Abstract
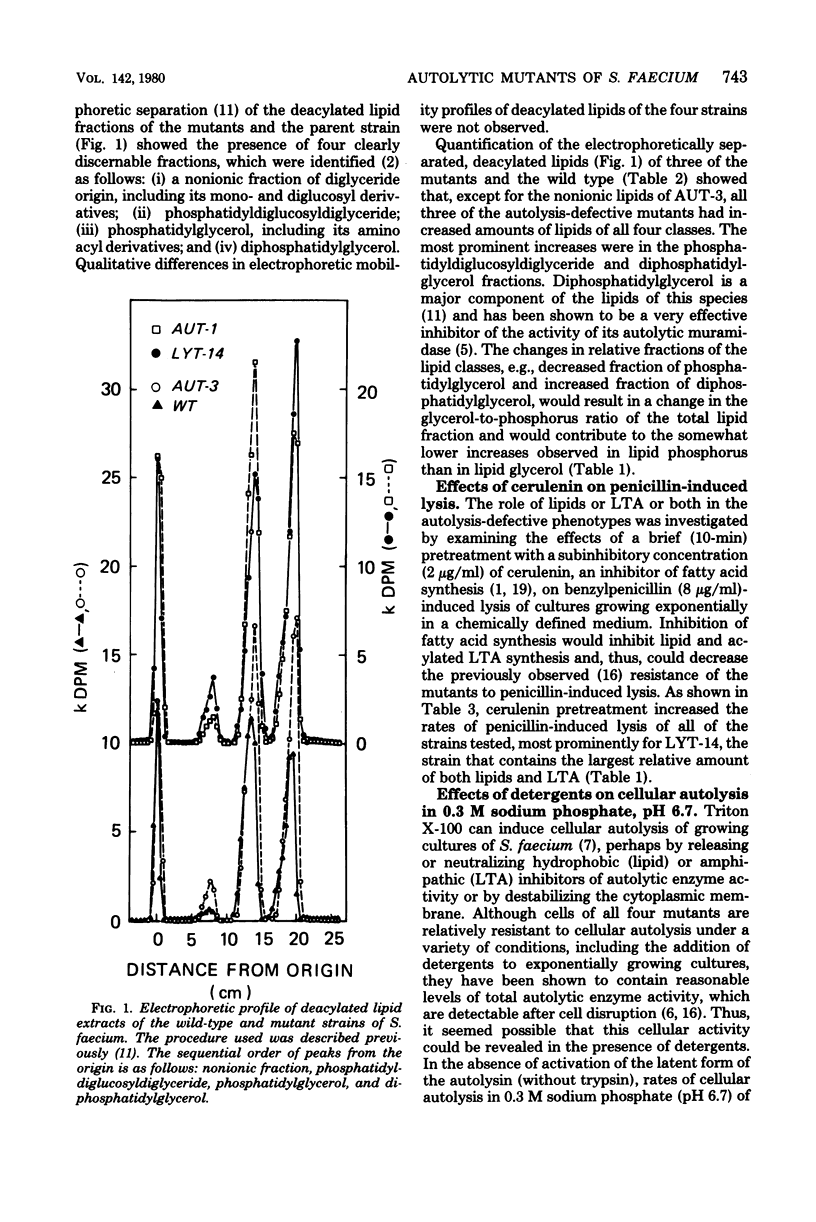
Two of four previously isolated autolysis-defective mutants of Streptococcus faecium (Streptococcus faecalis ATCC 9790) incorporated substantially more [14C]glycerol into lipids and lipoteichoic acid than did the parent strain. Consistent with increased accumulation of lipids and lipoteichoic acid, significantly higher levels of phosphorus were found in the corresponding fractions of the two mutant strains than in the wild type. Although the autolysis-defective mutant strains contained the same assortment of lipids as the wild type, the relative amount of [14C]glycerol incorporated into diphosphatidylglycerol increased, accompanied by a decreased fraction of phosphatidylglycerol. These results suggested that increased cellular content of two types of substances, acylated lipoteichoic acid and lipids (notably diphosphatidylglycerol), which previously had been shown to be potent inhibitors of the N-acetylmuramoylhydrolase of this species, contributed to the autolysis-defective phenotype of these mutants. Consistent with this interpretation are observations that (i) cerulenin inhibition of fatty acid synthesis increased the rates of benzylpenicillin-induced cellular lysis and that (ii) Triton X-100 or Zwittergent 3-14 treatment could reveal the presence of otherwise cryptic but substantial levels of the active form of the autolysin in cells of three of four mutants and of the proteinase-activable latent form in all four mutants.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carson D., Daneo-Moore L. Effect of cerulenin on Streptococcus faecalis macromolecular synthesis and cell division. J Bacteriol. 1978 Feb;133(2):472–476. doi: 10.1128/jb.133.2.472-476.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson D., Pieringer R. A., Daneo-Moore L. Effect of growth rate on lipid and lipoteichoic acid composition in Streptococcus faecium. Biochim Biophys Acta. 1979 Nov 21;575(2):225–233. doi: 10.1016/0005-2760(79)90024-9. [DOI] [PubMed] [Google Scholar]
- Cleveland R. F., Daneo-Moore L., Wicken A. J., Shockman G. D. Effect of lipoteichoic acid and lipids on lysis of intact cells of Streptococcus faecalis. J Bacteriol. 1976 Sep;127(3):1582–1584. doi: 10.1128/jb.127.3.1582-1584.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland R. F., Holtje J. V., Wicken A. J., Tomasz A., Daneo-Moore L., Shockman G. D. Inhibition of bacterial wall lysins by lipoteichoic acids and related compounds. Biochem Biophys Res Commun. 1975 Dec 1;67(3):1128–1135. doi: 10.1016/0006-291x(75)90791-3. [DOI] [PubMed] [Google Scholar]
- Cleveland R. F., Wicken A. J., Daneo-Moore L., Shockman G. D. Inhibition of wall autolysis in Streptococcus faecalis by lipoteichoic acid and lipids. J Bacteriol. 1976 Apr;126(1):192–197. doi: 10.1128/jb.126.1.192-197.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornett J. B., Redman B. E., Shockman G. D. Autolytic defective mutant of Streptococcus faecalis. J Bacteriol. 1978 Feb;133(2):631–640. doi: 10.1128/jb.133.2.631-640.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornett J. B., Shockman G. D. Cellular lysis of Streptococcus faecalis induced with triton X-100. J Bacteriol. 1978 Jul;135(1):153–160. doi: 10.1128/jb.135.1.153-160.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonenne A., Ernst R. Solubilization of membrane proteins by sulfobetaines, novel zwitterionic surfactants. Anal Biochem. 1978 Jun 15;87(1):28–38. doi: 10.1016/0003-2697(78)90565-1. [DOI] [PubMed] [Google Scholar]
- Kessler R. E., Shockman G. D. Precursor-product relationship of intracellular and extracellular lipoteichoic acids of Streptococcus faecium. J Bacteriol. 1979 Feb;137(2):869–877. doi: 10.1128/jb.137.2.869-877.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROBERTS N. R., LEINER K. Y., WU M. L., FARR A. L. The quantitative histochemistry of brain. I. Chemical methods. J Biol Chem. 1954 Mar;207(1):1–17. [PubMed] [Google Scholar]
- Pieringer R. A., Ambron R. T. A method for the specific labeling of the glycerol in glyceride-containing lipids of Streptococcus faecalis ATCC 9790. J Lipid Res. 1973 May;14(3):370–372. [PubMed] [Google Scholar]
- Pooley H. M., Shockman G. D. Relationship between the latent form and the active form of the autolytic enzyme of Streptococcus faecalis. J Bacteriol. 1969 Nov;100(2):617–624. doi: 10.1128/jb.100.2.617-624.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynor R. H., Scott D. F., Best G. K. Oxacillin-induced lysis of Staphylococcus aureus. Antimicrob Agents Chemother. 1979 Aug;16(2):134–140. doi: 10.1128/aac.16.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockman G. D., Daneo-Moore L., Cornett J. B., Mychajlonka M. Does penicillin kill bacteria?. Rev Infect Dis. 1979 Sep-Oct;1(5):787–796. doi: 10.1093/clinids/1.5.787. [DOI] [PubMed] [Google Scholar]
- Shungu D. L., Cornett J. B., Shockman G. D. Morphological and physiological study of autolytic-defective Streptococcus faecium strains. J Bacteriol. 1979 May;138(2):598–608. doi: 10.1128/jb.138.2.598-608.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toennies G., Das D. N., Feng F. New observations on the determination of bacterial lipid phosphorus. Can J Microbiol. 1968 Apr;14(4):484–485. doi: 10.1139/m68-079. [DOI] [PubMed] [Google Scholar]
- Vance D., Goldberg I., Mitsuhashi O., Bloch K. Inhibition of fatty acid synthetases by the antibiotic cerulenin. Biochem Biophys Res Commun. 1972 Aug 7;48(3):649–656. doi: 10.1016/0006-291x(72)90397-x. [DOI] [PubMed] [Google Scholar]
- Wicken A. J., Knox K. W. Studies on the group F antigen of lactobacilli: isolation of a teichoic acid-lipid complex from Lactobacillus fermenti NCTC 6991. J Gen Microbiol. 1970 Mar;60(3):293–301. doi: 10.1099/00221287-60-3-293. [DOI] [PubMed] [Google Scholar]


