Abstract
Background
Alleles of apolipoprotein E (APOE) are the major genetic risk factor for late onset Alzheimer's Disease (LOAD). Recently, an APOE splice variant that retains intron 3 (APOE-I3) was identified. To gain insight into the possible role of this isoform in LOAD, we quantified its expression in a cohort of 56 human brain specimens by using quantitative RT-PCR.
Results
We found that APOE-I3 generally represents a low percentage (< 0.5%) of overall APOE expression. However, in one specimen, the proportion of APOE-I3 was increased about ~13 fold. This specimen was unique in the cohort for possessing the minor allele of an intron 3 single nucleotide polymorphism (SNP), rs12982192. Additionally, an allelic expression imbalance study indicated that the rs12982192 minor allele was associated with increased APOE-I3 expression.
Conclusions
Overall, we interpret our results as suggesting that APOE-I3 represents a minor portion of APOE expression and that rs12982192 is associated with APOE intron 3 retention. Since the minor allele of this SNP is on the same haplotype as the minor allele of rs429358, which defines the APOE4 allele, we speculate that rs12982192 may reflect a modest loss of mRNA encoding functional APOE4.
Background
APOE variants are the single largest genetic factors impacting late onset Alzheimer Disease (LOAD), the most common neurodegenerative disease. In particular, the APOE4 allele confers increased risk, being present at a ~15% frequency in the general population and at 50% in LOAD [1,2]. APOE consists of 4 exons and 3 introns encoding a total of 317 amino acids. After cleavage of the signal peptide, the mature APOE protein is 299 amino acids. Protein translation begins in exon 2 and ends in exon 4. In the brain, APOE transports cholesterol and modulates Aß clearance [1,2]. APOE is primarily secreted by astrocytes [3,4] with microglia [5] and, possibly, stressed neurons also contributing to APOE production [6]. Overall, insights into APOE expression will provide further information on AD risk.
Recently, Xu et al reported an APOE isoform that retained intron 3 (APOE-I3) and was increased with neuronal stress in murine models [7]. To gain insights into this novel APOE isoform in humans, we quantified APOE-I3 as well as the normal length APOE (APOE-NL) in a cohort of 56 AD and non-AD brain samples. We report that APOE-I3 represents a low proportion of overall APOE expression. In addition, we identified an individual with significantly increased expression of the APOE-I3 isoform. Further analysis revealed that this individual was unique in the cohort for an intron 3 single nucleotide polymorphism (SNP), rs12982192. Moreover, APOE-I3 was in allelic expression imbalance (AEI) in this individual such that the minor allele of rs12982192 correlated with increased expression of the APOE-I3 isoform. Overall, we interpret our results as suggesting that APOE-I3 represents a minor portion of APOE expression and that rs12982192 is associated with APOE intron 3 retention.
Materials and methods
Human autopsy tissue
The human autopsy tissue specimens used here have been described elsewhere [8,9]. Briefly, anterior cingulate tissue was provided by the University of Kentucky AD Center. Diagnoses of AD and non-AD were based upon evaluation of both cognitive status, i.e., Clinical Dementia Rating and Mini-Mental State Examination scores, as well as neuropathology [10]. The age at death for individuals that were cognitively intact, i.e., non-AD, was 82 ± 9 years (mean ± SD, n = 29) while age at death for AD individuals was 82 ± 6 (n = 27). The average post-mortem interval for non-AD and AD individuals was 2.8 ± 0.8 hours (mean ± SD, n = 29) and 3.4 ± 0.6 hours (n = 27), respectively. The non-AD and AD individuals were well-balanced for sex, i.e., 15 of the 29 non-AD individuals were women while 14 of the 27 AD individuals were women.
PCR amplification
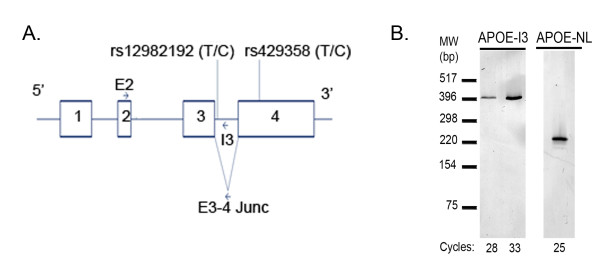
RNA was extracted and converted to cDNA as described previously [8,9]. To initially visualize the APOE isoforms (Figure 1A), APOE-I3 was amplified with primers that corresponded to sequences within exon 2 (primer E2: CGTTGCTGGTCACATTCCT) and intron 3 (I3: AGAGAGAGACAAAGCTGAGA). The normal length APOE isoform (APOE-NL) was amplified with the same exon 2 primer and a reverse primer that corresponded to the exon 3-4 junction (E3-4 Junc: CCATCAGCGCCCTCAGTT) (Figure 1A). The equivalent of 20 ng of template cDNA was amplified with 1 μM of each primer in a PCR profile consisting of pre-incubation for 3 minutes at 94°C, followed by cycles of 94°C for 30 sec, 60°C for 30 sec and 72°C for 30 sec (Perkin Elmer 9600). PCR products were separated by polyacrylamide gel electrophoresis and visualized by SYBR-gold staining and fluorescence imaging (FujiFLA-2000). The PCR products were isolated from the gel and identified by direct sequencing (Davis Sequencing). For real-time quantitative PCR (RT-qPCR), reaction mixtures consisting of 1 uM each primer, 1× SYBR-green Master Mix (Applied Biosystems) and 20 ng of template cDNA were pre-incubated for 10 minutes at 95°C followed by 40 cycles of 94°C for 30 sec, 60°C for 30 sec and 72°C for 30 sec (BioRad Chromo4). After completion of the RT-qPCR cycles, assay specificity was confirmed by melting curve analysis and by visualizing the products by polyacrylamide gel electrophoresis and SYBR-gold staining. Copy numbers were determined relative to standard curves that were amplified in parallel; the standards were generated from PCR-amplified products that were gel purified and quantified by UV absorbance. Each sample was analyzed twice by RT-qPCR. The limit of detection was five copies of APOE isoform per 20 ng of cDNA. Copy numbers were normalized to the geometric mean of the copy numbers of hypoxanthine-guanine phosphoribosyltransferase 1 and ribosomal protein L32 [11,12]. Samples were genotyped for rs12982192 by using a TaqMan approach (Applied Biosystems).
Figure 1.
PCR Detection of APOE-NL and APOE-I3. PCR primer positions are depicted relative to the four exons of APOE (A). Two SNPs are also shown: rs12982192 within intron 3 and rs429358, which determines APOE4 status, in exon 4. PCR of 25 or 28-33 cycles was used to detect APOE-NL and APOE-I3, respectively, in pooled brain cDNA samples (B).
Allelic expression imbalance
To determine whether each allele of rs12982192 and rs429358 contributed equally to APOE-I3 and APOE-NL, respectively, we cloned the APOE-I3 and APOE-NL PCR products from specimen #31 (TA-Cloning pcDNA3.1, Invitrogen). The identity of each clone was confirmed by direct sequencing (Davis Sequencing). We then estimated the proportion of each allele present in specimen #31 APOE-I3 and APOE-NL cDNA by comparing the relative sequencing electropherogram peak areas of each allele of rs12982192 and rs429358, respectively, essentially as described [13]; the results of this assay were normalized relative to standard curves derived from varying proportions of each allelic clone and validated by analysis of genomic DNA (gDNA).
Statistical Analysis
The association between APOE-I3 and/or APOE-NL and sex, age, PMI and APOE4 genotype was analyzed by linear regression (SPSS v 17.0). Differences in AEI in gDNA and cDNA were evaluated by a two-tailed T-test (SPSS, V17). A p < 0.05 was considered to be significant.
Results
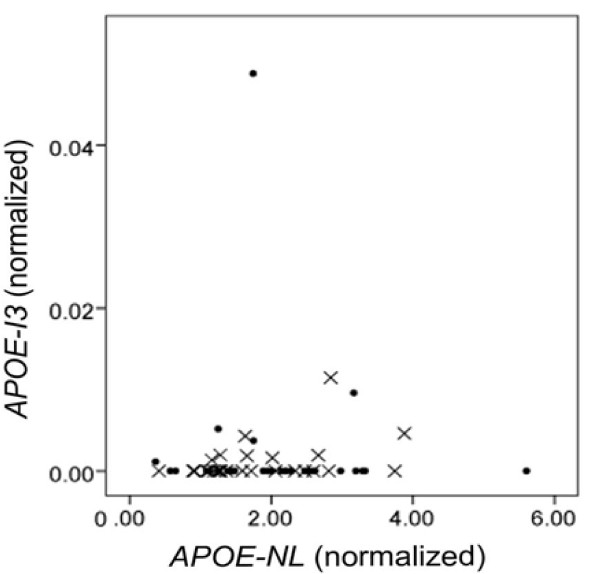
To begin to evaluate APOE-I3 and APOE-NL expression, we amplified human brain cDNA by using a forward primer within exon 2 (E2) and reverse primers within intron 3 (I3) or the junction of exon 3-4 (E3-4 Junc), respectively (Figure 1A). Both the APOE-I3 and the APOE-NL isoform were readily discernible (Figure 1B). We then used RT-qPCR to quantify each APOE isoform in 20 ng of cDNA prepared from 56 human brain samples and the results normalized for housekeeping gene expression. We found that APOE-I3 expression was low relative to APOE-NL expression (Figure 2). Indeed, the APOE-I3 isoform was not detected in 43 of the 56 specimens and was very low in another 12 of the samples, averaging 0.22% of APOE message. Interestingly, the cDNA from the remaining sample (specimen #31) had much higher APOE-I3, reaching 2.8% of total APOE expression (Figure 2). This autopsy brain specimen was from a 91 year old, cognitively-intact person with a Braak stage of II and an APOE3/4 genotype. Excluding this outlier and samples where APOE-I3 was not detected, there was a strong trend between the expression of APOE-NL and APOE-I3 (p = 0.053). The expression of neither isoform correlated with AD diagnosis, PMI, gender, APOE genotype or age (p > 0.05). Hence, APOE-I3 is a relatively rare APOE isoform in this cohort, with one specimen showing ~13-fold higher APOE-I3 levels.
Figure 2.
Quantification of APOE-I3 and APOE-NL Expression. Expression of APOE-I3 is shown as a function of APOE-NL in AD (X markers) and non-AD (circle markers) samples. Specimen #31 is the non-AD outlier with a high level of APOE-I3 expression. RT-qPCR data were normalized to the geometric mean of hypoxanthine-guanine phosphoribosyltransferase and ribosomal protein L32. There was no correlation of APOE-I3 or APOE-NL with APOE3 versus APOE4 genotype, or AD status (data not shown).
To identify SNPs that may underlie the increased APOE-I3 in specimen #31, we generated genomic APOE clones from exon 2 to exon 4 of this individual. These clones included the intervening 1,092 bp intron 2 and 580 bp intron 3. Sequencing revealed that this gDNA was heterozygous for (i) rs12982192, which is a rare T/C SNP located 50 bp into intron 3 (dbSNP Build 131), (ii) rs769449, which has a 6.5% minor allele frequency and is located 78 bp into intron 2, (iii) rs769450, which has a 39.9% minor allele frequency and is also located within intron 2, and (iv) rs429358, which is within exon 4 and determines APOE3/4 status (Figure 1A). The APOE4 haplotype (rs429358C) contained the minor rs12982192C and rs769449A alleles and the major rs769450G allele. Since rs12982192 is within the retained intron 3, we hypothesized that the minor rs12982192C allele may be associated with APOE-I3. To test this possibility, we first assessed whether the minor allele of rs12982192 was present in any of the other 55 DNA samples in this study; only specimen #31 possessed the minor rs12982192C allele. This finding supports the possibility that intron 3 retention is associated with rs12982192.
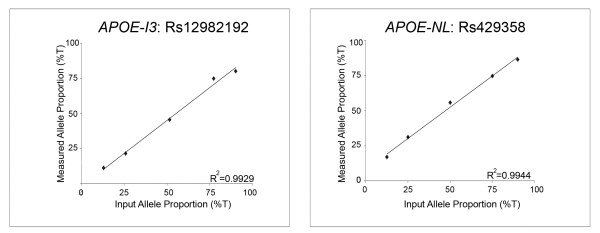
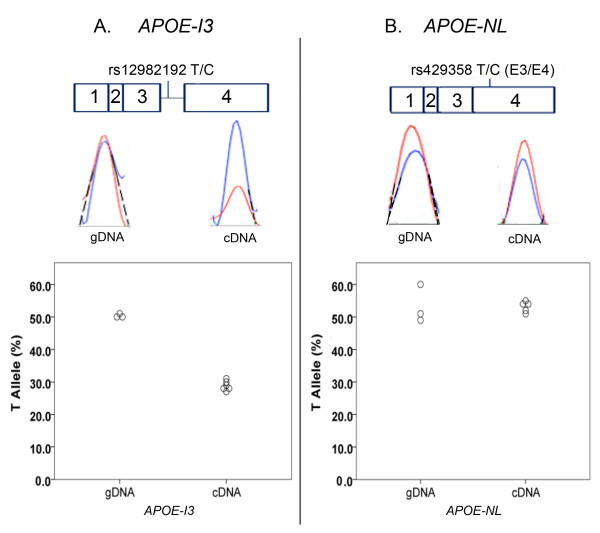
To evaluate this possibility further, we reasoned that APOE-I3 should be in allelic expression imbalance (AEI) with respect to rs12982192 genotype, that is, we hypothesized that the minor rs12982192C allele would be over-represented in APOE-I3. Hence, we compared the proportion of each rs12982192 allele in APOE-I3. In addition, to evaluate whether the bulk of APOE expression, as reflected by APOE-NL, was in AEI in specimen #31, we also analyzed APOE-NL by using rs429358 in exon 4. Standard curves demonstrated the linearity of the AEI assay (Figure 3). The assay was further validated by testing gDNA, which found that, as expected, each allele was present in equal proportions for both rs12982192 and rs429358 (Figure 4A-B). As previously reported for APOE expression generally [14], APOE-NL expression in specimen #31 did not show evidence of AEI (Figure 4B). In contrast, APOE-I3 showed robust AEI in specimen #31, with significant over-representation of the minor rs12982192C allele (Figure 4A). Hence, these results support the hypothesis that the minor rs12982192C allele is associated with increased APOE-I3.
Figure 3.
AEI Standard Curves. These standard curves were generated by subjecting the indicated proportions of allelic clones to AEI analysis. The resulting quantified allelic proportions were linear with respect to input proportions as noted by the indicated correlation coefficients. The slopes of the lines for rs12982192 and rs429358 were 0.94 and 0.88, respectively.
Figure 4.
APOE-I3 but not APOE-NL shows AEI in Specimen #31. The upper portion shows each APOE isoform and the placement of the SNP used to evaluate its AEI. The middle portion shows representative electropherograms for assessing AEI in APOE-I3 (A) and APOE-NL (B). The lower portion represents quantitative results for genomic DNA, which shows equal representation of each allele, and for cDNA, which shows unequal allelic expression for APOE-I3 but not APOE-NL. Electropherograms were analyzed for AEI by extrapolating the curves to baseline (dashed lines) and then estimating relative allelic areas. These data were normalized relative to standard curves generated from mixtures of allelic clones (Figure 3).
Discussion
The major findings of this paper are (i) APOE-I3 appears to be a rare APOE isoform compared to APOE-NL in the aged human brain, (ii) APOE-I3 was increased ~13-fold in one individual, (iii) rs12982192, an intron 3 SNP, was uniquely present in the specimen with high APOE-I3, and (iv) APOE-I3 expression showed significant AEI in this sample, with the minor rs12982192 allele contributing a greater proportion of APOE-I3 expression. Hence, rs12982192 appears to be associated with increased expression of a rare APOE isoform.
Although the role of apoE-I3 in the brain is unclear, this isoform is infrequent relative to APOE-NL. While APOE is expressed largely in astrocytes [3,4,15], Xu et al found that APOE-I3 expression was restricted to neurons in mice [7]. We did not discern a correlation between the expression of APOE-I3 and synaptophysin, an mRNA restricted to neurons, although this correlation may have been precluded by the generally low APOE-I3 expression such that only 13 of the 56 samples with detectable APOE-I3 contributed to this analysis. While APOE-NL encodes a 317 amino acid secreted protein, the sequence at the exon 3 - intron 3 junction results in a stop codon. Hence, APOE-I3 encodes a truncated 79 amino acid, amino-terminal APOE fragment that is apparently not detectable in transfected cells or brain [7]. Although we considered that APOE-I3 may be a candidate for nonsense-mediated decay, APOE-I3 levels were independent of PMI (data not shown), suggesting that APOE-I3 is not rapidly degraded. In summary, the role of APOE-I3 in the brain is unclear but, given that this isoform is rare and that its encoded protein is not detectable, we speculate that APOE-I3 represents a loss of APOE mRNA encoding functional protein.
Several lines of evidence suggest that rs12982192 modulates the proportion of APOE-I3, including (i) rs12982192 resides within intron 3 consistent with its possible role as a cis-acting SNP, (ii) this SNP was uniquely present in the specimen with relatively high APOE-I3 among the 56 brain specimens analyzed, (iii) rs12982192 was associated with significant AEI for APOE-I3 with the rare rs12982192C minor allele contributing a greater proportion of APOE-I3 expression and (iv) the APOE-NL isoform did not display AEI similar to the APOE-I3 isoform, ruling out the possibility of an overall APOE AEI in specimen #31 due to other mechanisms, e.g., a promoter SNP. While this reasoning appears robust to support a role for rs12982192 in APOE-I3 splicing, two lines of evidence suggest that rs129382192 does not act independently to modulate intron 3 splicing. First, rs129382192 did not alter APOE splicing in a transfected cell, minigene paradigm (Dieter and Estus, unpublished observations), suggesting in vivo elements may be necessary to discern the actions of the SNP. Second, while the main finding of the AEI study was that the minor allele of rs12982192 was associated with increased APOE-I3 expression, a secondary finding was that the ~3.5:1 allelic ratio was quantitatively insufficient to account for the ~13-fold increase in overall APOE-I3 expression in specimen #31. We interpret this result as possibly reflecting additional variables specific to specimen #31 that influence APOE-I3 or, more likely, that the difference is attributable to currently unclear technical differences between the RT-qPCR and AEI assays. In summary, although APOE-I3 levels may be modulated by additional variables, several lines of evidence support the association of rs12982192 with APOE-I3 expression.
Since APOE4 is the primary genetic risk factor for AD, SNPs that alter APOE4 expression are likely to modulate the association of APOE4 with AD. In this regard, we note that the minor rs12982192C allele is on the same haplotype as the minor rs429358C allele, which defines the APOE4 allele. Since our AEI study indicates that rs12982192 is associated in cis with retention of APOE intron 3, rs12982192 may emerge as a modulator of APOE4 allelic association with AD. Hence, although APOE-I3 represents only ~3% of total APOE mRNA in the rs12982192 heterozygous individual, the AEI study indicates that the majority of this APOE-I3 is derived from the APOE4 allele, and therefore could approach 5-6% of APOE4 expression. The extent that rs12982192 may be associated with AD risk will require a very large study population, given the rarity of this SNP, i.e., this SNP is listed within dbSNP Build 131 without a true frequency. We note that we have genotyped an additional ~800 DNA samples and have not identified another rs12982192 carrier.
In summary, our major findings are that APOE-I3 is a low abundance APOE isoform. Moreover, the minor C allele of rs12982192 appears associated with increased APOE-I3. Since the minor rs12982192C allele is on the same haplotype as APOE4, and since APOE-I3 appears to represent a loss of functional APOE4 mRNA, rs12982192 may modulate the association of APOE4 with AD risk. Future large case-control association studies are necessary to evaluate this possibility.
Abbreviations
(APOE): apolipoprotein E; (LOAD): late onset Alzheimer's Disease; (APOE-I3): APOE splice variant retaining intron 3; (SNP): single nucleotide polymorphism; (APOE-NL): normal length APOE; (AEI): allelic expression imbalance; (RT-qPCR): real-time quantitative PCR; (gDNA): genomic DNA.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
LSD performed the experiments under the supervision of SE. Both authors contributed to data analysis and writing the manuscript. Both authors read and approved the final manuscript.
Contributor Information
Laura S Dieter, Email: ljstephens@yahoo.com.
Steven Estus, Email: steve.estus@uky.edu.
Acknowledgements
The authors gratefully acknowledge tissue supplied by the University of Kentucky AD Center, which is supported by P30AG028383, as well as NIH for grant support (R01AG026147 and P01AG030128).
References
- Bu G. Apolipoprotein E and its receptors in Alzheimer's Disease: Pathways, pathogenesis and therapy. Nat Rev Neurosci. 2009;10:333–44. doi: 10.1038/nrn2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Basak JM, Holtzman DM. The role of Apolipoprotein E in Alzheimer's Disease. Neuron. 2009;63:287–303. doi: 10.1016/j.neuron.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyles JK, Pitas RE, Wilson E, Mahley RW, Taylor JM. Apolipoprotein E associated with astrocytic glia of the central nervous system and with nonmyelinating glia of the peripheral nervous system. J Clin Invest. 1985;76:1501–13. doi: 10.1172/JCI112130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitas RE, Boyles JK, Lee SH, Foss D, Mahley RW. Astrocytes synthesize apolipoprotein E and metabolize apolipoprotein E-containing lipoproteins. Biochim Biophys Acta. 1987;917:148–61. doi: 10.1016/0005-2760(87)90295-5. [DOI] [PubMed] [Google Scholar]
- Stone DJ, Rozovsky I, Morgan TE, Anderson CP, Hajian H, Finch CE. Astrocytes and microglia respond to estrogen with increased apoE mRNA in vivo and in vitro. Exp Neurol. 1997;143:313–8. doi: 10.1006/exnr.1996.6360. [DOI] [PubMed] [Google Scholar]
- Xu Q, Bernardo A, Walker D, Kanegawa T, Mahley RW, Huang Y. Profile and regulation of apolipoprotein E (apoE) expression in the CNS in mice with targeting of green fluorescent protein gene to the apoE locus. J Neurosci. 2006;26:4985–94. doi: 10.1523/JNEUROSCI.5476-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Walker D, Bernardo A, Brodbeck J, Balestra ME, Huang Y. Intron-3 retention/splicing controls neuronal expression of apolipoprotein E in the CNS. J Neurosci. 2008;28:1452–9. doi: 10.1523/JNEUROSCI.3253-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou F, Gopalraj RK, Lok J, Zhu H, Ling IF, Simpson JF, Tucker HM, Kelly JF, Younkin SG, Dickson DW, Petersen RC, Graff-Radford NR, Bennett DA, Crook JE, Estus S. Sex-dependent association of a common low-density lipoprotein receptor polymorphism with RNA splicing efficiency in the brain and Alzheimer's Disease. Hum Mol Genet. 2008;17:929–35. doi: 10.1093/hmg/ddm365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grear KE, Ling IF, Simpson JF, Furman JL, Simmons CR, Peterson SL, Schmitt FA, Markesbery WR, Liu Q, Crook JE, Younkin SG, Bu G, Estus S. Expression of SORL1 and a novel SORL1 splice variant in normal and Alzheimers Disease brain. Mol Neurodegener. 2009;4:46. doi: 10.1186/1750-1326-4-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Braak H, Markesbery WR. Neuropathology and cognitive impairment in Alzheimer Disease: A complex but coherent relationship. J Neuropathol Exp Neurol. 2009;68:1–14. doi: 10.1097/NEN.0b013e3181919a48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Ding L, Sandford AJ. Selection of reference genes for gene expression studies in human neutrophils by real-time PCR. BMC Mol Biol. 2005;6:4. doi: 10.1186/1471-2199-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heap GA, Yang JH, Downes K, Healy BC, Hunt KA, Bockett N, Franke L, Dubois PC, Mein CA, Dobson RJ, Albert TJ, Rodesch MJ, Clayton DG, Todd JA, van Heel DA, Plagnol V. Genome-wide analysis of allelic expression imbalance in human primary cells by high-throughput transcriptome resequencing. Hum Mol Genet. pp. 122–34. [DOI] [PMC free article] [PubMed]
- Growdon WB, Cheung BS, Hyman BT, Rebeck GW. Lack of allelic imbalance in APOE epsilon3/4 brain mRNA expression in Alzheimer's Disease. Neurosci Lett. 1999;272:83–6. doi: 10.1016/S0304-3940(99)00557-1. [DOI] [PubMed] [Google Scholar]
- Grehan S, Tse E, Taylor JM. Two distal downstream enhancers direct expression of the human apolipoprotein E gene to astrocytes in the brain. J Neurosci. 2001;21:812–22. doi: 10.1523/JNEUROSCI.21-03-00812.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]