (NCTSN; 2005). Latino/Hispanic victims of early trauma report higher rates of post-abuse depression (Sanders-Phillips et al., 1995; Roosa et al., 1999), and more psychiatric symptoms associated with anxiety, depression and dissociation compared to their trauma-free counterparts (Mennen, 2000; Martinez-Taboas and Bernal, 2000). In addition, childhood trauma is associated with overall poorer subjective health in Latinos/Hispanic men, and more muscular skeletal and gastrointestinal symptoms in females (Baker et al., 2009). Moreover, depression appears to mediate the relationship between childhood trauma and negative health outcomes for Latino/Hispanic men and women (Baker et al., 2009). Clearly, early exposure to traumatic stress represents a significant, pre-existing risk factor for the onset of certain mental and physical health problems in the Latino/Hispanic community.
In addition to pre-existing exposure to early traumatic stress, some high risk Latinos/Hispanics may be exposed to culturally unique sources of chronic stress as adults, including the stress associated with the challenges of acculturation (Cervantes and Castro, 1985; Mena et al., 1987; NCTSN, 2005; Bernal and Santiago, 2006). Acculturation is the process that involves adaptation into a host culture (Mena et al., 1987). The combination of psychological, somatic, and social stressors that may be associated with the process of adaptation into a host culture is acculturative stress (Berry, 1980; Mendoza, 1984; Sodowsky et al., 1991). The stressors associated with acculturation increase risk for the onset of negative health outcomes in high risk Mexican-Americans, and the challenges associated with acculturation modulate the relationship between childhood trauma and negative health outcomes (Matheson et al., 2008). However, the specific physiological mechanisms contributing to this relationship remain to be clarified (Williams and Berry, 1991; Hovey and King, 1996; Aranda et al., 2001). Given these findings, an examination of the interplay between early traumatic stress and unique cultural risk factors (i.e., acculturation) on crucial biological stress response systems may be of particular importance in this understudied and underserved minority group.
Stress-induced dysregulation of the Hypothalamic-Pituitary-Adrenal (HPA) axis is one neurobiological pathway that may link childhood trauma and acculturation to negative health outcomes (McEwen et al., 1998; Danese et al., 2007). HPA axis activation during early development following childhood trauma increases risk for mood and anxiety disorders (Goodwin and Stein, 2004; Heim et al., 2006). A series of seminal studies conducted by Heim and colleagues (1999, 2000a, 2000b, 2001a, 2001b) suggest that exposure to childhood trauma (sexual/physical abuse), during critical periods of neural plasticity, may alter the set point of the stress response system. This may result in adaptive counter-regulation of the adrenal cortex with HPA activation during early development leading to adult hypocortisolism. It is possible that for Hispanic children, exposure to childhood trauma during critical periods of neural plasticity, coupled with the stressors associated with acculturation as adults, may alter the set point of the stress response system. This may lead to adaptations that result in aberrant cortisol secretion and poor psychological and physical health outcomes.
Determination of the Cortisol Awakening Response (CAR) is increasingly being utilized as a means to interrogate stress-induced injury to the HPA Axis (Pruessner et al., 1999). Changes in the CAR can yield important information regarding the relationship between altered stress responsivity and injury to the awakening portion of the cortisol circadian rhythm. The sensitivity and capacity of the adrenal cortex is suggested to play a crucial role in the magnitude of the CAR (Pruessner et al., 1997, 1999; Schulz et al., 1998; Schmidt-Reinwald et al., 1999; Wust et al., 2000a; Kudielka and Kirschbaum, 2003). A lower CAR has been reported in adults with a history of childhood trauma, with psychopathology (Stein et al., 1997; Meinslschmidt and Heim, 2005), in patients with a history of childhood trauma and borderline personality disorder (Flory et al., 2009), and in women with a history of intimate partner violence (Johnson et al., 2008). However, to date, no studies have examined the effects of childhood trauma on the CAR in healthy subjects, while adequately screening out depression, which is known to alter the HPA axis and the CAR (Heim and Nemeroff, 2001a; Pruessner et al., 2003; Huber et al., 2006; Stetler and Miller, 2005). Therefore, it has been difficult to differentiate the effects of depression from the effects of trauma on the CAR. Moreover, to our knowledge, there has never been an examination of the effects of acculturation on the CAR. Therefore, it has been impossible to unravel the effects of childhood trauma from the effects of the psychosocial stress that is often associated with the challenges of acculturation.
The aims of the present investigation were to examine: 1) the effects of early traumatic stress on the CAR in Mexican-American adults screened for depression; 2) the effects of acculturation on the CAR; and 3) the effects of a trauma x acculturation interaction on the CAR. We hypothesized that childhood trauma and acculturation would be associated with an attenuated CAR.
MATERIALS AND METHODS
Participants and Study Design
The University of Texas Institutional Review Board approved the study, and all participants gave written, informed consent prior to participation. Participants of Mexican descent (n=59), aged 18 to 38, were recruited from the San Antonio metropolitan area, through advertisements in the community and local college campuses. During an initial visit to the laboratory, participants underwent an in-person screening interview and a battery of self-report assessments designed to identify and exclude factors known to potentially affect the HPA axis including: lifetime depression (Bhagwager et al., 2005; Shea et al., 2007), use of oral contraceptives in the past 60 days (Meulenberg and Hofman et al., 1990; Pruessner et al., 1997,1999), current pregnancy (Meulenberg and Hofman et al., 1990), menstrual cycle abnormalities in the past 60 days (Suh et al., 1988; Bao et al., 2003, 2004), strenuous aerobic exercise (more than 2 hours per day for 4 or more days per week in the past 60 days (Kanaley et al., 2001; Hansen et al., 2008; Kelly et al., 2008), reported medical conditions and previous head trauma, use of medications, severe obesity (defined as a body mass index of > 30.0 kg/m2) and, alcohol or other drug use disorders (Wand and Dobs, 1991; Huizink et al., 2006; Hansen et al., 2008). In addition, participants were excluded from the study who reported abnormal sleeping patterns, including disrupted sleep for more than one night per week, in the past 60 days, as determined by a 60-day sleep diary (Lasikiewicz et al., 2008), shift work including night shifts for the past week, overtime work for more than 8 hours per week in the past 30 days (Lundberg and Hellstrom, 2002; Clow et al., 2004; for review, see Hanrahan et al., 2006). To determine subjects’ economic status information regarding family income and parental occupational status was collected.
Psychometric Assessment
Depression
Participants were screened for depression using the Hamilton Depression Inventory Short Form (HDI-SF; Reynolds and Kobak, 1995), and excluded from participation based on a score of 10 or greater as recommended by Reynolds and Kobak (1995) indicating a strong likelihood of depression. The HDI-SF has strong psychometric properties and the reliability for the current Mexican-American sample was acceptable (α=.78).
Substance Abuse/Dependence
Participants were screened and excluded for alcohol and other drug use disorders using the World Health Organization Alcohol, Smoking and Substance Involvement Screening Test (WHO ASSIST; Humeniuk et al., 2008). The ASSIST assesses the frequency of lifetime and recent use (within the past 3 months) and problems associated with the use of alcohol and other drugs of abuse (e.g., cannabis, cocaine, stimulants, etc.). The ASSIST has strong psychometric properties and the reliability for the current subject sample was acceptable (α=.80).
Menstrual Irregularities, Premenstrual Dysphoric Disorder (PMDD)
The Calendar of Premenstrual Experiences (CPE; Mortola et al., 1990) was used to assess and exclude participants with PMDD, irregular menstrual cycles and premenopausal symptoms. The test-retest reliability of the calendar given in the same phase of two consecutive menstrual cycles was high (r=0.78, p <.0001).
Ethnicity/Nativity
Participants reporting Hispanic origin other than Mexican or Mexican-American were excluded from the study. Participants were determined to be of Mexican descent if they reported that both biological parents and both maternal and paternal grandparents were of Mexican descent.
Acculturation
The revised Acculturation Rating Scale for Mexican Americans (ARSMA-II; Cuéllar et al., 1995) was used to assess acculturation by measuring subjects’ orientation towards Anglo culture and Mexican culture. Scores on the ARSMA Linear Acculturation Scale (LAS) are based on the extent to which participants endorse Anglo and Mexican orientations. The ARSMA-II has strong psychometric properties with good reliability for the current sample (α = .89).
Childhood Trauma
The current study assessed exposure to severe childhood stress prior to the age of 16, using the Early Trauma Inventory–Short Form adapted from the clinician administered Early Trauma Inventory (ETISR-SF; Bremner et al., 2007). The ETI-SF is a 27 item questionnaire that is comprised of “yes/no” items designed to assess whether someone has been exposed to potential traumatic experiences. The inventory includes four subscales: General Trauma, Physical Punishment, Emotional Abuse and Sexual Events, and a total trauma score is calculated by summing the total number of endorsed items across subscales. Scores are a reflection of degree of diversity of early exposure to severe childhood stress, rather than severity or frequency of exposure to any single stressor. The ETISR-SF has strong psychometric properties (Hyman et al., 2005; Bremner et al., 2007) with good reliability for the current sample (α = .82).
Measurement of the CAR
Participants were free to wake up as usual (using an alarm or spontaneously), and instructed to start sampling immediately at awakening (T0), and T30, T45 and T60 minutes following awakening. Sampling was completed on two consecutive weekdays. Participants were instructed to log wake-up times along with sampling times for comparison with electronic monitoring data. Participants were instructed to refrain from strenuous exercise and from brushing their teeth, eating or drinking, and use of alcohol, caffeine and nicotine during sampling to avoid contamination of cortisol samples (Clow et al., 2004; Badrick et al., 2007). Participants placed a roll of cotton in their mouths, chewed on it until it became saturated, and then returned it to the salivette (Sarstedt; Rommelsdorft, Germany). Participants remained sitting upright in bed until the second saliva sample was obtained, when they were free to follow their normal weekday routine during the sampling period. To employ a conservative approach to control for possible effects of menstrual cycle phase on the CAR (Bao et al., 2003, 2004), cortisol was assessed in females during the early follicular phase of their menstrual cycle (determined by menstrual diary).
We utilized electronic monitoring devices together with self-reported time of sampling to determine concordance between monitored times and self-reported times, detect deviations from the protocol and maximize accuracy in the documentation of sample times (Jacobs et al., 2005). To further address accuracy, participants collected saliva samples on two separate, consecutive days. Salivettes were stored in a vial equipped with an electronic medication event monitoring system (MEMS; Aardex; Zug, Switzerland), as recommended for use in an ambulatory setting by (Kudielka et al., 2003). The MEMS is designed to compile the dosing/sampling-compliance histories of participants. This system includes a standard plastic vial with cap that contains a threaded opening and a closure that contains a micro-electronic circuit that registers times when opened and closed. Time-stamped medication events are stored in the unit for later software analyses to maximize accuracy of recorded awakening and sampling times included in later analyses.
Hormonal Assays
Participants stored the samples in their home freezers until they were returned to the laboratory the next day and stored at −80 C until analyzed, when they were thawed at room temperature and centrifuged at 2500g for 15 minutes at −4 C. The saliva samples were kept frozen until the start of the assay. Salivary cortisol levels were assayed in duplicate using high sensitivity enzyme immunoassays (Diagnostic Systems Laboratories; Webster, Texas), with a mean lower sensitivity limit of 0.11 ug/dL, standard curve range from 0.1 to 10ug/dL. The intra-assay and inter-assay coefficients of variation were less than 5% at all levels of the calibrator curve. The concentration of cortisol in saliva was expressed as ug/dL. To minimize the potential effects of exposure to stressful events during the sampling period, participants who were currently students were not sampled the week prior to scheduled class examinations. In addition, participants indicating daily hassles, or exposure to stressful daily events during the sampling period (van Eck et al., 1996), sleep disturbances (Lasikiewicz et al., 2008) and other unusual events or protocol noncompliance (teeth brushing, eating, etc), during sampling periods were excluded from the final analyses.
Statistical Analyses
Data were available from 59 participants. Descriptive statistics are reported to characterize the sample. Cortisol data were log-transformed to correct positive skew. The primary statistical design was a mixed effects regression model with repeated measures at four time points (awakening and 30, 45, and 60 minutes thereafter). Fixed design effects were self-reported Early Trauma (ET), used as a continuous, dimensional variable (scores ranged from 0-18), Time (a fixed classification factor with four levels), and their interaction. The ET x Time interaction was the primary focus as it tests the association between level of trauma and changes in cortisol over time. The same analysis design was used to assess effects of Acculturation (ARSMA-II; LAS) on the cortisol response. First, we looked at Acculturation, substituting the continuous measure of acculturation for the measure of early trauma in a mixed effects regression model with repeated measures as above. Then, early trauma and acculturation were examined together in a mixed regression model that included main effects of both factors and their interaction. Primary interest was again on the interactions of these terms with time, testing unique direct (main) and moderating (interaction) effects on the cortisol response. Continuous variables were centered by subtracting the mean to reduce confounding of lower and higher order effects.
Modeling time as a fixed classification factor simplified specification of the covariance structure and presentation of the results, and was supported by the observation of extremely little intra-individual variability in the timing of cortisol sampling: 93% of the 339 samples were obtained within five minutes of the scheduled time, and 97% within 10 minutes of protocol. Two samples were obtained 61 and 75 minutes later than scheduled. These were both from the same participant, and could have been recording errors as they are almost exactly one hour off. Omitting them had no meaningful effect on the analyses, so they were retained as recorded. We specified the Kenward-Rogers adjustment for degrees of freedom and a spatial power structure for the covariance matrix with measures at 0, 30, 45 and 60 minutes. This is a generalization of first-degree autoregressive structure to the case of unequally spaced measures, and all of the likelihood-criteria provided by SAS MIXED (AIC, AICC, BIC) indicated a better fit than with compound symmetry, autoregressive, or a fully unstructured matrix (Littell, et al., 1996, pp. 126-130).
All but three participants provided cortisol data following the same measurement protocol on two weekdays. Summary cortisol statistics are presented separately for days one and two to enable comparison with previous data in other samples, but for the regression analyses we used the average of the day one and day two data. Averaging over a period from two to six days and weekday sampling minimizes the effects of state factors, increases trait specificity (Clow et al., 2004; Kunz-Ebrecht et al., 2004, Scholtz et al., 2004; Hellhammer et al., 2007; Thorn et al., 2006, 2009), and considerably simplifies the covariance structure and analysis. We had no expectation of meaningful day-to-day variability, but confirmed this judgment by performing preliminary mixed effects analyses including Day as an additional design factor. These did not reveal any significant effects involving Day (all p>0.10) or meaningfully change the results, and are not reported further. Statistical analyses were done using the SAS 9.2 statistical library. Tests were at unadjusted two-tailed p=.05. Inferences are based on the results of the analyses using the continuous measures, but analyses were also done grouping both scales into classes (e.g., low, high) for the purpose of graphing results to aid interpretation.
RESULTS
Participant Characteristics
Table 1 presents demographic characteristics of the sample. Participants were single (98%), adult males (44%) and females (56%). Most were first or second year college students, ranging in age from 18-38 years (median=20 years, M = 22, SD = 5.4) with a normal Body Mass Index (BMI). A little over half the sample (56%) reported a family income below $40,000. Just over one-fourth (28%) of the sample were first generation Mexican-Americans (born in Mexico), 28% were born in the United States, and 44% were third generation (parents and self were born in the United States). The majority were bilingual. Participants were healthy, with mean scores on the General Health scale of the RAND-36 for the current sample (M = 79.90, SD = 20.10) in line with those previously reported for healthy, young adults (M = 77.60, SD = 20.10, Vander Zee et al., 1996). They reported no current psychiatric diagnoses or current use of psychotropic medication. Individuals with HDI scores higher than 10 were excluded from the study. The mean score on the HDI for the sample was lower than normative means reported for college-aged participants (M = 5.16, SD = 4.48), (Reynolds and Kobak, 1995;Vander Zee et al., 1996), most likely due to exclusion based on a diagnosis of depression. However, scores on the HDI ranged from 0 to 9.50 (M = 2.84, SD = 2.39.) for the sample, suggesting subjects endorsed a range of depressive symptoms.
Table 1.
Sample demographics for Mexican-American adult, males and females
| Overall Sample |
Females | Males | |
|---|---|---|---|
| Gender | (n=59) | 33 (56%) | 26 (44%) |
| Age (Median and Range) | 20 (18-38) | 21(18-38) | 19 (18-37) |
| Body Mass Index (Mean and SD) | 24.39 ± 4.19 | 24.54 ± 4.5 | 24.20 ± 3.8 |
| Parental/Participant Income | |||
| Below 40K | 33 (55.9%) | 22 (66.7%) | 11 (42.3%) |
| 40K-80K | 16 (27.1%) | 7 (21.2%) | 9 (34.6%) |
| 80K and above | 10(17.0%) | 4 (12.1%) | 6 (23.1%) |
| Parental Occupational Status | |||
| Executive | 10 (16.9%) | 5(15.2%) | 5 (19.2%) |
| Administrative/Management | 18 (30.5%) | 11 (33.3%) | 7 (27.0%) |
| Clerical/Skilled Manual Labor | 20 (34.0%) | 11 (33.3%) | 9 (34.6%) |
| Semi-skilled or Unskilled Labor | 11 (18.6%) | 6 (18.2%) | 5 (19.2%) |
| Participant Education Level | |||
| ≤12 years | 4 (6.8%) | 2 (6.1%) | 2(7.7%) |
| 13 years | 23 (39.0%) | 11 (33.3%) | 12 (46.1%) |
| 14 years | 13 (22.0%) | 11 (33.3%) | 2 (7.7%) |
| 15 years | 6 (10.2%) | 2 (6.1%) | 4 (15.4%) |
| ≥16 years | 13 (22.0%) | 7 (21.2%) | 6 (23.1%) |
Exposure to Early Trauma
The prevalence of exposure to trauma in this sample was high. Ninety-five percent of the participants reported exposure to at least one childhood trauma item. However epidemiological evidence suggests that over 56% of Americans experience a lifetime trauma, and risk of exposure to general or complex traumas are reported to be as high as 72% among Latino/Hispanic children (NCTSN; 2005). Consistent with this evidence, participants, on average, reported exposure to more than five different trauma items across all trauma dimensions. Table 2 depicts mean exposures to the dimensions of trauma (ETISR-SF; Bremner et al., 2007) for the male and female participants in the sample.
Table 2.
Number of exposures to different dimensions of trauma in adult, Mexican-Americans (Means and SD)
| Trauma | Overall Sample (59) |
Males (26) |
Females (33) |
P |
|---|---|---|---|---|
| Total Traumas | 5.32 (4.25) | 6.73(4.79) | 4.21(3.45) | p<.05 |
| General Traumas | 2.42 (1.78) | 2.96(1.89) | 2.00(1.60) | p<.05 |
| Physical Punishment | 1.29 (1.33) | 1.70(1.44) | 0.97(1.16) | p<.05 |
| Emotional Abuse | 1.19 (1.58) | 1.42(1.75) | 1.00(1.44) | NS |
| Sexual Events | .42 (0.86) | 0.65(1.02) | 0.24(0.66) | NS |
Males tended to report greater exposure to all dimensions of early trauma compared with females, with significantly greater total trauma exposures (F (1, 57) = 5.51, p<.05), greater exposure to general traumas (F (1, 57) = 4.48, p <.05), and physical punishment (F (1, 57) = 4.58, p<.05). Gender differences in exposure to sexual events approached significance, although was not statistically significant (F (1, 57) = 3.51, p =.066).
Characterization of the Cortisol Awakening Response
Salivary cortisol response for the overall sample was characterized with respect to overall cortisol secretory activity measured in the period after awakening and the dynamic of the response (change in concentration from awakening to peak levels) as recommended by Clow et al. (2004). Cortisol concentrations were assessed over 4 time points, and were averaged over two days for each person. Calculation of the changes in cortisol concentration from awakening to 30 min post-awakening for trial one (11.90 ± 8 nmol/l) and trial two (10.09 ± 9 nmol/l) were comparable to what is previously reported (9.3 ± 3.1 nmol/l) (Clow et al., 2004). The correlation between delta cortisol for days one and two was r = 0.47 (df=43, p=.0003; Spearman rho=0.34, p=.01). The simple correlation across all data points (i.e., using 4 values per person with N = 59) was r = 0.53 for raw cortisol, and r=0.50 for log (cort), both p<.0001, df=57.
Early Trauma Exposure and Awakening Cortisol Response
The mixed effects regression results indicated a significant Trauma x Time interaction (F=4.85, df=3, 170, p=.003) when the Trauma scale was included as a dimensional covariate. To clarify this interaction, the sample was divided into approximate thirds based on the Trauma Score (0-2=Low, N=17; 3-6=Middle, N=23; 7-18=High, N=19). This grouping (29%, 39%, 32% of the sample respectively) was the closest to equal possible. The mixed effects regression using this trichotomy in place of the dimensional score also yielded a significant Trauma x Time interaction (F=3.13, df=6,168, p=.0062). The finding is depicted in Figure 1. The analysis was based on log-transformed data, but the figure presents antilogs to display the data in its original scale and an easily recognizable metric (a graph of the logged data looks almost identical). Addition of Age (F=0.00, df=1, 59, p=0.99) and Sex (F=1.88, df=1, 59, p=0.18), as covariates had no effect on the Trauma x Time interaction (F=4.84, df=3, 170, p=.0029).
Figure 1.
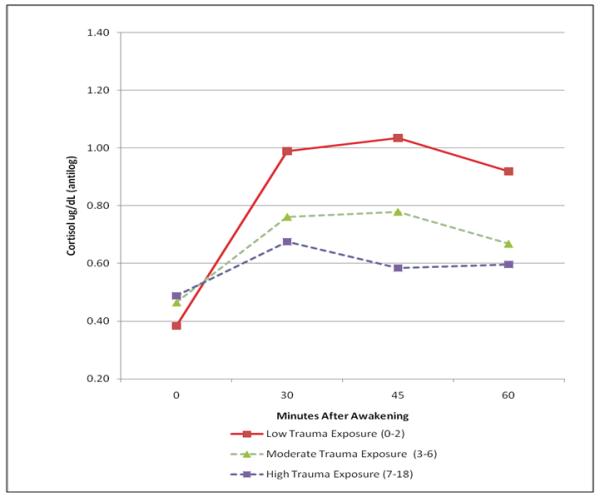
The effect of early trauma on the Cortisol Awakening Response (CAR)
The three groups defined by level of Trauma exposure show attenuated cortisol response in direct relation to the level of trauma. Follow-up analyses revealed that the significant interaction depicted in Figure 1 was largely due to the greater rise in CAR seen in the lowest Trauma group (0-2) 30 minutes after awakening. A contrast comparing the slope in the lowest Trauma group with the average of the other two was highly significant (t=3.55, df=184, p=.0005), while the rising slopes of the other two groups did not differ (t=1.02, df=184, p=0.31). Furthermore, the differences in cortisol levels observed by the 30 minute time point remained significant thereafter. A mixed effects model that included only the three post 30 minute-awakening measures showed a significant main effect of Trauma group (F=3.86, df=2, 59.2, p=0.027) and a non-significant Trauma x Time interaction (F=1.22, df=4,113, p=0.305). There was a significant difference, averaged across time, between the Low trauma and High trauma groups (t=2.76, p=0.008). The middle group fell between the others, and did not differ significantly from the low (t=1.76, p=.084) or high Trauma groups (t=1.16, p=0.250, all df=59.2).
Acculturation
The mixed effects regression using Acculturation as the independent variable also yielded a highly significant interaction with time (F=5.36, df=3, 169, p=.0015). Again, addition of Age and Sex as covariates had no effect (F=5.35, df=3, 169, p=.0015). Inspection of the distribution of acculturation scores (ARSMA-II; LAS), indicated a bimodal distribution with a natural split at zero, with scores less than zero generally indicating greater Mexican orientation, and scores greater than zero, indicating greater Anglo orientation. Figure 2 displays the cortisol response in the two groups defined by splitting scores on the ARSMA-II (LAS) at zero (Lower N=24 (41%), Higher N=35 (59%). Again, the analysis was based on log-transformed data, but Figure 2 presents antilogs to display an easily recognizable metric.
Figure 2.
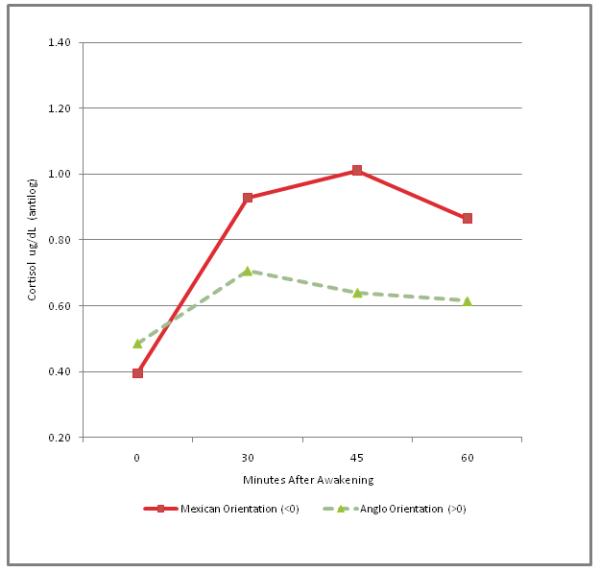
The effect of acculturation on the Cortisol Awakening Response (CAR)
Combined effects of Early Trauma and Acculturation
The results with Acculturation were strikingly similar to those obtained with Early Trauma. This raises the question, were these same or distinct effects? The Trauma and Acculturation measures were not significantly correlated (r=0.20, df=57, p=0.13), suggesting the latter. The combined effects were assessed in a fully crossed mixed effects regression. Interest focused on the 2-way interactions of Trauma and Acculturation with Time, which tests the impact of these variables on the awakening cortisol response, and the three-factor interaction (Trauma x Acculturation x Time), which tests for moderating effects. Because of the presence of multiple interactions involving continuous measures in this model, the Trauma and Acculturation measures were both centered at zero (Kraemer and Blasey, 2004).
Both the Early Trauma x Time interaction (F=2.72, df=3, 164, p=.046) and the Acculturation x Time interaction (F=3.95, df=3, 164, p=.009) were again significant. The three-factor interaction (Acculturation x Trauma x Time) was not significant (F=1.27, df=3, 164, p=0.29), suggesting that these two effects were independent and additive (i.e., cumulative).
These results are displayed in Figure 3 which is based on a mixed effects regression model analysis that used the categorical versions of the Early Trauma (3 groups) and Acculturation (2 groups) variables described above. Early Trauma (F=2.29, df=6, 159 p=0.04) and Acculturation interactions with Time (F=3.97, df=3, 158, p=0.01) were again significant.
Figure 3.
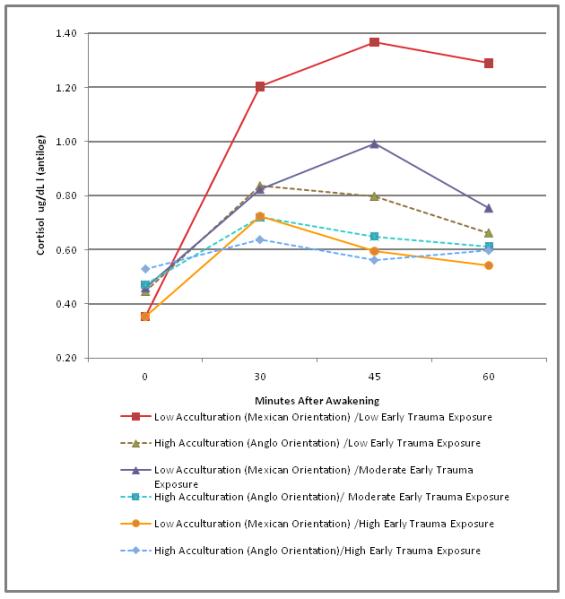
The effects of acculturation and trauma on the Cortisol Awakening Response (CAR).
The three-factor interaction was not significant, but Figure 3 gives the impression of larger differences between the Low Acculturation-Low Trauma classification and the other five groups than a simple additive model would suggest. The non-significant interaction (a complex effect with df=6) is probably due to the fact that almost all of this effect is in a single df=1 contrast, namely, the larger rise between 0 and 30 minutes in the Low Acculturation-Low Trauma group than in the other five groups, which accounts for almost all of the group differences. An omnibus test of the differences in the cortisol rise, during the first interval, among the other five groups yielded F=1.66, df=4, p=0.16, indicating that the rise in cortisol did not differ among these groups. Therefore, these groups were combined and then compared to the Low Acculturation-Low Trauma group. A test of the change in cortisol during the first 30 minutes in the Low Acculturation-Low Trauma group showed that it was significantly greater compared to the average change in the same time interval in the other five groups combined.(t=3.80, df=174, p=.0002). Figure 3 presents antilogs of the estimated means of the logged data, and there is no standard error for these exponentiated values. However, to estimate the standard errors of those exponentiated values we bootstrapped the mixed effects regression using 1000 replications, sampling cases with replacement. We saved the estimated means of the logged data in each of the 6 groups at each of the 4 time points. We exponentiated the estimated means to achieve values like those depicted in the graphic, and calculated their standard deviations over bootstrap 1000 replications sampling cases with replacement to get a bootstrap estimate of their standard errors. Table 3 presents these estimates to provide an idea of the variability of the means depicted in the graphic.
Table 3.
Estimated mean log cortisol by time after awakening in the six trauma-acculturation groups
| Minutes after awakening | |||||||
|---|---|---|---|---|---|---|---|
| Trauma | Acculturation | N | 0 | 30 | 45 | 60 | ML SEs |
| Low | Low | 9 | −1.04 (0.19) | 0.19 (0.20) | 0.31 (0.19) | 0.25 (0.22) | 0.20 |
| High | 8 | −0.81 (0.19) | −0.18 (0.16) | −0.23 (0.18) | −0.41 (0.16) | 0.21 | |
| Mid | Low | 10 | −0.78 (0.17) | −0.19 (0.07) | −0.01 (0.12) | −0.28 (0.12) | 0.19 |
| High | 12 | −0.75 (0.15) | −0.33 (0.18) | −0.43 (0.20) | −0.49 (0.19) | 0.17 | |
| High | Low | 5 | −1.04 (0.29) | −0.32 (0.16) | −0.52 (0.26) | −0.61 (0.23) | 0.27 |
| High | 15 | −0.64 (0.18) | −0.45 (0.20) | −0.58 (0.21) | −0.51 (0.18) | 0.15 | |
Note: Bootstrap standard errors in body of table based on 1000 replications sampling cases with replacement. Figure 3 depicts antilogs of these values. Rightmost column presents ML estimated standard errors by group (SAS MIXED reports one SE for all four data points per group).
We examined a summary measure of the rising limb of the CAR, namely Delta cortisol, (defined as the Peak cortisol concentration minus the cortisol concentration at time point zero), to further clarify the result. We examined the six groups formed by crossing the 3 (Trauma) x 2 (Acculturation) groups and performed a one-way analysis of variance on Delta cortisol. There was a significant interaction of Trauma x Acculturation on Delta cortisol, with higher Delta cortisol in the Low Trauma/Low Acculturation group (F=6.21, df=5, 53, p=.0001). The 5 pair wise t-tests comparing this group with the others were all significant at p<.001. None of the 10 pairwise t-tests between the other groups were significant (all p>.15).
DISCUSSION
To our knowledge this is the first examination of the effects of childhood trauma and acculturation on the CAR in Mexican-Americans. In addition, this study is distinguished by its examination of the effects of childhood trauma in healthy adults, without depression, while utilizing a carefully constructed monitoring system to determine sampling compliance. Findings from the current study show that greater exposure to early trauma is associated with attenuation of the CAR, even after controlling for age and sex. Higher levels of acculturation (greater Angloorientation) were also associated with attenuation of the CAR. This effect was largely due to group differences in the slope of the CAR from awakening to thirty minutes post-awakening. Although the initial interaction (Trauma x Acculturation x Time) was not significant, subsequent analyses showed that the Low Acculturation/Low Trauma group demonstrated a significantly greater rise in cortisol during the first 30 minutes compared to the average change for the same time interval in the other groups. Apparently, anything more than minimal exposure to either risk factor was associated with attenuation of the awakening cortisol response. High exposure to both risk factors appeared to be only incrementally worse than exposure to either one.
Our findings have an additional important novel observation. It has long been recognized that depression alters HPA axis dynamics including the CAR (Heim and Nemeroff, 2001a; Pruessner et al., 2003; Huber et al., 2006; Stetler and Miller, 2005). It has also been reported that early life trauma increases the risk for depression in adulthood (Heim and Nemeroff, 2001a; Weiss et al., 1999; Duncan et al., 1996; Bernet and Stein, 1999). However, the subjects in this study were carefully screened and excluded if they met diagnostic criteria for depression. Therefore, our findings show that both early life trauma and the stress of acculturation can attenuate the CAR independent of the influences of depression.
The findings in this study are not attributable to major methodological sources of variability and sampling error observed in some previous studies. For example, our findings cannot be attributed to the effects of depression or antidepressant use on the CAR, given that both conditions were carefully screened and excluded (Aihara et al., 2007). It is unlikely that our findings are attributable to the known effects of substance abuse on the CAR, since we assessed and excluded participants for current alcohol, nicotine and other drug use disorders to minimize the potential influence of these confounds. The HPA axis response to stress is affected by levels of estrogen and progesterone that vary due to gender (Kudielka and Kirschbaum, 2005), the use of oral contraceptives, and menstrual phase cycle (Kirschbaum et al., 1999), producing potential sources of variability and error in previous findings. In the current study, female participants were screened and excluded for use of oral contraceptives prior to enrollment, and sampled during the early follicular phase. We also performed our measurements with strict reference to time of awakening (Wust et al., 2000b; Federenko et al., 2004; Williams et al., 2005), to minimize the influence of time on measurement of the CAR (Edwards et al., 2001; Federenko et al, 2004). We minimized sampling error through the use of electronic monitoring devices (Kudielka et al., 2003; Broderick et al., 2004; review in Hansen et al., 2008). Moreover, the current study measured the CAR across two separate sampling sessions to minimize the influence of fluctuating situational factors on the CAR, and to maximize trait specificity (Clow et al., 2004; Kunz-Ebrecht et al., 2004; Scholtz et al., 2004; Thorn et al., 2006; Hellhammer et al., 2006, 2007).
Our findings show an unusually robust rise in cortisol in the lowest risk group (low acculturation/low exposure to childhood trauma), with an averaged, percent difference of 238%, compared to a relatively normal cortisol excursion in the other five subject groups. We are uncertain why the CAR was more robust in this sample. The design was carefully crafted, sampling compliance was monitored through the use of MEMS caps, and subjects were carefully trained in in-home salivary collection techniques. Therefore, we do not have any reason to suspect technical issues related to sample acquisition or cortisol measurement. However, few studies have measured the CAR in Mexican-American samples, and to our knowledge the CAR has never been examined in a similar subgroup of healthy subjects free from lifetime mood disorders and history of childhood trauma.
Our finding that greater exposure to early trauma is associated with an attenuated CAR in Mexican Americans lends support to an expanding body of preclinical and clinical evidence showing that early exposure to adversity during a time of high neuronal plasticity, may contribute to changes in several brain systems including the HPA axis, leading to persistent behavioral and neuroendocrine dysregulation in adulthood (Plotsky and Meaney, 1993; Caldji et al., 2000; Sanchez et al., 1998, 2001, 2005, 2006). Results are consistent with findings demonstrating reduced adrenocortical secretion in female survivors of sexual and physical abuse with and without major depressive disorder (Heim et al., 2001b), in pregnant females with a history of childhood abuse (Shea et al., 2007), in a sample of adult offspring of holocaust survivors (Yehuda et al., 2001), in PTSD patients (Rohleder et al., 2004; Wessa et al., 2006), and in adults with early loss experience (Meinslschmidt and Heim, 2005).
Although the majority of evidence suggests severe childhood stress is associated with diminished cortisol concentrations and a lower CAR, it should be noted that two studies report an enhanced CAR associated with early life adversity in postpartum females (Gonzalez et al., 2009) and enhanced cortisol levels throughout the day in adult men exposed to early parental loss (Nicolson, 2004). However, in one of these studies the CAR was calculated based on a limited time course, without respect to time of awakening, in the absence of a systematic monitoring of protocol compliance, while the other study relied on a small subject sample, did not monitor protocol compliance and did not screen for previous exposures to other forms of childhood adversities.
Our finding that a higher degree of acculturation (greater Anglo-orientation) is associated with an attenuation of the CAR, is of particular interest. A number of studies suggest many Mexican-Americans face unique stressors associated with some aspects of acculturation (Cervantes and Castro, 1985; Mena, et al., 1987; Hovey and King, 1996; Finch, et al., 2000; Miranda and Matheny, 2000; Huebner, et al., 2005), that can lead to greater stresses, psychological disorders and health problems (Salgado de Snyder, 1987; Hovey and King, 1996; Escobar et al., 2000; Finch, et al., 2000; Hovey, 2000a, 2000b; Aranda, et al., 2001; Gonzalez et al., 2001; Finch and Vega, 2003; Thoman and Suris, 2004). Some have argued that greater acculturation with more Anglo-orientation may diminish protective factors associated with the culture of origin (familism). As protective factors erode, individuals may become more vulnerable to the effects of continuous challenges and chronic psychosocial stressors associated with acculturation (Kaplan et al., 1990; Rogler et al., 1991; Gil et al., 1994; Vega and Amaro, 1994; Finch et al., 2001; Finch and Vega, 2003).
Our findings suggest that for some individuals, acculturation with greater Anglo-orientation may lead to injury in crucial physiological stress response systems. It is possible that greater Anglo-orientation, may contribute to allostatic load, and over time, lead to injury of the circadian rhythm and decreased adrenal capacity, similar to that observed with other chronic stressors (Pruessner et al., 1999). Similar effects have been observed in teachers with burnout symptoms who report living under chronic stress (Pruessner et al., 1999), in individuals reporting job stress (Caplan et al., 1979) and in Romanian orphans with a history of social deprivation (Carlson and Earls, 1997).
Determination of the precise developmental mechanisms that contribute to the association between a lower CAR and early exposure to trauma or greater acculturation (Anglo-orientation) is beyond the scope of the current study. However, it has been hypothesized that early exposure to stress alters the development of the hypothalamic CRF system, leading to enhanced stress reactivity (Meaney et al., 1993; Plotsky and Meaney, 1993; van Oers et al., 1998; Wigger and Neuman, 1999; Caldji et al., 2000; Ladd et al., 2000) which can then undergo desensitization with subsequent exposure to chronic stress. The model proposes that the HPA axis transitions from a system with increased stress responsivity to a system with a lower CAR (Heim and Nemeroff, 1999; Ehlert et al., 2001; Heim et al., 2001b; Meinslschmidt and Heim, 2005). The mechanism for this transition is unclear. Alternatively it is also plausible that early childhood stress or persistent chronic stress produces a blunted CAR with no antecedent HPA axis hyperactivity.
There are some limitations of the current study worth noting. The current study used a linear assessment of acculturation that assesses levels of acculturation based on patterns and mastery of language, demographic factors, and participation in either ethnic group or host culture (Marin and Marin, 1991; Cuellar et al., 1995). This approach assumes that as an individual increases their orientation in one culture there is a corresponding reduction in orientation in the other culture. It is more likely that some bicultural individuals may embrace aspects of both cultures. The effects of a highly bicultural adaptation to acculturation on the CAR will need to be examined in future studies.
It is also possible that unreported or undiagnosed PTSD in our subject sample influenced our findings because we did not specifically assess for the presence of a diagnosis of PTSD. We believe this is unlikely given that while 56% of Americans experience a lifetime trauma, only 8% subsequently develop Post Traumatic Stress Disorder (PTSD), (Perkonigg et al., 2000). Moreover, the odds of an abused and neglected child developing PTSD are only 1.75 times higher than the odds for a matched comparison subject (Widom, 1999). In addition, the conditional risk for PTSD is highest for females exposed to assault-related violence (Breslau et al., 1998, 2002), while in our sample, mean number of exposures to assault-related violence for female participants was less than one, decreasing the probability that PTSD influenced our findings. Also, depression is one of the most commonly co-occurring disorders with PTSD, and among individuals who have or have had a diagnosis of PTSD; approximately 48% also have current or past depression (Breslau, 2002). The assessment and exclusion of participants for depression in our sample, further minimizes the potential influence of unreported or undiagnosed PTSD on current findings.
About half of the sample reported a relatively low family income; therefore it is possible that a lower socioeconomic status contributed to a diminished CAR, possibly due to material hardship, and increased burden (Anderson and Armstead, 1995; Ranjit et al., 2005). However, we believe it is unlikely that our findings are due to the overall lower income in our sample because there were no differences in parental income among trauma groups (F=0.58, df=2, 54, p=.56) or between acculturation groups (F=0.41, df=1, 55, p=.53). Moreover, the effects of socioeconomic parameters on cortisol secretion and the CAR generally indicate an inverse relationship (Lupien et al., 2000, 2001; Rosmond and Bjorntorp, 2000; Kapuku et al., 2002; Bennett et al., 2004; Kunz-Ebrecht et al., 2004; Wright and Steptoe, 2005). However, it is possible that prolonged exposure to the increased material hardship and burden associated with low SES could result in an enhanced HPA axis response to stress transitioning to a system with a lower CAR.
Taken together, these findings show that both moderate and extreme exposure to early traumatic stress is associated with attenuation of the CAR in a sample of healthy, adult Mexican-Americans. These findings also show that higher levels of acculturation (greater Anglo-orientation) may be associated with culturally unique, chronic stressors that lead to injury of the HPA axis and attenuation of the CAR. The effects of early childhood trauma and acculturation on the CAR were not additive. Early childhood trauma and higher levels of acculturation modulate the CAR independent of the influences of depression on cortisol dynamics. It will be important to determine if injury to the HPA axis induced by early childhood trauma and the stress of acculturation is one possible mechanism accounting for the reported higher incidence of negative health outcomes in this population.
Acknowledgements
This work was supported by NIH grant R24-MH070636-01A1 (DLM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aihara IN, Yuuki A, Oshima H, Kumano K, Takahashi M, Fukuda N, Oriuchi K, Endo H, Matsuda, Mikuni M. HPA axis dysfunction in unmedicated major depressive disorder and its normalization by pharmacotherapy correlates with alteration of neural activity in prefrontal cortex and limbic/paralimbic regions. Psychiatry. Res. 2007;155:245–256. doi: 10.1016/j.pscychresns.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Altman DG. Practical statistics for medical research. Chapman and Hall; New York: 1991. p. 611. [Google Scholar]
- Anderson NB, Armstead CA. Toward understanding the association of socioeconomic status and health: a new challenge for the biopsychosocial approach. Psychosom. Med. 1995;57:213–225. doi: 10.1097/00006842-199505000-00003. [DOI] [PubMed] [Google Scholar]
- Arand MP, Castaneda I, Lee PJ, Sobel E. Stress, social support, and coping as predictors of depressive symptoms: Gender differences among Mexican Americans. Social Work Res. 2001;25(1):37–48. [Google Scholar]
- Ayuso-Mateos JL, Lasa L, Vazquez-Barquero JL, Oviedo A, Diez-Manrique JF. Measuring health status in psychiatric community surveys: internal and external validity of the Spanish version of the SF-36. Acta Psychiatr. Scand. 1999;99:26–32. doi: 10.1111/j.1600-0447.1999.tb05381.x. [DOI] [PubMed] [Google Scholar]
- Badrick E, Kirschbaum C, Kumari M. The relationship between smoking status and cortisol secretion. J. Clin. Endocrinol. Metab. 2007;92:819–824. doi: 10.1210/jc.2006-2155. [DOI] [PubMed] [Google Scholar]
- Baker CK, Norris FH, Jones EC, Murphy AD. Childhood trauma and adulthood physical health in Mexico. J. Behav. Med. 2009;32(3):255–69. doi: 10.1007/s10865-009-9199-2. [DOI] [PubMed] [Google Scholar]
- Bao AM, Ji YF, Van Someren EJW, Hofman MA, Liu RY, Zhou JN. Diurnal rhythms of free estradiol and cortisol during the normal menstrual cycle in women with major depression. Hormones and Beh. 2004;45:93–102. doi: 10.1016/j.yhbeh.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Bao AM, Rong-Yu L, Van Someren EJW, Hofman MA, Jiang-Ning Z. Changes in diurnal rhythms of free cortisol secretion during the different phases of the menstrual cycle. Acta Physiologica Sinica. 2003;55:547–553. [PubMed] [Google Scholar]
- Bendixen M, Muus KM, Schei B. The impact of child sexual abuse - a study of a random sample of Norwegian students. Child Abuse Negl. 1994;18:837–847. doi: 10.1016/0145-2134(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Bennett GG, Merritt MM, Wolin KY. Ethnicity, education, and the cortisol response to awakening: a preliminary investigation. Ethn. Health. 2004;9:337–347. doi: 10.1080/1355785042000285366. [DOI] [PubMed] [Google Scholar]
- Bernet CZ, Stein MB. Relationship of childhood maltreatment to the onset and course of major depression in adulthood. Depression and Anxiety. 1999;9(4):169–174. [PubMed] [Google Scholar]
- Berry JW. Acculturation as varieties of adaptation. In: Padilla A, editor. Acculturation: Theory, models and some new findings. Boulder, CO; Westview: 1980. [Google Scholar]
- Bhagwager Z, Hafizi S, Cowen PJ. Increased salivary cortisol after waking in depression. Psychopharmacology (Berl) 2005;182:54–57. doi: 10.1007/s00213-005-0062-z. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Bolus R, Mayer E. Psychometric properties of the early trauma inventory-self report. J. Nerv. Ment. Dis. 2007;195:211–218. doi: 10.1097/01.nmd.0000243824.84651.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N. Epidemiologic studies of trauma, posttraumatic stress disorder and other psychiatric disorders. Can J Psychiatry. 2002;47:923–929. doi: 10.1177/070674370204701003. [DOI] [PubMed] [Google Scholar]
- Breslau N, Kessler R, Chilcoat HD, Schultz LR, Davis GC, Andreski P. Trauma and posttraumatic stress disorder in the community: the 1996 Detroit area survey of trauma. Arch. Gen. Psychiatry. 1998;55:626–632. doi: 10.1001/archpsyc.55.7.626. [DOI] [PubMed] [Google Scholar]
- Broderick JE, Arnold D, Kudielka BM, Kirschbaum C. Salivary cortisol sampling compliance: comparison of patients and healthy volunteers. Psychoneuroendocrinology. 2004;29:636–650. doi: 10.1016/S0306-4530(03)00093-3. [DOI] [PubMed] [Google Scholar]
- Caldji C, Diorio J, Meaney MJ. Variations in maternal care in infancy regulate the development of stress reactivity. Biol. Psychiatry. 2000;48:1164–1174. doi: 10.1016/s0006-3223(00)01084-2. [DOI] [PubMed] [Google Scholar]
- Caplan RD, Cobb S, French JR. White collar workload and cortisol: disruption of a circadian rhythm by job stress? J. Psychosom. Res. 1979;23:181–92. doi: 10.1016/0022-3999(79)90003-5. [DOI] [PubMed] [Google Scholar]
- Carlson M, Earls F. Psychological and neuroendocrinological sequelae of early social deprivation in institutionalized children in Romania. Ann. NY Acad. Sci. 1997;807:419–428. doi: 10.1111/j.1749-6632.1997.tb51936.x. [DOI] [PubMed] [Google Scholar]
- Cervantes RC, Castro FG. Stress, coping and exican-American mental health: A systematic review. Hispanic J. Behav. Sci. 1985;7(1):1–73. [Google Scholar]
- Chrousos GP. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. New Eng. J. Med. 1995;332:1351–1363. doi: 10.1056/NEJM199505183322008. [DOI] [PubMed] [Google Scholar]
- Clow A, Thorn L, Evans P, Hucklebridge F. The awakening cortisol response: methodological issues and significance. Stress. 2004;7:29–37. doi: 10.1080/10253890410001667205. [DOI] [PubMed] [Google Scholar]
- Cuéllar, et al. Arnold B, Maldonado R. Acculturation Rating Scale for Mexican Americans-II: A revision of the original ARSMA scale. Hispanic J.Behav. Sci. 1995;17(3):275–304. [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc. Natl. Acad. Sci. USA. 2007;104:1321–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong M, Guiles WH, Felitti VJ, Dube SR, Williams JE, Chapman DP, Anda RF. Insights into causal pathways for ischemic heart disease. Circulation. 2004;110:1761–1766. doi: 10.1161/01.CIR.0000143074.54995.7F. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Gold PW. Evidence for impaired activation of the hypothalamic-pituitary-adrenal axis in patients with chronic fatigue syndrome. J. Clin. Endocrinol. Metab. 1991;73:1224–1234. doi: 10.1210/jcem-73-6-1224. [DOI] [PubMed] [Google Scholar]
- De Santis AS, Adam EK, Leah DD, Mineka S, Zinbarg RE, Craske MG. Racial/ethnic differences in cortisol diurnal rhythms in a community sample of adolescents. J. Adolesc. Health. 2007;41:3–13. doi: 10.1016/j.jadohealth.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Dong M, Giles WH, Felitti VJ, Dube SR, Williams JE, Chapman DP, Anda RF. Insights into causal pathways for ischemic heart disease: adverse childhood experiences study. Circulation. 2004;110:1761–1766. doi: 10.1161/01.CIR.0000143074.54995.7F. [DOI] [PubMed] [Google Scholar]
- Duncan RD, Saunders BE, Kilpatrick DG. Childhood physical assault as a risk factor for PTSD, depression and substance abuse: Findings from a national survey. Am J Orthospychiatry. 1996;66(3):437–448. doi: 10.1037/h0080194. [DOI] [PubMed] [Google Scholar]
- Edwards S, Evans P, Hucklebridge F, Clow A. Association between time of awakening and diurnal cortisol secretory activity. Psychoneuroendocrinology. 2001;26:613–622. doi: 10.1016/s0306-4530(01)00015-4. [DOI] [PubMed] [Google Scholar]
- Edwards S, Hucklebridge F, Clow A, Evans P. Components of the diurnal cortisol cycle in relation to upper respiratory symptoms and perceived stress. Psychosom. Med. 2003;65:320–327. doi: 10.1097/01.psy.0000033123.70631.8e. [DOI] [PubMed] [Google Scholar]
- Elhert U, Gaab J, Heinrichs M. Psychoneuroendocrinological contributions to the etiology of depression, posttraumatic stress disorder, and stress-related bodily disorders: the role of the hypothalamic-pituitary-adrenal axis. Biol. Psychol. 2001;57:141–152. doi: 10.1016/s0301-0511(01)00092-8. [DOI] [PubMed] [Google Scholar]
- Epstein JN, Saunders BE, Kilpatrick DG, Resnick HS. PTSD as a mediator between childhood rape and alcohol use in adult women. Child Abuse Negl. 1998;22:223–235. doi: 10.1016/s0145-2134(97)00133-6. [DOI] [PubMed] [Google Scholar]
- Federenko I, Wust S, Hellhammer DK, Dechoux R, Kumsta R, Kirschbaum C. Free cortisol awakening responses are influenced by awakening time. Psychoneuroendocrinology. 2004;29:174–184. doi: 10.1016/s0306-4530(03)00021-0. [DOI] [PubMed] [Google Scholar]
- Felitti VJ. Long-term medical consequences of incest, rape, and molestation. South Med. J. 1991;84:328–331. doi: 10.1097/00007611-199103000-00008. [DOI] [PubMed] [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults: the adverse childhood experiences (ACE) study. Am. J. Prev. Med. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Finch BK, Hummer RA, Kolody B. The role of discrimination and acculturative stress in the physical health of Mexican-origin adults. Hispanic J. Behav. Sci. 2001;23:399–429. [Google Scholar]
- Finch B, Vega W. Acculturation stress, social support, and self-rated health among Latinos in California. J. Immigrant Health. 2003;5:109–117. doi: 10.1023/a:1023987717921. [DOI] [PubMed] [Google Scholar]
- Fleming J, Mullen PE, Sibthorpe B, Bammer G. The long-term impact of childhood sexual abuse in Australian women. Child Abuse Negl. 1999;23:145–160. doi: 10.1016/s0145-2134(98)00118-5. [DOI] [PubMed] [Google Scholar]
- Flores E, Tschann JM, Dimas JM, Bachen EA, Pasch LA, de Groat CL. Perceived discrimination, perceived stress, and mental and physical health among Mexican-origin adults. Hispanic J. Behav. Sci. 2008;30:401–424. [Google Scholar]
- Flory JD, Yehuda R, Grossman R, New AS, Mitropoulou V, Siever LJ. Childhood trauma and basal cortisol in people with personality disorders. Compr. Psychiatry. 2009;50:34–37. doi: 10.1016/j.comppsych.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil A, Vega W, Dimas J. Acculturative stress and personal adjustment among Hispanic adolescent boys. J. Commun. Psychol. 1994;22:43–53. [Google Scholar]
- Glaser R, Robles TF, Sheridan J, Malarkey WB, Kiecolt-Glaser JK. Mild depressive symptoms are associated with amplified and prolonged inflammatory responses after influenza virus vaccination in older adults. Arch. Gen. Psychiatry. 2003;60:1009–1014. doi: 10.1001/archpsyc.60.10.1009. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Jenkins JM, Steiner M, Fleming AS. The relation between early life adversity, cortisol awakening response and diurnal salivary cortisol levels in postpartum women. Psychoneuroendocrinology. 2009;34:76–86. doi: 10.1016/j.psyneuen.2008.08.012. [DOI] [PubMed] [Google Scholar]
- Goodwin RD, Stein MB. Association between childhood trauma and physical disorders among adults in the United States. Psychol. Med. 2004;34:509–520. doi: 10.1017/s003329170300134x. [DOI] [PubMed] [Google Scholar]
- Goodwin RD, Stein MB. Association between childhood trauma and physical disorders among adults in the United States. Psychol. Med. 2004;34(3):509–520. doi: 10.1017/s003329170300134x. [DOI] [PubMed] [Google Scholar]
- Griep EN, Boersma JW, Lentjes EG, Prins AP, ven der Korst JK, de Kloet ER. Function of the hypothalamic–pituitary–adrenal axis in patients with fibromyalgia and low back pain. J. Rheumatol. 1998;25:1374–1381. [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Low cortisol and a flattening of expected daytime rhythm: potential indices of risk in human development. Dev. Psychopathol. 2001;13:515–538. doi: 10.1017/s0954579401003066. [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Vazquez DM. Stress neurobiology and developmental psychopathology. In: Cicchetti D, Cohen DJ, editors. Developmental Neuroscience. Vol. 2. John Wiley & Sons; New Jersey: 2006. [Google Scholar]
- Hanrahan K, McCarthy AM, Kleiber C, Lutgendorf S, Tsalikian E. Strategies for salivary cortisol collection and analysis in research with children. Appl. Nurs. Res. 2006;19:95–101. doi: 10.1016/j.apnr.2006.02.001. [DOI] [PubMed] [Google Scholar]
- Hansen M, Garde AH, Persson R. Sources of biological and methodological variation in salivary cortisol and their impact on measurement among healthy adults: A review. Scand. J. Clin. Lab. Invest. 2008;68:448–458. doi: 10.1080/00365510701819127. [DOI] [PubMed] [Google Scholar]
- Hays RD, Sherbourne CD, Mazel RM. The RAND 36-item health survey 1.0. Health Econ. 1993;2:217–27. doi: 10.1002/hec.4730020305. [DOI] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hanker JP, Hellhammer DH. Abuse-related posttraumatic stress disorder and alterations of the hypothalamic-pituitary-adrenal axis in women with chronic pelvic pain. Psychosom. Med. 1998;60:309–318. doi: 10.1097/00006842-199805000-00017. [DOI] [PubMed] [Google Scholar]
- Heim C, Ehlert U, Hellhammer DH. The potential role of hypocortisolism in the pathophysiology of stress-related bodily disorders. Psychoneuroendocrinology. 2000a;25:1–35. doi: 10.1016/s0306-4530(99)00035-9. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The impact of early adverse experiences on brain systems involved in the pathophysiology of anxiety and affective disorders. Biol. Psychiatry. 1999;46:1509–1522. doi: 10.1016/s0006-3223(99)00224-3. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff C. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol. Psychiatry. 2001a;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB. Altered pituitary adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Am. J. Psychiatry. 2001b;158:575–581. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Heit S, Graham YP, Wilcox M, Bonsall R, Miller AH, Nemeroff CB. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000b;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- Heim C, Wagner D, Maloney W, Papanicolaou D, Solomon L, Jones JF, Unger E, Reeves W. Early adverse experience and risk for chronic fatigue syndrome. Arch. Gen. Psychiatry. 2006;63:1258–1266. doi: 10.1001/archpsyc.63.11.1258. [DOI] [PubMed] [Google Scholar]
- Hellhammer J, Fries E, Schweisthal OW, Schlotz W, Stone AA, Hagemann D. Several daily measurements are necessary to reliably assess the cortisol rise after awakening: State- and trait components. Psychoneuroendocrinology. 2006;32:80–86. doi: 10.1016/j.psyneuen.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Hellhammer DH, Buske-Kirschbaum A, Kern S, Ebrecht M. Altered distribution of leukocyte subsets and cytokine production in response to acute psychosocial stress in patients with psoriasis vulgaris. Brain Behav. Immun. 2007;21:92–99. doi: 10.1016/j.bbi.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Hovey JD, King CA. Acculturative stress, depression and suicidal ideation among immigrant and second-generation Latino Adolescents. J. Am. Acad. of Child and Adolescent Psychiat. 1996;35:1183–1192. doi: 10.1097/00004583-199609000-00016. [DOI] [PubMed] [Google Scholar]
- Huber TJ, Issa K, Schik G, Wolf OT. The cortisol awakening response is blunted in psychotherapy in-patients suffering from depression. Psychoneruyoendocrinology. 2006;31(7):900–9004. doi: 10.1016/j.psyneuen.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Huizink AC, Ferdinand RF, Ormel J, Verhulst FC. Hypothalamic-pituitary-adrenal axis activity and early onset of cannabis use. Addiction. 2006;101:1581–1588. doi: 10.1111/j.1360-0443.2006.01570.x. [DOI] [PubMed] [Google Scholar]
- Humeniuk R, Ali R, Babor TF, Farrell M, Formigoni ML, Jittiwutikarn J, de Lacerda RB, Ling W, Marsden J, Monteiro M, Nhiwatiwa S, Pal H, Poznyak V, Simon S. Validation of the alcohol, smoking and substance involvement screening test (ASSIST) Addiction. 2008;103:1039–47. doi: 10.1111/j.1360-0443.2007.02114.x. [DOI] [PubMed] [Google Scholar]
- Hyman SM, Garcia M, Kemp K, Mazure CM, Sinha R. A gender specific psychometric analysis of the early trauma inventory short form in cocaine dependent adults. Addict. Behav. 2005;30:847–852. doi: 10.1016/j.addbeh.2004.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irish L, Kobayashi I, Delahanty DL. Long-term physical health consequences of childhood sexual abuse: A meta-analytic review. J Pediatric Psychol. 2009;118(1):847–852. doi: 10.1093/jpepsy/jsp118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs N, Nicolson NA, Derom C, Delespaul P, van Os J, Myin-Germeys I. Electronic monitoring of salivary cortisol sampling compliance in daily life. Life Sci. 2005;76:2431–2443. doi: 10.1016/j.lfs.2004.10.045. [DOI] [PubMed] [Google Scholar]
- Johnson DM, Delahanty DL, Pinna K. The cortisol awakening response as a functionof PTSD severity and abuse chronicity in sheltered battered women. J. Anxiety Disord. 2008;22:793–800. doi: 10.1016/j.janxdis.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanaley JA, Sames C, Swisher L, Swick AG, Ploutz-Snyder LL, Steppan CM, Sagendorf KS, Feiglin D, Jaynes EB, Meyer RA, Weinstock RS. Abdominal fat distribution in pre- and post-menopausal women: the impact of physical activity, age, and menopausal status. Metabolism. 2001;50:976–982. doi: 10.1053/meta.2001.24931. [DOI] [PubMed] [Google Scholar]
- Kaplan M, Marks G. Adverse effects of acculturation: Psychological distress among Mexican-American young adults. Soc. Sci. Med. 1990;31:1313–1319. doi: 10.1016/0277-9536(90)90070-9. [DOI] [PubMed] [Google Scholar]
- Kapuku GK, Treiber FA, Davis HC. Relationships among socioeconomic status, stress induced changes in cortisol, and blood pressure in African American males. Ann. Behav. Med. 2002;24:320–325. doi: 10.1207/S15324796ABM2404_08. [DOI] [PubMed] [Google Scholar]
- Kelly SJ, Young R, Sweeting H, Fischer JE, West P. Levels and confounders of morning cortisol collected from adolescents in a naturalistic (school) setting. Psychoneuroendocrinology. 2008;33:1257–1268. doi: 10.1016/j.psyneuen.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Thornton LM, Prescott CA. Gender differences in the rates of exposure to stressful life events and sensitivity to their depressogenic effects. Am. J. Psychiatry. 2001:587–593. doi: 10.1176/appi.ajp.158.4.587. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Davis CG, Kendler KS. Childhood adversity and adult psychiatric disorder in the US national comorbidity survey. Psychol. Med. 1997;27:1101–1119. doi: 10.1017/s0033291797005588. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Magee WJ. Childhood adversities and adult depression: basic patters of association in a US national survey. Psychol. Med. 1993;23:679–690. doi: 10.1017/s0033291700025460. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C, Kudielka BM, Gaab J, Schommer NC, Hellehammer DH. Impact of gender, menstrual cycle phase, and oral contraceptives on the activity of the hypothalamic-pituitary-adrenal axis. Psychosom. Med. 1999;61:154–162. doi: 10.1097/00006842-199903000-00006. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Blasey CM. Centering in regression analyses: A strategy to prevent errors in statistical inference. Int J Methods Pscyhiatr. Res. 2004;13(3):141–51. doi: 10.1002/mpr.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudielka BM, Broderick JE, Kirschbaum C. Compliance with saliva sampling protocols: Electronic monitoring reveals invalid cortisol daytime profiles in noncompliant participants. Psychosom. Med. 2003;65:313–319. doi: 10.1097/01.psy.0000058374.50240.bf. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Awakening cortisol responses are influenced by health status and awakening time but not by menstrual cycle phase. Psychoneuroendocrinology. 2003;28:35–47. doi: 10.1016/s0306-4530(02)00008-2. [DOI] [PubMed] [Google Scholar]
- Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol. Psychol. 2005;69:113–132. doi: 10.1016/j.biopsycho.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Kunz-Ebrecht SR, Kirschbaum C, Marmot M, Steptoe A. Differences in cortisol awakening response on work days and weekends in women and men from the Whitehall II cohort. Psychoneuroendocrinology. 2004;29:516–528. doi: 10.1016/s0306-4530(03)00072-6. [DOI] [PubMed] [Google Scholar]
- Kupper N, de Geus E, van den Berg M, Kirschbaum C, Boomsma DI, Willemsen G. Familial influences on basal salivary cortisol in an adult population. Psychoneuroendocrinology. 2005;30:857–868. doi: 10.1016/j.psyneuen.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Lara M, Gamboa C, Kahramanian MI, Morales LS, Bautista DE. Acculturation and Latino health in the United States: A review of the literature and its sociopolitical context. Annu. Rev. Public Health. 2005;26:367–397. doi: 10.1146/annurev.publhealth.26.021304.144615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasikiewicz N, Hendrickx H, Talbot D, Dye L. Exploration of basal diurnal salivary cortisol profiles in middle-aged adults: Associations with sleep quality and metabolic parameters. Psychoneuroendocrinology. 2008;33:143–151. doi: 10.1016/j.psyneuen.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Lechner ME, Vogel ME, Garcia-Shelton LM, Leichter JL, Steibel KR. Self-reported medical problems of adult female survivors of childhood sexual abuse. J Fam Pract. 1993;36:633–638. [PubMed] [Google Scholar]
- Littell RC, Milliken GA, Stroup WW. SAS System for Mixed Models. SAS; Cary, NC: 1996. [Google Scholar]
- Lundberg U, Hellstrom B. Workload and morning salivary cortisol in women. Work and Stress. 2002;16:976–980. [Google Scholar]
- Lupien S, King S, Meaney M, McEwen B. Can poverty get under your skin? Basal cortisol levels and cognitive function in children from low and high socioeconomic status. Dev. Psychopathol. 2001;13:653–676. doi: 10.1017/s0954579401003133. [DOI] [PubMed] [Google Scholar]
- Martinez-Taboas A, Bernal G. Dissociation, psychopathology, and abusive experiences in a nonclinical Latino university student group. Psychol. Med. 2000;38:1419–1426. doi: 10.1037/1099-9809.6.1.32. [DOI] [PubMed] [Google Scholar]
- Matheson K, Jorden S, Anisman H. Relations between trauma experiences and psychological, physical and neuroendocrine functioning among Somali refugees: mediating role of coping with acculturation stressors. J. Immigrant and Minority Health. 2008;10:291–305. doi: 10.1007/s10903-007-9086-2. [DOI] [PubMed] [Google Scholar]
- McCauley J, Kern DE, Kolodner K, Dill L, Schroeder AF, DeChant HK, Ryden J, Derogatis LR, Bass EB. Clinical characteristics of women with a history of childhood abuse: unhealed wounds. JAMA. 1997;277:1362–1368. [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators. N. Engl. J. Med. 1998:171–180. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- Meinlschmidt G, Heim C. Decreased cortisol awakening response after early loss experience. Psychoneuroendocrinology. 2005;30:568–576. doi: 10.1016/j.psyneuen.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Mena F, Padilla AM, Maldonado M. Acculturative stress and specific coping strategies among immigrant and later generation college students. Hispanic J. Behav. Sci. 1987;9(2):207–225. [Google Scholar]
- Mendoza R. Acculturation and sociocultural variability. In: Martiniez JL, Mendoza R, editors. Chicano Psychology. 2nd ed. Academic Press; Orlando: 1984. [Google Scholar]
- Mennen FE. Psychological Symptoms in a sample of Latino abused children. J Ethnic and Cultural Diversity in Social Work. 2000;8(3):193–213. [Google Scholar]
- Messina N, Grella C. Childhood trauma and women’s health in a California prison population. Am. J. of Public Health. 2006;96:1842–1848. doi: 10.2105/AJPH.2005.082016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meulenberg PM, Hofman JA. The effect of oral contraceptive use and pregnancy on the daily rhythm of cortisol and cortisone. Clin. Chim. Acta. 1990;190:211–221. doi: 10.1016/0009-8981(90)90175-r. [DOI] [PubMed] [Google Scholar]
- Moeller TP, Bachmann GA, Moeller JR. The combined effects of physical, sexual, and emotional abuse during childhood: long-term health consequences for women. Child Abuse Negl. 1993;17:623–640. doi: 10.1016/0145-2134(93)90084-i. [DOI] [PubMed] [Google Scholar]
- Mortola JF, Girton L, Beck L, Yen SS. Diagnosis of premenstrual syndrome by a simple, prospective, reliable instrument: the calendar of premenstrual experiences. Obstet. Gynecol. 1990;76:302–307. [PubMed] [Google Scholar]
- National Child Abuse and Neglect Data System (NCANDS) Detailed case data component, 1995–1999. Cornell University Family Life Development Center; Ithaca, NY: 2002. [Google Scholar]
- National Child Traumatic Stress Network (NCTSN) Culture and trauma brief: promoting culturally competent trauma-informed practices (Report) Washington, DC: 2005. [Google Scholar]
- Newman MG, Clayton L, Zuellig A, Cashman L, Arnow B, Dea R, Taylor CB. The relationship of childhood sexual abuse and depression with somatic symptoms and medical utilization. Psychol. Med. 2000;30:1063–1077. doi: 10.1017/s003329179900272x. [DOI] [PubMed] [Google Scholar]
- Nicolson NA, Storms C, Ponds R, Sulon J. Salivary cortisol levels and stress reactivity in human aging. J. Gerontol.: Med. Sci. 1997;52A:M68–M75. doi: 10.1093/gerona/52a.2.m68. [DOI] [PubMed] [Google Scholar]
- Nicolaidis C, McFarland B, Curry M, Berrity M. Differences in physical and mental health symptoms and mental health utilization associated with intimate Partner violence versus childhood abuse. Psychosomatics. 2009;50(4):340–346. doi: 10.1176/appi.psy.50.4.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkonigg A, Kessler RC, Storz S, Wittchen HU. Traumatic events and post-traumatic stress disorder in the community: prevalence, risk factors and comorbidity. Acta Psychiatr. Scan. 2000;101:46–59. doi: 10.1034/j.1600-0447.2000.101001046.x. [DOI] [PubMed] [Google Scholar]
- Plotsky PM, Meany MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Mol. Brain Res. 1993;18:195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Wolf OT, Hellhammer DH, Buske-Kirschbaum A, von Auer K, Jobst S, Kaspers F, Kirschbaum C. Free cortisol levels after awakening: a reliable biological marker for the assessment of adrenocortical activity. Life Sci. 1997;61:2539–2549. doi: 10.1016/s0024-3205(97)01008-4. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Hellhammer DH, Kirschbaum C. Burnout, perceived stress, and cortisol responses to awakening. Psychosom. Med. 1999;61:197–204. doi: 10.1097/00006842-199903000-00012. [DOI] [PubMed] [Google Scholar]
- Pruessner M, Jellhammer DH, Pruessner JC, Lupien SJ. Self-reported depressive symptoms and stress levels in healthy young men: Associations with the cortisol response to awakening. Psychosomatic Medicine. 2003;65:92–99. doi: 10.1097/01.psy.0000040950.22044.10. [DOI] [PubMed] [Google Scholar]
- Raison CL, Miller AH. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am. J. Psychiatry. 2003;160:1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- Ranjit N, Young E, Kaplan GA. Material hardship alters the diurnal rhythm of salivary cortisol. Int. J. Epidemiol. 2005;34:1138–1143. doi: 10.1093/ije/dyi120. [DOI] [PubMed] [Google Scholar]
- Reynolds W,M, Kobak KA. Reliability and validity of the Hamilton Depression Inventory: a paper-and-pencil version of the Hamilton Depression Rating Scale Clinical Interview. Psychol. Assess. 1995;7:472–483. [Google Scholar]
- Rohleder N, Joksimovic L, Wolf JM, Kirschbaum C. Hypocortisolism and increased glucocorticoid sensitivity of pro-inflammatory cytokine production in Bosnian war refugees with posttraumatic stress disorder. Biol. Psychiatry. 2004;55:745–751. doi: 10.1016/j.biopsych.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Roosa M, Reinholtz C, Angelini PJ. The relation of child sexual abuse and depression in young women: comparisons across four ethnic groups. J. Abnorm. Child Psychol. 1999;27:65–76. [PubMed] [Google Scholar]
- Rosmond R, Bjorntorp P. The hypothalmic-pituitary-adrenal axis activity as a predictor of cardiovascular disease, type 2 diabetes and stroke. J. Intern. Med. 2000;247:188–97. doi: 10.1046/j.1365-2796.2000.00603.x. [DOI] [PubMed] [Google Scholar]
- Safren SA, Gershuny BS, Marzol P, Otto MW, Pollack MH. History of childhood abuse in panic disorder, social phobia, and generalized anxiety disorder. J. Nerv. Ment. Dis. 2002;190:453–456. doi: 10.1097/00005053-200207000-00005. [DOI] [PubMed] [Google Scholar]
- Sanchez MM. The impact of early adverse care on HPA axis development: nonhuman primate models. Horm. Behav. 2006;50:623–631. doi: 10.1016/j.yhbeh.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Ladd CO, Plotsky PM. Early adverse experience as a developmental risk factor for later psychopathology: evidence from rodent and primate models. Dev. Psychopathol. 2001;13:419–449. doi: 10.1017/s0954579401003029. [DOI] [PubMed] [Google Scholar]
- Sánchez MM, Noble PM, Lyon CK, Plotsky PM, Davis M, Nemeroff CB, Winslow JT. Alterations in diurnal cortisol rhythm and acoustic startle response in nonhuman primates with adverse rearing. Biol. Psychiatry. 2005;57:373–381. doi: 10.1016/j.biopsych.2004.11.032. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Aguado F, Sanchez-Toscano F, Saphier D. Neuroendocrine and immunocytochemical demonstrations of decreased hypothalamo-pituitary-adrenal axis responsiveness to restraint stress after long-term social isolation. Endocrinology. 1998;139:579–587. doi: 10.1210/endo.139.2.5720. [DOI] [PubMed] [Google Scholar]
- Sanders-Phillips K, Moisan P, Wadlington S, Morgan S, English K. Ethnic differences in psychological functioning among Black and Latino sexually abused girls. Child Abuse Negl. 1995;19:691–706. doi: 10.1016/0145-2134(95)00027-6. [DOI] [PubMed] [Google Scholar]
- Schmidt-Reinwald A, Pruessner JC, Hellhammer DH, Federenko I, Rohleder N, Schurneyer TH, Kirschbaum C. The cortisol response to awakening in relation to different challenge tests and a 12-hour cortisol rhythm. Life Sci. 1999;64(18):1653–1660. doi: 10.1016/s0024-3205(99)00103-4. [DOI] [PubMed] [Google Scholar]
- Scholtz W, Hellhammer J, Schulz P, Stone AA. Perceived work overload and chronic worrying predict weekend-weekday differences in the cortisol awakening response. Psychosom. Med. 2004;66:207–214. doi: 10.1097/01.psy.0000116715.78238.56. [DOI] [PubMed] [Google Scholar]
- Schulz P, Kirschbaum C, Prüßner J, Hellhammer D. Increased free cortisol secretion after awakening in chronically stressed individuals due to work overload. Stress Med. 1998;14:91–97. [Google Scholar]
- Shaw JA, Lewis JE, Loeb A, Rosado J, Rodriguez RA. A comparison of Hispanic and African-American sexually abused girls and their families. Child Abuse Neg. 2001;25:1363–1367. doi: 10.1016/s0145-2134(01)00272-1. [DOI] [PubMed] [Google Scholar]
- Shea AK, Streiner DL, Fleming A, Kamath MV, Broad K, Steiner M. The effect of depression, anxiety and early life trauma on the cortisol awakening response during pregnancy: preliminary results. Psychoneuroendocrinology. 2007;32:1013–1020. doi: 10.1016/j.psyneuen.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Shea AK, Streiner DL, Fleming A, Kamath MV, Broad K, Steiner M. The effect of depression, anxiety and early life trauma on the cortisol awakening response during pregnancy: Preliminary results. Psychoneuroendocrinology. 2007;32(8-10):1013–1020. doi: 10.1016/j.psyneuen.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Sodowsky G, Lai E, Plake B. Moderating effects of sociocultural variables on acculturation attitudes of Hispanics and Asian Americans. J. Counseling Develop. 1991;70:194–204. [Google Scholar]
- Spertus IL, Yehuda R, Wong CM, Halligan S, Seremetis SV. Childhood emotional abuse and neglect as predictors of psychological and physical symptoms in women presenting to a primary care practice. Child Abuse Negl. 2003;27:1247–1258. doi: 10.1016/j.chiabu.2003.05.001. [DOI] [PubMed] [Google Scholar]
- Stetler C, Miller GE. Blunted cortisol response to awakening in mild to moderate depression: regulatory influences of sleep patterns and social contracts. J. Abn. Psychol. 2005;114(4):697–705. doi: 10.1037/0021-843X.114.4.697. [DOI] [PubMed] [Google Scholar]
- Suh BY, Liu JH, Berga SL, Quigley ME, Laughlin GA, Yen SS. Hypercortisolism in patients with functional hypothalamic-amenorrhea. J. Clin. Endocrinol. Metab. 1988;66:733–739. doi: 10.1210/jcem-66-4-733. [DOI] [PubMed] [Google Scholar]
- Thorn L, Hucklebridge F, Evans P, Clow A. Suspected non-adherence and weekend versus weekday differences in awakening cortisol response. Psychoneuroendocrinology. 2006;31:1009–1018. doi: 10.1016/j.psyneuen.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Thorn L, Hucklebridge F, Evans P, Clow A. The cortisol awakening response, seasonality, stress and arousal: a study of trait and state influences. Psychoneuroendocrinology. 2009;34:299–306. doi: 10.1016/j.psyneuen.2008.11.005. [DOI] [PubMed] [Google Scholar]
- VanderZee K, Sanderman R, Heyink J, de Haes H. Psychometric qualities of the RAND 36-item health survey 1.0: a multidimensional measure of general health status. Int. J. Behav, Med. 1996;3:104–122. doi: 10.1207/s15327558ijbm0302_2. [DOI] [PubMed] [Google Scholar]
- van Eck M, Nicolson NA, Berkhof H, Sulon J. Individual differences in cortisol responses to a laboratory speech task and their relationship to responses to stressful daily events. Biol. Psychol. 1996;43:69–84. doi: 10.1016/0301-0511(95)05159-7. [DOI] [PubMed] [Google Scholar]
- Wand GS, Dobs AS. Alterations in the hypothalamic-pituitary-adrenal axis in actively drinking alcoholics. J. Clin. Endocrinol. Metab. 1991;72:1290–1295. doi: 10.1210/jcem-72-6-1290. [DOI] [PubMed] [Google Scholar]
- Weissbecker I, Floyd A, Dedert E, Salmon P, Sephton S. Childhood trauma and diurnal cortisol disruption in fibromyalgia syndrome. Psychoneuroendocrinology. 2005;31:312–324. doi: 10.1016/j.psyneuen.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Weiss EL, Longhurst CM, Mazure CM. Childhood sexual abuse as a risk factor for depression in women: Psychosocial and neurobiological correlates. Am J Psychiatry. 1999;156:816–828. doi: 10.1176/ajp.156.6.816. [DOI] [PubMed] [Google Scholar]
- Wessa M, Rohleder N, Kirschbaum C, Flor H. Altered cortisol awakening response in posttraumatic stress disorder. Psychoneuroendocrinology. 2006;31:209–215. doi: 10.1016/j.psyneuen.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Widom CS. Posttraumatic stress disorder in abused and neglected children grown up. Am. J. Psychiatry. 1999;156:1223–1229. doi: 10.1176/ajp.156.8.1223. [DOI] [PubMed] [Google Scholar]
- Williams DR. Race, SES, and health: the added effects of racism and discrimination. Ann. N. Y. Acad. Sci. 1999;896:173–188. doi: 10.1111/j.1749-6632.1999.tb08114.x. [DOI] [PubMed] [Google Scholar]
- Williams C, Berry JW. Primary prevention of acculturative stress among refugees: Applicationof psychological theory and practice. American Psychologist. 1991;46(6):632–641. doi: 10.1037//0003-066x.46.6.632. [DOI] [PubMed] [Google Scholar]
- Williams E, Magid K, Steptoe A. The impact of time of waking and concurrent subjective stress on the cortisol response to awakening. Psychoneuroendocrinology. 2005;30:139–148. doi: 10.1016/j.psyneuen.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Wright CE, Steptoe A. Subjective socioeconomic position, gender and cortisol responses to waking in an elderly population. Psychoneuroendocrinology. 2005;30:582–590. doi: 10.1016/j.psyneuen.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Wust S, Federenko I, Hellhammer DH, Kirschbaum C. Genetic factors, perceived chronic stress, and the free cortisol response to awakening. Psychoneuroendocrinology. 2000a;25:707–720. doi: 10.1016/s0306-4530(00)00021-4. [DOI] [PubMed] [Google Scholar]
- Wust S, Wolf J, Hellhammer DH, Federenko IS, Schommer N, Kirschbaum C. The cortisol response to awakening - normal values and confounds. Noise Health. 2000b;7:77–85. [PubMed] [Google Scholar]
- Yehuda R, Halligan SL, Grossman R. Childhood trauma and risk for PTSD: relationship to intergenerational effects of trauma, parental PTSD, and cortisol excretion. Dev. Psychopathol. 2001;13:733–753. doi: 10.1017/s0954579401003170. [DOI] [PubMed] [Google Scholar]
- Young EA, Abelson JL, Curtis GC, Nesse RM. Childhood adversity and vulnerability to mood and anxiety disorders. Depress. Anxiety. 1997;5:66–72. [PubMed] [Google Scholar]


