Abstract
Glioblastoma multiforme (GBM) is a highly invasive and vascularized aggressive brain tumor. Less than 10% of GBM patients survive more than 5 years after diagnosis. Angiogenesis plays an important role in GBM growth and anti-angiogenesis based therapies have demonstrated clinical efficacy for GBM patients. Unfortunately, therapeutic resistance often develops in these patients, suggesting GBM cells are capable of switching their dependency on one pro-angiogenic signaling pathway to an alternative one. Therefore, it is important to identify novel angiogenic factors that play essential roles in tumor angiogenesis and GBM progression. Angiopoietins (angiopoietin-1, -2, and -4) are the ligands of Tie-2 receptor tyrosine kinase (RTK). The roles of angiopoietin-1 and -2 (Ang-1 and -2) in tumor angiogenesis have been established. However, little is known about how angiopoietin-4 (Ang-4) affects tumor angiogenesis and GBM progression and the mechanism underlying its effects. In our current study, we establish that Ang-4 is up-regulated in human GBM tissues and cells. We demonstrate that like endothelial cells, human GBM cells express Tie-2 RTK. We first establish that Ang-4 promotes in vivo growth of human GBM cells by promoting tumor angiogenesis and directly activating Erk1/2 kinases in GBM cells. Our results establish the novel effects of Ang-4 on tumor angiogenesis and GBM progression and suggest that this pro-GBM effect of Ang-4 is mediated by promoting tumor angiogenesis and activating Erk1/2 kinase in GBM cells. Together, our results suggest that the Ang-4-Tie-2 functional axis is an attractive therapeutic target for GBM.
Keywords: Angiopoietin-4, Tumor Angiogenesis, Glioblastoma, Therapeutic Target
Introduction
Despite of advances in surgery, radiation, chemotherapy, and targeted therapy, the majority of patients with malignant glioma have poor prognosis and approximately one year of median survival length. Malignant glioma is highly vascularized and angiogenesis is known to play an important role in the progression of this deadly disease (1). Therapeutic agents targeting VEGF and VEGFR have shown clinical benefit for patients with malignant glioma and other cancer types (2–3). However, response duration of patients varied and at least 50% of patients failed to respond to the anti-angiogenesis treatments (3–4). Resistance to anti-VEGF/VEGFR therapy suggests that malignant glioma is capable of switching its dependence or addiction of the VEGF-VEGFR signaling pathway to other alternative pro-angiogenic signaling pathways. In order to overcome the resistance to current anti-angiogenesis therapies and achieve better therapeutic efficacy, it is essential to first identify other important pro-angiogenic factors that play critical roles in the progression of malignant glioma.
Tumor angiogenesis is regulated by numerous molecules (5–8). Angiopoietins are among the factors that play important roles in the process and are often up-regulated in the tumors that have developed resistance to anti-VEGF/VEGFR agents (9–10), suggesting their potential roles in mediating the resistance to anti-VEGF/VEGFR therapy. Angiopoietins are the ligands of Tie-2 RTK that is reportedly expressed primarily by endothelial cells (ECs) (11–16). Tie-2 plays an important role in tumor angiogenesis (17–18). Three angiopoietins have been identified in humans: angiopoietin-1, -2, and –4 (Ang-1, -2, and –4) (19–21). All three angiopoietins have a similar protein domain organization, which consists of a signal peptide, an amino terminal coiled-coil domain, a linker peptide region, and a carboxyl terminal fibrinogen homology domain (FHD). The coiled-coil domain is responsible for dimerization/mulimerization of angiopoietins, whereas the FHD binds to Tie-2 receptor (19–23). At least tetrameric aggregation of angiopoietins is required to activate Tie-2 (24). The contributions of Ang-1 and Ang-2 to tumor angiogenesis are relatively well understood and they display context dependent pro- or anti-angiogenic effects (11–12, 16, 25). We have previously shown that cell-surface tethered monomeric mouse Ang-3 inhibits tumor angiogenesis and metastasis (14, 26). However, the role of Ang-4 in tumor angiogenesis and progression has not been established.
In the current study, we investigate how Ang-4 affects tumor angiogenesis, and the growth and progression of human glioblastoma or glioblastoma multiforme (GBM), the stage IV and most aggressive form of glioma, and the mechanism underlying Ang-4 bioactivity. We establish for the first time that Ang-4 is up regulated in human GBM tissue and cells and show that human GBM cells express aggregated multimeric Ang-4, which can be dissociated into monomers under reducing conditions. We demonstrate that like ECs, human GBM cells express Tie-2, the angiopoietin receptor, and that Ang-4 overexpression promotes in vivo growth of human GBM by promoting tumor angiogenesis and directly activating Erk1/2 kinases in GBM cells whereas knockdown of Ang-4 expression inhibits GBM growth in vivo. Our results establish a novel effect of Ang-4 on tumor angiogenesis and glioma progression and suggest the pro-glioma effect of Ang-4 is mediated through enhancing tumor angiogenesis and directly activating Erk1/2 kinase in GBM cells. Together, these results suggest that the Ang-4-Tie-2 functional axis is an attractive therapeutic target for GBM.
Materials and Methods
Patient Glioma Samples, Cells, and Reagents
GBM and normal brain tissues were obtained from the Cooperative Human Tissue Network (CHTN) at University of Pennsylvania and the Ohio State University. Human umbilical vein endothelial cells (HUVECs) and normal human astrocytes (NHAs) were from ALLCELLS, Inc. U87MG was obtained from ATCC and U251 was obtained from the Neurosurgery Tissue Bank at University of California, San Francisco (https://gnome.ucsf.edu/btrc/cell_line.php). These cells were tested and authenticated by the providers, maintained according to the providers’ instructions, and the cumulative culture length of these cells were less than 6 months after reception of the cells.
Anti-v5 epitope (Invitrogen), -Tie-2, and -Ang-2 (Santa Cruz), -Ang-1, and Ang–4 (R&D Systems), - CD31 (Chemicon, BD Biosciences, ThermoScientific—for fluorescence), -smooth muscle actin-Cy3 (Sigma), -Erk1/2, -phospho-Erk1/2, -Akt, and -phospho-Akt (Santa Cruz) antibodies, and Cell Titer-Glo Luminescent Cell Viability Assay kit (Promega) were used in the experiments. Purified Ang-1, Ang-2, and Ang-4 were obtained from R & D systems.
Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR), and Expression and Knockdown Constructs
RT-PCR was performed and full-length human Ang-1 and human Ang-4 cDNAs were generated as described (11, 14, 27). hAng-1 and hAng-4 along with their COOH-terminal v5-epitope tags from the expression vector (pEF6/v5-HisTOPO, Invitrogen) were cloned into the retroviral expression vector pQCXIP (BD Bioscience) as described (28). All expression constructs were verified by DNA sequencing. Retroviruses were generated using these expression constructs and pVSVG in GP2-293 cells following the manufacturer’s instructions (BD Bioscience). To knock down expression of human Ang-1 or Ang-4, several hAng-1 or hAng-4 specific shRNA-TRC constructs and a non-targeting control shRNA were obtained from Open Biosystems and Addgene. Lentiviruses carrying these shRNAs were generated following the manufacturer’s instructions.
Retrovirus and Lentivirus Transduction
U87MG-Luc and U251-Luc human GBM cells expressing luciferase were established as described (28–29). These cells were transduced with the retroviruses carrying the empty retroviral expression vector, hAng-1, or hAng-4. Infected cells were selected for their resistance to puromycin. Anti-v5 mAb (Invitrogen) was used to detect expression of exogenous v5-tagged hAng-1 and hAng-4. To knock down expression of endogenous Ang-1 or Ang-4, these GBM cells were transduced with the lentiviruses carrying shRNAs against hAng-1 or hAng-4. Knockdown expression of Ang-1 or Ang-4 in these GBM cells was validated by western blotting using anti-hAng-1 or anti-hAng-4 antibodies, respectively.
Real-Time Quantitative PCR (qPCR)
Real-time qPCR was performed as described (28) by using SYBR Green PCR Master Mix (Roche) and the Mx3005 Real-Time PCR Machine (Stratagene). The cycling parameters used were 95°C for 10 min. followed by 42 cycles of 95°C (30 s), 60°C (1 min.), and 72°C (1 min.), and a melting curve analysis. Relative quantification of the targets were normalized with a endogenous housekeeping gene (GAPDH) and data analyses were performed using a comparative (ΔΔCt) method using the manufacturer’s (Stratagene) software and according to manufacturer’s instructions.
Western Blot Analysis
Serum free cell culture supernatants (SFM) were collected from the cultured GBM cells. 1ml of SFM was concentrated by NanoSep Centricon-3K (Life Science) to 100µl. Cells were extracted with 8 × SDS Laemmli sample buffer without the dye. Protein concentrations from all the samples were determined using Bio-Rad Dc Protein Assay Reagents. Equal amounts of proteins were analyzed by western blotting as described previously (27).
Erk and Akt Phosphorylation
U87MG cells were cultured until subconfluence and switched to serum-free medium (SFM) for 72 hours. Purified Ang-1, Ang-4 (R & D Systems), or 10% FBS were applied to the serum-starved U87MG cells for 30 min or 24 hours as detailed in the figure legend. The cells were then lysed and equal amounts of extracted proteins were analyzed by western blotting with anti-phospho-Erk1/2 or - phospho-Akt and anti-Erk1/2 or anti-Akt to detect phosphorylated and total amounts of Erk1/2 and Akt proteins, respectively.
Cell Viability Assay
Cell viability assays were performed using the Cell Titer-Glo Luminescent Cell Viability Assay kit (Promega) following manufacturer’s instructions. U87MG cells were plated in triplicate (2×105 cells per well) in 96-well plates and switched to SFM for 24 hours before treatment for 24 hours with purified 200ng/ml of angiopoietins or 20ng/ml of VEGF (R & D Systems) (14, 26, 30–31) and as detailed in the panels. The cell viability was measured by Modulus™ Microplate Multimode Reader (Turner Biosystems).
Endothelial Cell Proliferation, Survival, Migration, and Tube Formation Assay
Endothelial cell proliferation assay was performed by seeding HUVECS at 2.5 × 104 cells/well in 6-well plates in 1:1 HUVEC medium: Ham’s F-12 medium (Invitrogen). After 12 hours, the cells were switched to fresh 1:1 HUVEC medium: Ham’s F-12 medium (Invitrogen) in the absence or presence of 200ng/ml of angiopoietins (14, 26, 30–31) and numbers of the cells were counted every day for four days. Endothelial cell survival assays were performed by switching confluent HUVECs to SFM in the presence or absence of 200ng/ml Ang-1, Ang-2, or Ang-4, or 20ng/ml of VEGF (14, 26, 30–31). The cells were cultured for an additional 48 hours and viable cells were detected using the Cell Titer-Glo Luminescent Cell Viability Assay kit (Promega) following manufacturer’s instructions.
To assess the effects of angiopoietins on tube formation, Matrigel tubulogenesis assays were performed. 300 µL of Matrigel (~10.5 mg/ml, BD Biosciences) was coated onto each well of a 24-well plate and allowed to polymerize at 37°C for 30 min. 5×104 HUVECS in 200ul of 1:1 HUVEC medium: Ham’s F-12 medium (Invitrogen) with or without 200ng/ml of angiopoietins (14, 26, 30–31) was seeded on Matrigel and cultured for 12 hours to allow tubes to form. Photographs of 5 randomly selected 100x microscopic fields were taken for each type of treatment. Tube length and the number of branch points of tubes were counted in these fields to assess the extent of tubulogenesis.
Subcutaneous and Intracranial Tumor Growth Experiments and Bioluminescence Imaging Analysis of Intracranial Gliomas
Pooled populations of transduced U87MG and U251 cells were used for subcutaneous tumor growth experiments as described previously (28–29). Briefly, 1×106 GBM cells were injected subcutaneously into each immunocompromised B6.129S7-Rag1tmMom mouse (Rag1, Jackson Laboratories). Six mice were used for each type of the infected GBM cell line. After solid tumors became visible, the longest and shortest diameters of the solid tumors were measured using a digital caliper every other day for four to six weeks. Tumor volumes were calculated using the following formula: tumor volume=1/2 × (shortest diameter)2 × longest diameter (mm3). At the end of the experiments, tumors were fixed and sectioned for histological and immunological analyses.
For intracranial tumor growth, U87MG or U251 cells (2×105 cells in 10µl HBSS/Rag1 mouse) were injected stereotactically as described (28–29). Following injection, mice were closely monitored and the duration of their survival was recorded. Mice that showed signs of morbidity were euthanized and considered as if they had died on that day, and the number of surviving mice was recorded. The survival rates were calculated as follows: survival rate (%) = (number of mice still alive/total number of experimental mice) × 100%. At the end of the experiments, mouse brains were removed, fixed, and sectioned for further analysis. Bioluminescence imaging was used to monitor growth of intracranial gliomas in live animals as described (28–29). Images were acquired at 7, 12, and 15 days after the intracranial injections of the GBM cells using IVIS-200 imaging system (Xenogen) at the In Vivo Molecular Imaging Shared Facility (Mount Sinai School of Medicine).
Histology, Immunohistochemistry (IHC), and Scoring of IHC Results
Histology was performed as described (28–29). Paraffin sections derived from GBM patients and normal brains were reacted with anti-Ang-1, -Ang-2, or Ang-4 antibody (Santa Cruz). The intensity of immunoreactivity in six randomly selected 200x microscopic fields for each case were scored by two people independently as the following: score 0 = negative, 1 = weak, 2 = intermediate, and 3 = strong staining (32). The scores were averaged and standard deviations and p values were calculated.
The frozen and paraffin sections derived from gliomas grown in experimental mice were reacted with anti-mouse CD31 (Chemicon, BD Biosciences, ThermoScientific), FITC-conjugated anti- hamster secondary antibody, and then Cy3-conjugated anti-smooth muscle actin (SMA, Sigma) antibody to assess tumor angiogenesis and pericyte coverage and with anti-phospho-Erk1/2 antibody to assess phosphor-Erk1/2 levels in GBM cells in vivo. To determine microvessel density (MVD), CD31+ vessels within 5 randomly selected 400x microscopic fields from each of 4 vascular hot spots were counted per group. A blood vessel was counted as positive for pericyte coverage when at least 50% of the CD31-positive vessel was covered by SMA-positive pericytes (33). Peri-vascular coverage was assessed in 10 randomly selected 200x microscopic fields per group.
Statistics
Other than survival experiments, one tailed student’s t test was used to analyze statistical differences between the control and experimental groups. For mouse survival experiments, LogRank statistic analysis (SigmaPlot) was used. Differences were considered statistically significant at p<0.05.
Results
Ang-4 is up-regulated in GBM tissues and in GBM cells
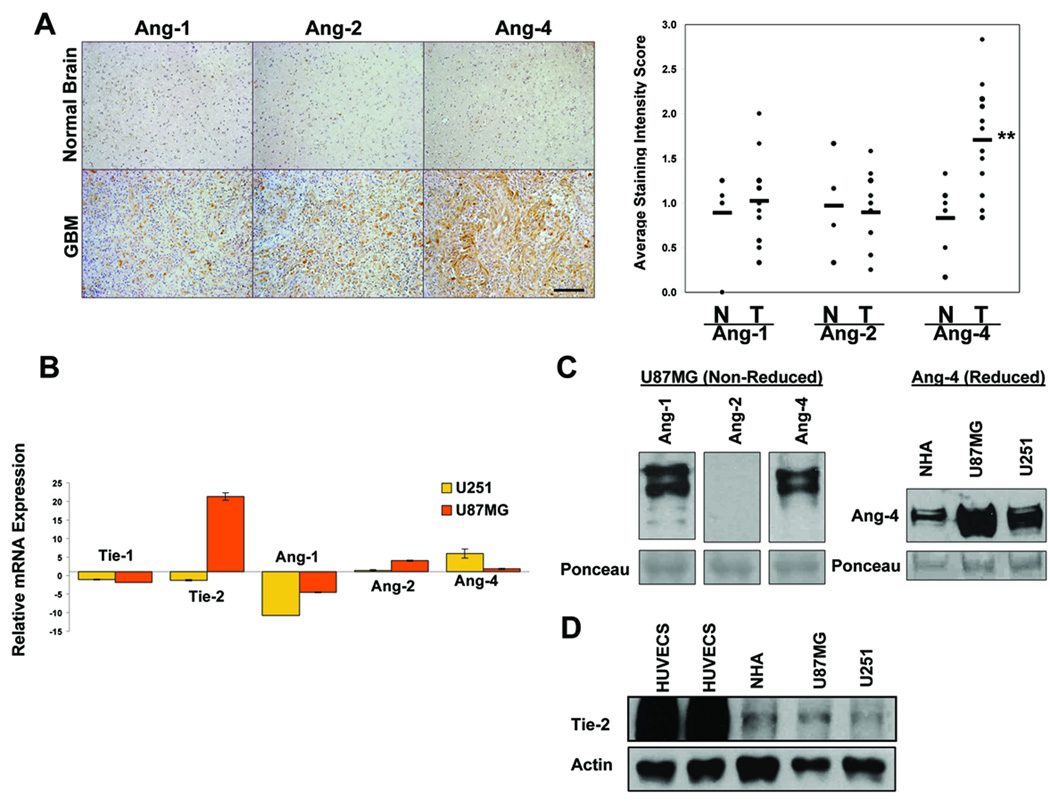
Analyses of the available public datasets (www.oncomine.org) indicated that compared to normal brain, Ang-1 (Beroukhim, Sun, and TCGA Brain) (34–35) and Ang-2 (Shai and Bredel Brain)(36–37) mRNA are up-regulated in GBM whereas Ang-4 mRNA is either unchanged (Beroukhim Brain) (34) or slightly up-regulated in GBM (TCGA Brain 2). To further determine expression levels of endogenous angiopoietin proteins in GBM tissues, we performed immunohistochemistry analysis of paraffin sections derived from 14 GBM tissues and 6 normal brain tissues. Intensity of immunoreactivity was scored as described in Materials and Methods. Our results showed that protein levels of Ang-4 but not Ang-1 or Ang-2 are significantly up regulated in GBM tissues compared to normal human brain (Fig 1A).
Figure 1. Expression levels of angiopoietins and Tie-2 in GBM tissues and cells.
A, Angiopoietin expression was assessed by immunohistochemistry (IHC) using anti-human Ang-1, Ang-2, or Ang-4 antibodies (Santa Cruz). Left panels show representative pictures of 14 GBM samples and 6 normal human brain samples. Bar, 200µm. Right panel shows the plots of intensity scores of the IHC results as determined following the description in Materials and Methods. N = Normal, T = Tumor. B. Real-time qPCR assessing relative transcript levels of human Ang-1, Ang-2, Ang-4, Tie-1, and Tie-2 in U87MG and U251 GBM cells compared to normal human astrocytes (NHAs). C. Endogenous Ang-1, Ang-2, and Ang-4 secreted by U87MG cells (left panel) and endogenous Ang-4 produced by NHAs, U251, and U87MG (right panel) were detected by western blotting with anti-Ang-1 (R&D), Ang-2 (Santa Cruz), or Ang-4 (R&D) antibodies. The SDS-PAGEs were run either under non-reducing conditions (left panel) or reducing conditions (right panel). 160µg of proteins from concentrated serum-free cell culture media was loaded in each lane. The intensities of ~50kDa ponceau-stained bands on the transferred membranes were used as the controls for protein loading and transferring efficiency. D. Expression of endogenous Tie-2 RTK was determined by Western blotting using anti-Tie-2 antibody (Santa Cruz) and the cell lysates derived from human umbilical vein endothelial cells (HUVECs), NHAs, and U87MG and U251 GBM cells (upper panel). 25 µg of total protein was loaded in each lane. Actin was used as a control for protein loading (lower panel).
We then compared the levels of angiopoietins, Tie-2, and Tie-1 transcripts in U87MG and U251 GBM cells to that of normal human astrocytes (NHA) and found that the GBM cells express higher levels of Ang-2, Ang-4, and Tie-2 than normal human astrocytes (NHAs, Fig 1B). The real-time qPCR results reveal only the relative levels of mRNAs. To further confirm that human GBM cells produce endogenous angiopoietin proteins, we collected and concentrated serum-free cell culture supernatants derived from U87MG cells and showed that U87MG cells readily secrete detectable levels of multimeric forms of Ang-1 and Ang-4 but a low level of Ang-2 into the culture media (Fig 1C, left panel), which can be dissociated into monomers under reducing conditions (supplemental Fig 1A). To assess Ang-4 levels in U87MG and U251 GBM cells, and NHAs, we performed Western blotting using the proteins derived from the concentrated serum-free culture supernatants. Our result showed that U87MG and U251 GBM cells express higher levels of Ang-4 then NHAs (Fig 1C, right panel, supplemental Figure 1B). Slight differences in the molecular weight of Ang-4 produced by different cells may reflect differences in the glycosylation status of angiopoietins.
Even though Tie-2 is thought to be expressed largely by ECs and bone marrow progenitor cells (38–39), our real-time qPCR results indicated that the Tie-2 transcript is present in the GBM cells tested (Fig 1B). To establish that Tie-2 protein is expressed by these GBM cells, we performed Western blotting using cell lysates derived from U87MG and U251 cells. We found that U87MG and U251 clearly express endogenous Tie-2, albeit at the levels much lower than that expressed by ECs (Fig 1D).
Ang-4 promotes subcutaneous and intracranial growth of human GBM
To determine how Ang-4 affects tumor angiogenesis and GBM growth and progression, we first transduced U87MG-Luc and U251-Luc cells that express luciferase (28) with retroviruses carrying v5 epitope tagged human Ang-1 (U87MG-Luc/U251-LucAng-1) or human Ang-4 (U87MG-Luc/U251-LucAng-4), or with empty expression vector (U87MG-Luc/U251-Lucctl). The latter cells were used as the controls. U87MG-Luc/U251-LucAng-1 and U87MG-Luc/U251-LucAng-4 but not U87MG-Luc/U251-Lucctl cells express high levels of v5-tagged aggregated Ang-1 and Ang-4, respectively (Fig 2A, upper panels), which can be dissociated into monomers under the reducing condition (Fig 2A, bottom panels).
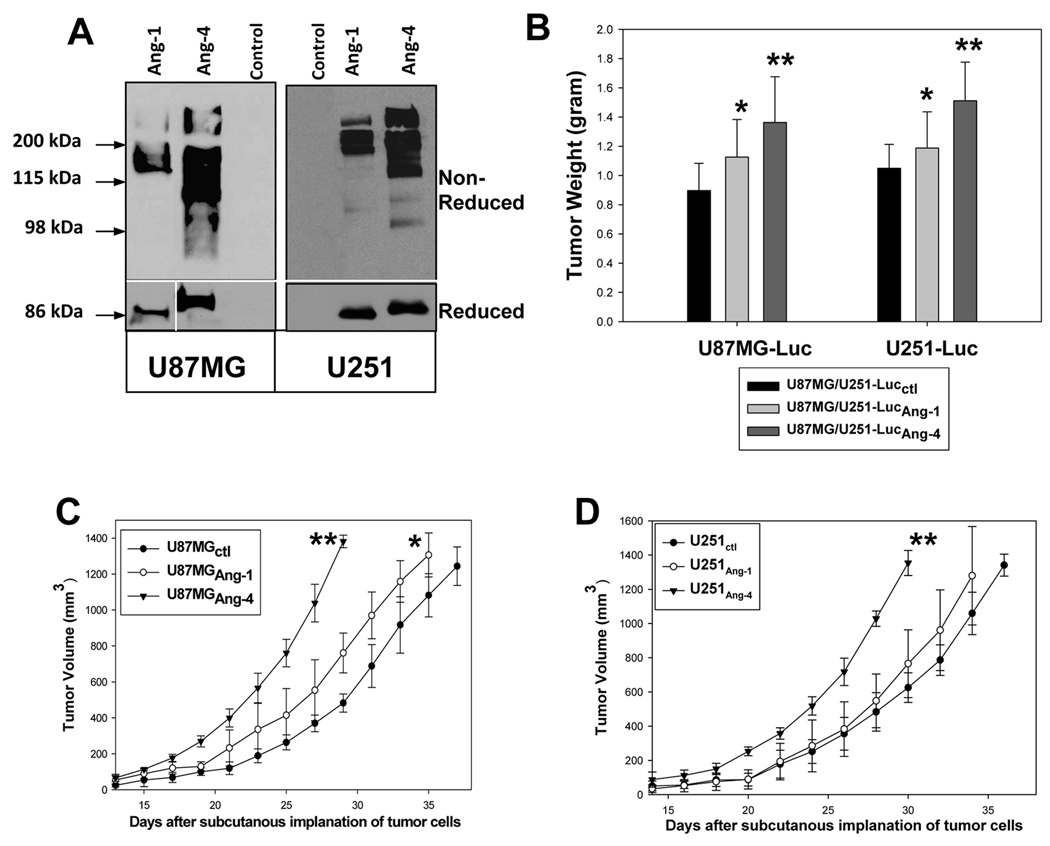
Figure 2. Ang-4 promotes subcutaneous growth of the GBM cells.
A, Establishment of U87MG-Luc and U251-Luc GBM cells expressing v5 epitope tagged Ang-1 (Ang-1v5) or Ang-4 (Ang-4v5). Secreted Ang-1v5 and Ang-4v5 was detected by anti-v5 mAb (Invitrogen) under non-reducing (upper panel) and reducing conditions (bottom panel). B, The effects of Ang-1 and Ang-4 on subcutaneous growth of U87MG and U251 cells were assessed by tumor weight 5 weeks after tumor cell implantation. Six mice were used to implant each type of transduced GBM cells. C–D, Growth rates of the subcutaneous tumors derived from U87MG-Luc (C) and U251-Luc (D) cells expressing Ang-1 or Ang-4 or transduced with empty expression vector. The growth rates are expressed as the mean of tumor volumes (mm3) +/− SD. Six mice were used for each type of transduced GBM cells.
The pooled populations of transduced U87MG-Luc and U251-Luc cells were assessed for their capacity to grow subcutaneously and intracranially. Our results showed that similar to Ang-1, Ang-4 promotes subcutaneous growth of U87MG and U251 GBM cells (Fig 2 B–D). In addition, Ang-4, and to a lesser extent Ang-1, promoted intracranial growth of the GBM cells and Ang-4 significantly reduced survival length of the mice bearing these intracranial gliomas (Fig 3 A–B).
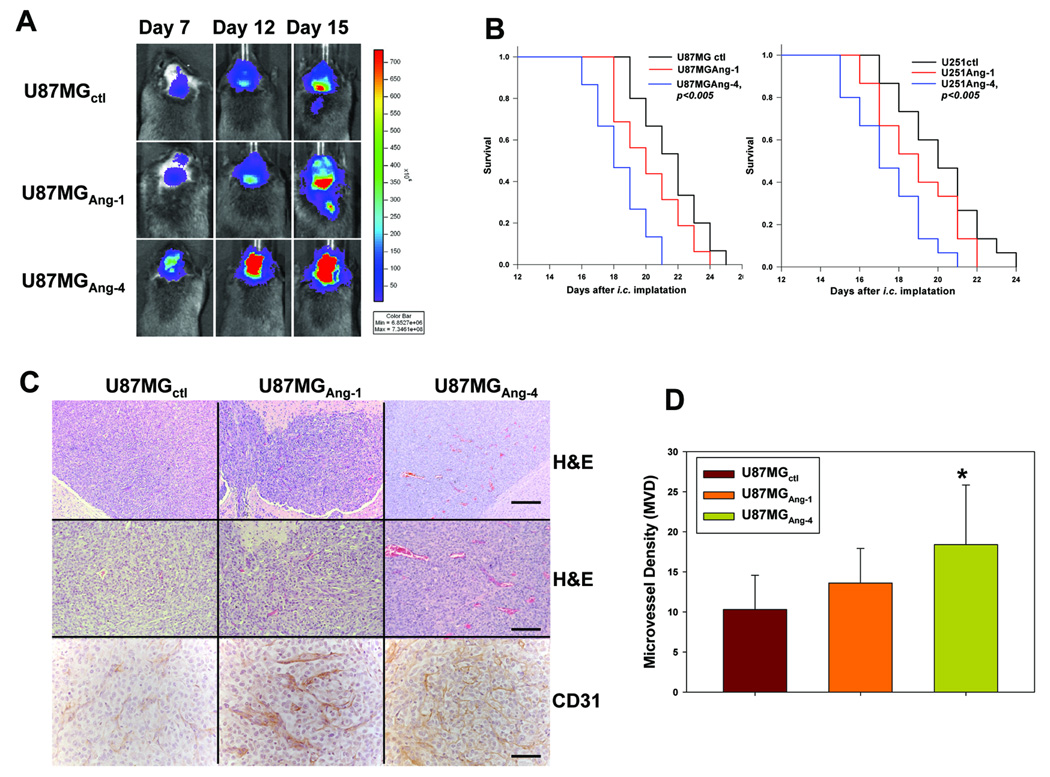
Figure 3. Ang-4 promotes intracranial growth of U87MG-Luc and U251-Luc gliomas.
A, Progression of intracranial GBMs was monitored through bio-luminescence imaging at 7, 12, and 15 days after the intracranial injection of the transduced U87MG cells. The images were obtained 12min after injection of D-luciferin using the same intensity scale. B, Survival rates of mice following intracranial injections of the transduced U87MG-Luc (left panel) and U251-Luc (right panel) cells are shown. 15 mice were used for each type of the transduced GBM cells. C, Histologic (H&E) and immunological (anti-CD31) analyses of the GBM sections derived from U87MG-Lucctl, U87MG-LucAng-1, and U87MG-LucAng-4 cells were performed. Bar: 100µm in the upper panels, 50µm in the middle panels, and 25µm in the bottom panels. D, Microvessel density (MVD) in each type of intracranial gliomas was determined by counting the CD31-positive blood vessels in 12 randomly selected 400X microscopic fields within 4 vascular hot-spots.
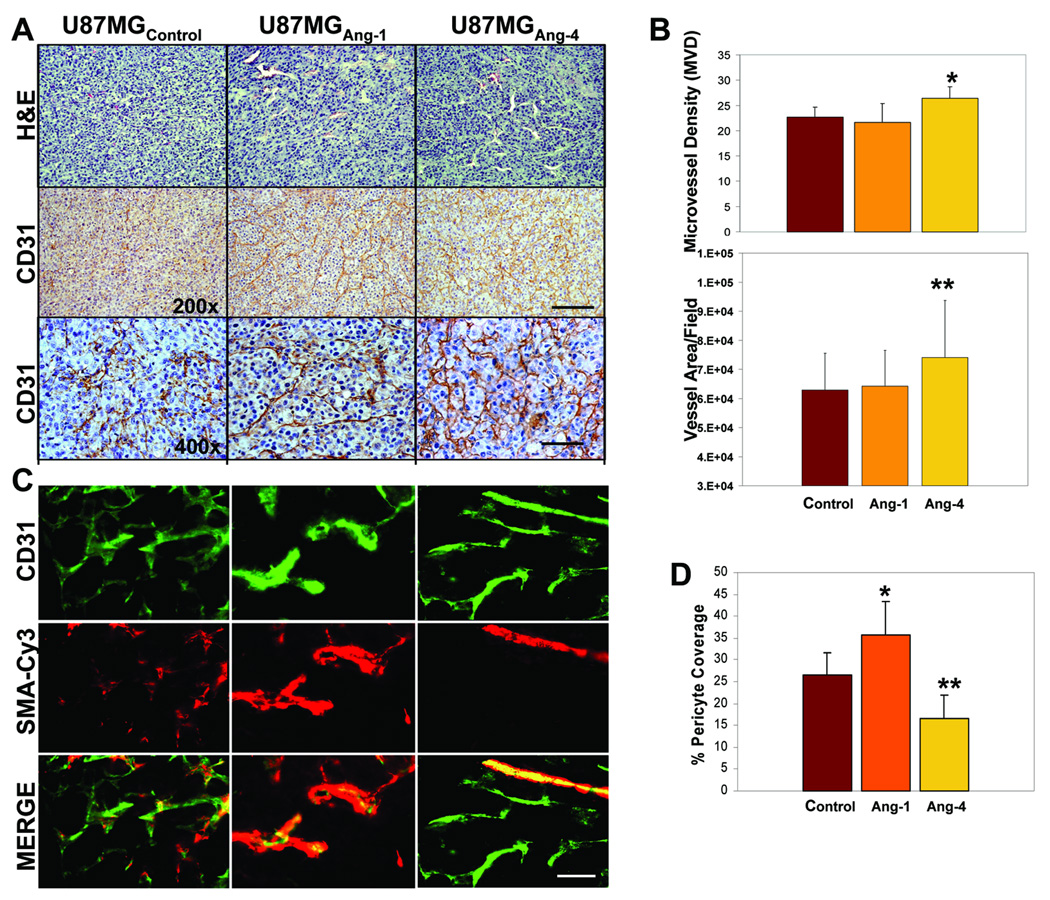
To further investigate the potential mechanism underlying the pro-GBM growth effect of Ang-4, we assessed the extent of angiogenesis in the gliomas derived from U87MG-LucAng-1,U87MG-LucAng-4, or U87MG-Lucctl cells. To achieve that, the GBM sections were analyzed for the presence of CD31-positive blood vessels in 400x microscopic fields as detailed in materials and methods. The microvessel density (MVD) values in the control GBM is consistent with published glioma MVD data (40–41). Our results showed that the intracranial and subcutaneous tumors derived from the GBM cells expressing Ang-4 are more angiogenic than the control tumors and the tumors expressing Ang-1 as determined by MVD (Fig 3C–D, Fig 4A–B), suggesting that Ang-4 is the predominant angiopoietin that promotes tumor angiogenesis during GBM progression. We also assessed coverage of these blood vessels by peri-vascular smooth muscle cells by co-staining the FITC-CD31 positive blood vessels with Cy3-conjugated anti-smooth muscle actin antibody. We found that Ang-1 promotes pericyte coverage of blood vessels whereas Ang-4 reduces the coverage in the gliomas expressing exogenous Ang-1 or Ang-4, respectively when compared to the controls (Fig 4-C–D).
Figure 4. Ang-4 promotes GBM progression by stimulating tumor angiogenesis.
A, Representative H&E and anti-CD31 immunoreactivity pictures of the GBM sections derived from subcutaneous tumors of U87MG-LucAng-1, U87MG-LucAng-4, or U87MG-Lucctl. Bar: 50µm in the upper and middle panels and 25µm in the bottom panels. B, Assessment of tumor angiogenesis: in the top panel, to determine microvessel density (MVD), CD31+ blood vessels within 5 randomly selected 400x microscopic fields in each of 4 vascular hot spots of each type glioma were counted. In the bottom panel, to assess blood vessel area per microscopic field, the perimeter of CD31+ blood vessels from 4 randomly selected 400x microscopic fields in each of 3 vascular hot-spots of each type glioma were outlined and the area was calculated using Image-Pro Plus software. C, The representative pictures of the GBM sections derived from subcutaneous gliomas of U87MG-LucAng-1, U87MG-LucAng-4, or U87MG-Lucctl that show CD31+ ECs and Cy3-smooth muscle actin (SMA)+ peri-vascular cells. Merged pictures of the top and middles panels are shown in the bottom panels. Bar: 20µm. D, Peri-vascular pericyte coverage was determined by assessing numbers of CD 31 positive blood vessels that are covered by Cy3-SMA-positive pericytes in 10 randomly selected 400x microscopic fields of each glioma type. A blood vessel was counted as positive for pericyte coverage when at least 50% of a CD31-positve vessel is covered by SMA-positive pericytes.
Knocking down Ang-4 expression inhibits GBM growth in vivo
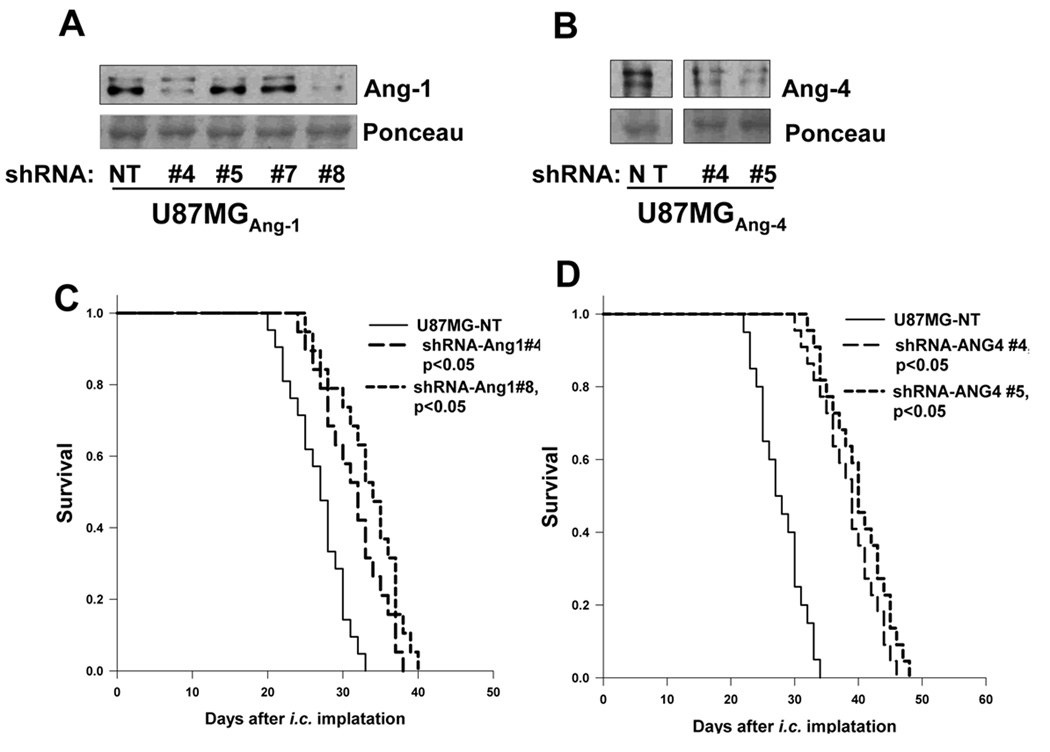
Since the GBM cells express sufficient amount of endogenous Ang-1 and Ang-4 (Fig 1C), we investigated how knockdown of Ang-1 or Ang-4 expression affects GBM growth in vivo. We established that knockdown of Ang-4 expression and to a lesser extent knockdown of Ang-1 inhibited intracranial growth of U87MG glioma (Fig 5). We did not obtain sufficient Ang-4 knockdown in U251 cells to assess the effect of Ang-4 knockdown on U251 cell growth in vivo, however, we obtained the similar growth inhibitory result when Ang-1 was knocked down in U251 cells (data not shown). Together, these results suggest that Ang-4 constitutes an essential pro-angiogenic factor that promotes tumor angiogenesis during GBM progression and is a potential target for GBM therapy.
Figure 5. Knocking down expression of Ang-4 and to a less extent of Ang-1 inhibits GBM growth in vivo.
A–B, Western blots, using anti-Ang-1 (Santa Cruz) or Ang-4 (R&D Systems) antibody, were performed to assess the effectiveness of Ang-1 (A) or Ang-4 (B) knockdown by a panel of TRC-shRNAs (#4, #5, #7, and #8) against human Ang-1 and two TRC-shRNAs (#4 and #5) against human Ang-4. A non-targeting (NT) TRC-shRNA was used as a negative control. 120µg of proteins in concentrated serum free culture supernatants was loaded in each lane. The intensities of ~50kDa ponceau-stained bands were used as the controls for protein loading and transferring efficiency (lower panels). C–D, Survival rates of mice following intracranial injections of the transduced U87MG-Luc with Ang-1 knockdown or NT shRNA (C) or with Ang-4 knockdown or NT shRNA (D). 15 mice were used for each type of transduced GBM cells.
Ang-4 directly affects GBM cell viability and activates Erk1/2 kinase in GBM cells in vivo
To better understand the cellular mechanisms that mediate Ang-4 bioactivity, we first assessed the effects of Ang-4 on endothelial cell functions by performing endothelial proliferation, survival, migration, and tubulogenesis assays in the presence or absence of Ang-4. Angiopoietins and VEGF are often used ranging from 100ng–400ng/ml and 10–40ng/ml, respectively, for in vitro assays (14, 26, 30–31). We have found that 200ng/ml of angiopoietins sufficiently affect Erk1/2 activation and EC proliferation and survival (14). This dose of angiopoietin (200ng/ml) was therefore used in the following assays. We found that Ang-1 and Ang-4 weakly enhance EC proliferation (Supplemental Fig 2A), and significantly promotes EC survival, migration, and formation of branches in the tubulogenesis assay (Supplemental Fig 2B–D).
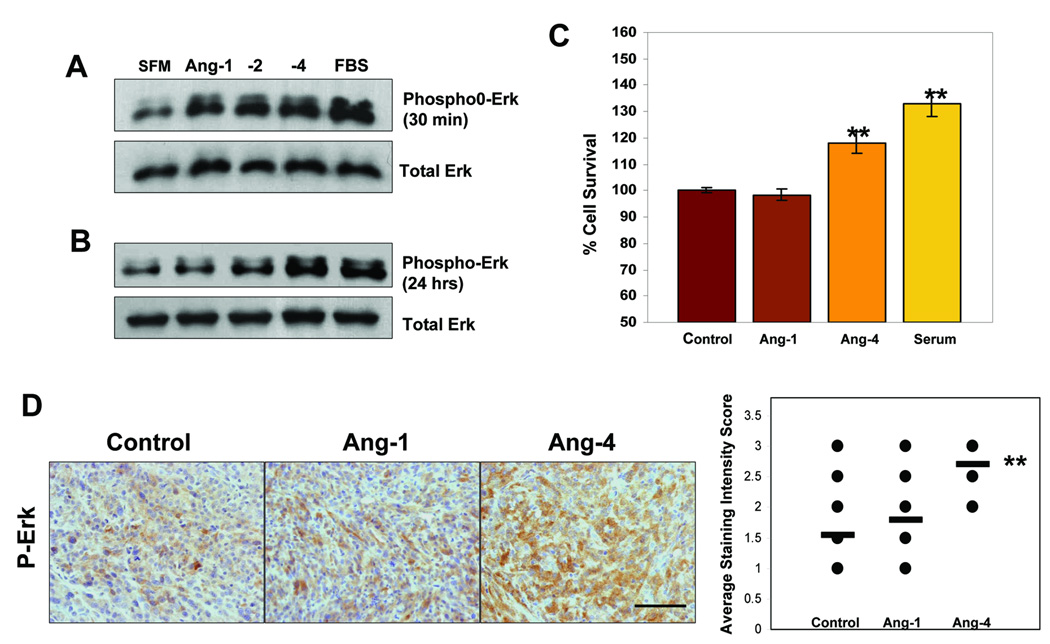
Like ECs, GBM cells express Tie-2, we therefore investigated whether GBM cells can respond to angiopoietins. It is well appreciated in the research field that it is much easier to assess activation of the Tie-2 downstream signaling molecules, Erk1/2 and AKT kinase, than to directly assess the phosphorylation status of Tie-2 (42–45). To determine whether and how angiopoietins affect the Tie-2 signaling pathway in the GBM cells, we treated serum starved U87MG cells with Ang-1, -2, or -4 for various lengths of time as detailed in Fig 6A–B. Our results showed that Ang-4 as well as Ang-1 and Ang-2 activates Erk1/2 kinases (Fig 6A) but not Akt kinase (data not shown) and that the activation occurred at 30 min and sustained throughout 24 hours after administrating different angiopoietins (Fig 6A–B). We then assessed GBM cell viability in the absence (control) or presence of Ang-1, Ang-4, or 10% FBS under serum withdrawn conditions and found that Ang-4 but not Ang-1 significantly promoted U87MG viability (Fig 6C), suggesting an important and direct pro-survival effect of Ang-4 on the GBM cells.
Figure 6. Ang-4 enhances GBM cell viability and actives Erk1/2 kinase in vitro and in vivo.
A–B, Serum-starved U87MG cells were supplied with serum free medium (SFM), 10%FBS containing medium, or SFM containing 200ng/ml of Ang-1 or Ang-4 for 30min (A) and 24 hours (B). The cells were lysed and proteins were analyzed by Western blotting with anti-phospho-Erk1/2 (upper panels) or with anti-Erk1/2 (bottom panels) antibodies. C, Cell viability assays were performed. U87MG cells were plated at 2 × 104/well of 96-well plates in triplicates and allowed to grow overnight. The cells were switched to SFM and cultured for an additional 48 hrs and then treated with 200ng/ml of Ang-1 or Ang-4, 10%FBS, or SFM for another 24 hrs before cell viability was measured using the Cell Titer-Glo Luminescent Cell Viability Assay kit (Promega) following the manufacturer’s instructions. D, Levels of phosphorylated Erk1/2 in the GBM sections derived from subcutaneous tumors of U87MG-LucAng-1, U87MG-LucAng-4, or U87MG-Lucctl were detected using anti-phopho-Erk1/2 antibody (Santa Cruz). The immunoreactivity intensity was scored in ten randomly selected 400x microscopic filed using a 0–3 scoring system described in Materials and Methods.
To further establish that Ang-4 enhances Erk1/2 kinase activity in GBM cells in vivo, we assessed phosphorylated Erk1/2 levels in the GBM tumors derived from U87MG-LucAng-1, U87MG-LucAng-4, or U87MG-Lucctl cells. We found that phospho-Erk1/2 levels and numbers of phosphor-Erk1/2 positive GBM cells were significantly increased when Ang-4 but not Ang-1 was expressed by these cells (Fig 6D), suggesting that Ang-4-induced Erk1/2 activation occurs in growing GBM tumors in situ, further implying that this novel effect of Ang-4 contributes to the pro-GBM activity of Ang-4.
Discussion
Tumor angiogenesis is regulated by pro- and anti-angiogenic factors produced by tumor cells and the surrounding and infiltrating host cells (8, 46). Sufficient angiogenesis is essential for GBM growth and progression and anti-angiogenesis based therapies such as anti-VEGF/VEGFR therapies have demonstrated clinical benefit for GBM patients. However, the resistance to anti-VEGF/VEGFR therapies often occurs or develops in a large fraction of patients (1–3), which demands better understanding about other angiogenic factors and their downstream signaling pathways that play essential roles and/or overlapping roles with VEGF/VEGFR in regulating tumor angiogenesis during progression of this deadly disease. In our present study, we establish the novel pro-angiogenic and pro-GBM growth effects of Ang-4 (Fig 2–3). We demonstrate that Ang-4 is up regulated in human GBM tissues and cells compared to their normal counterparts (Fig 1) and that increased expression of Ang-4 promotes, whereas knockdown of Ang-4 inhibits, GBM growth in vivo. We further demonstrate that GBM cells express Tie-2 RTK and that Ang-4 induces activation of Erk1/2 kinases in GBM cells in vitro and in vivo. Together, these results suggest for the first time that theAng-4-Tie-2 functional axis plays an important role in promoting tumor angiogenesis and GBM growth and is an attractive therapeutic target for GBM.
The effect of Ang-4 on tumor angiogenesis: the tales of monomeric and aggregated forms of Ang-4
Little has been done to determine the role of Ang-4 in tumor growth and angiogenesis. One published study demonstrated that Ang-4 inhibited angiogenesis in tumors derived from a small cell lung cancer cell line, GLC19 (47). Even though the authors didn’t describe in their paper the aggregation status of Ang-4 expressed by GLC19 tumor cells, the migration pattern of the produced Ang-4 resembles monomeric Ang-4 (47) (see Fig 2A for the patterns of monomeric and oligomeric Ang-4). It has been established that tetrameric or higher orders of aggregation of angiopoietins is required to induce Tie-2 activation (24), suggesting that monomeric or dimeric angiopoietins may bind to their receptor but are incapable of inducing its activation, and therefore serve as dominant negative inhibitors of Tie-2. We have shown in our previous studies that Ang-3, the mouse ortholog of human Ang-4, is tethered on tumor cell surface by heparan sulfate proteoglycans, which maintains Ang-3 in a monomeric form (26) to exert anti-angiogenic and anti-cancer activity (14). Both endogenous and exogenous Ang-4 expressed by the GBM cells is in the aggregated forms (Fig 1C and 2A). We showed that the aggregated Ang-4 promoted tumor angiogenesis and GBM progression and significantly reduced survival of the mice bearing the glioblastomas. Together, these results suggest that apparent discrepant results derived from different studies may reflect different aggregation statuses of Ang-4 expressed by different tumor cells and/or modified by the different tumor microenvironments and that monomeric/dimeric Ang-4 inhibits tumor angiogenesis whereas oligomeric Ang-4 promotes tumor angiogenesis.
The effects of Ang-4 on tumor angiogenesis and GBM cell viability
We showed for the first time that Ang-4 promotes GBM progression by promoting tumor angiogenesis and Ang-4 seems to display a more potent pro-angiogenic activity than Ang-1 (Fig 2–4). There were higher levels of exogenous Ang-4 than Ang-1 secreted into culture medium by the GBM cells used in our in vivo experiments (Fig 2A), which may account for the more potent pro-angiogenic and pro-GBM growth activity of Ang-4 observed. However, it has been established that a significant proportion of Ang-1 is incorporated into the insoluble extracellular matrix (ECM) (23). Furthermore, knockdown of Ang-4 in these GBM cells resulted in more dramatic anti-GBM and anti-angiogenic effects than knockdown of Ang-1 (Fig 5 and data not shown). These in vivo angiopoietin knockdown results may reflect the fact that Ang-1 is predominantly produced by host cells, Ang-1 expression in which was not eliminated by the knockdown strategy used in our study, or support a potentially more predominant role of Ang-4 in GBM angiogenesis, growth, and progression. Further studies are required to distinguish these possibilities and to reach a definitive conclusion.
We established that GBM cells express Tie-2 and demonstrated a novel role of Ang-4 in promoting Erk1/2 kinase activation in GBM cells and in enhancing the GBM cell viability. These results suggest that Ang-4 can not only stimulate tumor angiogenesis but also promote GBM cell survival directly through Tie-2 RTK expressed by these cells. This dual function of Ang-4 makes it an ideal target for anti-GBM therapy.
Erk1/2 kinases are well-established downstream effectors of Tie-2. It has been well appreciated in the research field that Tie-2 phosphorylation is difficult to detect and that activation of the downstream effectors of Tie-2 have been often used as the indicator of activation of the Tie-2 signaling pathway (42–45). We have tried to determine whether Ang-4 directly induces Tie-2 phosphorylation in GBM cells using several different strategies and antibodies but had difficulties to obtain positive results, which may merely reflect a technical difficulty or suggest that the effect of Ang-4 on the GBM cells is Tie-2 independent. Studies have shown that Ang-1 induces PC12 neurite outgrowth in a Tie2-independent and beta1-integrin-dependent manner (48) and that Ang-1 monomers reduce cardiac hypertrophy through integrins but not Tie-2 (49). Furthermore, Ang-2 can stimulate breast cancer metastasis through the alpha(5)beta(1) integrin- but not Tie-2-mediated pathway (50). The precise mechanism underlying the effect of Ang-4 on viability of GBM cells requires further study.
Supplementary Material
Acknowledgments
This work was supported by the grants from NIH (RO1HL074117 to QY) and AHA (0555420U to QY). We thank Dr. Yu Zhou at the Molecular Imaging Shared Facility (Mount Sinai School of Medicine) for performing bioluminescence imaging analysis and Mr. Yin Xu for his excellent technical assistance.
References
- 1.Norden AD, Drappatz J, Wen PY. Novel anti-angiogenic therapies for malignant gliomas. Lancet Neurol. 2008;7:1152–1160. doi: 10.1016/S1474-4422(08)70260-6. [DOI] [PubMed] [Google Scholar]
- 2.Norden AD, Drappatz J, Wen PY. Antiangiogenic therapy in malignant gliomas. Curr Opin Oncol. 2008;20:652–661. doi: 10.1097/CCO.0b013e32831186ba. [DOI] [PubMed] [Google Scholar]
- 3.Norden AD, Young GS, Setayesh K, et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70:779–787. doi: 10.1212/01.wnl.0000304121.57857.38. [DOI] [PubMed] [Google Scholar]
- 4.Masabumi S. Brain angiogenesis in developmental and pathological processes: therapeutic aspects of vascular endothelial growth factor. FEBS Journal. 2009;276:4636–4643. doi: 10.1111/j.1742-4658.2009.07175.x. [DOI] [PubMed] [Google Scholar]
- 5.Woo-Young K, Ho-Young L. Brain angiogenesis in developmental and pathological processes: mechanism and therapeutic intervention in brain tumors. FEBS Journal. 2009;276:4653–4664. doi: 10.1111/j.1742-4658.2009.07177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 7.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 8.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 9.Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer. 2008;8:592–603. doi: 10.1038/nrc2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jain RK. Lessons from multidisciplinary translational trials on anti-angiogenic therapy of cancer. Nat Rev Cancer. 2008;8:309–316. doi: 10.1038/nrc2346. [DOI] [PubMed] [Google Scholar]
- 11.Yu Q, Stamenkovic I. Angiopoietin-2 is implicated in the regulation of tumor angiogenesis. Am J Pathol. 2001;158:563–570. doi: 10.1016/S0002-9440(10)63998-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu Q. The dynamic roles of angiopoietins in tumor angiogenesis. Future Oncol. 2005;1:475–484. doi: 10.2217/14796694.1.4.475. [DOI] [PubMed] [Google Scholar]
- 13.Hayes AJ, Huang WQ, Yu J, et al. Expression and function of angiopoietin-1 in breast cancer. Br J Cancer. 2000;83:1154–1160. doi: 10.1054/bjoc.2000.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu Y, Liu YJ, Yu Q. Angiopoietin-3 inhibits pulmonary metastasis by inhibiting tumor angiogenesis. Cancer Res. 2004;64:6119–6126. doi: 10.1158/0008-5472.CAN-04-1054. [DOI] [PubMed] [Google Scholar]
- 15.Holopainen T, Huang H, Chen C, et al. Angiopoietin-1 overexpression modulates vascular endothelium to facilitate tumor cell dissemination and metastasis establishment. Cancer Res. 2009;69:4656–4664. doi: 10.1158/0008-5472.CAN-08-4654. [DOI] [PubMed] [Google Scholar]
- 16.Hu B, Cheng SY. Angiopoietin-2: development of inhibitors for cancer therapy. Curr Oncol Rep. 2009;11:111–116. doi: 10.1007/s11912-009-0017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin P, Buxton JA, Acheson A, et al. Antiangiogenic gene therapy targeting the endothelium-specific receptor tyrosine kinase Tie2. Proc Natl Acad Sci U S A. 1998;95:8829–8834. doi: 10.1073/pnas.95.15.8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lin P, Polverini P, Dewhirst M, Shan S, Rao PS, Peters K. Inhibition of tumor angiogenesis using a soluble receptor establishes a role for Tie2 in pathologic vascular growth. J Clin Invest. 1997;100:2072–2078. doi: 10.1172/JCI119740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis S, Aldrich TH, Jones PF, et al. Isolation of angiopoietin-1, a ligand for the TIE2 receptor, by secretion-trap expression cloning. Cell. 1996;87:1161–1169. doi: 10.1016/s0092-8674(00)81812-7. [DOI] [PubMed] [Google Scholar]
- 20.Maisonpierr PC, Suri C, Jones PF, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 21.Valenzuela DM, Griffiths JA, Rojas J, et al. Angiopoietins 3 and 4: diverging gene counterparts in mice and humans. Proc Natl Acad Sci U S A. 1999;96:1904–1909. doi: 10.1073/pnas.96.5.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barton WA, Tzvetkova-Robev D, Miranda EP, et al. Crystal structures of the Tie2 receptor ectodomain and the angiopoietin-2-Tie2 complex. Nat Struct Mol Biol. 2006;13:524–532. doi: 10.1038/nsmb1101. [DOI] [PubMed] [Google Scholar]
- 23.Xu Y, Yu Q. Angiopoietin-1, unlike angiopoietin-2, is incorporated into the extracellular matrix via its linker peptide region. J Biol Chem. 2001;276:34990–34998. doi: 10.1074/jbc.M103661200. [DOI] [PubMed] [Google Scholar]
- 24.Kim KT, Choi HH, Steinmetz MO, et al. Oligomerization and multimerization are critical for angiopoietin-1 to bind and phosphorylate Tie2. J Biol Chem. 2005;280:20126–20131. doi: 10.1074/jbc.M500292200. [DOI] [PubMed] [Google Scholar]
- 25.Shim WS, Ho IA, Wong PE. Angiopoietin: a TIE(d) balance in tumor angiogenesis. Mol Cancer Res. 2007;5:655–665. doi: 10.1158/1541-7786.MCR-07-0072. [DOI] [PubMed] [Google Scholar]
- 26.Xu Y, Liu YJ, Yu Q. Angiopoietin-3 is tethered on the cell surface via heparan sulfate proteoglycans. J Biol Chem. 2004;279:41179–41188. doi: 10.1074/jbc.M400292200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bai Y, Liu YJ, Wang H, Xu Y, Stamenkovic I, Yu Q. Inhibition of the hyaluronan-CD44 interaction by merlin contributes to the tumor-suppressor activity of merlin. Oncogene. 2007;26:836–850. doi: 10.1038/sj.onc.1209849. [DOI] [PubMed] [Google Scholar]
- 28.Lau YK, Murray LB, Houshmandi SS, Xu Y, Gutmann DH, Yu Q. Merlin is a potent inhibitor of glioma growth. Cancer Res. 2008;68:5733–5742. doi: 10.1158/0008-5472.CAN-08-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu Y, Stamenkovic I, Yu Q. CD44 attenuates activation of the hippo signaling pathway and is a prime therapeutic target for glioblastoma. Cancer Res. 2010;70:2455–2464. doi: 10.1158/0008-5472.CAN-09-2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oubaha M, Gratton JP. Phosphorylation of endothelial nitric oxide synthase by atypical PKC zeta contributes to angiopoietin-1-dependent inhibition of VEGF-induced endothelial permeability in vitro. Blood. 2009;114:3343–3351. doi: 10.1182/blood-2008-12-196584. [DOI] [PubMed] [Google Scholar]
- 31.Lee HJ, Cho CH, Hwang SJ, et al. Biological characterization of angiopoietin-3 and angiopoietin-4. FASEB J. 2004;18:1200–1208. doi: 10.1096/fj.03-1466com. [DOI] [PubMed] [Google Scholar]
- 32.Harvey JM, Clark GM, Osborne CK, Allred DC. Estrogen receptor status by immunohistochemistry is superior to the ligand-binding assay for predicting response to adjuvant endocrine therapy in breast cancer. J Clin Oncol. 1999;17:1474–1481. doi: 10.1200/JCO.1999.17.5.1474. [DOI] [PubMed] [Google Scholar]
- 33.Lu C, Kamat AA, Lin YG, et al. Dual targeting of endothelial cells and pericytes in antivascular therapy for ovarian carcinoma. Clin Cancer Res. 2007;13:4209–4217. doi: 10.1158/1078-0432.CCR-07-0197. [DOI] [PubMed] [Google Scholar]
- 34.Beroukhim R, Getz G, Nghiemphu L, et al. Assessing the significance of chromosomal aberrations in cancer: methodology and application to glioma. Proc Natl Acad Sci U S A. 2007;104:20007–20012. doi: 10.1073/pnas.0710052104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun L, Hui AM, Su Q, et al. Neuronal and glioma-derived stem cell factor induces angiogenesis within the brain. Cancer Cell. 2006;9:287–300. doi: 10.1016/j.ccr.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Shai R, Shi T, Kremen TJ, et al. Gene expression profiling identifies molecular subtypes of gliomas. Oncogene. 2003;22:4918–4923. doi: 10.1038/sj.onc.1206753. [DOI] [PubMed] [Google Scholar]
- 37.Bredel M, Bredel C, Juric D, et al. Functional network analysis reveals extended gliomagenesis pathway maps and three novel MYC-interacting genes in human gliomas. Cancer Res. 2005;65:8679–8689. doi: 10.1158/0008-5472.CAN-05-1204. [DOI] [PubMed] [Google Scholar]
- 38.Sato TN, Qin Y, Kozak CA, Audus KL. Tie-1 and tie-2 define another class of putative receptor tyrosine kinase genes expressed in early embryonic vascular system. Proc Natl Acad Sci U S A. 1993;90:9355–9358. doi: 10.1073/pnas.90.20.9355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schnurch H, Risau W. Expression of tie-2, a member of a novel family of receptor tyrosine kinases, in the endothelial cell lineage. Development. 1993;119:957–968. doi: 10.1242/dev.119.3.957. [DOI] [PubMed] [Google Scholar]
- 40.Benny O, Kim SK, Gvili K, et al. In vivo fate and therapeutic efficacy of PF-4/CTF microspheres in an orthotopic human glioblastoma model. FASEB J. 2008;22:488–499. doi: 10.1096/fj.07-8801com. [DOI] [PubMed] [Google Scholar]
- 41.Folkins C, Shaked Y, Man S, et al. Glioma tumor stem-like cells promote tumor angiogenesis and vasculogenesis via vascular endothelial growth factor and stromal-derived factor 1. Cancer Res. 2009;69:7243–7251. doi: 10.1158/0008-5472.CAN-09-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Papapetropoulos A, Fulton D, Mahboubi K, et al. Angiopoietin-1 inhibits endothelial cell apoptosis via the Akt/survivin pathway. J Biol Chem. 2000;275:9102–9105. doi: 10.1074/jbc.275.13.9102. [DOI] [PubMed] [Google Scholar]
- 43.Kim I, Kim HG, So JN, Kim JH, Kwak HJ, Koh GY. Angiopoietin-1 regulates endothelial cell survival through the phosphatidylinositol 3'-Kinase/Akt signal transduction pathway. Circ Res. 2000;86:24–29. doi: 10.1161/01.res.86.1.24. [DOI] [PubMed] [Google Scholar]
- 44.Kim I, Ryu YS, Kwak HJ, et al. EphB ligand, ephrinB2, suppresses the VEGF- and angiopoietin 1-induced Ras/mitogen-activated protein kinase pathway in venous endothelial cells. Faseb J. 2002;16:1126–1128. doi: 10.1096/fj.01-0805fje. [DOI] [PubMed] [Google Scholar]
- 45.Abou-Khalil R, Le Grand F, Pallafacchina G, et al. Autocrine and Paracrine Angiopoietin 1/Tie-2 Signaling Promotes Muscle Satellite Cell Self-Renewal. Cell Stem Cell. 2009;5:298–309. doi: 10.1016/j.stem.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 47.Olsen MW, Ley CD, Junker N, Hansen AJ, Lund EL, Kristjansen PE. Angiopoietin-4 inhibits angiogenesis and reduces interstitial fluid pressure. Neoplasia. 2006;8:364–372. doi: 10.1593/neo.06127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen X, Fu W, Tung CE, Ward NL. Angiopoietin-1 induces neurite outgrowth of PC12 cells in a Tie2-independent, [beta]1-integrin-dependent manner. Neuroscience Research. 2009;64:348–354. doi: 10.1016/j.neures.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dallabrida SM, Ismail NS, Pravda EA, et al. Integrin binding angiopoietin-1 monomers reduce cardiac hypertrophy. FASEB J. 2008;22:3010–3023. doi: 10.1096/fj.07-100966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Imanishi Y, Hu B, Jarzynka MJ, et al. Angiopoietin-2 Stimulates Breast Cancer Metastasis through the {alpha}5{beta}1 Integrin-Mediated Pathway. Cancer Res. 2007;67:4254–4263. doi: 10.1158/0008-5472.CAN-06-4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.