Abstract
Adenosine uptake into cells by nucleoside transporters plays a significant role in governing extracellular adenosine concentration. Extracellular adenosine is an important signaling molecule that modulates many cellular functions via four G-protein-coupled receptor subtypes (A1, A2A, A2B, and A3). Previously, we demonstrated that adenosine is critical in maintaining airway homeostasis and airway repair and that airway host defenses are impaired by alcohol. Taken together, we hypothesized that ethanol impairs adenosine uptake via the nucleoside transport system. To examine ethanol-induced alteration on adenosine transport, we used a human bronchial epithelial cell line (BEAS-2B). Cells were preincubated for 10 min in the presence and absence of varying concentrations of ethanol (EtOH). In addition, some cells were pretreated with S - (4-Nitrobenzyl)-6-thioinosine (100 μM: NBT), a potent adenosine uptake inhibitor. Uptake was then determined by addition of [3H]-adenosine at various time intervals. Increasing EtOH concentrations resulted in increasing inhibition of adenosine uptake when measured at 1 min. Cells pretreated with NBT effectively blocked adenosine uptake. In addition, short-term EtOH revealed increased extracellular adenosine concentration. Conversely, adenosine transport became desensitized in cells exposed to EtOH (100 mM) for 24 hr. To determine the mechanism of EtOH-induced desensitization of adenosine transport, cAMP activity was assessed in response to EtOH. Short-term EtOH exposure (10 min) had little or no effect on adenosine-mediated cAMP activation, whereas long-term EtOH exposure (24 hr) blocked adenosine-mediated cAMP activation. Western blot analysis of lysates from unstimulated BEAS-2B cells detected a single 55 kDa band indicating the presence of hENT1 and hENT2, respectively. Real-time RT-PCR of RNA from BEAS-2B revealed transcriptional expression of ENT1 and ENT2. Collectively, these data reveal that acute exposure of cells to EtOH inhibits adenosine uptake via a nucleoside transporter, and chronic exposure of cells to EtOH desensitizes the adenosine transporter to these inhibitory effects of ethanol. Furthermore, our data suggest that inhibition of adenosine uptake by EtOH leads to an increased extracellular adenosine accumulation, influencing the effect of adenosine at the epithelial cell surface, which may alter airway homeostasis.
Keywords: ethanol, adenosine, airway diseases, nucleoside transporter
INTRODUCTION
Extracellular adenosine levels are dynamically regulated by various mechanisms involving enzymes and transporters (Kobayashi et al., 2000). In mammalian cells, adenosine is transported by facilitated diffusion. The diffusion transporter has a broad specificity for nucleosides and mediates both influx and efflux (Nagy et al., 1990). The influx of nucleoside(s) across the plasma membrane regulates extracellular adenosine concentration. After influx through the nucleoside transporter, particularly the equilibrative nucleoside transporter (ENT), adenosine is subject to intracellular metabolism (Choi et al., 2004; Farre et al., 2004). However, multiple pathways exist to produce appropriate extracellular levels of adenosine. The classical intracellular ATP pathway, entails sequential dephosphorylation of intracellular ATP to adenosine (ATP → ADP → AMP → adenosine; Jackson and Dubey, 2001). According to this paradigm, extracellular release of adenosine occurs during hypoxia, ischemia, or exercise. Adenosine is viewed as a central regulatory molecule that serves to balance cellular oxygen supply and demand during metabolic stress. We previously demonstrated that adenosine plays a critical role in maintaining airway homeostasis and airway repair in human bronchial epithelial cells (Allen-Gipson et al., 2006). We observed that adenosine accelerates wound closure via occupancy of the A2AAR(s); whereas, occupancy of either the A1AR or A3AR slows wound closure. Occupancy of A2BAR has little to no effect on wound closure (Allen-Gipson et al., 2006). Understanding that adenosine exerts multiple modulatory effects through activation of many different (A1, A2A, A2B, and A3) receptors (Fredholm et al., 2001) and because many cell types contain bidirectional nucleoside transporters that facilitate diffusion of adenosine across the cell membrane (Cass et al., 1999), we investigated the role of ethanol on adenosine-mediated uptake in the bronchial epithelium.
Ethanol has been proposed to stimulate adenosine receptors by two mechanisms. The first involves metabolism of ethanol by the liver, that can be further metabolized to adenosine in many different tissues including the lung (Carmichael et al., 1991). The second mechanism has been observed only in cultured cells and involves ethanol inhibition of nucleoside transport (Gordon et al., 1986; Nagy et al., 1990). These earlier studies in cultured neural cells demonstrated that acute alcohol exposure increases extracellular adenosine by selectively inhibiting the adenosine type 1 equilibrative nucleoside transporter, ENT1 (Melendez and Kalivas, 2004; Nagy et al., 1989). Conversely, chronic exposure to ethanol has also been shown to decrease ENT1 expression, thus reducing the ability of acute ethanol to inhibit the transporter such that ethanol no longer increases extracelluar adenosine (Nagy et al., 1989). As an extension to these earlier findings, Choi et al., demonstrated that adenosine or adenosine transport mediates ethanol drinking behavior using knockout mice lacking the ENT1 gene (Choi et al., 2004). The data revealed that the ENT1-null mice were less sensitive to the acute effects of ethanol and showed an increase in alcohol consumption (Choi et al., 2004).
Previously, we demonstrated other differential cAMP pathway responses of airway epithelial cells for acute vs. chronic exposure to ethanol (Wyatt et al., 2003). Acute ethanol exposure of airway epithelial cells produced a rapid and transient stimulation of ciliary beat frequency, whereas longer exposures of ethanol produced desensitization of cAMP-dependent cilia beating (Sisson et al., 1999; Wyatt and Sisson, 2001). Supporting evidence implies that ethanol exposure increases susceptibility to lung infections and pulmonary diseases thereby altering overall lung function (Heinemann, 1977; Tabak et al., 2001). Understanding that adenosine is an important mediator of ethanol intoxication in which extensive studies have characterized ethanol-induced increases in extracellular adenosine in the nervous system (Coe et al., 1996; Gordon et al., 1986; Gordon et al., 1990; Nagy et al., 1990; Sapru et al., 1994), there is relatively little information of ethanol-induced increases of extracellular adenosine in the lung. In this context, we hypothesized that ethanol impairs adenosine uptake via nucleoside transport system in airway epithelial cells. Such findings would suggest inhibition of adenosine uptake by ethanol leads to an increased extracellular adenosine accumulation, influencing the effect of adenosine at the epithelial cell surface, which may prove critical in maintaining the balance of adenosine utilization by the lung.
MATERIAL and METHODS
Reagents/materials
LHC (Laboratory of Human Carcinogenesis) basal medium was purchased from Biofluids (Rockville, MD). RPMI 1640 (Roswell Park Memorial Institute), Dulbecco’s Modified Eagle Medium (DMEM), F-12 Nutrient Mixture (Hams), streptomycin, penicillin, protease (type IV), fetal calf serum and fungizone were purchased from Invitrogen (Carlsbad, CA). The Type I collagen gel matrix, Vitrogen 100, was purchased from Cohesion (Palo Alto, CA). Adenosine, CPCA, (5′-(N-cyclopropyl)-carboxamido-adenosine; A2A receptor agonist), S - (4-Nitrobenzyl)-6-thioinosine (potent adenosine uptake inhibitor; NBT), [3H]-Adenosine and all other reagents not otherwise indicated were purchased from Sigma (St Louis, MO).
Cell preparation
The transformed human BEAS-2B bronchial epithelial cell line was purchased from the American Type Culture Collection (Rockville, MD). Cells were cultured on type I collagen (Vitrogen 100) coated dishes in serum-free medium, LHC-9/RPMI (Lechner and LaVeck, 1985). Cells were maintained in culture at 37°C in humidified 95% air-5% CO2 for 48–72 hr before the experiment. Trypan blue exclusion was conducted to assured cellular viability under all experimental conditions.
Adenosine uptake measurement
Cells were grown on 24-well plates until confluent in serum-free, LHC-9/RPMI (1:1). Cells were pretreated at 37°C for 10 minutes with 0.25 ml/well of assay media [DMEM: F-12 (HAM), 3:1; 25mM HEPES, pH 7.4] in the presence and absence of varying concentrations of ethanol (0, 25, 50, 100 and 200 mM). Following pretreatment, 0.025 ml of 0.3 μCi [3H] adenosine (31.5 Ci/mM) was added to each well. The assay was stopped at various time intervals (0–1 min) by rapidly aspirating the medium, following by washing the cells with 0.5 ml of ice-cold DMEM:F12 medium. The cells were dissolved in 0.5 ml of 0.5 N NaOH by overnight incubation at room temperature, an aliquot of the extract was taken for protein determination by the method of Lowry et al. (Lowry et al., 1951), and 0.2 ml aliquots were counted in a liquid scintillation counter. Specific uptake was determined by subtracting nonspecific uptake in the presence of 100 μM NBT, a potent adenosine uptake inhibitor (Poucher et al., 1994), from the total uptake. At 1 min, nonspecific uptake was less than 10% of the total uptake. All the samples were assayed in quadruplicate, and no fewer than three separate experiments (n=12) were performed per unique parameter.
Determination of cAMP concentration
cAMP production was determined utilizing a cAMP immunoassay kit (R&D Systems; Minneapolis, MN). To prevent degradation of ADO, CPCA or cAMP, cells were pretreated with EHNA (10 μM; an adenosine deaminase inhibitor) and IBMX (0.2 mM; non-selective phosphodiesterase inhibitor) for 1 h. Following this incubation, cells were pretreated for 10 min or 24 hr in the presence or absence of 100 mM EtOH, and then stimulated for 30 min with ADO (10 μM) or CPCA (an A2A agonist; 10 μM). Positive control groups were treated with Forskolin (100 μM; adenylyl cyclase activator) for 30 min in the presence or absence of EtOH. Cells were lysed, centrifuged at 600g for 10 min at 4°C and supernatants were assayed as described by the manufacturer. All the samples were assayed in triplicate and no fewer than three separate experiments (n=9) were performed per unique parameter. Samples were read at wavelength of 450 nm using Biorad Benchmark microplate reader (Biorad-Life Science Research; Hercules CA). Data were analyzed for significance using one-way ANOVA followed by Tukey multiple comparison test. Significance was assigned at p ≤ 0.05.
Preparation of membranes
Cell membranes from BEAS-2B were prepared using a modified method as described (Massague and Czech, 1982). Cell lysates were sonicated and particulate centrifuged at 5,000g for 10 min at 4°C and the resulting pooled supernatants were centrifuged at 50,000g for 45 min. The pelleted materials were resuspended in 10 mM Tris (pH 7.4) and 1 mM EDTA. Protease inhibitors (1 μg/ml each of leupeptin, aprotinin and 1 mM phenylmethylsulfonyl fluoride) were used during membrane preparation. Protein concentrations were determined as described (Bradford, 1976).
SDS-PAGE and Western blot analysis
Proteins were separated by SDS-PAGE under reducing conditions on a 10% polyacrylamide gel. The resolved proteins were electroblotted to Immun-Blot PVDF membranes (Bio-Rad, Hercules, CA). The membranes were blocked with buffer containing 20 mM Tris, 150 mM NaCl, and 5% BSA (pH 7.4). Transferred proteins were probed with monospecific polyclonal rabbit antibody against human equilibrative nucleoside transporter protein, ENT1 and ENT2 (1:1000), overnight at 4°C (Gift provided by Dr. Pastor-Anglada, Universitat De Barcelona, Barcelona, Spain). Membranes were washed several times and incubated with HRP-conjugate goat anti-rabbit IgG (1:40,000) for 90 min at room temperature (Rockland, Gilbertsville, PA). An enhanced chemiluminescence kit (Amersham, Arlington Heights, IL) was used to visualize the blotted proteins on x-ray film (Kodak, Rochester, NY).
RNA extraction
BEAS-2B cells were grown to 60–70% and then exposed to 100 mM ethanol at various time points (4, 6, 24 h). Cell monolayers were rinsed twice in HEPES solution and then trypsinized and stored in RNA Later (Applied Biosystems, Goster City, CA) until RNA extraction could be performed. RNA was extracted and genomic DNA was removed using the Magmax 96 kit (Applied Biosystems) according to the manufacturer’s instructions. As part of this process, genomic DNA was removed using DNAse. Concentration and purity of the RNA were determined using the NanoDrop spectrophotometer (NanoDrop Technologies, Wilmington, DE). All RNA samples had a A260/A280 ratio of 1.9–2.0.
Reverse transcription
First strand cDNA was synthesized using 100 ng of template mRNA from the mRNA using the Taqman reverse transcription kit (Applied Biosystems). Reactions (25 μl) were prepared using the following: 1X Taqman RT buffer, 5.5 mM Magnesium chloride, 500μM of each DNTP, 2.5 uM random hexamers, 0.4 U/μl RNAse inhibitor, and 1.25 U/μl of MultiScribe reverse transcriptase. Samples were incubated in a themocycler at 25°C for 10 min, then 48°C for 30 min then 95° for 5 min.
Real-time PCR for ENT1 and ENT2
Real-time PCR reactions were prepared using the following: 1X Taqman master mix (Applied biosystems) and 1X human ENT1 (Applied Biosystems Hs 0185706) and ENT2 (Applied Biosystems Hs01546959). Ribosomal RNA was used as an endogenous control. PCR was performed using the ABI PRISM 7500 Sequence Detection System (Applied Biosystems) Reactions were thermocycled as follows: 50°C for 2 min, 95° for 10 min, then 40 cycles of 95°C for 15 sec and 60°C for 1 min. Each reaction was carried out in duplicate, and the mean cycle threshold (Ct) of the two wells was calculated. For relative comparison of ENT1 and ENT2 to ribosomal RNA endogenous control, we used the ΔΔCt method as described (Livak and Schmittgen, 2001).
Determination of extracellular adenosine via reverse-phase high-performance liquid chromatography
BEAS-2B cells were grown to 90–95% confluency, trypsinized and resuspended in 1.5 ml microcentrifuged tube at 1 × 10 6 cells/ml in LHC-9/RPMI (1:1). Cells were maintained in suspension, and rocked to prevent cell attachment at 37 °C in humidified 95% air-5% C02 for 15 min before experiment. LHC-9/RPMI (0.1 ml) containing 100 mM was then added to 1 ml aliquots of cells, rocked and incubated at various time points (0, 10, 12.5 min and 24 h). After incubation, the media was removed and treated with perchlorate acid (HClO4; 0.5 mol/L). The resulting supernatants following centrifugation were filtered through a 0.22 μm millipore membrane. Aliquots of the filtered acid extracts were directly applied to HPLC for analysis of adenosine levels using a modification of procedures previously described (Kharbanda et al., 2005).
The samples were analyzed using a Luna 100A-C18(2) column (250 × 4.6 mm, 5 μm; Phenomenex, Torrance, CA). The mobile phase consisted of 0.1 mol/L sodium acetate, 2.4 mmol sodium heptanesulfonic acid, 4.2 % acetonitrile and 50 mmol/L sodium perchlorate and pH was adjusted to 3.5 with 70% perchloric acid. The isocratic elution was carried out at 1.3 ml/min and the injection volume was 100 μl and the UV detector (Model Prostar 323; Varian Inc., Victoria, Australia) was set at 254 nm. During HPLC analysis, adenosine was co-chromatographed with authentic standard and identified according to their retention time. Standard adenosine (0.01 mmol/L; Sigma, St. Louis, MO) was dissolved in 0.05 mol/L HClO4 and then diluted to final concentrations.
Statistical analysis
The adenosine uptake assays (24-well format) were performed in quadruplicate (in separate wells) and repeated in three separate experiments with similar results (n=12). The data represent means ± SEM. Data were analyzed for significance using one-way ANOVA followed by Tukey multiple comparison test. Significance was assigned at p ≤.05. Other assays were performed in triplicate and repeated in three separate experiments with similar results (n = 3). To pool data from multiple independent experiments, the effect was tested with paired t-tests to account for varying control results. For the PCR assay, all samples were assayed in duplicate and no fewer than three separate experiments were performed per unique parameter (n = 6). Data were analyzed for significance using Student’s t-test. Significance was assigned at p < 0.05.
RESULTS
Identification of the human adenosine Type 1 and/or Type 2 equilibrative nucleoside transporter (ENT1 and/or ENT2) on human bronchial epithelium
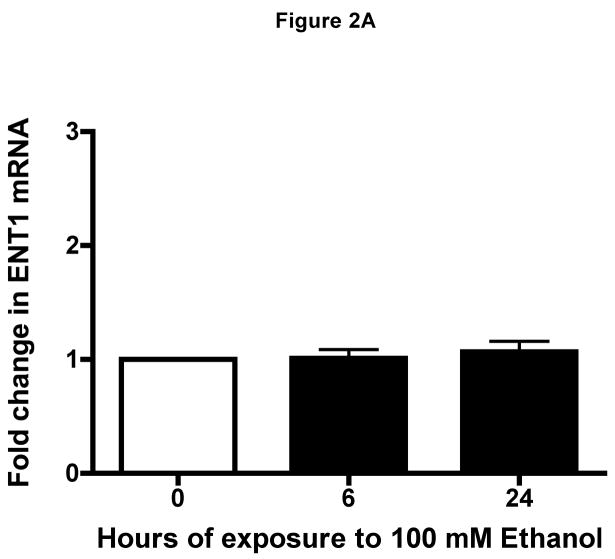
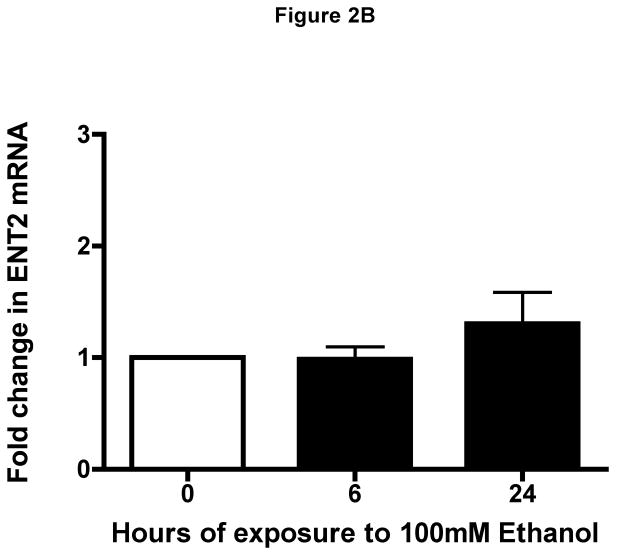
We first determined if equilibrative nucleoside transporter (ENT1 and/or ENT2) was present in airway epithelium. Western blot analysis revealed a single band of 55 kDa representative of ENT1 and ENT2 in lysates from unstimulated human bronchial epithelial cells (Fig 1). To support this finding, real-time quantitative PCR was conducted to demonstrate the transcriptional levels of ENT1 and/or ENT2 in the presence or absence of ETOH at various time points (Fig 2A & B). Transcriptional levels of ENT1 and/or ENT2 remained constant at all time points in the presence or absence of ETOH. These findings indicate that ENT1 and ENT2 are present on human airway epithelial cells and are not directly modified by ethanol.
Figure 1. Western blot analysis of hENT1 and hENT2 expression.
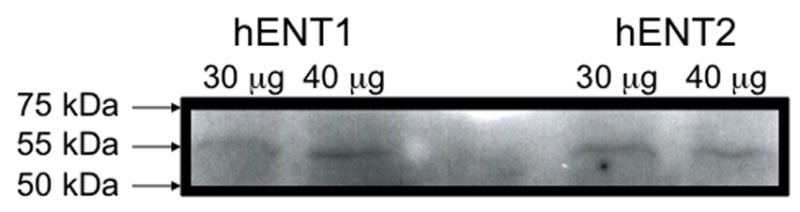
Representative Western blots identifying a single band at 55 kda of hENT1 and hENT2 proteins in crude extracts from human bronchial epithelial cells, BEAS-2B.
Figure 2. Identification of human equilibrative nucleoside transporters in human epithelial cells.
Transcriptional Level of (A) hENT1 and of hENT2 (B) were quantified using real-time PCR in RNA isolated from BEAS-2B cells exposed to ethanol at indicated time points (0, 6 and 24 h). Ethanol-treated cells transcriptional level was unchanged as compared to RNA isolated from control cells during the 0, 6 and 24 h (media: 6 h: 26.35 ± 0.2616; 24 h: 26.5 ± 0.2262, P > 0.05). Values were normalized to (human) in-house ribosomal RNA with a mean average of 12.43 ± 0.287. Data are expressed as mean fold change from control cells ± S.E. of three experiments, each performed in duplicate.
Ethanol inhibits adenosine uptake in bronchial epithelial cells
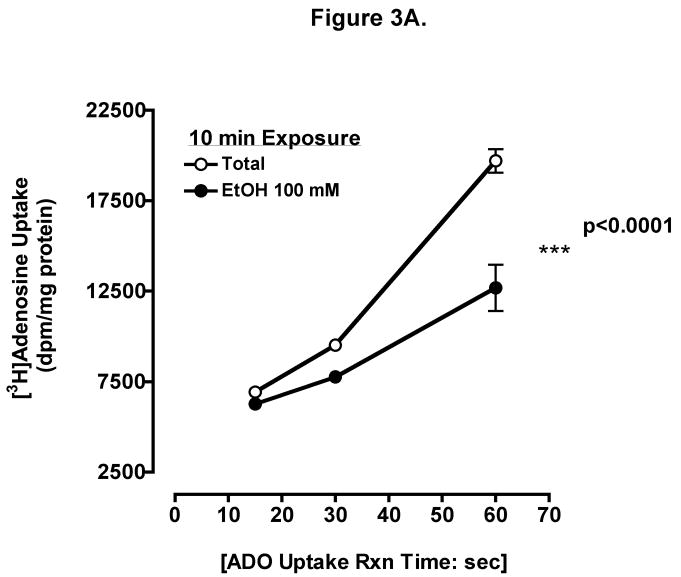
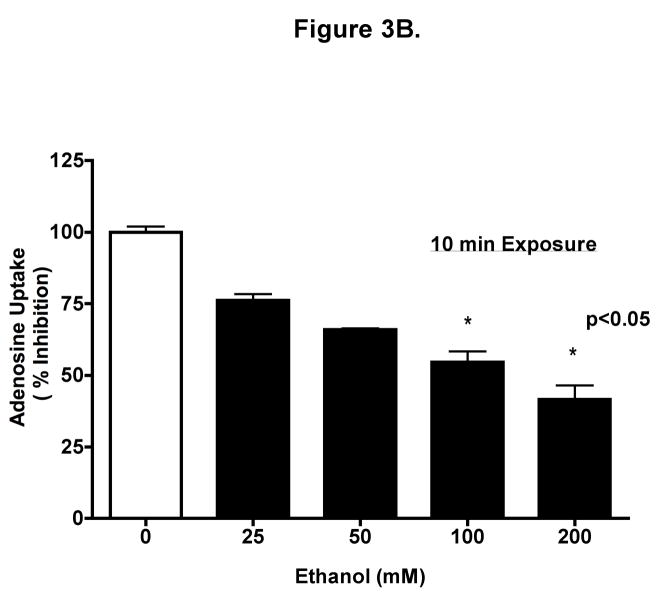
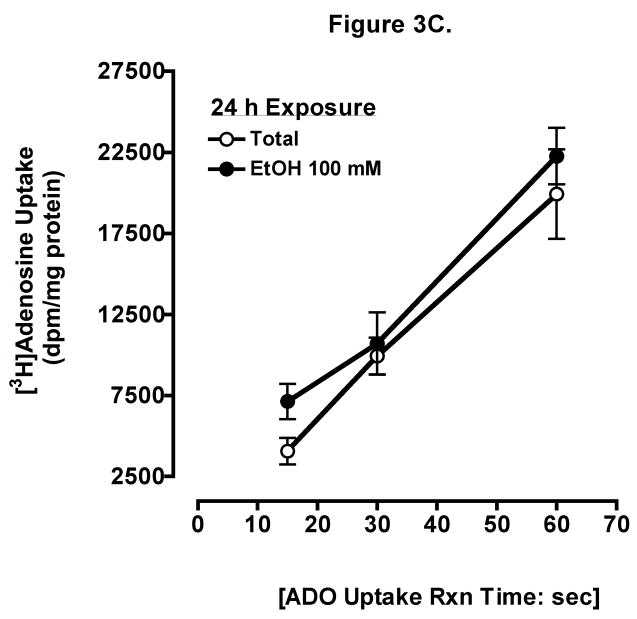
To determine if ethanol inhibits adenosine uptake, we measured the direct uptake of adenosine in BEAS-2B cells. Cells were preincubated for 10 min with 100 mM ethanol, and uptake of 0.3 μCi [3H] adenosine at indicated times was determined (Fig 3A). Uptake was linear to 1 min and there were significant differences between control and ethanol-treated cells at 15 sec, 30 sec, and 1 min (mean ± SEM control vs. mean ± SEM EtOH at 1 min, p < 0.001). Increasing ethanol concentrations resulted in progressive inhibition of adenosine uptake when measured at 1 min (Fig 3B) using biologically relevant concentrations. Adenosine uptake was maximally inhibited at 1 min by 100 mM ethanol. Prolonged ethanol exposure (24-hour pretreatment) did not block adenosine uptake when measured at 1 min (Fig 3C). These data suggest that acute exposure of ethanol effectively slows adenosine uptake in bronchial epithelial cells.
Figure 3. Ethanol inhibits adenosine uptake.
(A) Time course of adenosine uptake in the presence and absence of ethanol. BEASs-2B cells were preincubated for 10 min with 0 and 100 mM ethanol and uptake of 0.3 μCi [3H] adenosine was measured in the presence (●) or absence (○) of 100 mM ethanol at the indicated times as described. (B) Concentration-response for ethanol inhibition of adenosine uptake in 1 min. Cells were preincubated for 10 min with various concentration of ethanol (0, 25, 50, 100, and 200 mM). (C) Times course of adenosine uptake in the presence and absence of ethanol after 24 h pretreatment. Values represent mean ± S.E.M. of three experiments, each performed in quadruplet.
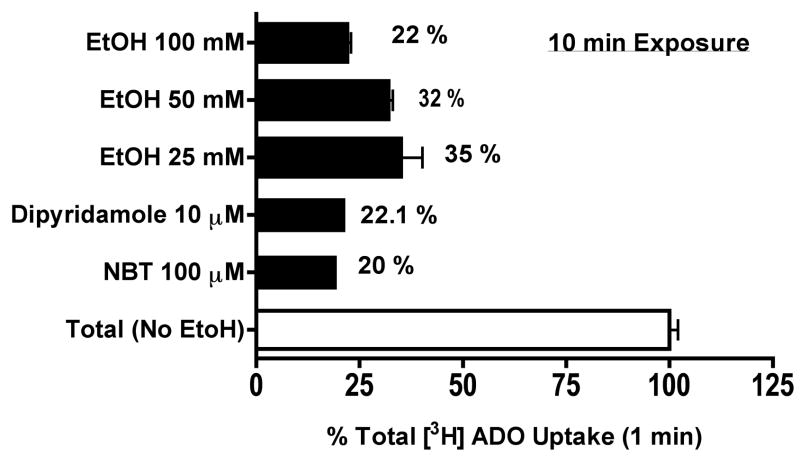
Comparison of ethanol to adenosine transporter inhibitors in adenosine uptake
To further characterize the effect of ethanol on adenosine uptake via nucleoside transporter(s), some cells were pretreated for 10 min with either 10 μM dipyridamole or 100 μM NBT, two potent inhibitors of facilitated nucleoside transporter(s) (Paterson et al., 1980), in the presence or absence of ethanol exposure and uptake was measured at 1 min. Cells preincubated with 25–100 mM ethanol blocked adenosine uptake as effectively as the adenosine transporter inhibitors (Fig 4). There was a 78% reduction in adenosine reuptake in cells acutely exposed to 100 mM of ethanol. Similarly, both dipyridamole and NBT competitively blocked adenosine reuptake by 77.9% and 80%, respectively. Interestingly, we did not observe an additive influence when cells were exposed concomitantly with ethanol and the selective transporter inhibitor (data not shown). These findings suggest ethanol blocks adenosine reuptake via inhibiting the nucleoside transport system in bronchial epithelial cells.
Figure 4. Ethanol blocks adenosine uptake as effectively as the adenosine transporter inhibitors, Dipyridamole and NBT.
Some cells were pretreated for 10 min with either 10μM dipyridamole or 100 μM NBT, two potent inhibitors of facilitated nucleoside transporter in the presence or absence of ethanol exposure and uptake was measured at 1 min. Cells preincubated with 25–100 mM ethanol blocked adenosine uptake as effectively as the adenosine transporter inhibitors. Values represent mean ± S.E.M. of three experiments, each performed in quadruplet.
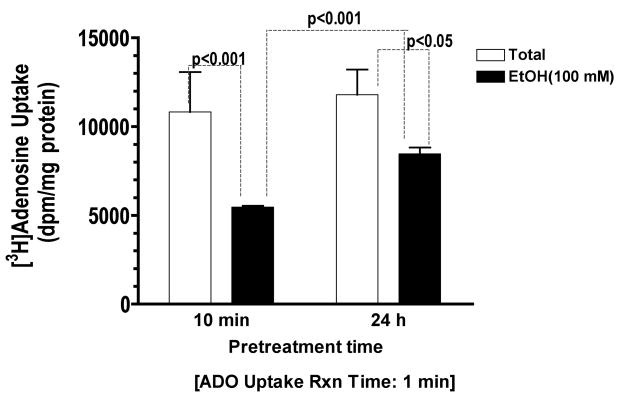
Prolonged exposure to ethanol has less impact than brief exposure on adenosine uptake in BEAS-2B cells
Studies previously conducted in our lab demonstrated that prolonged exposure of ethanol desensitized airway epithelium to stimulants that otherwise accelerate epithelial wound closure and repair (Spurzem et al., 2005). We investigated the effects of prolonged exposure to ethanol on adenosine reuptake via nucleoside transporter in BEAS-2B cells. Cells were preincubated with or without 100 mM ethanol for either 10 min or 24 h and uptake was measured for 1 min. Consistent with earlier studies in neural cells (Sapru et al., 1994), we found that prolonged exposure to ethanol caused less reduction of adenosine uptake than brief exposure (Fig 5). These findings suggest that the ethanol effect on adenosine uptake diminishes with prolonged exposure in bronchial epithelial cells.
Figure 5. Prolonged effect of ethanol exposure on adenosine uptake in BEAS-2B cells.
Monolayers of Beas-2B were either pretreated for 10 min or 24 h with either 100 mM Ethanol or control medium followed by uptake of 0.3 μCi [3H] adenosine for 1 min. Prolonged exposure to ethanol, caused less reduction of adenosine uptake than brief exposure. Values represent mean ± S.E.M. of three experiments, each performed in quadruplet.
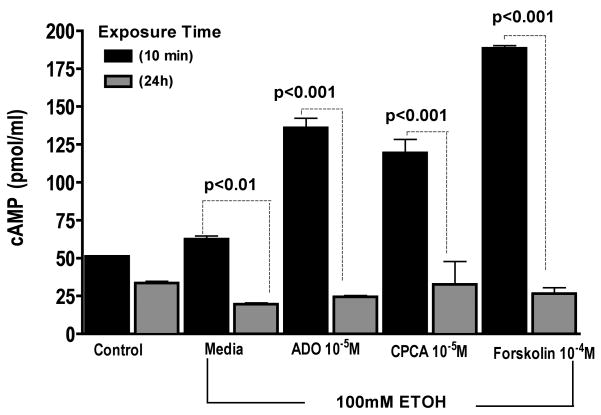
Ethanol exposure down-regulates adenosine-mediated activation of cAMP
To determine the potential role of cAMP in the loss of ethanol’s effect on adenosine transport following prolonged exposure, the cAMP activation state was assessed in response to EtOH. Cells were preincubated with or without 100 mM EtOH for either 10 min or 24 h and then stimulated for 30 min with 10 μM ADO or 10 μM CPCA. Positive control groups were treated with 100 μM Forskolin for 30 min. Cells pretreated with EtOH for 10 min and further stimulated with ADO or CPCA demonstrated significant increases in cAMP levels (Fig 6). In sharp contrast, pretreatment of cells with EtOH for 24 h blocked changes in cAMP. Interestingly, the prolonged exposure to 100 mM EtOH also inhibited the ability of 100 μM Forskolin, a known activator of cAMP. Consistent with our earlier finding (Wyatt and Sisson, 2001), prolonged ethanol exposure blunts airway epithelial responsiveness to additional challenges by agents that stimulate cAMP increases in vitro.
Figure 6. Prolonged ethanol exposure blunts adenosine-mediated activation of cAMP.
Monolayers of BEAS-2B were either pretreated for 10 min or 24 h with either 100 mM Ethanol or control medium followed by 30 min treatment with 10 μM ADO (adenosine), 10 μM CPCA (A2A receptor agonist), and 100 μM forskolin (cAMP activator) and cAMP activity was assayed. Each data point represents the average of triplicate measurements of 3 or more samples within an experiment.
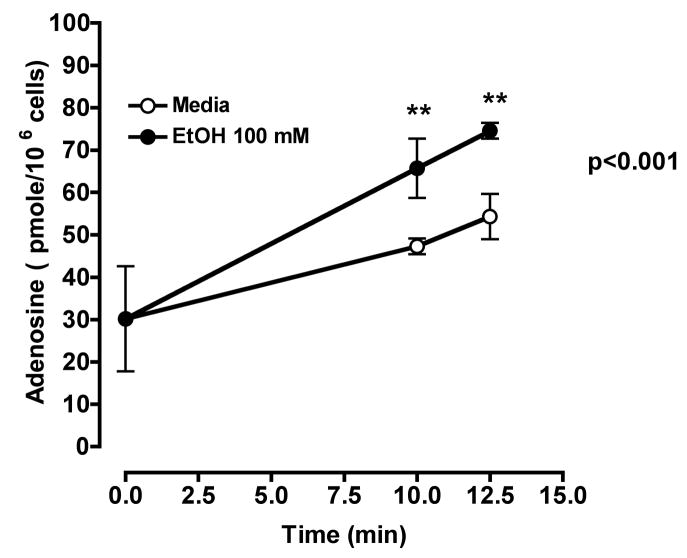
Ethanol exposure increases extracellular adenosine
To demonstrate that acute ethanol exposure increases extracellular adenosine as a result of inhibition of adenosine uptake, cells were incubated in the absence of ethanol and the concentration of extracellular adenosine increased with time. However, addition of 100 mM ethanol for 10–12.5 min resulted in increased extracellular adenosine concentrations above those found in cells not exposed to ethanol (Fig 7). This ethanol-induced increase in extracellular adenosine concentrations was not observed when cells were exposed to ethanol (36 ± 2.265 pM/106 cells) over a 24 h period as compared to media control (32 ± 2.265 pM/106 cells). These data demonstrate that acute ethanol exposure inhibits adenosine uptake and leads to an increase in extracellular adenosine. In addition, the adenosine nucleoside transporter becomes unresponsive to the acute inhibitory effects of ethanol after prolonged exposure to ethanol. Our data support earlier studies that suggest that accumulated extracellular adenosine is required for the development of ethanol-induced heterologous desensitization (Nagy et al., 1989).
Figure 7. Acute ethanol exposure increases extracellular adenosine in bronchial epithelial cells.
BEAS-2B cells were incubated at 37° C in the presence (●) or absence (○) of 100 mM ethanol at indicated times. Extracellular adenosine concentrations were measured by HPLC as described. Values represent mean ± S.E.M. of three experiments, each performed in triplicate.
DISCUSSION
The present study provides evidence that ethanol blocks adenosine uptake by inhibiting the nucleoside transport system in bronchial epithelial cells. Consistent with work performed in neural cells (Nagy et al., 1990; Nagy et al., 1991), our data revealed that brief ethanol exposure inhibits adenosine reuptake via a nucleoside transporter. We also found that adenosine uptake was less inhibited by prolonged exposure to ethanol, suggesting that ethanol desensitized adenosine receptor(s) in bronchial epithelial cells. In addition, we demonstrated that acute ethanol exposure stimulated cAMP activity and when bronchial epithelial cells were preincubated with ethanol for 24 h, the stimulation of cAMP by adenosine, CPCA and forskolin was abrogated. To our knowledge, this is the first demonstration that the adenosine nucleoside transport system in the airway can be modulated by ethanol.
Our data indicate that hENT1 and hENT2 are present in human bronchial epithelial cells and that ethanol exposure did not altered hENT1 and/or hENT2 mRNA. Both hENT1 and hENT2 are members of the equilibrative nucleoside transporters, also known as the SLC29 family (Baldwin et al., 2004; Baldwin et al., 1999). They are classified on their sensitivity to inhibition by nitrobenzylthioinosine (NBMPR), as es (equilibrative-sensitive) or ei (equlibrative-insensitve), respectively (Baldwin et al., 1999). To further distinguish the predominant equilibrative nucleotide transporter affected by ethanol we used NBT, a potent adenosine uptake inhibitor as well as dipyridamole. Both NBT and dipyridamole effectively blocked adenosine uptake as comparable to ethanol. It has been well characterized that hENT1 has ~ 100–1,000 fold more sensitivity to dipyridamole than hENT2 (Baldwin et al., 2005; Visser et al., 2002 ). Interestingly, when we incubated the cells simultaneously with ethanol and NBT we did not see an additive effect, suggesting that ethanol inhibition is independent from the selective nucleoside transporter. Additionally, earlier studies demonstrated the hENT2 is insensitive to ethanol (Choi et al., 2004; Mailliard and Diamond, 2004). Therefore, our data support that alcohol alters the hENT1 equilibrative nucleoside transporter in bronchial epithelial cells. We recognize that our findings in cultural airway epithelial cells may have limited application to other model systems.
The mechanism of ethanol-induced hENT1 inhibition is unclear in the airway. Earlier studies that demonstrate inhibition of hENT1 transporter appear to account exclusively for ethanol-induced increase in extracellular adenosine in cultured cells (Krauss et al., 1993; Nagy et al., 1990). Our data revealed that acute ethanol exposure inhibits adenosine uptake resulting in increased extracellular adenosine concentrations. We postulate that the ethanol-induced increase in extracellular adenosine subsequently activates adenosine cell-surface receptors. We previously demonstrated all four adenosine receptor subtypes are expressed in bronchial epithelial cells (Allen-Gipson et al., 2006). Adenosine A1 and A3 receptors are coupled to the inhibitory G-protein, Gi/o, while adenosine A2 receptors are coupled to the stimulatory G- protein, Gs or Golf (Kull et al., 2000). It is evident that the A2A adenosine receptor plays a pivotal role in promoting wound closure in the airway (Allen-Gipson et al., 2006). The current findings support that ethanol exposure inhibits hENT1 leading to an increase in extracellular adenosine and may alter airway epithelial functions.
Alternatively, we observed that adenosine uptake is not as inhibited with prolonged ethanol exposure, suggesting an adaptive response. This adaptive or restorative response results in a decrease in ethanol-mediated inhibition of hENT1, so that ethanol no longer blocked adenosine uptake ensuing decreases in extracellular adenosine concentration. The decreased extracellular adenosine accumulation also suggests that adenosine transport became desensitized to ethanol. In many systems (Sibley and Lefkowitz, 1985;Nagy et al., 1989), heterologous desensitization is an adaptive response to continued receptor activation and is characterized by an initial increase in cAMP (Sibley and Lefkowitz, 1985). We observed that acute ethanol exposure initiated an increased cAMP activation that was not apparent after prolong ethanol exposure. This immediate increase in cAMP observed with acute ethanol exposure also suggests activation of receptor-dependent cAMP production by the activation of adenosine via its receptor coupled to adenylyl cyclase. It is well documented that extracellular adenosine accumulation is required to induce heterologous desensitization (Nagy et al., 1989). Our HLPC data revealed acute ethanol exposure increased extracellular adenosine concentrations resulting in adenosine-mediated activation of cAMP and subsequently desensitizing cAMP production. Therefore, ethanol-induced inhibition of adenosine uptake is responsible for the heterologous desensitization of receptors that occurs after chronic exposure to ethanol.
It has been proposed that ethanol stimulates adenosine receptors by two mechanisms: 1) the metabolism of ethanol via the liver; and 2) ethanol’s ability to block the type 1 equilibrative nucleoside transport (Choi et al., 2004). For the past decade our research has focused on the effects of ethanol exposure on pulmonary function particularly as it relates to the ability of the airway epithelium to repair itself (Sisson, 2007). We have documented that ethanol exposure modulates the cyclic nucleotide signaling systems in bronchial epithelial cells (Forget et al., 2003; Spurzem et al., 2005; Wyatt and Sisson, 2001). We observed that ethanol acutely elevates ciliary motility and requires 3′5′-cyclic monophosphate (cAMP) and nitric oxide-dependent signaling pathway (Sisson et al., 1999; Wyatt et al., 2003). In this study, we report that acute ethanol exposure increases adenosine receptor-stimulated accumulation of cAMP. However, after prolonged ethanol exposure (24 h) there was a diminished response in adenosine receptor-stimulated cAMP level as well as a diminished response in both chronic ethanol- and forskolin-stimulation of receptor-dependent cAMP production. Consistent with our earlier finding (Wyatt and Sisson, 2001), these data reveal that prolonged ethanol exposure blunts airway epithelial responsiveness to additional challenges by agents that stimulate cAMP increases in vitro. In addition, our data support that ethanol induces changes in cAMP signal transduction, which subsequently affect adenosine reuptake via the nucleoside transport system.
In summary, we have shown that both hENT1 and hENT2 nucleoside transporters exist in the human bronchial epithelium and that ethanol regulates the hENT1 nucleoside transporter. Acute exposure to ethanol blocks adenosine reuptake via nucleoside transporter(s), which increases the concentration of extracellular adenosine resulting in an increase in intracellular cAMP levels. In cells treated chronically with ethanol, adenosine uptake is no longer fully inhibited by ethanol. We have shown that ethanol inhibition of adenosine uptake requires activation of the cAMP signaling system. In addition, chronic exposure of ethanol results in an adaptive response in which ethanol no longer blocks adenosine reuptake via nucleoside transporter. These data suggest that ethanol plays a role in modulating nucleoside transport processes, which regulate both endogenous and extracellular adenosine levels. This regulation ultimately influences the effect of adenosine at the epithelial cell surface, which may prove critical in maintaining adenosine-mediated airway homeostasis and wound repair in airway epithelium. Because ethanol consumption has been associated with significant lung disease including pneumonia, bronchitis and chronic obstructive pulmonary disease (COPD; Heinemann, 1977; Suadicani et al., 2001; Tabak et al., 2001), further studies are needed to evaluate ethanol’s role in adenosine-mediated airway wound repair processes.
Supplementary Material
Acknowledgments
The authors like to thank Dr. Pastor-Anglada, Universitat De Barcelona, Barcelona Spain for his generous gift of monospecific polyclonal antibody against human equilibrative nucleoside transporter protein, ENT1 and ENT2, Jane M. DeVasure for her exemplary technical support and Lisa Chudomelka for her editorial and administrative support on this paper. This work was supported by the Department of Health and Human Services, National Institutes of Health, National Heart, Lung and Blood Institute- K01-HL084684-01.
References
- Allen-Gipson DS, Wong J, Spurzem JR, Sisson JH, Wyatt TA. Adenosine A2A receptors promote adenosine-stimulated wound healing in bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2006;290(5):L849–55. doi: 10.1152/ajplung.00373.2005. [DOI] [PubMed] [Google Scholar]
- Baldwin SA, Beal PR, Yao SY, King AE, Cass CE, Young JD. The equilibrative nucleoside transporter family, SLC29. Pflugers Arch. 2004;447(5):735–43. doi: 10.1007/s00424-003-1103-2. [DOI] [PubMed] [Google Scholar]
- Baldwin SA, Mackey JR, Cass CE, Young JD. Nucleoside transporters: molecular biology and implications for therapeutic development. Mol Med Today. 1999;5(5):216–24. doi: 10.1016/S1357-4310(99)01459-8. [DOI] [PubMed] [Google Scholar]
- Baldwin SA, Yao SY, Hyde RJ, Ng AM, Foppolo S, Barnes K, Ritzel MW, Cass CE, Young JD. Functional characterization of novel human and mouse equilibrative nucleoside transporters (hENT3 and mENT3) located in intracellular membranes. J Biol Chem. 2005;280(16):15880–7. doi: 10.1074/jbc.M414337200. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Carmichael FJ, Israel Y, Crawford M, Minhas K, Saldivia V, Sandrin S, Campisi P, Orrego H. Central nervous system effects of acetate: contribution to the central effects of ethanol. J Pharmacol Exp Ther. 1991;259(1):403–8. [PubMed] [Google Scholar]
- Cass CE, Young JD, Baldwin SA, Cabrita MA, Graham KA, Griffiths M, Jennings LL, Mackey JR, Ng AM, Ritzel MW, Vickers MF, Yao SY. Nucleoside transporters of mammalian cells. Pharm Biotechnol. 1999;12:313–52. doi: 10.1007/0-306-46812-3_12. [DOI] [PubMed] [Google Scholar]
- Choi DS, Cascini MG, Mailliard W, Young H, Paredes P, McMahon T, Diamond I, Bonci A, Messing RO. The type 1 equilibrative nucleoside transporter regulates ethanol intoxication and preference. Nat Neurosci. 2004;7(8):855–61. doi: 10.1038/nn1288. [DOI] [PubMed] [Google Scholar]
- Coe IR, Dohrman DP, Constantinescu A, Diamond I, Gordon AS. Activation of cyclic AMP-dependent protein kinase reverses tolerance of a nucleoside transporter to ethanol. J Pharmacol Exp Ther. 1996;276(2):365–9. [PubMed] [Google Scholar]
- Farre X, Guillen-Gomez E, Sanchez L, Hardisson D, Plaza Y, Lloberas J, Casado FJ, Palacios J, Pastor-Anglada M. Expression of the nucleoside-derived drug transporters hCNT1, hENT1 and hENT2 in gynecologic tumors. Int J Cancer. 2004;112(6):959–66. doi: 10.1002/ijc.20524. [DOI] [PubMed] [Google Scholar]
- Forget MA, Sisson JH, Spurzem JR, Wyatt TA. Ethanol increases phosphodiesterase 4 activity in bovine bronchial epithelial cells. Alcohol. 2003;31(1–2):31–8. doi: 10.1016/j.alcohol.2003.06.005. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, AP IJ, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53(4):527–52. [PMC free article] [PubMed] [Google Scholar]
- Gordon AS, Collier K, Diamond I. Ethanol regulation of adenosine receptor-stimulated cAMP levels in a clonal neural cell line: an in vitro model of cellular tolerance to ethanol. Proc Natl Acad Sci U S A. 1986;83(7):2105–8. doi: 10.1073/pnas.83.7.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon AS, Nagy L, Mochly-Rosen D, Diamond I. Chronic ethanol-induced heterologous desensitization is mediated by changes in adenosine transport. Biochem Soc Symp. 1990;56:117–36. [PubMed] [Google Scholar]
- Heinemann HO. Alcohol and the lung. A brief review. Am J Med. 1977;63(1):81–5. doi: 10.1016/0002-9343(77)90120-6. [DOI] [PubMed] [Google Scholar]
- Jackson EK, Dubey RK. Role of the extracellular cAMP-adenosine pathway in renal physiology. Am J Physiol Renal Physiol. 2001;281(4):F597–612. doi: 10.1152/ajprenal.2001.281.4.F597. [DOI] [PubMed] [Google Scholar]
- Kharbanda KK, Rogers DD, 2nd, Mailliard ME, Siford GL, Barak AJ, Beckenhauer HC, Sorrell MF, Tuma DJ. A comparison of the effects of betaine and S-adenosylmethionine on ethanol-induced changes in methionine metabolism and steatosis in rat hepatocytes. J Nutr. 2005;135:519–24. doi: 10.1093/jn/135.3.519. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Zimmermann H, Millhorn DE. Chronic hypoxia enhances adenosine release in rat PC12 cells by altering adenosine metabolism and membrane transport. J Neurochem. 2000;74(2):621–32. doi: 10.1046/j.1471-4159.2000.740621.x. [DOI] [PubMed] [Google Scholar]
- Krauss SW, Ghirnikar RB, Diamond I, Gordon AS. Inhibition of adenosine uptake by ethanol is specific for one class of nucleoside transporters. Mol Pharmacol. 1993;44(5):1021–6. [PubMed] [Google Scholar]
- Kull B, Svenningsson P, Fredholm BB. Adenosine A(2A) receptors are colocalized with and activate g(olf) in rat striatum. Mol Pharmacol. 2000;58(4):771–7. doi: 10.1124/mol.58.4.771. [DOI] [PubMed] [Google Scholar]
- Lechner JF, LaVeck MA. A serum-free method for culturing normal human bronchial epithelial cells at clonal density. J Tissue Culture Methods. 1985;9:43–48. [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;5:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Fan AL, Randall RJ. Protein measurement with the Folin Phenol Reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Mailliard WS, Diamond I. Recent advances in the neurobiology of alcoholism: the role of adenosine. Pharmacol Ther. 2004;101(1):39–46. doi: 10.1016/j.pharmthera.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Massague J, Czech MP. The subunit structures of two distinct receptors for insulin-like growth factors I and II and their relationship to the insulin receptor. J Biol Chem. 1982;257(9):5038–45. [PubMed] [Google Scholar]
- Melendez RI, Kalivas PW. Last call for adenosine transporters. Nat Neurosci. 2004;7(8):795–6. doi: 10.1038/nn0804-795. [DOI] [PubMed] [Google Scholar]
- Nagy LE, Diamond I, Casso DJ, Franklin C, Gordon AS. Ethanol increases extracellular adenosine by inhibiting adenosine uptake via the nucleoside transporter. J Biol Chem. 1990;265(4):1946–51. [PubMed] [Google Scholar]
- Nagy LE, Diamond I, Collier K, Lopez L, Ullman B, Gordon AS. Adenosine is required for ethanol-induced heterologous desensitization. Mol Pharmacol. 1989;36(5):744–8. [PubMed] [Google Scholar]
- Nagy LE, Diamond I, Gordon AS. cAMP-dependent protein kinase regulates inhibition of adenosine transport by ethanol. Mol Pharmacol. 1991;40(5):812–7. [PubMed] [Google Scholar]
- Paterson AR, Lau EY, Dahlig E, Cass CE. A common basis for inhibition of nucleoside transport by dipyridamole and nitrobenzylthioinosine? Mol Pharmacol. 1980;18(1):40–4. [PubMed] [Google Scholar]
- Poucher SM, Brooks R, Pleeth RM, Conant AR, Collis MG. Myocardial infarction and purine transport inhibition in anaesthetised ferrets. Eur J Pharmacol. 1994;252(1):19–27. doi: 10.1016/0014-2999(94)90570-3. [DOI] [PubMed] [Google Scholar]
- Sapru MK, Diamond I, Gordon AS. Adenosine receptors mediate cellular adaptation to ethanol in NG108–15 cells. J Pharmacol Exp Ther. 1994;271(1):542–8. [PubMed] [Google Scholar]
- Sibley DR, Lefkowitz RJ. Molecular mechanisms of receptor desensitization using the beta-adrenergic receptor-coupled adenylate cyclase system as a model. Nature. 1985;317(6033):124–9. doi: 10.1038/317124a0. [DOI] [PubMed] [Google Scholar]
- Sisson JH. Alcohol and airways function in health and disease. Alcohol. 2007;41(5):293–307. doi: 10.1016/j.alcohol.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisson JH, May K, Wyatt TA. Nitric oxide-dependent ethanol stimulation of ciliary motility is linked to cAMP-dependent protein kinase (PKA) activation in bovine bronchial epithelium. Alcohol Clin Exp Res. 1999;23(9):1528–33. [PubMed] [Google Scholar]
- Spurzem JR, Veys T, Devasure J, Sisson JH, Wyatt TA. Ethanol treatment reduces bovine bronchial epithelial cell migration. Alcohol Clin Exp Res. 2005;29(4):485–92. doi: 10.1097/01.alc.0000158830.21657.bb. [DOI] [PubMed] [Google Scholar]
- Suadicani P, Hein HO, Meyer HW, Gyntelberg F. Exposure to cold and draught, alcohol consumption, and the NS-phenotype are associated with chronic bronchitis: an epidemiological investigation of 3387 men aged 53–75 years: the Copenhagen Male Study. Occup Environ Med. 2001;58(3):160–4. doi: 10.1136/oem.58.3.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabak C, Smit HA, Rasanen L, Fidanza F, Menotti A, Nissinen A, Feskens EJ, Heederik D, Kromhout D. Alcohol consumption in relation to 20-year COPD mortality and pulmonary function in middle-aged men from three European countries. Epidemiology. 2001;12(2):239–45. doi: 10.1097/00001648-200103000-00018. [DOI] [PubMed] [Google Scholar]
- Visser F, Vickers MF, Ng AM, Baldwin SA, Young JD, Cass CE. Mutation of residue 33 of human equilibrative nucleoside transporters 1 and 2 alters sensitivity to inhibition of transport by dilazep and dipyridamole. J Biol Chem. 2002;277(1):395–401. doi: 10.1074/jbc.M105324200. [DOI] [PubMed] [Google Scholar]
- Wyatt TA, Forget MA, Sisson JH. Ethanol stimulates ciliary beating by dual cyclic nucleotide kinase activation in bovine bronchial epithelial cells. Am J Pathol. 2003;163(3):1157–66. doi: 10.1016/S0002-9440(10)63475-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt TA, Sisson JH. Chronic ethanol downregulates PKA activation and ciliary beating in bovine bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2001;281(3):L575–81. doi: 10.1152/ajplung.2001.281.3.L575. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.