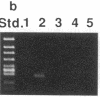
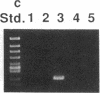
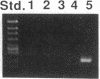
Abstract
The presence of somatostatin receptors has been demonstrated in various endocrine tumors as well as in normal tissues. We recently have cloned five human somatostatin receptor subtypes (SSTR1-SSTR5). These mRNAs are expressed in a tissue-specific manner. In this study, we have determined the somatostatin receptor subtypes expressed in various endocrine tumors using a reverse transcriptase polymerase chain reaction method. In two cases of glucagonoma and its metastatic lymph nodes in one case, all the SSTR subtype mRNAs except SSTR5 mRNA were expressed. In four cases of insulinoma, SSTR1 and SSTR4 mRNAs were detected, but SSTR2 mRNA was not detected in one case and SSTR3 mRNA was not detected in two cases, indicating a heterogeneous expression of SSTR subtypes in insulinomas. Interestingly, SSTR3 mRNA, which is highly expressed in rat pancreatic islets, is not expressed in normal human pancreatic islets, while SSTR1, SSTR2, and SSTR4 mRNAs are expressed. In three cases of pheochromocytoma, SSTR1 and SSTR2 mRNAs were detected, showing an expression pattern identical to that of normal adrenal gland. In a carcinoid, SSTR1 and SSTR4 mRNAs were detected. We have also found that human SSTR2 shows a high affinity for SMS 201-995, which has been used clinically for the treatment of endocrine tumors. Since SMS 201-995 was effective in the treatment of a patient with glucagonoma in which SSTR2 mRNA was present, but had no effect in a patient with carcinoid in which SSTR2 mRNA was not detected, this study suggests that the efficacy of SMS 201-995 may depend, at least in part, on the expression of SSTR2 in tumors.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer W., Briner U., Doepfner W., Haller R., Huguenin R., Marbach P., Petcher T. J., Pless SMS 201-995: a very potent and selective octapeptide analogue of somatostatin with prolonged action. Life Sci. 1982 Sep 13;31(11):1133–1140. doi: 10.1016/0024-3205(82)90087-x. [DOI] [PubMed] [Google Scholar]
- Brazeau P., Vale W., Burgus R., Ling N., Butcher M., Rivier J., Guillemin R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973 Jan 5;179(4068):77–79. doi: 10.1126/science.179.4068.77. [DOI] [PubMed] [Google Scholar]
- Cai R. Z., Szoke B., Lu R., Fu D., Redding T. W., Schally A. V. Synthesis and biological activity of highly potent octapeptide analogs of somatostatin. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1896–1900. doi: 10.1073/pnas.83.6.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Evers B. M., Parekh D., Townsend C. M., Jr, Thompson J. C. Somatostatin and analogues in the treatment of cancer. A review. Ann Surg. 1991 Mar;213(3):190–198. doi: 10.1097/00000658-199103000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurose T., Seino Y., Ishida H., Fujita J., Taminato T., Matsukura M., Imura H. Successful treatment of metastatic glucagonoma with dacarbazine. Lancet. 1984 Mar 17;1(8377):621–622. doi: 10.1016/s0140-6736(84)91011-0. [DOI] [PubMed] [Google Scholar]
- Lamberts S. W., Krenning E. P., Reubi J. C. The role of somatostatin and its analogs in the diagnosis and treatment of tumors. Endocr Rev. 1991 Nov;12(4):450–482. doi: 10.1210/edrv-12-4-450. [DOI] [PubMed] [Google Scholar]
- O'Carroll A. M., Lolait S. J., König M., Mahan L. C. Molecular cloning and expression of a pituitary somatostatin receptor with preferential affinity for somatostatin-28. Mol Pharmacol. 1992 Dec;42(6):939–946. [PubMed] [Google Scholar]
- Reichlin S. Somatostatin (second of two parts). N Engl J Med. 1983 Dec 22;309(25):1556–1563. doi: 10.1056/NEJM198312223092506. [DOI] [PubMed] [Google Scholar]
- Reichlin S. Somatostatin. N Engl J Med. 1983 Dec 15;309(24):1495–1501. doi: 10.1056/NEJM198312153092406. [DOI] [PubMed] [Google Scholar]
- Reisine T., He H. T., Rens-Domiano S., Martin J. M., Raynor K., Borislow S., Thermos K. Biochemical properties of somatostatin receptors. Metabolism. 1990 Sep;39(9 Suppl 2):70–73. doi: 10.1016/0026-0495(90)90215-x. [DOI] [PubMed] [Google Scholar]
- Rens-Domiano S., Law S. F., Yamada Y., Seino S., Bell G. I., Reisine T. Pharmacological properties of two cloned somatostatin receptors. Mol Pharmacol. 1992 Jul;42(1):28–34. [PubMed] [Google Scholar]
- Reubi J. C., Häcki W. H., Lamberts S. W. Hormone-producing gastrointestinal tumors contain a high density of somatostatin receptors. J Clin Endocrinol Metab. 1987 Dec;65(6):1127–1134. doi: 10.1210/jcem-65-6-1127. [DOI] [PubMed] [Google Scholar]
- Reubi J. C., Krenning E., Lamberts S. W., Kvols L. Somatostatin receptors in malignant tissues. J Steroid Biochem Mol Biol. 1990 Dec 20;37(6):1073–1077. doi: 10.1016/0960-0760(90)90468-z. [DOI] [PubMed] [Google Scholar]
- Reubi J. C., Maurer R., von Werder K., Torhorst J., Klijn J. G., Lamberts S. W. Somatostatin receptors in human endocrine tumors. Cancer Res. 1987 Jan 15;47(2):551–558. [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Schally A. V. Oncological applications of somatostatin analogues. Cancer Res. 1988 Dec 15;48(24 Pt 1):6977–6985. [PubMed] [Google Scholar]
- Wynick D., Bloom S. R. Clinical review 23: The use of the long-acting somatostatin analog octreotide in the treatment of gut neuroendocrine tumors. J Clin Endocrinol Metab. 1991 Jul;73(1):1–3. doi: 10.1210/jcem-73-1-1. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Kagimoto S., Kubota A., Yasuda K., Masuda K., Someya Y., Ihara Y., Li Q., Imura H., Seino S. Cloning, functional expression and pharmacological characterization of a fourth (hSSTR4) and a fifth (hSSTR5) human somatostatin receptor subtype. Biochem Biophys Res Commun. 1993 Sep 15;195(2):844–852. doi: 10.1006/bbrc.1993.2122. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Post S. R., Wang K., Tager H. S., Bell G. I., Seino S. Cloning and functional characterization of a family of human and mouse somatostatin receptors expressed in brain, gastrointestinal tract, and kidney. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):251–255. doi: 10.1073/pnas.89.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y., Reisine T., Law S. F., Ihara Y., Kubota A., Kagimoto S., Seino M., Seino Y., Bell G. I., Seino S. Somatostatin receptors, an expanding gene family: cloning and functional characterization of human SSTR3, a protein coupled to adenylyl cyclase. Mol Endocrinol. 1992 Dec;6(12):2136–2142. doi: 10.1210/mend.6.12.1337145. [DOI] [PubMed] [Google Scholar]