Abstract
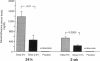
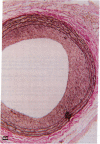
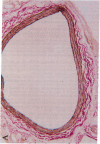
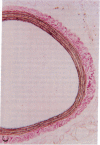
A periadventitial polymer system is an alternative local drug delivery technique to obtain and maintain high tissue levels of the drug at the site of vascular injury. To determine if local periadventitial delivery of dexamethasone decreases neointimal proliferation after balloon vascular injury, in three groups of Sprague-Dawley rats, 5% dexamethasone, 0.5% dexamethasone, and placebo silicone polymers were implanted around the left common carotid artery after balloon injury. In a fourth group, placebo polymers were implanted without balloon injury. Dexamethasone serum and tissue levels after polymer implantation were significantly higher in the 5% dexamethasone group compared with the 0.5% dexamethasone group. There was no neointima formation in any of the arterial segments covered with placebo polymers for 3 wk, but without balloon injury. In the arterial segments covered by the 5 and 0.5% dexamethasone polymers, there was a 76 and 75% reduction in intima/media ratios, respectively, compared with the placebo group (5% dexamethasone, 0.26 +/- 0.04; 0.5% dexamethasone, 0.27 +/- 0.03; placebo, 1.09 +/- 0.16, respectively; P < 0.0001). These results suggest that: (a) silicone polymers wrapped around the common carotid arteries for 3 wk did not, without balloon injury, stimulate neointimal proliferation in the rat model; (b) the activity of the drug-eluting polymer for suppressing intimal proliferation was chiefly, but not exclusively, site specific; and (c) transadventitial local delivery of dexamethasone at two different doses markedly inhibits neointimal proliferation after balloon vascular injury.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Austin G. E., Ratliff N. B., Hollman J., Tabei S., Phillips D. F. Intimal proliferation of smooth muscle cells as an explanation for recurrent coronary artery stenosis after percutaneous transluminal coronary angioplasty. J Am Coll Cardiol. 1985 Aug;6(2):369–375. doi: 10.1016/s0735-1097(85)80174-1. [DOI] [PubMed] [Google Scholar]
- Barker S. G., Talbert A., Cottam S., Baskerville P. A., Martin J. F. Arterial intimal hyperplasia after occlusion of the adventitial vasa vasorum in the pig. Arterioscler Thromb. 1993 Jan;13(1):70–77. doi: 10.1161/01.atv.13.1.70. [DOI] [PubMed] [Google Scholar]
- Beatt K. J., Serruys P. W., Hugenholtz P. G. Restenosis after coronary angioplasty: new standards for clinical studies. J Am Coll Cardiol. 1990 Feb;15(2):491–498. doi: 10.1016/s0735-1097(10)80081-6. [DOI] [PubMed] [Google Scholar]
- Berk B. C., Rao G. N. Angiotensin II-induced vascular smooth muscle cell hypertrophy: PDGF A-chain mediates the increase in cell size. J Cell Physiol. 1993 Feb;154(2):368–380. doi: 10.1002/jcp.1041540221. [DOI] [PubMed] [Google Scholar]
- Betz E., Fallier-Becker P., Wolburg-Buchholz K., Fotev Z. Proliferation of smooth muscle cells in the inner and outer layers of the tunica media of arteries: an in vitro study. J Cell Physiol. 1991 Jun;147(3):385–395. doi: 10.1002/jcp.1041470302. [DOI] [PubMed] [Google Scholar]
- Bolling S. F., Lin H., Annesley T. M., Boyd J. A., Gallagher K. P., Levy R. J. Local cyclosporine immunotherapy of heart transplants in rats enhances survival. J Heart Lung Transplant. 1991 Jul-Aug;10(4):577–583. [PubMed] [Google Scholar]
- Clark S. D., Kobayashi D. K., Welgus H. G. Regulation of the expression of tissue inhibitor of metalloproteinases and collagenase by retinoids and glucocorticoids in human fibroblasts. J Clin Invest. 1987 Nov;80(5):1280–1288. doi: 10.1172/JCI113203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole C. W., Hagen P. O., Lucas J. F., Mikat E. M., O'Malley M. K., Radic Z. S., Makhoul R. G., McCann R. L. Association of polymorphonuclear leukocytes with sites of aortic catheter-induced injury in rabbits. Atherosclerosis. 1987 Oct;67(2-3):229–236. doi: 10.1016/0021-9150(87)90283-8. [DOI] [PubMed] [Google Scholar]
- Crabbé P., Archer S., Benagiano G., Diczfalusy E., Djerassi C., Fried J., Higuchi T. Long-acting contraceptive agents: design of the WHO Chemical Synthesis Programme. Steroids. 1983 Mar;41(3):243–253. doi: 10.1016/0039-128x(83)90095-8. [DOI] [PubMed] [Google Scholar]
- Cronstein B. N., Kimmel S. C., Levin R. I., Martiniuk F., Weissmann G. A mechanism for the antiinflammatory effects of corticosteroids: the glucocorticoid receptor regulates leukocyte adhesion to endothelial cells and expression of endothelial-leukocyte adhesion molecule 1 and intercellular adhesion molecule 1. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):9991–9995. doi: 10.1073/pnas.89.21.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran T., Franza B. R., Jr Fos and Jun: the AP-1 connection. Cell. 1988 Nov 4;55(3):395–397. doi: 10.1016/0092-8674(88)90024-4. [DOI] [PubMed] [Google Scholar]
- Edelman E. R., Adams D. H., Karnovsky M. J. Effect of controlled adventitial heparin delivery on smooth muscle cell proliferation following endothelial injury. Proc Natl Acad Sci U S A. 1990 May;87(10):3773–3777. doi: 10.1073/pnas.87.10.3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman E. R., Nugent M. A., Karnovsky M. J. Perivascular and intravenous administration of basic fibroblast growth factor: vascular and solid organ deposition. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1513–1517. doi: 10.1073/pnas.90.4.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman E. R., Nugent M. A., Smith L. T., Karnovsky M. J. Basic fibroblast growth factor enhances the coupling of intimal hyperplasia and proliferation of vasa vasorum in injured rat arteries. J Clin Invest. 1992 Feb;89(2):465–473. doi: 10.1172/JCI115607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis S. G., Roubin G. S., Wilentz J., Douglas J. S., Jr, King S. B., 3rd Effect of 18- to 24-hour heparin administration for prevention of restenosis after uncomplicated coronary angioplasty. Am Heart J. 1989 Apr;117(4):777–782. doi: 10.1016/0002-8703(89)90612-1. [DOI] [PubMed] [Google Scholar]
- Ferns G. A., Raines E. W., Sprugel K. H., Motani A. S., Reidy M. A., Ross R. Inhibition of neointimal smooth muscle accumulation after angioplasty by an antibody to PDGF. Science. 1991 Sep 6;253(5024):1129–1132. doi: 10.1126/science.1653454. [DOI] [PubMed] [Google Scholar]
- Forrester J. S., Fishbein M., Helfant R., Fagin J. A paradigm for restenosis based on cell biology: clues for the development of new preventive therapies. J Am Coll Cardiol. 1991 Mar 1;17(3):758–769. doi: 10.1016/s0735-1097(10)80196-2. [DOI] [PubMed] [Google Scholar]
- Gay C. G., Winkles J. A. Interleukin 1 regulates heparin-binding growth factor 2 gene expression in vascular smooth muscle cells. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):296–300. doi: 10.1073/pnas.88.1.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebrane J., Roland J., Orcel L. Experimental diffuse intimal thickening of the femoral arteries in the rabbit. Virchows Arch A Pathol Anat Histol. 1982;396(1):41–59. doi: 10.1007/BF00428499. [DOI] [PubMed] [Google Scholar]
- Golomb G., Dixon M., Smith M. S., Schoen F. J., Levy R. J. Controlled-release drug delivery of diphosphonates to inhibit bioprosthetic heart valve calcification: release rate modulation with silicone matrices via drug solubility and membrane coating. J Pharm Sci. 1987 Apr;76(4):271–276. doi: 10.1002/jps.2600760402. [DOI] [PubMed] [Google Scholar]
- Guyton J. R., Rosenberg R. D., Clowes A. W., Karnovsky M. J. Inhibition of rat arterial smooth muscle cell proliferation by heparin. In vivo studies with anticoagulant and nonanticoagulant heparin. Circ Res. 1980 May;46(5):625–634. doi: 10.1161/01.res.46.5.625. [DOI] [PubMed] [Google Scholar]
- Hass R., Brach M., Kharbanda S., Giese G., Traub P., Kufe D. Inhibition of phorbol ester-induced monocytic differentiation by dexamethasone is associated with down-regulation of c-fos and c-jun (AP-1). J Cell Physiol. 1991 Oct;149(1):125–131. doi: 10.1002/jcp.1041490116. [DOI] [PubMed] [Google Scholar]
- Jonat C., Rahmsdorf H. J., Park K. K., Cato A. C., Gebel S., Ponta H., Herrlich P. Antitumor promotion and antiinflammation: down-modulation of AP-1 (Fos/Jun) activity by glucocorticoid hormone. Cell. 1990 Sep 21;62(6):1189–1204. doi: 10.1016/0092-8674(90)90395-u. [DOI] [PubMed] [Google Scholar]
- Järveläinen H., Halme T., Rönnemaa T. Effect of cortisol on the proliferation and protein synthesis of human aortic smooth muscle cells in culture. Acta Med Scand Suppl. 1982;660:114–122. doi: 10.1111/j.0954-6820.1982.tb00367.x. [DOI] [PubMed] [Google Scholar]
- Krane S. M., Amento E. P. Glucocorticoids and collagen diseases. Adv Exp Med Biol. 1984;171:61–71. [PubMed] [Google Scholar]
- Lee S. W., Tsou A. P., Chan H., Thomas J., Petrie K., Eugui E. M., Allison A. C. Glucocorticoids selectively inhibit the transcription of the interleukin 1 beta gene and decrease the stability of interleukin 1 beta mRNA. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1204–1208. doi: 10.1073/pnas.85.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R. J., Wolfrum J., Schoen F. J., Hawley M. A., Lund S. A., Langer R. Inhibition of calcification of bioprosthetic heart valves by local controlled-release diphosphonate. Science. 1985 Apr 12;228(4696):190–192. doi: 10.1126/science.3919445. [DOI] [PubMed] [Google Scholar]
- Libby P. Do vascular wall cytokines promote atherogenesis? Hosp Pract (Off Ed) 1992 Oct 15;27(10):51–58. doi: 10.1080/21548331.1992.11705508. [DOI] [PubMed] [Google Scholar]
- Libby P., Warner S. J., Friedman G. B. Interleukin 1: a mitogen for human vascular smooth muscle cells that induces the release of growth-inhibitory prostanoids. J Clin Invest. 1988 Feb;81(2):487–498. doi: 10.1172/JCI113346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longenecker J. P., Kilty L. A., Johnson L. K. Glucocorticoid influence on growth of vascular wall cells in culture. J Cell Physiol. 1982 Nov;113(2):197–202. doi: 10.1002/jcp.1041130203. [DOI] [PubMed] [Google Scholar]
- Macdonald R. G., Panush R. S., Pepine C. J. Rationale for use of glucocorticoids in modification of restenosis after percutaneous transluminal coronary angioplasty. Am J Cardiol. 1987 Jul 31;60(3):56B–60B. doi: 10.1016/0002-9149(87)90486-3. [DOI] [PubMed] [Google Scholar]
- Manthorpe R., Garbarsch C., Lorenzen I. Glucocorticoid effect on repair processes in vascular connective tissue. Morphological examination and biochemical studies on collagen RNA and DNA in rabbit aorta. Acta Endocrinol (Copenh) 1975 Oct;80(2):380–397. [PubMed] [Google Scholar]
- Martin J. F., Booth R. F., Moncada S. Arterial wall hypoxia following hyperfusion through the vasa vasorum is an initial lesion in atherosclerosis. Eur J Clin Invest. 1990 Dec;20(6):588–592. doi: 10.1111/j.1365-2362.1990.tb01905.x. [DOI] [PubMed] [Google Scholar]
- Mond H., Stokes K., Helland J., Grigg L., Kertes P., Pate B., Hunt D. The porous titanium steroid eluting electrode: a double blind study assessing the stimulation threshold effects of steroid. Pacing Clin Electrophysiol. 1988 Feb;11(2):214–219. doi: 10.1111/j.1540-8159.1988.tb04543.x. [DOI] [PubMed] [Google Scholar]
- Muller D. W., Topol E. J., Abrams G. D., Gallagher K. P., Ellis S. G. Intramural methotrexate therapy for the prevention of neointimal thickening after balloon angioplasty. J Am Coll Cardiol. 1992 Aug;20(2):460–466. doi: 10.1016/0735-1097(92)90118-7. [DOI] [PubMed] [Google Scholar]
- Muller D., Quantin B., Gesnel M. C., Millon-Collard R., Abecassis J., Breathnach R. The collagenase gene family in humans consists of at least four members. Biochem J. 1988 Jul 1;253(1):187–192. doi: 10.1042/bj2530187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols N. R., Olsson C. A., Funder J. W. Steroid effects on protein synthesis in cultured smooth muscle cells from rat aorta. Endocrinology. 1983 Sep;113(3):1096–1101. doi: 10.1210/endo-113-3-1096. [DOI] [PubMed] [Google Scholar]
- Nobuyoshi M., Kimura T., Nosaka H., Mioka S., Ueno K., Yokoi H., Hamasaki N., Horiuchi H., Ohishi H. Restenosis after successful percutaneous transluminal coronary angioplasty: serial angiographic follow-up of 229 patients. J Am Coll Cardiol. 1988 Sep;12(3):616–623. doi: 10.1016/s0735-1097(88)80046-9. [DOI] [PubMed] [Google Scholar]
- Okada T., Bark D. H., Mayberg M. R. Local anticoagulation without systemic effect using a polymer heparin delivery system. Stroke. 1988 Dec;19(12):1470–1476. doi: 10.1161/01.str.19.12.1470. [DOI] [PubMed] [Google Scholar]
- Okada T., Bark D. H., Mayberg M. R. Localized release of perivascular heparin inhibits intimal proliferation after endothelial injury without systemic anticoagulation. Neurosurgery. 1989 Dec;25(6):892–898. doi: 10.1097/00006123-198912000-00007. [DOI] [PubMed] [Google Scholar]
- Olanoff L. S., Anderson J. M., Jones R. D. Sustained release of gentamicin from prosthetic heart valves. Trans Am Soc Artif Intern Organs. 1979;25:334–338. doi: 10.1097/00002480-197902500-00062. [DOI] [PubMed] [Google Scholar]
- Parrillo J. E., Fauci A. S. Mechanisms of glucocorticoid action on immune processes. Annu Rev Pharmacol Toxicol. 1979;19:179–201. doi: 10.1146/annurev.pa.19.040179.001143. [DOI] [PubMed] [Google Scholar]
- Powell J. S., Clozel J. P., Müller R. K., Kuhn H., Hefti F., Hosang M., Baumgartner H. R. Inhibitors of angiotensin-converting enzyme prevent myointimal proliferation after vascular injury. Science. 1989 Jul 14;245(4914):186–188. doi: 10.1126/science.2526370. [DOI] [PubMed] [Google Scholar]
- Raines E. W., Dower S. K., Ross R. Interleukin-1 mitogenic activity for fibroblasts and smooth muscle cells is due to PDGF-AA. Science. 1989 Jan 20;243(4889):393–396. doi: 10.1126/science.2783498. [DOI] [PubMed] [Google Scholar]
- Ross R., Glomset J. A. The pathogenesis of atherosclerosis (first of two parts). N Engl J Med. 1976 Aug 12;295(7):369–377. doi: 10.1056/NEJM197608122950707. [DOI] [PubMed] [Google Scholar]
- Schüle R., Rangarajan P., Kliewer S., Ransone L. J., Bolado J., Yang N., Verma I. M., Evans R. M. Functional antagonism between oncoprotein c-Jun and the glucocorticoid receptor. Cell. 1990 Sep 21;62(6):1217–1226. doi: 10.1016/0092-8674(90)90397-w. [DOI] [PubMed] [Google Scholar]
- Shapiro S. D., Campbell E. J., Kobayashi D. K., Welgus H. G. Dexamethasone selectively modulates basal and lipopolysaccharide-induced metalloproteinase and tissue inhibitor of metalloproteinase production by human alveolar macrophages. J Immunol. 1991 Apr 15;146(8):2724–2729. [PubMed] [Google Scholar]
- Simons M., Edelman E. R., DeKeyser J. L., Langer R., Rosenberg R. D. Antisense c-myb oligonucleotides inhibit intimal arterial smooth muscle cell accumulation in vivo. Nature. 1992 Sep 3;359(6390):67–70. doi: 10.1038/359067a0. [DOI] [PubMed] [Google Scholar]
- Welgus H. G., Campbell E. J., Cury J. D., Eisen A. Z., Senior R. M., Wilhelm S. M., Goldberg G. I. Neutral metalloproteinases produced by human mononuclear phagocytes. Enzyme profile, regulation, and expression during cellular development. J Clin Invest. 1990 Nov;86(5):1496–1502. doi: 10.1172/JCI114867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilensky R. L., March K. L., Hathaway D. R. Direct intraarterial wall injection of microparticles via a catheter: a potential drug delivery strategy following angioplasty. Am Heart J. 1991 Oct;122(4 Pt 1):1136–1140. doi: 10.1016/0002-8703(91)90482-w. [DOI] [PubMed] [Google Scholar]
- Wolinsky H., Thung S. N. Use of a perforated balloon catheter to deliver concentrated heparin into the wall of the normal canine artery. J Am Coll Cardiol. 1990 Feb;15(2):475–481. doi: 10.1016/s0735-1097(10)80079-8. [DOI] [PubMed] [Google Scholar]