Abstract
Purpose
To determine the extent and time course of upper limb impairment and dysfunction in women being treated for breast cancer, and followed prospectively, using a novel physical therapy surveillance model post-treatment.
Patients and Methods
Subjects included adult women with newly diagnosed, untreated, unilateral, Stage I to III BC and normal physiological and biomechanical shoulder function. Subjects were excluded if they had a previous history of BC, or prior injury or surgery of the affected upper limb. Measurements included body weight, shoulder ranges of motion (ROM), manual muscle tests, pain levels, upper limb volume, and an upper limb disability questionnaire (ULDQ). Measurements were taken at baseline (pre surgery), and one, three-six, and 12 months post surgery. All subjects received pre-operative education and exercise instruction and specific physical therapy (PT) protocol after surgery including ROM and strengthening exercises.
Results
All measures of function were significantly reduced one month post surgery, but most recovered to baseline levels by one year post surgery. Some subjects developed signs of lymphedema 3–12 months post surgery, but this did not compromise function. Shoulder abduction, flexion, and external rotation, but not internal rotation ROM, were associated with the ULDQ.
Conclusion
Most women in this cohort undergoing surgery for BC who receive PT intervention may expect a return to baseline ROM and strength by three months. Those who do not reach baseline, often continue to improve and reach their pre-operative levels by one year post surgery. Lymphedema develops independently of shoulder function three to 12 months post surgery, necessitating continued monitoring. A prospective physical therapy model of surveillance allows for detection of early and later onset of impairment following surgery for BC in this specific cohort of patients.
Keywords: Breast Cancer, Shoulder, Physical Therapy
INTRODUCTION
Upper limb (UL) dysfunction and decreased quality of life (QOL) are frequently reported sequelae of early stage breast cancer treatment (BC) [1–10]. Surgical trauma and/or radiation therapy may lead to UL impairments, functional limitations and disabilities including pain, stiffness, lymphedema, seroma, cording, decreased strength and range of motion (ROM) and decreased activity tolerance [1, 11–14]. Preventative exercise and education are recommended to reduce the incidence of BC-related UL dysfunction and to enhance QOL [15–18]. Although long term benefits of these treatments have been reported in a few longitudinal studies, [19–21] we found no studies that tracked specific shoulder impairments and associated functional limitations.
Many reliable and valid outcome instruments used to measure UL and shoulder dysfunction [22–32] are generic scales not designed to specifically assess BC sequelae and therefore are likely not sensitive enough to detect early BC treatment related changes in UL function. Few studies assess rate and predictors of UL functional recovery in this population because no instrument exists that incorporates all domains of UL physical disabilities such as symptom distress, shoulder impairment, and general functional limitations [33–34].
The main purpose of this study was to determine the extent and time course of UL dysfunction in subjects seen pre-operatively and followed prospectively using a novel physical therapy (PT) surveillance model post BC and treatment. Secondary purposes were to determine if pain was a factor in recovery and to assess self report of functional task difficulty 12 months post surgery using the Upper Limb Disability Questionnaire (ULDQ).
PATIENTS AND METHODS
Subject Selection and Study Design
A large prospective, observational IRB approved study, (NIH 02-CC-0044; NNMC B01-052) was conducted at the National Naval Medical Center Breast Care Center (NNMC-BCC), in conjunction with NIH from 2001–2006. Women newly diagnosed with unilateral, Stage I to III BC, and eligible for care in a military facility were screened pre-operatively to determine appropriateness for this prospective trial. Subjects were excluded if less than 18 years of age, male, or with a history of BC, bilateral BC, injury or surgery of the affected UL. Informed consent in accordance with NIH and NNMC IRBs was obtained.
Analyses were based on repeated measures for subjects who attended all four visits on the following schedule: a pre-operative visit (month 0) and three post-surgical visits (1, 3–6, and 12+ months).
Over the five year recruitment period for this study, close to 200 women met eligibility criteria. However, because of the nature of repeated measures analysis, women who deviated from our required follow up schedule could not be included in our analysis. 94 subjects completed all of the required visits on schedule and were included. Demographics and outcomes of the general population were similar to those of this subgroup. Subject demographics are shown in Table 1.
Table 1.
Subject Demographics and Characteristics
| Demographics and Characteristics | Number of subjects = 94 N (%) | |
|---|---|---|
| Age at Diagnosis, years |
Mean±SD: 53.39±11.8 Minimum: 30 Maximum: 82 |
|
| < 45 | 24 (25.53) | |
| 45 – 54 | 27 (28.72) | |
| 55 – 64 | 25 (26.59) | |
| 65+ | 18 (19.15) | |
| Ethnicity | ||
| Caucasian | 75 (79.78) | |
| African American | 16 (17.02) | |
| Asian /Pacific Islander | 2 ( 2.13) | |
| Hispanic | 1 (1.06) | |
| Side Involved | ||
| Right | 52 (55.32) | |
| Left | 42 (44.68) | |
| Hand Dominance | ||
| Right | 85 (90.42) | |
| Left | 9 (9.57) | |
| Involved Side to Hand Dominance | ||
| Ipsilateral side | 55 (58.51) | |
| Contralateral side | 39 (41.49) | |
| Body Mass Index, Baseline, kg/m2 |
Mean±SD: 27.16±6.54 Minimum: 19.1 Maximum: 55.6 |
|
| Normal | 18.5 – 24.9 | 43 (45.74) |
| Overweight | 25 – 29.9 | 32 (34.04) |
| Obese | ≥ 30 | 19 (20.21) |
| Type of Breast Cancer | ||
| Ductal carcinoma-in-situ (DCIS) | 11 (11.70) | |
| Invasive Ductal Carcinoma (IDC) | 44 (46.81) | |
| DCIS & IDC | 31 (32.98) | |
| Other | 8 (8.51) | |
| Stage of Breast Cancer | ||
| 0 | 11 (11.70) | |
| I | 40 (42.55) | |
| II | 30 (31.91) | |
| III | 13 (13.83) | |
| Lymph Node Dissection | ||
| None | 8 (8.51) | |
| ALND | 66 (70.21) | |
| SLNB | 20 (21.28) | |
| Number of Lymph Nodes Sampled |
Mean±SD:12.60±9.78 Minimum: 0 Maximum: 48 |
|
| 0 | 8 (8.51) | |
| 1–3 | 15 (15.96) | |
| 4 – 20 | 56 (59.57) | |
| > 20 | 15 (15.96) | |
| Number of Positive Lymph Nodes |
Mean±SD: 1.40±3.53 Minimum: 0 Maximum: 28 |
|
| 0 | 60 (63.83) | |
| 1 – 3 | 23 (24.47) | |
| > 3 | 11 (11.70) | |
| Surgery | ||
| Breast Conservation Therapy (BCT) | 41 (43.62) | |
| Modified Radical Mastectomy (MRM) | 50† (53.19) | |
| Simple Mastectomy | 3† (3.19) | |
| Reconstruction (N=53)† | †note: N = sum of MRM + Simple mastectomy | |
| No | 23 (43.40) | |
| Yes | 30 (56.60) | |
| ChemotherapyTherapy | ||
| No | 37 (39.36) | |
| Yes | 57 (60.64) | |
| Hormone Therapy | ||
| No | 27 (28.72) | |
| Yes | 67 (71.28) | |
| AI | 15 | |
| SERM | 51 | |
| Other | 1 | |
| Immunotherapy | ||
| No | 28 (29.79) | |
| Yes | 66 (70.21) | |
| Radiation Therapy | ||
| No | 30 (31.91) | |
| Yes | 64 (68.09) | |
Abbreviations: ALND, axillary lymph node dissection; SLNB, sentinel lymph node biopsy; AI, aromatase inhibitor; SERM, selective estrogen receptor modulator
Data Collection
All subjects were recruited from NNMC-BCC. Subjects were interviewed and evaluated by a physical therapist for their initial (baseline) examination and for all follow-up appointments. The physical therapist conducted an UL screening examination. The physical therapist recorded employment status, physical activity, pain, and fatigue followed by an UL examination including visual inspection and palpation for postural asymmetries, cording, swelling, and seromas; and tested bilateral shoulder ROM and strength. If an active ROM deficit was identified, the subject was positioned supine and measurement of the ROM deficit recorded using a standard goniometer. Shoulder muscle strength was determined by performing break testing of the ULs [35].
Volume and girth measurements for both ULs were taken in a standard position as described by Stout Gergich et al [36] using an optoelectronic volumeter containing a framed infrared scanning system, the Perometer®1. The Perometer® was designed specifically to measure girth (cm) and volume (mL) of the upper or lower extremities and has been validated for clinical use [37–39].
The ULDQ (Appendix A) was administered to the subjects at 12+ months post-surgery. Specifically, the ULDQ is a tool developed by our research team to measure UL function and disability and includes items from several existing published tools such as the Quick Dash [26–28]. Validation of the ULDQ was not completed at the time of the study. To assess shoulder functional limitations, three physical therapists collectively chose all items related to shoulder function from the ULDQ (n = 27 items), then independently categorized and rank-ordered the shoulder function tasks into four degree of difficulty ratings as described previously (2005) [40]: (1) Hard (eg. overhead height task), (2) Medium (eg. sliding a box), (3) Easy (eg. reaching for salt shaker), and (4) Routine (eg. shoulder height task).
Early PT Intervention
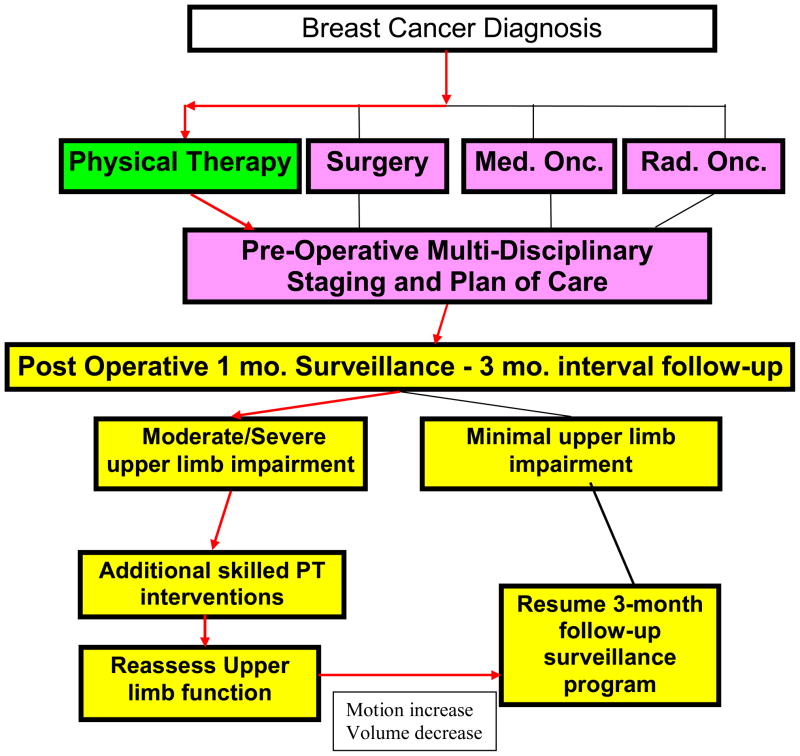
During the pre-operative examination, subjects were instructed in a postoperative UL ROM exercise program and were educated regarding UL lymphedema precautions and physical exercise initiation and progression. At the one-month post-operative visit, the exercise program was reviewed and additional individualized home program instructions were provided as needed. Subjects exhibiting shoulder girdle strength deficits were provided a program of resistance band exercises. When moderate-severe shoulder impairment was diagnosed, subjects received additional skilled PT intervention. Subjects were seen for continued surveillance at the scheduled intervals as described above regardless of the presence or absence of impairment (Figure 1).
Figure 1.
Algorithm
If subjects were diagnosed with UL lymphedema, treatment was initiated according to a protocol described by Stout Gergich et al [36]. Subjects with lymphedema were not placed on activity restrictions.
Measurements and Statistical Analyses
Range of motion (ROM) was recorded for shoulder flexion (FLEX), abduction (ABD), internal rotation (IR), and external rotation (ER). A single composite score (sumROM) was defined as the total of these four motions. A composite score for manual muscle test (sumMMT) was defined as the sum of six individual shoulder strength tests (scored individually 1 – 10, composite sumMMT scored 6 – 60) [41–43]. The six shoulder MMTs included shoulder flexors (FLEX), internal rotators (IR), external rotators (ER), abductors (ABD), and individual muscle tests for pectoralis major (PEC) and serratus anterior (SA). Cording (axillary web syndrome) was rated by the physical therapist at each visit as none = 0, mild (present, but asymptomatic) = 1, moderate (mild ROM restriction) = 2, or severe (major ROM restriction) = 3. Pain severity and frequency of pain for chest and shoulder were reported by numerical ratings at each session with 0 = no pain and 10 = extreme pain. For the purposes of analysis, pain scales were blocked into four categories: 0 = none; 1, 2, 3 = Mild; 4, 5, 6 = Moderate; and 7, 8, 9, 10 = Severe. Cording (an ordinal variable) and pain were analyzed with Chi-Square.
At each visit UL volume and girths were measured using the Perometer® and body weight was taken at each visit with a standard scale2. UL volumes were compared between affected and unaffected upper extremities with protected t-tests, and were correlated with body weight.
ROM in each shoulder plane (FLEX, ABD, IR and ER), the sumROM, sumMMT, and UL Volume were analyzed with One-way Repeated ANOVA protected by Greenhouse-Geisser correction for non-normal data. Post hoc testing was done using Within-Subjects Contrasts. Analyses were with SPSS software3. The individual shoulder ROMs were also compared to normal ranges as defined by the American Academy of Orthopedic Surgeons (AAOS) [44]. Level of shoulder impairment was expressed as deficit from full ROM using the American Medical Association (AMA) scale for regional impairment. The percent of subjects at each level of shoulder impairment were then determined for each time interval [45–46].
The total score as well as the four task subcategories of shoulder function from the ULDQ (Hard, Medium, Easy, and Routine) were each correlated with the shoulder ROM results to determine if affected UL function was related to one or another plane of movement, or if tasks of different effort levels had discriminative value.
RESULTS
Subject demographics and clinical-surgical characteristics are shown in Table 1. Approximately half the subjects had normal body mass index (BMI) <25 kg/m2 (20% were classified as obese≥30 kg/m2). The majority of subjects (53.2%) underwent a modified radical mastectomy (MRM) or simple mastectomy (3.2%), the remaining group (43.6%) had breast conserving surgery. Approximately half (57%) of the subjects who had an MRM also had reconstruction. This includes both immediate and late reconstruction. The majority of subjects (70%) had axillary lymph node dissection. Treatments included radiation (68%), hormonal therapy (71%), and chemotherapy (61%).
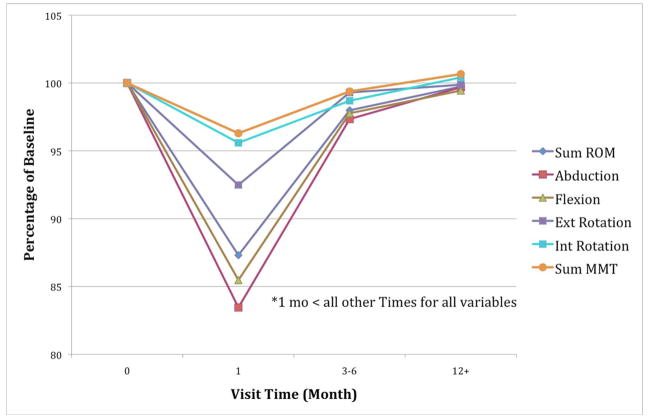
Repeated ANOVA results are summarized in Figure 2. Shoulder ABD, ER, FLEX, and sum ROM decreased from the 0 visit (baseline) to 1 month (p < 0.0001), improved from one month to three-six months (p <0.0001), and further improved from three-six month to 12+ month visits (p < 0.0001). Analysis of IR ROM yielded a significant decrease from 0 visit to one month (p<.04), however no significant difference was noted in the change from one month to three-six months, and a significant improvement from one and three-six to 12+ months (p < 0.03). Results for shoulder sumMMT followed the same pattern as the shoulder ROM data with significant decrement in shoulder strength at one month (p<.001) and general recovery for most subjects by three-six and 12+. This pattern is illustrated in Figure 2. Over 80% of pain responses by subjects at baseline, three-six, and 12 months were at the 0 (no pain) level (on a scale of 0–10), over 60% at one month were at 0. Most of the remaining responses were less than 4 reporting mild pain. Distributions for severity of pain are shown in Table 2. Incidence of pain distributions and tests and severity results were similar. In both cases, distributions showed greater pain at one month as identified by Chi-squared analysis (p<.001). However, nearly all of the subjects with pain at 12 months had no pain at the previous three month visit.
Figure 2.
Relative Ranges of Motion and Sum of MMT
Table 2.
Chest and Shoulder Pain Severity
| Time Point (Months) | ||||
|---|---|---|---|---|
| 0 | 1* | 3–6 | 12+ | |
| Chest Pain† | ||||
| None | 83 | 62 | 81 | 85 |
| Mild | 4 | 18 | 5 | 2 |
| Moderate | 1 | 7 | 2 | 1 |
| Severe | 0 | 1 | 0 | 0 |
| Shoulder Pain† | ||||
| None | 83 | 60 | 80 | 76 |
| Mild | 4 | 15 | 6 | 6 |
| Moderate | 1 | 8 | 2 | 5 |
| Severe | 0 | 5 | 0 | 1 |
p<.001, Chi Sq
Pain scale 0–10, as follows:
None = 0
Mild pain = 1, 2, 3
Moderate pain = 4, 5, 6
Severe pain = 7, 8, 9, 10
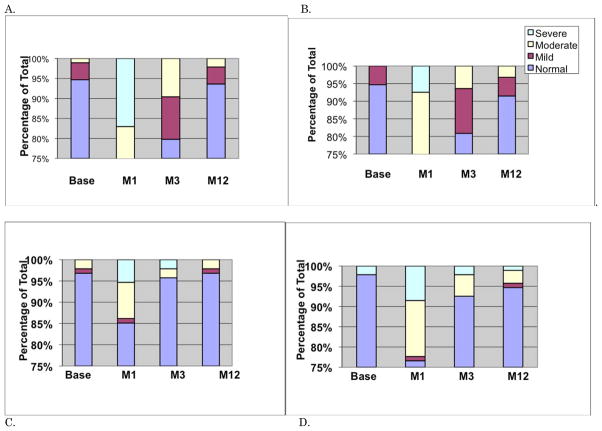
The majority of subjects demonstrated normal shoulder ROMs for all movements at all four visits except at the one month assessment. Relative impairments are shown in Figure 3. While few cases of shoulder ER impairment were noted at 12+ months, these cases were not apparent until between three and 12 months.
Figure 3.
Relative Impairment for each Movement. A. Abduction. B. Flexion. C. Int Rot. D. Ext Rot.
At 12 months, 92% of the subjects reported no or slight limitation performing hard functional tasks of the UL (none =42%, slight = 50%) and 89% reported no or slight limitation with medium UL tasks (none =42%, slight = 47%). Easy and Routine UL tasks were reported to be performed with no or slight limitation by 96% and 98% respectively. Correlations among the four ROM variables, sumROM, sumMMT, and the four ULDQ subcategories are shown in Table 3. ABD, FLEX, ER, and sumROM significantly correlated with all subcategories of the ULDQ (p<.02), but IR did not. The Quick Dash, as embedded in the ULDQ, correlated with shoulder ER (p < .05) and no other shoulder ROM. These results contribute to the validation of the ULDQ for studying this population.
Table 3.
Correlations Among Subcategories of the Upper Limb Disability Questionnaire (ULDQ) and Shoulder Range of Motion (ROM) at 12+ months
| N = 66 Significance levels shown in parentheses | |||||
|---|---|---|---|---|---|
| ABD | FLEX | ER | IR | sumROM | |
| Hard | .49(.00) | .42(.00) | .43(.01) | NS | .47(.00) |
| Medium | .32(.01) | .27(.01) | .38(.01) | NS | .32(.00) |
| Easy | .30(.01) | .29(.01) | .46(.00) | NS | .37(.00) |
| Routine | .25(.02) | .28(.01) | .40(.01) | NS | .32(.00) |
Abbreviations: ABD, abduction; FLEX, flexion; ER, external rotation; IR, internal rotation; sumROM, single composite score (total of all four ROMs)
Cording with associated movement restrictions was present in 29% (minor restriction = 21%, major restriction = 7%) at one month and in 5% (all minor) at three-six months. At one month, additional asymptomatic cording (total 40%) was noted.
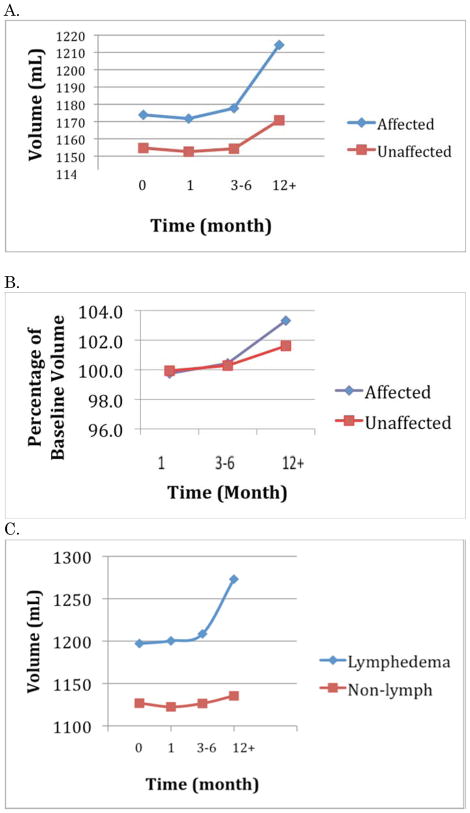
Overall limb volume increased over the course of follow-up, with the affected UL volume increasing slightly more than the unaffected limb volume. However, statistical significance for change over time in UL volume was only noted at 12+ months. These differences were significant both for the affected and the unaffected UL, but the differences between the ULs were not significant suggesting an overall weight gain for the cohort over time.
Subjects in this cohort who were diagnosed with sub-clinical lymphedema were identified for comparison to subjects without lymphedema. A significant difference in limb volume was found between these two groups at 12+ months (p<.045). Volume comparisons can be seen in Figure 4. Mean body weight was relatively unchanged (0.68 Kg increase) for these subjects from baseline to 12+ months, and correlations among body weight, and limb volume were not significant. There was no difference in pain report between the lymphedema subset and the rest of the cohort, but subjects in the non-lymphedema group reported higher levels of physical activity at 12+ months (82% vs 60%, p<.05).
Figure 4.
Limb Volumes. A. Absolute. B. Relative. C. With or Without Lymphedema.
DISCUSSION
In general, subjects exhibited shoulder impairments in ROM and MMT at one month following BC surgery, but experienced full recovery of shoulder function by 12 months post surgery. All subjects received a pre-operative PT assessment with education for post-operative ROM exercises and lymphedema identification and were followed prospectively to identify impairments and to provide further intervention as needed. Pre-operative and post-operative PT intervention has been reported to augment movement recovery after BC treatment [20, 21, 47]. We believe this prospective PT management enhanced the recovery of our subjects, but we are unable to definitively conclude this in absence of a control group.
Consistent with other studies, shoulder FLEX and ABD ROM showed the greatest change from baseline measures at one month. Functional impairment in the month following surgery should not be surprising. Damaged tissue must heal, and reduced activity levels subsequent to surgery would be expected to result in deficits. Studies by Box et al [20–21] and Gutman et al [47] demonstrated good shoulder motion outcome from three up to 24 months post surgery. These studies also incorporated PT management of their subjects. However, Hladiuk et al [48]reported that 12% of their subjects did not regain full shoulder ROM by 12 months. Therapeutic intervention was not described in this 1992 study. Because of this apparent range in time frame for recovery, periodic PT assessment is advised to detect any developing functional impairments.
Individual shoulder MMT are ordinal scaled as 1–5, or 1–10 (as in this study). By adding the MMT scores of six different muscle groups, we were able to establish an acceptable distribution for testing via ANOVA. This was supported by Greenhouse-Geisser correction for non-normal data. This composite representation of shoulder muscle strength followed the same pattern as shoulder ROM scores: a significant drop at one month post surgery followed by ascent to baseline values by 12 months after surgery.
Higher scores on the sub-components of the ULDQ were related to increased shoulder ROM. Although the correlations were slightly higher for the more difficult UL tasks, task difficulty did not seem to demonstrate discriminative value. SumROM correlations were very similar to values for shoulder ABD, FLEX and ER, individually, but IR did not correlate significantly with any of the sub-components of ULDQ. These findings suggest that shoulder ABD, FLEX and ER closely correlated with self report of UL function, were well represented by the sub-components of the ULDQ, and this relationship was not dependent on task difficulty. Clinicians should monitor all levels of UL functional tasks because conclusions cannot be made about overall UL function by only assessing patient’s ability to complete “hard” tasks. More patients reported deficits with medium tasks, such as pushing a vacuum, than with hard tasks, such as reaching overhead. Altered scapulothoracic motion as well as reduced shoulder complex muscle activity after treatment for breast cancer as noted by Shamley [9] may contribute to greater difficulty in performance of these functional tasks.
Chest and shoulder pain did not seem to be a problem for these subjects at one year. Only 30% of the sample reported levels higher than 0 at one month post surgery, and most were lower than 6 on a 10 point scale. We postulate perceived pain might be explained as a post-surgical effect. Subjects continued to recover, and by three-six months, less than 10% of these subjects reported any pain greater than mild. At 12 months, chest pain was nearly resolved with only three subjects reporting any pain. However, some subjects (13.6%) began to report shoulder pain at 12 months. This was not associated with changes in shoulder ROM. There is evidence that after completion of BC treatment that includes chemotherapy and radiation therapy, biomechanical changes/impairments may occur from 12 months to 3 years later [9, 19].
Patients receiving chemotherapy and radiation usually complete these treatments approximately 8–9 months after surgery. At 12 months after baseline, we may be observing increasing pain as an effect of these treatments. In this cohort, 92% of subjects with onset of pain at 12 months had received radiation therapy. The protracted time-to-onset of late effects suggests continued follow-up of patients beyond one year is necessary for periodic screening for UL impairments and functional limitations. The UL volumes for these subjects did not change for several months post surgery. Only late in the first year did we detect measurable changes. While the UL impairments detected late in the year were at a greater volume than those noted in the unaffected arm, these differences were not significant. We suspect this non-significance may be due to large variability in the sample.
As not all subjects appear to develop lymphedema, we identified a subgroup as having subclinical lymphedema at 12 months based on our previously reported criterion: difference of 3% or more from baseline in upper extremity volume [36]. The resulting significant difference in limb volume represents a validation of our criterion. However, a factor to discriminate these groups earlier in their progression would be of value. Weight seemed to be a reasonable supposition, but we found no significant relationships between weight or weight gain and limb volume with our grouped statistics. This was similar to the findings of Stout Gergich et al [36]. We also examined basic physical activity levels. Subjects without lymphedema were more likely to report regular physical activity. Whether physical activity prevented exacerbation of existing lymphedema (as suggested by Schmitz 2009 [49]), prevented lymphedema (as suggested by Swenson 2009 [50]), or absence of lymphedema allows more activity, cannot be established by these data. However, a recent analysis of lymphedema risk factors demonstrated an inverse correlation between exercise and the development of lymphedema in a breast cancer cohort [50].
Historically, many health care providers believed that lymphedema was directly related to UL functional impairment. When our subjects began to manifest lymphedema, it was identified in the latter half of the year post surgery. By that time their UL function, as determined by shoulder ROM and MMT, was significantly improved from those levels observed one month post surgery. There is a possibility that UL function may deteriorate between one and two years post surgery if not monitored on a periodic basis due to intermediate and late effects of radiation therapy. Two studies from the early 1990s [51–52] found impaired shoulder function up to two years post surgery, and a recent three year study [53]reported that shoulder disability seems to be a frequent late complication after treatment of early breast cancer. These studies suggest that normal shoulder ROM, MMT and absence of UL lymphedema is not assured, and those recovering from primary medical treatment should continue to be monitored for possible UL impairments and dysfunction beyond their initial medical treatment.
We suggest that appropriate intervention and treatment including UL ROM and strengthening exercises should be introduced to patients pre-operatively with instruction to begin shortly after surgery for BC. The current study was not controlled to test intervention, but our subjects did receive pre-operative examination and education for exercise and prospective surveillance with PT intervention, and demonstrated excellent return to UL function beginning about three-six months after their surgeries. This is a remarkable finding when considered in the context of previous literature. Reports of UL impairments have been documented in as many as 76% of subjects following breast cancer treatment interventions [54]. Most reports range from 28% to 50% [53, 55–58]. A prospective surveillance plan of care, in this cohort, may have prompted higher rates of recovery at the one year mark.
Limitations of this study relate to the fact that our subjects were all drawn from a cohort with a military background or association. Many subjects were active or retired military members or their family members. Although we included a wide range of ages in our sample, the mean age of the cohort was younger than most similar studies, possibly due to earlier diagnosis due to periodic monitoring as is often required in military facilities. Because subjects resided a distance from our medical center, they often sought after-care closer to their homes. This resulted in loss of follow-up numbers and less control related to their specific therapies. We also recognize that UL functional activity involves components in addition to Shoulder ROM and strength, such as fatigue and coordination. Finally, the ULDQ while using previously published items has not yet been studied psychometrically. This study represents one of its first applications to a post-surgical cohort and these results contribute to the validation of the ULDQ for studying this population. This data cannot be accepted as standard of care until this paradigm of screening/surveillance is tested in a prospective, randomized controlled design with a large sample size that includes an age distribution that would be more representative of the breast cancer population.
CONCLUSIONS
Subjects treated for BC and followed prospectively by a physical therapist providing pre-operative examination and post-operative surveillance recovered shoulder function in a consistent and timely pattern. Deficits in UL function exist one month after BC surgery, but recovery of objective function occurs in most subjects by three months with the majority of subjects achieving full recovery one year post surgery. Upper limb lymphedema and other late effects of treatment impacting the shoulder complex and UL function may still develop in patients anywhere from three months to more than one year post-surgery so periodic screening is recommended as their UL functional recovery progresses. Absence of pain is not sufficient indication of UL functional recovery because further pain may develop several months after cessation of medical treatment. A new UL functional assessment instrument, the ULDQ, appears to correlate well with improvement noted in shoulder ABD, FLEX and ER.
Supplementary Material
Acknowledgments
The authors thank Chingyi A. Shieh, Violeta Gutierrez, Gloria Furst, Wendy Chen, and Beth Rasch for their statistical contributions; and Leighton Chan and Ismail Jatoi for their support of this project.
This research was funded by the National Naval Medical Center, 8901 Wisconsin Avenue, Building 10, Breast Care Center, 4th Floor West, Bethesda, MD 20889-5600 (protocol NNMC #B01-052) and the National Institutes of Health, Mark O. Hatfield Clinical Research Center, Rehabilitation Medicine Department, Physical Therapy Section, MSC 1604, 10 Center Dr, Bethesda, MD 20892-1604 (protocol NIH #02-CC-0044)
Footnotes
Pero-System Messgerate, Am Tescher Busch 9 D-42327, Wuppertal, Germany
Detecto Model CN20, Webb City, MO
Version 15.0; SPSS Inc., Chicago, IL
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the US Army, the US Navy, the Department of Defense, the US PHS, the NIH, or the US Government.
References
- 1.Kuroi K, Shimozuma K, Taguchi T, et al. Evidence-based risk factors for seroma formation in breast surgery. Jpn J Clin Oncol. 2006;36:197–206. doi: 10.1093/jjco/hyl019. [DOI] [PubMed] [Google Scholar]
- 2.Sugden EM, Rezvani M, Harrison JM, et al. Shoulder movement after the treatment of early stage breast cancer. Clin Oncol (R Coll Radiol) 1998;10:173–181. doi: 10.1016/s0936-6555(98)80063-0. [DOI] [PubMed] [Google Scholar]
- 3.Tasmuth T, von Smitten K, Hietanen P, et al. Pain and other symptoms after different treatment modalities of breast cancer. Ann Oncol. 1995;6:453–459. doi: 10.1093/oxfordjournals.annonc.a059215. [DOI] [PubMed] [Google Scholar]
- 4.Yap KP, McCready DR, Narod S, et al. Factors influencing arm and axillary symptoms after treatment for node negative breast carcinoma. Cancer. 2003;97:1369–1375. doi: 10.1002/cncr.11218. [DOI] [PubMed] [Google Scholar]
- 5.Tengrup I, Nittby LT, Christiansson I, et al. Problems with arms are common after breast surgery. Lymphedema is a frequent complication in elderly women treated for breast cancer. Lakartidningen. 1999;96:5089–5091. [PubMed] [Google Scholar]
- 6.Tengrup I, Tennvall-Nittby L, Christiansson I, et al. Arm morbidity after breast-conserving therapy for breast cancer. Acta Oncol. 2000;39:393–397. doi: 10.1080/028418600750013177. [DOI] [PubMed] [Google Scholar]
- 7.Herd-Smith A, Russo A, Muraca MG, et al. Prognostic factors for lymphedema after primary treatment of breast carcinoma. Cancer. 2001;92:1783–1787. doi: 10.1002/1097-0142(20011001)92:7<1783::aid-cncr1694>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 8.Vinokur AD, Threatt BA, Vinokur-Kaplan D, et al. The process of recovery from breast cancer for younger and older patients. Changes during the first year. Cancer. 1990;65:1242–1254. doi: 10.1002/1097-0142(19900301)65:5<1242::aid-cncr2820650535>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 9.Shamley DR, Srinanaganathan R, Weatherall R, et al. Changes in shoulder muscle size and activity following treatment for breast cancer. Breast Cancer Res Treat. 2007;106:19–27. doi: 10.1007/s10549-006-9466-7. [DOI] [PubMed] [Google Scholar]
- 10.Ridner SH. Quality of life and a symptom cluster associated with breast cancer treatment-related lymphedema. Support Care Cancer. 2005;13:904–911. doi: 10.1007/s00520-005-0810-y. [DOI] [PubMed] [Google Scholar]
- 11.Isaksson G, Feuk B. Morbidity from axillary treatment in breast cancer: a follow-up study in a district hospital. Acta Oncol. 2000;39:335–336. doi: 10.1080/028418600750013104. [DOI] [PubMed] [Google Scholar]
- 12.Ivens D, Hoe AL, Podd TJ, et al. Assessment of morbidity from complete axillary dissection. Br J Cancer. 1992;66:136–138. doi: 10.1038/bjc.1992.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bentzen SM, Dische S. Morbidity related to axillary irradiation in the treatment of breast cancer. Acta Oncol. 2000;39:337–347. doi: 10.1080/028418600750013113. [DOI] [PubMed] [Google Scholar]
- 14.Ryttov N, Holm NV, Qvist N, et al. Influence of adjuvant irradiation on the development of late arm lymphedema and impaired shoulder mobility after mastectomy for carcinoma of the breast. Acta Oncol. 1988;27:667–670. doi: 10.3109/02841868809091766. [DOI] [PubMed] [Google Scholar]
- 15.Gosselink R, Rouffaer L, Vanhelden P, et al. Recovery of upper extremity function after axillary dissection. J Surg Oncol. 2003;83:204–211. doi: 10.1002/jso.10271. [DOI] [PubMed] [Google Scholar]
- 16.Lee TS, Kilbreath SL, Refshauge KM, et al. Pectoral stretching program for women undergoing radiotherapy for breast cancer. Breast Cancer Res Treat. 2007;102:313–321. doi: 10.1007/s10549-006-9339-0. [DOI] [PubMed] [Google Scholar]
- 17.Wingate L. Efficacy of physical therapy for patients who have undergone mastectomies. A prospective study. Phys Ther. 1985;65:896–900. doi: 10.1093/ptj/65.6.896. [DOI] [PubMed] [Google Scholar]
- 18.Demark-Wahnefried W, Pinto BM, Gritz ER. Promoting health and physical function among cancer survivors: potential for prevention and questions that remain. J Clin Oncol. 2006;24:5125–5131. doi: 10.1200/JCO.2006.06.6175. [DOI] [PubMed] [Google Scholar]
- 19.Lauridsen MC, Christiansen P, Hessov I. The effect of physiotherapy on shoulder function in patients surgically treated for breast cancer: a randomized study. Acta Oncol. 2005;44:449–457. doi: 10.1080/02841860510029905. [DOI] [PubMed] [Google Scholar]
- 20.Box RC, Reul-Hirche HM, Bullock-Saxton JE, et al. Physiotherapy after breast cancer surgery: results of a randomised controlled study to minimise lymphoedema. Breast Cancer Res Treat. 2002;75:51–64. doi: 10.1023/a:1016591121762. [DOI] [PubMed] [Google Scholar]
- 21.Box RC, Reul-Hirche HM, Bullock-Saxton JE, et al. Shoulder movement after breast cancer surgery: results of a randomised controlled study of postoperative physiotherapy. Breast Cancer Res Treat. 2002;75:35–50. doi: 10.1023/a:1016571204924. [DOI] [PubMed] [Google Scholar]
- 22.Michener LA, McClure PW, Sennett BJ. American Shoulder and Elbow Surgeons Standardized Shoulder Assessment Form, patient self-report section: reliability, validity, and responsiveness. J Shoulder Elbow Surg. 2002;11:587–594. doi: 10.1067/mse.2002.127096. [DOI] [PubMed] [Google Scholar]
- 23.Heald SL, Riddle DL, Lamb RL. The shoulder pain and disability index: the construct validity and responsiveness of a region-specific disability measure. Phys Ther. 1997;77:1079–1089. doi: 10.1093/ptj/77.10.1079. [DOI] [PubMed] [Google Scholar]
- 24.Roach KE, Budiman-Mak E, Songsiridej N, et al. Development of a shoulder pain and disability index. Arthritis Care Res. 1991;4:143–149. [PubMed] [Google Scholar]
- 25.Kocher MS, Horan MP, Briggs KK, et al. Reliability, validity, and responsiveness of the American Shoulder and Elbow Surgeons subjective shoulder scale in patients with shoulder instability, rotator cuff disease, and glenohumeral arthritis. J Bone Joint Surg Am. 2005;87:2006–2011. doi: 10.2106/JBJS.C.01624. [DOI] [PubMed] [Google Scholar]
- 26.Jester A, Harth A, Wind G, et al. Disabilities of the arm, shoulder and hand (DASH) questionnaire: Determining functional activity profiles in patients with upper extremity disorders. J Hand Surg [Br] 2005;30:23–28. doi: 10.1016/j.jhsb.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 27.Gummesson C, Atroshi I, Ekdahl C. The disabilities of the arm, shoulder and hand (DASH) outcome questionnaire: longitudinal construct validity and measuring self-rated health change after surgery. BMC Musculoskelet Disord. 2003;4:11. doi: 10.1186/1471-2474-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gummesson C, Ward MM, Atroshi I. The shortened disabilities of the arm, shoulder and hand questionnaire (QuickDASH): validity and reliability based on responses within the full-length DASH. BMC Musculoskelet Disord. 2006;7:44. doi: 10.1186/1471-2474-7-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Macdermid JC, Solomon P, Prkachin K. The Shoulder Pain and Disability Index demonstrates factor, construct and longitudinal validity. BMC Musculoskelet Disord. 2006;7:12. doi: 10.1186/1471-2474-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dowrick AS, Gabbe BJ, Williamson OD, et al. Outcome instruments for the assessment of the upper extremity following trauma: a review. Injury. 2005;36:468–476. doi: 10.1016/j.injury.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 31.Paul A, Lewis M, Shadforth MF, et al. A comparison of four shoulder-specific questionnaires in primary care. Ann Rheum Dis. 2004;63:1293–1299. doi: 10.1136/ard.2003.012088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bot SD, Terwee CB, van der Windt DA, et al. Clinimetric evaluation of shoulder disability questionnaires: a systematic review of the literature. Ann Rheum Dis. 2004;63:335–341. doi: 10.1136/ard.2003.007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lash TL, Silliman RA. Long-term follow-up of upper-body function among breast cancer survivors. Breast J. 2002;8:28–33. doi: 10.1046/j.1524-4741.2002.08006.x. [DOI] [PubMed] [Google Scholar]
- 34.Gerber L, Lampert M, Wood C, et al. Comparison of pain, motion, and edema after modified radical mastectomy vs. local excision with axillary dissection and radiation. Breast Cancer Res Treat. 1992;21:139–145. doi: 10.1007/BF01836960. [DOI] [PubMed] [Google Scholar]
- 35.Kendall F, McCreary E, Provance P. Muscles Testing and Function. 4. Baltimore: Williams and Wilkins; 1993. [Google Scholar]
- 36.Stout Gergich NL, Pfalzer LA, McGarvey C, et al. Pre-operative assessment enables early diagnosis and successful treatment of lymphedema. Cancer. 2008;112:2809–2819. doi: 10.1002/cncr.23494. [DOI] [PubMed] [Google Scholar]
- 37.Stanton AW, Northfield JW, Holroyd B, et al. Validation of an optoelectronic limb volumeter (Perometer) Lymphology. 1997;30:77–97. [PubMed] [Google Scholar]
- 38.Hayes S, Cornish B, Newman B. Comparison of methods to diagnose lymphoedema among breast cancer survivors: 6-month follow-up. Breast Cancer Res Treat. 2005;89:221–226. doi: 10.1007/s10549-004-2045-x. [DOI] [PubMed] [Google Scholar]
- 39.Armer JM. The problem of post-breast cancer lymphedema: impact and measurement issues. Cancer Invest. 2005;23:76–83. [PubMed] [Google Scholar]
- 40.Lin JJ, Hanten WP, Olson SL, et al. Functional activity characteristics of individuals with shoulder dysfunctions. J Electromyogr Kinesiol. 2005;15:576–586. doi: 10.1016/j.jelekin.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 41.Siegel KL, Hicks JE, Koziol DE, et al. Walking ability and its relationship to lower-extremity muscle strength in children with idiopathic inflammatory myopathies. Arch Phys Med Rehabil. 2004;85:767–771. doi: 10.1016/j.apmr.2003.07.005. [DOI] [PubMed] [Google Scholar]
- 42.Rudhe C, van Hedel HJ. Upper extremity function in persons with tetraplegia: Relationships between strength, capacity, and the spinal cord independence measure. Neurorehabil Neural Repair. 2009;23:413–421. doi: 10.1177/1545968308331143. [DOI] [PubMed] [Google Scholar]
- 43.Harris-Love MO, Shrader JA, Koziol D, et al. Distribution and severity of weakness among patients with polymyositis, dermatomyositis and juvenile dermatomyositis. Rheumatology. 2009;48:134–139. doi: 10.1093/rheumatology/ken441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Joint Motion: Method of Measuring and Recording. American Academy of Orthopedic Surgery; Chicago, IL: 1985. [Google Scholar]
- 45.Okunieff P, Augustine E, Hicks J, et al. Pentoxifylline in the treatment of radiation-induced fibrosis. J Clin Oncol. 2004;22:2207–2213. doi: 10.1200/JCO.2004.09.101. [DOI] [PubMed] [Google Scholar]
- 46.Gerber LH, Hoffman K, Chaudhry U, et al. Functional outcomes and life satisfaction in long-term survivors of pediatric sarcomas. Arch Phys Med Rehabil. 2006;87:1611–1617. doi: 10.1016/j.apmr.2006.08.341. [DOI] [PubMed] [Google Scholar]
- 47.Gutman H, Kersz T, Barzilai T, et al. Achievements of physical therapy in patients after modified radical mastectomy compared with quadrantectomy, axillary dissection and radiation for carcinoma of the breast. Arch Surg. 1990;125:389–391. doi: 10.1001/archsurg.1990.01410150111020. [DOI] [PubMed] [Google Scholar]
- 48.Hladiuk M, Huchcroft S, Temple W, et al. Arm function after axillary dissection for breast cancer: a pilot study to provide parameter estimates. J Surg Oncol. 1992;50:47–52. doi: 10.1002/jso.2930500114. [DOI] [PubMed] [Google Scholar]
- 49.Schmitz KH, Ahmed R, Troxel A, et al. Weight lifting in women with breast-cancer-related lymphedema. N Engl J Med. 2009;361:664–673. doi: 10.1056/NEJMoa0810118. [DOI] [PubMed] [Google Scholar]
- 50.Swenson KK, Nissen MJ, Leach JW, et al. Case-control study to evaluate predictors of lymphedema after breast cancer surgery. Oncol Nurs Forum. 2009;36:185–193. doi: 10.1188/09.ONF.185-193. [DOI] [PubMed] [Google Scholar]
- 51.Maunsell E, Brisson J, Deschenes L. Arm problems and psychological distress after surgery for breast cancer. Can J Surg. 1993;36:315–320. [PubMed] [Google Scholar]
- 52.Keramopoulos A, Tsionou C, Minaretzis D, et al. Arm morbidity following treatment of breast cancer with total axillary dissection: a multivariate approach. Oncology. 1993;50:445–449. doi: 10.1159/000227227. [DOI] [PubMed] [Google Scholar]
- 53.Lauridsen MC, Overgaard M, Overgaard J, et al. Shoulder disability and late symptoms following surgery for early breast cancer. Acta Oncol. 2008;47:569–575. doi: 10.1080/02841860801986627. [DOI] [PubMed] [Google Scholar]
- 54.Leidenius M, Leivonen M, Vironen J, et al. The consequences of long-time arm morbidity in node-negative breast cancer patients with sentinel node biopsy or axillary clearance. J Surg Oncol. 2005;92:23–31. doi: 10.1002/jso.20373. [DOI] [PubMed] [Google Scholar]
- 55.Ronka R, von Smitten K, Tasmuth T, et al. One-year morbidity after sentinel node biopsy and breast surgery. Breast. 2005;14:28–36. doi: 10.1016/j.breast.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 56.Chachaj A, Malyszczak K, Pyszel K, et al. Physical and psychological impairments of women with upper limb lymphedema following breast cancer treatment. Psychooncology. 2009 doi: 10.1002/pon.1573. [DOI] [PubMed] [Google Scholar]
- 57.Helms G, Kuhn T, Moser L, et al. Shoulder-arm morbidity in patients with sentinel node biopsy and complete axillary dissection--data from a prospective randomised trial. Eur J Surg Oncol. 2009;35:696–701. doi: 10.1016/j.ejso.2008.06.013. [DOI] [PubMed] [Google Scholar]
- 58.Thomas-Maclean RL, Hack T, Kwan W, et al. Arm morbidity and disability after breast cancer: new directions for care. Oncol Nurs Forum. 2008;35:65–71. doi: 10.1188/08.ONF.65-71. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.