Abstract
Ghrelin and peptide YY (PYY) stimulate hunger and satiety, respectively. The physiology of these hormones during normal meal intake remains unclear. The present study was designed to compare the responses of these two hormones to meal intake between lean and obese Hispanic adolescents. Ten obese and seven lean Hispanic youth, aged 11–14 yr, consumed two mixed meals, one small and one large, during which plasma measurements of active and total ghrelin and total PYY were obtained. Obese subjects tended to consume more calories during the small meal than lean subjects, although this did not reach statistical significance. Intake of the small meal significantly suppressed active ghrelin and stimulated PYY levels in the lean subjects, and these changes were further accentuated by the large meals. In obese subjects, the suppression of active ghrelin and stimulation of PYY by caloric intake were blunted. Interestingly, a paradoxical stimulation of active ghrelin levels was noted during the small meals in both lean and obese subjects. This stimulation was not seen during the larger meals in lean subjects, but remained present in the obese subjects. Thus, meal-related changes in active ghrelin and PYY are blunted in obese as compared to lean Hispanic subjects. This blunting could contribute to the development or worsening of obesity.
Keywords: Ghrelin, Adolescents, Appetite Regulation, Obesity
Introduction
Obesity is associated with alterations in levels of some appetite-regulating hormones, such as ghrelin and peptide YY (PYY), and apparent resistance to others, such as leptin and insulin (1). However, the precise roles of these hormones in energy balance remain unclear.
Ghrelin is secreted by endocrine cells in the gastrointestinal tract, primarily the fundus of the stomach (2, 3), and stimulates hunger by activating NPY/AgRP neurons in the arcuate nucleus of the hypothalamus (4). It circulates in both acylated (active) and de-acylated forms; the former appears to be responsible for signaling hunger (5). Both active and total ghrelin levels increase during fasting and are suppressed by intake of calories (6), particularly carbohydrates (7, 8). However, obese animals, including humans, have lower fasting levels of total and active ghrelin than do their lean counterparts (9). Additionally, the sensitivity of both forms of ghrelin to suppression by caloric intake appears to be blunted in obese adults (10, 11). There are conflicting data as to whether ghrelin is suppressed by meal intake in children (12–16).
PYY is secreted by neuroendocrine L-cells in the ileum, colon, and rectum, and appears to signal satiety by suppressing NPY neurons in the arcuate nucleus (17, 18). PYY increases with caloric intake, particularly fat (19, 20). Obese adults have lower fasting levels of PYY, and also demonstrate a blunted response to caloric intake (21, 22) compared to lean adults. Obese children also have low fasting PYY, which is at least partially corrected by weight loss (23).
Elucidating the roles of these two hormones in the development of obesity in humans is difficult. Most studies have been performed in adults, who likely have been obese for many years, and, thus, alterations in these hormones may not be directly relevant to the developing obese state. In addition, most studies measure the total rather than active (acylated) form of ghrelin. Since the active form accounts for only ~10% of circulating levels and the ratio of active to total ghrelin changes after calorie consumption (24), the relevance of post-prandial total ghrelin levels is questionable. Finally, human studies have used either a meal of fixed caloric size or an ad libitum meal, and, therefore, a dose response to caloric intake cannot be derived.
The present study was designed to examine ghrelin and PYY responses to small and large meals in lean and obese individuals. Our primary hypotheses were that obese adolescents would have less suppression of ghrelin and less stimulation of PYY in response to meal intake compared to their lean counterparts. Adolescents were studied in order to investigate the roles of these hormones in a state of obesity that may still be developing, and has not been chronically present (i.e. not for decades). Studies were performed in Hispanic Americans, a group at increased risk of obesity. Cafeteria meals of fixed nutrient composition were used to more closely simulate free-living meals. Two different meal sizes were used, so that the changes in hormones as a function of caloric intake could be observed. Finally, both active and total ghrelin were measured, so that differential changes in these two isoforms could be evaluated.
Methods and Procedures
Subjects
Ten obese (BMI ≥95th percentile for age and gender) and seven control (BMI between 25th and 85th percentiles) Hispanic adolescents were recruited from the Childrens Hospital Los Angeles (CHLA) Endocrinology Clinic and Emergency Department. We chose to study Hispanic youth, as obesity and diabetes is prominent in this population, and it is not known whether appetite hormones may respond differently in different ethnicities and races. Ethnicity was based on self-report. Subjects were in pubertal Tanner stage II, III, or IV, as documented by a pediatric endocrinologist. Control subjects were excluded if they had acanthosis nigricans or a first-degree relative with type 2 diabetes. Subjects were excluded from either group if they had a random serum glucose >126 mg/dL, significant illness, or were taking medications. All subjects provided informed assent and their parent/guardian provided informed consent for their participation in the study. All research activities were approved by the CHLA Committee on Clinical Investigations (Institutional Review Board).
Experimental Design
Subjects’ heights were measured on a stadiometer accurate to 0.1 cm and the average of three readings was used. Weight was recorded while subjects wore light clothing. Waist and hip circumference were measured by a trained observer using a measuring tape. Body fat percentage was measured via bioelectric impedance using a BIA 310e (Biodynamics, Seattle, WA) with subjects lying supine. Subjects then chose their meal for the future meal tolerance tests (MTTs) from a list of four choices: chicken salad sandwich, tuna salad sandwich, hamburger, or grilled ham and cheese sandwich. All meals included milk and fruit cocktail, and were designed to provide 50% of calories from carbohydrate, 30% from fat (10% saturated/20% unsaturated), and 20% from protein.
Subjects participated in a small and a large MTT in random order. Meal tests occurred on different days no less than 1 week and no more than 1 month apart, and started between 0800 and 0900. The small meal was designed to provide 25% of US Recommended Daily Allowance (RDA) of caloric intake adjusted for height, but not weight [daily caloric intake = 15.9 kcal/cm for boys and 14.0 kcal/cm for girls, (25)]. The large meal consisted of the same meal choice, but subjects were presented with 62.5% of the US RDA caloric intake. Subjects were instructed to refrain from consuming any foods or liquids after midnight the night before each MTT, with the exception of sips of water in the morning. An intravenous (IV) catheter was placed and 0.45% normal saline run at 5 cc/hr to keep the line patent throughout the experiment. Blood samples were drawn through the IV starting at least 30 min after placement, at t = −30, −1, 15, 30, 45, 60, 90, and 120 min relative to the introduction of the meal. Subjects were presented with the meal at t = 0, and instructed to eat to satiation. Remaining food was removed at t = 30 min and weighed by a registered dietitian who calculated the amounts consumed. Blood samples were drawn into chilled EDTA Vacutainers (BD, Franklin Lakes NJ). Tubes for total and active ghrelin contained 500 units of aprotinin per mL of blood (Celliance, Toronto Ontario, Canada). Tubes for PYY, insulin, and glucose contained 25 units aprotinin per mL blood (1 μg/mL blood). Samples were maintained on ice, centrifuged, and plasma transferred to −80° C within 30 min of collection. Plasma for ghrelin measurements was acidified with 100 μL 1N HCl per mL of plasma to inhibit degradation (26).
Measurements
Glucose was measured on a YSI 3000 (Yellow Springs Instruments, Yellow Springs, OH) and insulin was measured using a radioimmunoassay from Linco, both performed in the USC GCRC Laboratory. Total ghrelin, active ghrelin, and total PYY were measured using RIA kits from Linco Research (now owned by Millipore, Billerica MA).
Calculations
Basal hormone levels were defined as the average of the t = −30 and t = −1 min samples. Steady-state levels were defined as the average of the t = 90 and t = 120 min samples. Percent suppression and stimulation was calculated from basal and steady-state values. HOMAIR, a measure of insulin resistance, is defined as (fasting glucose in mM × fasting insulin in μU/mL)/22.5 (27). All results are reported as mean ± standard error. Anthropomorphic measures and hormone levels (fasting, steady-state, and deltas) were compared between lean and obese groups using t-tests for normally distributed variables and the Wilcoxon non-parametric test for non-normally distributed variables. Univariate correlations were performed between the changes in appetite hormones and BMI z-score, glucose AUC, insulin AUC, and incremental glucose and insulin AUC, and Pearson correlation coefficients are reported. A mixed model analysis was used to relate the appetite hormone levels to both body type (lean vs. obese), meal stimulus (fasting, small, large), and the interaction between body type and meal type, assuming either compound symmetry (i.e., a constant correlation between successive within-subject measurements), an unstructured or an auto-regressive covariance pattern, whichever was optimal for each dependent variable. A random effects mixed model was used to derive and compare slopes of ghrelin and PYY versus calories consumed. Statistical tests were 2-sided and a p-value <0.05 was considered statistically significant. Subjects who completed only one meal test were included in analyses as permitted by specific statistical methods. Statistical analyses were performed using SAS (version 9) under the UNIX operating system.
Results
Subjects
Characteristics of the 7 obese and 10 lean subjects are presented in Table 1. There were relatively more males in the obese group compared to the lean group, while the Tanner stage tended to be higher on average in the lean group, but these were not significantly different. Obese subjects had higher body fat percentage, fasting insulin and glucose levels, and HOMAIR.
Table 1.
Subject characteristics
| Obese | Lean | p | |
|---|---|---|---|
| n (M/F) | 10 (7/3) | 7 (3/4) | 0.35a |
| Age (yr) | 12.8 ± 0.4 | 12.8 ± 0.4 | 0.97 |
| Height (cm) | 160.4 ± 2.6 | 156.9 ± 3.9 | 0.22 |
| Tanner Stage | 2.9 ± 3 | 3.7 ± 3 | 0.14a |
| BMI (kg/m2) | 36.3 ± 2.5 | 19.7 ± 0.6 | n/a |
| Waist:hip ratio | 0.97 ± 0.05 | 0.84 ± 0.03 | 0.089 |
| Body Fat (%) | 38 ± 2 | 22 ± 2 | <0.001 |
| Fasting Insulin (μU/mL) | 40 ± 7 | 13 ± 2 | 0.006 |
| Fasting Glucose (mg/dL) | 91 ± 2 | 85 ± 2 | 0.053 |
| HOMAIR | 9.3 ± 1.5 | 2.7 ± 0.4 | 0.002 |
Fisher exact test
Two of the obese subjects completed only one of the two meals (one small and the other large). Due to assay problems, active ghrelin measurements were not obtained for one lean and two obese subjects. All subjects consumed more calories during the large meal than the small meal (Table 2, p<0.0001), and there were no significant differences in caloric intake between lean and obese subjects (small meal p=0.13, large meal p=0.54).
Table 2.
Caloric intake during meal tolerance tests by nutrient
| Small | Large | |||||||
|---|---|---|---|---|---|---|---|---|
| Lean | Obese | Lean | Obese | |||||
| Offered | Consumed | Offered | Consumed | Offered | Consumed | Offered | Consumed | |
| Total | 589±24 | 450±53 | 618±18 | 547±33 | 1460±64 | 777±89 | 1437±46 | 860±94 |
| Carbohydrate | 289±10 | 201±22 | 302±9 | 255±19 | 726±25 | 328±32 | 702±22 | 371±45 |
| Fat | 175±8 | 146±21 | 185±6 | 175±12 | 429±25 | 266±46 | 429±16 | 293±40 |
| Protein | 116±5 | 95±11 | 122±4 | 108±7 | 284±14 | 166±20 | 284±9 | 180±20 |
| % Carb | 49±1 | 45±2 | 49±1 | 47±2 | 50±1 | 44±4 | 49±1 | 43±3 |
| % Fat | 30±0 | 32±2 | 30±0 | 32±1 | 29±1 | 33±3 | 30±0 | 34±2 |
| % Protein | 20±0 | 21±1 | 20±0 | 20±1 | 19±0 | 21±1 | 20±0 | 21±1 |
Glucose & Insulin
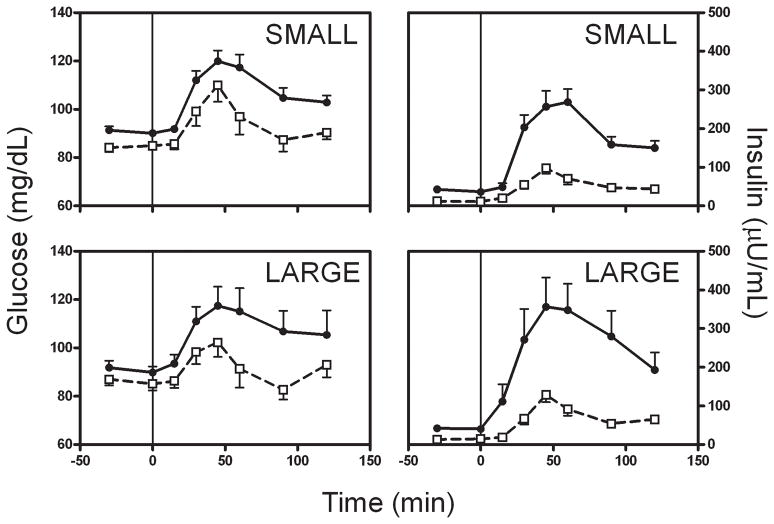
As expected, obese individuals had higher fasting and post-prandial glucose and insulin levels (Figure 1, Table 3). One obese subject had impaired fasting glucose on the day of one meal test (108 mg/dL), but not the other (98 mg/dL); this subject’s appetite hormone levels did not differ from the rest of the group. Post-prandial glucose levels in the lean subjects returned to within 4 mg/dL of baseline by 90 minutes after the start of the meal. Glucose levels in the obese subjects still remained on average more than 10 mg/dL higher than their baseline at 120 minutes.
Figure 1.
Glucose and Insulin levels during the small (top) and large meals (bottom). Meals were given at t=0, and food removed at t=30 min. Lean subjects = open squares with dashed line; obese subjects = closed circles with solid line.
Table 3.
Hormone levels
| Small | Large | |||||
|---|---|---|---|---|---|---|
| Lean | Obese | p | Lean | Obese | p | |
| Glucose | ||||||
| SSa | 88.8±3.6 | 103.7±3.4 | 0.009 | 87.9±4.5 | 106.1±9.3 | 0.108 |
| AUC (mg/dL * min * 103) | 11.2±0.5 | 12.9±3.8 | 0.018 | 10.9±0.5 | 12.9±0.9 | 0.079 |
| Insulin | ||||||
| SS | 45.2±5.7 | 154.2±18.2 | <0.001 | 59.8±5.0 | 236.6±49.9 | 0.009 |
| AUC (μU/mL * min * 103) | 6.3±0.8 | 20.9±2.0 | <0.001 | 8.0±0.9 | 30.5±5.8 | 0.001 |
| Total Ghrelin (pg/mL) | ||||||
| Basal | 1407±67 | 1441±128 | 0.835 | 1400±73 | 1393±176 | 0.977 |
| SS | 1490±138 | 1439±167 | 0.822 | 1195±38 | 1178±139 | 0.916 |
| Delta (SS – Basal) | 83±102 | −2±96 | 0.556 | −205±46 | −215±62 | 0.907 |
| % Change | 5.3±7.0 | −0.7±6.6 | 0.550 | −13.8±3.2 | −13.9±3.1 | 0.993 |
| Active Ghrelin (pg/mL) | ||||||
| Basal | 123.3±25.7 | 80.7±16.0 | 0.175 | 121.4±25.1 | 81.5±13.6 | 0.174 |
| SS | 83.8±19.3 | 66.9±12.4 | 0.463 | 67.4±12.0 | 71.6±10.1 | 0.796 |
| Delta (SS – Basal) | −39.5±11.6 | −13.8±5.1 | 0.055 | −54.0±15.4 | −9.9±6.6 | 0.018 |
| % Change | −31±6 | −15±5 | 0.059 | −40±7 | −7±9 | 0.017 |
| PYY (pg/mL) | ||||||
| Basal | 132.4±17.6 | 123.1±17.9 | 0.720 | 136.4±24.9 | 135.0±15.6 | 0.960 |
| SS | 190.2±23.9 | 151.5±20.7 | 0.239 | 193.9±27.7 | 173.4±21.5 | 0.563 |
| Delta (SS – Basal) | 57.8±13.7 | 28.4±8.2 | 0.074 | 57.5±15.3 | 38.5±9.3 | 0.286 |
| % Change | 46±11 | 25±8 | 0.134 | 54±19 | 29±5 | 0.180 |
SS = steady state
Ghrelin
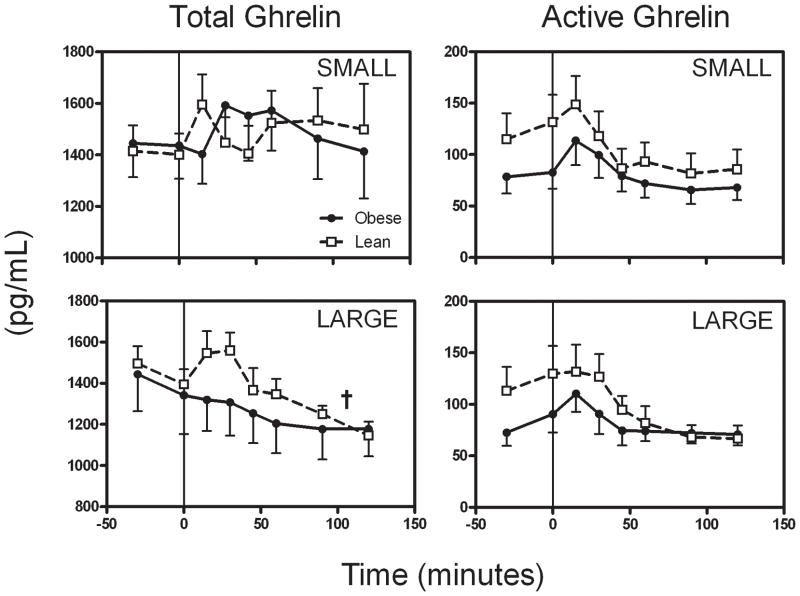
Fasting total ghrelin levels were similar between the lean and obese groups at the start of the small and large meals (p=0.92, repeated measure ANOVA including levels from both meals, Table 3). Total ghrelin levels did not decrease during or after the small meal, but did decline after the large meal (p<0.005 vs. basal for each group, Figure 2 and Table 3). Mixed model analysis showed a significant effect of meal type on total ghrelin level (i.e. total ghrelin was suppressed more by a large meal, p<0.0001), but no effect of body type (p=0.97) or interaction between body type and meal type (p=0.80).
Figure 2.
Total plasma ghrelin levels (left two panels) during the small (top) and large meals (bottom). Only large meals suppressed total ghrelin levels significantly in either obese or lean subjects (†p<0.005), and mixed model analysis showed a significant effect of meal type on these levels (p<0.001). However, there were no significant effects of obesity or interactions between obesity and meal type (p=n.s.). Active (acylated) ghrelin levels (right two panels) were suppressed in the lean subjects after intake of either meal (p<0.01 for both meal types), while in the obese subjects, only the large meal suppressed these levels (p=n.s. for small, p<0.05 for large). Symbols are defined in Figure 1
Fasting levels of active ghrelin (Figure 2 and Table 3) tended to be lower in the obese than in the lean subjects (p=0.05, including measures from both meals), and were slowly rising prior to meal initiation. This rise continued or was accentuated slightly during the first 30 minutes of the MTTs. Thereafter, active ghrelin fell in all groups. In the lean subjects, active ghrelin levels in the steady-state period were suppressed well below their fasting levels by the small meal (p=0.019) and further suppressed by the large meal (p=0.017). In contrast, active ghrelin levels were modestly suppressed below fasting levels by the small meal in the obese subjects (p=0.034) but not by the large meal (p=0.19). The change in active ghrelin levels (steady state minus basal) after the large meal was correlated with BMI z-score (r = 0.67, p=0.013, Figure S1), but not after the small meal (r = 0.46, p=0.12). This change in active ghrelin was not associated with the incremental insulin AUC in univariate analysis for the small meal (r = 0.41, p=0.17), though the association was nearly significant for the large meal (r = 0.56, p=0.058). Thus, subjects with highest BMI and insulin AUC had the smallest meal-related decreases in active ghrelin. The change in active ghrelin level was not correlated with % body fat or HOMAIR. Overall, while active ghrelin levels started lower in the obese than the lean subjects, the steady-state levels were similar in both groups. Mixed model analysis revealed a significant effect of meal type to suppress active ghrelin levels, with large meals suppressing the hormone level further than small meal (p<0.0001). There was also a significant interaction between body and meal type on active ghrelin levels (p=0.0039, see Figure 4), such that this effect of increasing meal size to suppress active ghrelin was less pronounced in the obese subjects.
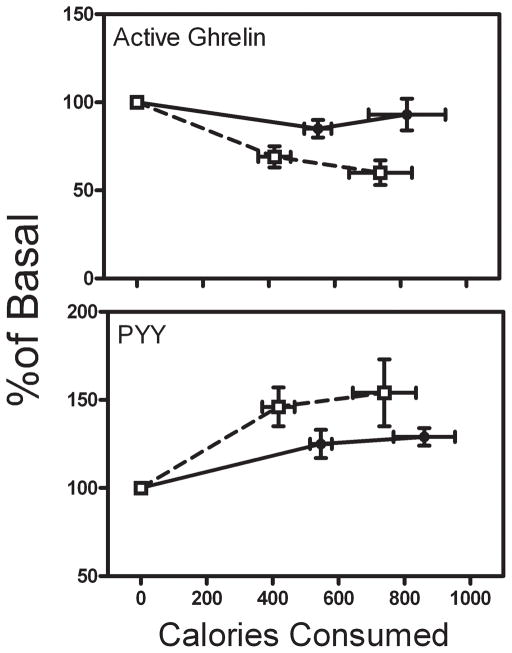
Figure 4.
Dose response of active ghrelin and total PYY (expressed as percentage of basal levels) vs. calories consumed during small and large meals. Symbols are defined in Figure 1.
A transient increase in active ghrelin levels was noted in lean and obese subjects, most notably in response to the small meal. Within 15 min of starting the small meal, active ghrelin levels increased significantly from basal in the obese (from 80.7±16.0 to 116.6±24.9 pg/mL, p=0.037) and lean subjects (from 123.3±25.7 to 148.7±27.8 pg/mL, p=0.012). The absolute increase in active ghrelin levels was not different between groups (obese 32.8±12.2 vs. lean 25.4±6.6 pg/mL, p=0.312). During the large meal, the active ghrelin levels increased significantly by 15 min in the obese group (from 81.5±13.6 to 111.3±17.5 pg/mL, p=0.005), but not in the lean group (from 121.4±25.1 to 137.7±24.5 pg/mL, p=0.413).
PYY
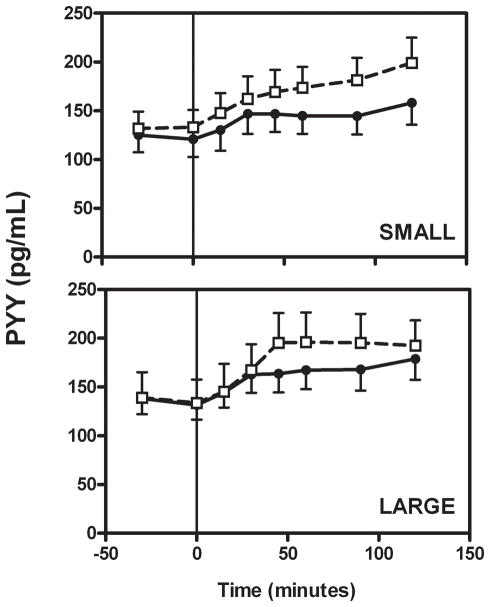
Fasting PYY levels (Figure 3, Table 3) were similar in the lean and obese groups (p=0.77, including measures from both meals). The absolute increase in PYY after the small meal was larger in the lean subjects than in the obese subjects, though not significantly (p=0.074). Interestingly, the PYY response to the large meal in the lean subjects was similar to the small meal, with no further stimulation by the increased caloric intake (i.e. steady state PYY values were no different between meals, p=0.801). On the other hand, the large meal led to higher steady-state PYY levels in the obese subjects than did the small meal (p=0.011). Thus, the absolute change in PYY levels after the large meal was not statistically different between the groups (p=0.143). The increase in PYY induced by the small meal correlated inversely with BMI z-score (r = −0.49, p=0.054, Figure S2) and the incremental insulin AUC (r = −0.56, p=0.025), but not with % body fat or HOMAIR (not shown). The change in PYY after the large meal did not correlate with any of these variables. Mixed model analysis revealed no overall effect of body type on PYY level (p=0.45), although there was a significant effect of meal type (large meals resulting in higher PYY levels than small meals, p<0.0001) and a borderline significant interaction between body and meal type (the effect of meals being more pronounced in lean subjects, particularly with the small meal, p=0.068) on this hormone.
Figure 3.
Total plasma PYY levels were increased in both lean and obese subjects during either meal type (p<0.05 for all comparisons). Symbols are defined in Figure 1.
Response to Caloric Intake
Because the groups of subjects tended to consume different amounts of calories (obese subjects ate more than lean subjects), we analyzed the change in hormone levels as a function of calories consumed (Figure 4), with a random effects mixed model to estimate and compare linear slopes between groups. The responses of total ghrelin levels to caloric intake were not different between groups (not shown; slope=−16.6 for lean vs. −18.0 for obese, p=0.94). In contrast, active ghrelin showed a steeper calorie-dependent suppression in the lean subjects compared to the obese subjects (slope = −7.4 for lean vs. −1.3 pg/mL/100 kCal for obese, p<0.0001). PYY levels were nearly maximally stimulated by the small meal in the lean subjects, while there was a non-significant rightward shift in the PYY vs. calorie curve in the obese subjects (slope = 8.0 for lean vs. 5.0 pg/mL/100 kCal for obese, p=0.11).
Discussion
In the present study, we show for the first time that obese Hispanic adolescents have impairments in the ability of a mixed meal to suppress active ghrelin and stimulate PYY. These impairments appear to be related to the degree of obesity and/or hyperinsulinemia, as the changes in these hormones in response to meals correlated to varying degrees with BMI z-score and insulin AUC. As active ghrelin is believed to signal hunger and PYY satiety, these alterations may contribute to the development and or worsening of obesity in this ethnic group.
There are several aspects to the present study which make it unique compared to prior studies of effects of food intake on ghrelin and PYY. First, we studied adolescents, an approach chosen to minimize any confounding effects of long-standing obesity. Second, we used solid mixed meals, rather than liquid meals or oral glucose, to more closely approximate natural physiological stimuli. Third, we measured not only total, but also active ghrelin levels, allowing us to examine the effects of meals on the form of this hormone believed to be most strongly involved in appetite regulation [reviewed in (28)]. Finally, we used two different-sized meals to evaluate whether alterations in the hormone responses to fixed meals could be overcome with a larger meal, which may more closely reflect what individuals are presented with on a regular basis.
We observed that the responses of both active ghrelin and PYY to caloric intake were significantly blunted in the obese adolescents. Both hormones changed less in response to the small meal in the obese than in the lean subjects, despite the tendency for the obese subjects to consume more calories. The increased caloric intake during the large meal in the obese subjects did not correct the suppression of active ghrelin or stimulation of PYY toward that observed in the lean subjects. Thus, differences in the responses of these hormones to meals could not simply be overcome by increasing calories over the range consumed in this study.
While resistance of the active form of ghrelin to caloric intake has previously been demonstrated in obese adults (29, 30), the meal response of ghrelin in children and adolescents is less clear. Some studies reported that normal-weight children had little suppression of ghrelin levels by meal intake (31–34). Others found significant suppression of ghrelin by meals (35, 36). Stock et al reported a blunting of the suppression of total ghrelin in obese compared to lean youth (37). Differences in meal size or type may help explain some of these conflicting results. Studies which used standard-sized, smaller meals tended to find no suppression of ghrelin in youth, while those that used ad libitum meals observed significant ghrelin suppression. In the present study, we found that the small meal did not suppress total ghrelin levels in either lean or obese subjects, while the large meal did. Additionally, studies in the literature which utilized oral glucose loads or liquid meals generally reported suppression of both total and active ghrelin levels in non-obese children, with variable suppression in obese children (38–42). However, these liquid stimuli could expose the ghrelin-secreting cells in the stomach to more rapid and dramatic increases in simple carbohydrates, which could suppress ghrelin levels more rapidly than do mixed solid meals (43). Since there are no studies that directly compare mixed and liquid meals in adolescents, the differential effects of these two stimuli on appetite hormones remain to be determined.
An unexpected finding in the present study was the transient increase in active ghrelin levels in the first 15 min after starting the meal. The active ghrelin was higher than basal in the 15-min sample in 22 out of the 25 meal tests for which active ghrelin levels were measured. We are not aware of any studies to date which reported acylated ghrelin levels within 15 min of starting a mixed meal. Erdmann et al found that total ghrelin levels transiently increased in this time period during meals consisting primarily of fat, protein, or vegetables, and transient increases were also noted in the mixed meal given to all subjects 4 hr later (44). These authors propose a gastric response to meal intake involving increased ghrelin secretion, a response which is only suppressed once insulin secretion is stimulated by carbohydrate absorption. Indeed, the insulin levels did not increase during most meals in our study until the 30-min time points, and so our data are also consistent with this hypothesis.
Our finding that PYY levels were resistant to stimulation by caloric intake in obese adolescents is consistent with other studies which examined PYY response to meals in youth (45, 46). However, we further found that this resistance was not completely overcome by large meal intake (Figure 3). The increase in PYY with the large meal was not significantly different between the obese subjects (+38.5±9.3) and the lean subjects (+57.5±15.3, p=0.286), though we only had sufficient power (0.8) to detect a difference greater than 44 pg/mL. Thus, the precise role of PYY in satiety in youth is unclear. If PYY levels truly signal satiety in obese children, then a shifted dose response could indicate that meal intake does not fully satiate obese adolescents.
One caveat to the present study is that the subjects were studied as outpatients, and therefore exercise and food intake on the days prior to the investigations were not controlled. This may have added increased variability to the hormonal responses to meal intake in these subjects. However, this also means that subjects were studied in as close to their usual state as possible, and thus changes in appetite hormones should most closely resemble those that occur on a day-to-day basis. It should also be noted that alterations in ghrelin and PYY responses to caloric intake may or may not be causally related to obesity. Indeed, food intake is a complex process involving numerous intrinsic and extrinsic factors. A significant portion of caloric intake in youth may be due to consumption of sweetened beverages or food eaten in the absence of hunger.
In conclusion, we find in the present study that active ghrelin and PYY levels are resistant to caloric intake in obese Hispanic adolescents. While active ghrelin was suppressed in a dose-dependent manner with caloric intake in lean subjects, this response was absent in the obese youth. Further, active ghrelin levels demonstrate a transient increase during meal intake, the relevance of which on hunger and caloric intake warrants further investigation.
Supplementary Material
Acknowledgments
The authors would like to thank Elza Dimerchyan, Rita Thomas, Tom Wright, and James W. Behan for their assistance with assays, and Linda Heller and Sohair Saad for help with planning and preparing meals. This work was supported by the General Clinical Research Center Grant MO1 RR00046, from the National Center for Research Resources (NCRR), NIH. SDM was supported by a Research Career Development Fellowship from the Saban Research Institute at CHLA, and a K12 Award, National Institute of Child Health, National Institute of Health. TAB was supported by a Distinguished Clinical Scientist Award from the American Diabetes Association. Portions of this study were published in abstract form (Obesity Research 13 Suppl. S:A49, 2005; Obesity 14: A133, 2006).
Footnotes
Disclosure – The authors have no conflict of interest to declare
Reference List
- 1.Schwartz MW. Central Nervous System Regulation of Food Intake. Obesity. 2006;14(2S):1S–8S. doi: 10.1038/oby.2006.275. [DOI] [PubMed] [Google Scholar]
- 2.Date Y, Kojima M, Hosoda H, et al. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000;141(11):4255–61. doi: 10.1210/endo.141.11.7757. [DOI] [PubMed] [Google Scholar]
- 3.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656–60. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 4.Nakazato M, Murakami N, Date Y, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409(6817):194–8. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 5.Hosoda H, Kojima M, Matsuo H, Kangawa K. Ghrelin and des-acyl ghrelin: two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochem Biophys Res Commun. 2000;279(3):909–13. doi: 10.1006/bbrc.2000.4039. [DOI] [PubMed] [Google Scholar]
- 6.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50(8):1714–9. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 7.Erdmann J, Topsch R, Lippl F, Gussmann P, Schusdziarra V. Postprandial response of plasma ghrelin levels to various test meals in relation to food intake, plasma insulin, and glucose. J Clin Endocrinol Metab. 2004;89(6):3048–54. doi: 10.1210/jc.2003-031610. [DOI] [PubMed] [Google Scholar]
- 8.Tentolouris N, Kokkinos A, Tsigos C, et al. Differential effects of high-fat and high-carbohydrate content isoenergetic meals on plasma active ghrelin concentrations in lean and obese women. Horm Metab Res. 2004;36(8):559–63. doi: 10.1055/s-2004-825761. [DOI] [PubMed] [Google Scholar]
- 9.Ariyasu H, Takaya K, Hosoda H, et al. Delayed short-term secretory regulation of ghrelin in obese animals: evidenced by a specific RIA for the active form of ghrelin. Endocrinology. 2002;143(9):3341–50. doi: 10.1210/en.2002-220225. [DOI] [PubMed] [Google Scholar]
- 10.English PJ, Ghatei MA, Malik IA, Bloom SR, Wilding JPH. Food fails to suppress ghrelin levels in obese humans. J Clin Endocrinol Metab. 2002;87(6):2984–7. doi: 10.1210/jcem.87.6.8738. [DOI] [PubMed] [Google Scholar]
- 11.Tschop M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML. Circulating Ghrelin levels are decreased in human obesity. Diabetes. 2001;50(4):707–9. doi: 10.2337/diabetes.50.4.707. [DOI] [PubMed] [Google Scholar]
- 12.Bellone S, Castellino N, Broglio F, et al. Ghrelin secretion in childhood is refractory to the inhibitory effect of feeding. J Clin Endocrinol Metab. 2004;89(4):1662–5. doi: 10.1210/jc.2003-031207. [DOI] [PubMed] [Google Scholar]
- 13.Lomenick JP, Clasey JL, Anderson JL. Meal-related Changes in Ghrelin, Peptide YY, and Appetite in Normal Weight and Overweight Children. Obesity. 2008;16(3):547–52. doi: 10.1038/oby.2007.129. [DOI] [PubMed] [Google Scholar]
- 14.Maffeis C, Bonadonna RC, Consolaro A, et al. Ghrelin, insulin sensitivity and postprandial glucose disposal in overweight and obese children. Eur J Endocrinol. 2006;154(1):61–8. doi: 10.1530/eje.1.02055. [DOI] [PubMed] [Google Scholar]
- 15.Bizzarri C, Rigamonti AE, Giannone G, et al. Maintenance of a normal meal-induced decrease in plasma ghrelin levels in children with Prader-Willi syndrome. Horm Metab Res. 2004;36(3):164–9. doi: 10.1055/s-2004-814340. [DOI] [PubMed] [Google Scholar]
- 16.Stock S, Leichner P, Wong AC, et al. Ghrelin, peptide YY, glucose-dependent insulinotropic polypeptide, and hunger responses to a mixed meal in anorexic, obese, and control female adolescents. J Clin Endocrinol Metab. 2005;90(4):2161–8. doi: 10.1210/jc.2004-1251. [DOI] [PubMed] [Google Scholar]
- 17.Halatchev IG, Ellacott KL, Fan W, Cone RD. Peptide YY3-36 inhibits food intake in mice through a melanocortin-4 receptor-independent mechanism. Endocrinology. 2004;145(6):2585–90. doi: 10.1210/en.2003-1754. [DOI] [PubMed] [Google Scholar]
- 18.Adrian TE, Ferri GL, Bacarese-Hamilton AJ, Fuessl HS, Polak JM, Bloom SR. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology. 1985;89(5):1070–7. doi: 10.1016/0016-5085(85)90211-2. [DOI] [PubMed] [Google Scholar]
- 19.Adrian TE, Ferri GL, Bacarese-Hamilton AJ, Fuessl HS, Polak JM, Bloom SR. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology. 1985;89(5):1070–7. doi: 10.1016/0016-5085(85)90211-2. [DOI] [PubMed] [Google Scholar]
- 20.Essah PA, Levy JR, Sistrun SN, Kelly SM, Nestler JE. Effect of Macronutrient Composition on Postprandial Peptide YY Levels. J Clin Endocrinol Metab. 2007;92(10):4052–5. doi: 10.1210/jc.2006-2273. [DOI] [PubMed] [Google Scholar]
- 21.Batterham RL, Cohen MA, Ellis SM, et al. Inhibition of food intake in obese subjects by peptide YY3-36. N Engl J Med. 2003;349(10):941–8. doi: 10.1056/NEJMoa030204. [comment] [DOI] [PubMed] [Google Scholar]
- 22.Le Roux CW, Batterham RL, Aylwin SJ, et al. Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology. 2006;147(1):3–8. doi: 10.1210/en.2005-0972. [DOI] [PubMed] [Google Scholar]
- 23.Roth CL, Enriori PJ, Harz K, Woelfle J, Cowley MA, Reinehr T. Peptide YY Is a Regulator of Energy Homeostasis in Obese Children before and after Weight Loss. J Clin Endocrinol Metab. 2005;90(12):6386–91. doi: 10.1210/jc.2005-1357. [DOI] [PubMed] [Google Scholar]
- 24.Nakai Y, Hosoda H, Nin K, et al. Short-term secretory regulation of the active form of ghrelin and total ghrelin during an oral glucose tolerance test in patients with anorexia nervosa. Eur J Endocrinol. 2004;150(6):913–4. doi: 10.1530/eje.0.1500913. [DOI] [PubMed] [Google Scholar]
- 25.Food and Nutrition Board NRCN. Recommended Dietary Allowances. Washington D.C.: National Academy Press; 1989. [Google Scholar]
- 26.Hosoda H, Doi K, Nagaya N, et al. Optimum collection and storage conditions for ghrelin measurements: octanoyl modification of ghrelin is rapidly hydrolyzed to desacyl ghrelin in blood samples. Clin Chem. 2004;50(6):1077–80. doi: 10.1373/clinchem.2003.025841. [DOI] [PubMed] [Google Scholar]
- 27.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis Model Assessment - Insulin Resistance and Beta-Cell Function from Fasting Plasma-Glucose and Insulin Concentrations in Man. Diabetologia. 1985;28(7):412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 28.van der Lely AJ, Tschop M, Heiman ML, Ghigo E. Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin. Endocr Rev. 2004;25(3):426–57. doi: 10.1210/er.2002-0029. [DOI] [PubMed] [Google Scholar]
- 29.English PJ, Ghatei MA, Malik IA, Bloom SR, Wilding JPH. Food fails to suppress ghrelin levels in obese humans. J Clin Endocrinol Metab. 2002;87(6):2984–7. doi: 10.1210/jcem.87.6.8738. [DOI] [PubMed] [Google Scholar]
- 30.Carroll JF, Kaiser KA, Franks SF, Deere C, Caffrey JL. Influence of BMI and Gender on Postprandial Hormone Responses[ast][ast] Obesity. 2007;15(12):2974–83. doi: 10.1038/oby.2007.355. [DOI] [PubMed] [Google Scholar]
- 31.Bellone S, Castellino N, Broglio F, et al. Ghrelin secretion in childhood is refractory to the inhibitory effect of feeding. J Clin Endocrinol Metab. 2004;89(4):1662–5. doi: 10.1210/jc.2003-031207. [DOI] [PubMed] [Google Scholar]
- 32.Bizzarri C, Rigamonti AE, Giannone G, et al. Maintenance of a normal meal-induced decrease in plasma ghrelin levels in children with Prader-Willi syndrome. Horm Metab Res. 2004;36(3):164–9. doi: 10.1055/s-2004-814340. [DOI] [PubMed] [Google Scholar]
- 33.Lomenick JP, Clasey JL, Anderson JL. Meal-related Changes in Ghrelin, Peptide YY, and Appetite in Normal Weight and Overweight Children. Obesity. 2008;16(3):547–52. doi: 10.1038/oby.2007.129. [DOI] [PubMed] [Google Scholar]
- 34.Misra M, Tsai PM, Mendes N, Miller KK, Klibanski A. Increased Carbohydrate Induced Ghrelin Secretion in Obese vs. Normal-weight Adolescent Girls. Obesity. 2009 doi: 10.1038/oby.2009.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maffeis C, Bonadonna RC, Consolaro A, et al. Ghrelin, insulin sensitivity and postprandial glucose disposal in overweight and obese children. Eur J Endocrinol. 2006;154(1):61–8. doi: 10.1530/eje.1.02055. [DOI] [PubMed] [Google Scholar]
- 36.Stock S, Leichner P, Wong AC, et al. Ghrelin, peptide YY, glucose-dependent insulinotropic polypeptide, and hunger responses to a mixed meal in anorexic, obese, and control female adolescents. J Clin Endocrinol Metab. 2005;90(4):2161–8. doi: 10.1210/jc.2004-1251. [DOI] [PubMed] [Google Scholar]
- 37.Stock S, Leichner P, Wong AC, et al. Ghrelin, peptide YY, glucose-dependent insulinotropic polypeptide, and hunger responses to a mixed meal in anorexic, obese, and control female adolescents. J Clin Endocrinol Metab. 2005;90(4):2161–8. doi: 10.1210/jc.2004-1251. [DOI] [PubMed] [Google Scholar]
- 38.Bacha F, Arslanian SA. Ghrelin suppression in overweight children: a manifestation of insulin resistance? J Clin Endocrinol Metab. 2005;90(5):2725–30. doi: 10.1210/jc.2004-1582. [DOI] [PubMed] [Google Scholar]
- 39.Baldelli R, Bellone S, Castellino N, et al. Oral glucose load inhibits circulating ghrelin levels to the same extent in normal and obese children. Clin Endocrinol (Oxf) 2006;64(3):255–9. doi: 10.1111/j.1365-2265.2006.02441.x. [DOI] [PubMed] [Google Scholar]
- 40.Lanyi E, Csernus K, Erhardt E, et al. Plasma levels of acylated ghrelin during an oral glucose tolerance test in obese children. J Endocrinol Invest. 2007;30(2):133–7. doi: 10.1007/BF03347411. [DOI] [PubMed] [Google Scholar]
- 41.Paik KH, Jin DK, Song SY, et al. Correlation between fasting plasma ghrelin levels and age, body mass index (BMI), BMI percentiles, and 24-hour plasma ghrelin profiles in Prader-Willi syndrome. J Clin Endocrinol Metab. 2004;89(8):3885–9. doi: 10.1210/jc.2003-032137. [DOI] [PubMed] [Google Scholar]
- 42.Stock S, Leichner P, Wong AC, et al. Ghrelin, peptide YY, glucose-dependent insulinotropic polypeptide, and hunger responses to a mixed meal in anorexic, obese, and control female adolescents. J Clin Endocrinol Metab. 2005;90(4):2161–8. doi: 10.1210/jc.2004-1251. [DOI] [PubMed] [Google Scholar]
- 43.Tieken SM, Leidy HJ, Stull AJ, Mattes RD, Schuster RA, Campbell WW. Effects of solid versus liquid meal-replacement products of similar energy content on hunger, satiety, and appetite-regulating hormones in older adults. Horm Metab Res. 2007;39(5):389–94. doi: 10.1055/s-2007-976545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Erdmann J, Topsch R, Lippl F, Gussmann P, Schusdziarra V. Postprandial response of plasma ghrelin levels to various test meals in relation to food intake, plasma insulin, and glucose. J Clin Endocrinol Metab. 2004;89(6):3048–54. doi: 10.1210/jc.2003-031610. [DOI] [PubMed] [Google Scholar]
- 45.Lomenick JP, Clasey JL, Anderson JL. Meal-related Changes in Ghrelin, Peptide YY, and Appetite in Normal Weight and Overweight Children. Obesity. 2008;16(3):547–52. doi: 10.1038/oby.2007.129. [DOI] [PubMed] [Google Scholar]
- 46.Stock S, Leichner P, Wong AC, et al. Ghrelin, peptide YY, glucose-dependent insulinotropic polypeptide, and hunger responses to a mixed meal in anorexic, obese, and control female adolescents. J Clin Endocrinol Metab. 2005;90(4):2161–8. doi: 10.1210/jc.2004-1251. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.