Abstract
Few studies have examined the extent to which structural and functional MRI, alone and in combination with genetic biomarkers, can predict future cognitive decline in asymptomatic elders. This prospective study evaluated individual and combined contributions of demographic information, genetic risk, hippocampal volume, and fMRI activation for predicting cognitive decline after an 18-month retest interval. Standardized neuropsychological testing, an fMRI scans semantic memory task (famous name discrimination), and structural MRI (sMRI) were performed on 78 healthy elders (73% female; mean age = 73 years, range = 65 to 88 years). Positive family history of dementia and presence of one or both apolipoprotein E (APOE) ε4 alleles occurred in 51.3% and 33.3% of the sample, respectively. Hippocampal volumes were traced from sMRI scans. At follow-up, all participants underwent a repeat neuropsychological examination. At 18 months, 27 participants (34.6%) declined by at least 1 SD on one of three neuropsychological measures. Using logistic regression, demographic variables (age, years of education, gender) and family history of dementia did not predict future cognitive decline. Greater fMRI activity, absence of an APOE ε4 allele, and larger hippocampal volume were associated with reduced likelihood of cognitive decline. The most effective combination of predictors involved fMRI brain activity and APOE ε4 status. Brain activity measured from task-activated fMRI, in combination with APOE ε4 status, was successful in identifying cognitively intact individuals at greatest risk for developing cognitive decline over a relatively brief time period. These results have implications for enriching prevention clinical trials designed to slow AD progression.
Keywords: Aging, apolipoprotein E, cognitive decline, fMRI, hippocampal volume, neuroimaging, memory
INTRODUCTION
Alzheimer’s disease (AD) neuropathology begins decades before the onset of observable symptoms [1]. Initiating interventions after symptom onset may be too late to make a meaningful impact on disease course. Clinical trials designed to prevent or slow AD progression have dramatically intensified the search for valid preclinical biomarkers. Extant biomarker studies have demonstrated success in predicting conversion from mild cognitive impairment (MCI) to AD using neuropsychological testing [2–5]; structural magnetic resonance imaging (sMRI) measurement of hippocampal volume [6–8] and rate of atrophy [9–11]; sMRI of entorhinal cortex volume [11–14]; cerebrospinal fluid (CSF) indices including elevated isoprostane [15–17], elevated total tau and phosphorylated tau [18–20], and low amyloid-β (Aβ)42 levels [15,21–23]; positron emission tomography(PET) involving regional glucose metabolism [24,25] and amyloid imaging using the 11C Pittsburgh Compound B [26–28]; and task-activated functional magnetic resonance imaging (fMRI) [29, 30]. A more challenging task for prevention trials, however, is to identify biomarkers capable of identifying asymptomatic older persons at-risk for developing cognitive decline within the time frame required of a prevention trial (2–3 years).
The apolipoprotein E (APOE) ε4 allele is a well-known risk factor for late onset AD [31,32], and healthy APOE ε4 carriers have demonstrated faster cognitive decline than non-carriers [33–35]. However, the biomarker potential for APOE alone is limited, given that the APOE ε4 allele frequency is less than 40% among AD cases [36,37], and it has a low positive predictive value for AD diagnosis [38–40]. Using test-retest intervals of approximately three years, studies using fluorodeoxyglucose (FDG) PET [34] and CSF tau181/Aβ42 and ptau181/Aβ42 ratios [41] have shown promise for predicting cognitive decline in otherwise healthy older adults. The relative invasiveness of these latter two approaches may preclude their routine use in screening large numbers of cognitively intact participants for inclusion in prevention trials.
Less invasive magnetic resonance imaging (MRI) techniques provide more practical alternatives for identifying cognitively intact older adults at risk for future cognitive decline. sMRI studies have demonstrated that smaller hippocampal and entorhinal cortex volumes at baseline predict cognitive decline in healthy elders [42–47]. A task-activated fMRI study [48] has also shown that increased number and spatial extent of activated regions at baseline can predict memory decline after a two-year retest interval. Genetic risk in middle aged women (family history of AD and at least one APOE ε4 allele) has been associated with decreased fMRI activation in extrastriate and posterior inferotemporal cortex at baseline, together with further decreases after four years in these regions as well as left inferior frontal and premotor cortex [49]. However, this decreased fMRI signal was not associated with cognitive decline in this study. In contrast, using a word categorization task during fMRI with APOE ε4 carriers, nine older adults showing cognitive stability on episodic memory testing after five years demonstrated increased left inferior parietal activation at baseline relative to nine participants who demonstrated episodic memory decline; greater blood oxygen level dependent (BOLD) fMRI response in this region was associated with better memory performance after five years [50]. However, no hippocampal volume differences were observed at baseline between stable and declining participants. No study to date has directly compared the relative sensitivity of sMRI and fMRI approaches, particularly over a relatively brief interval (e.g., 1–2 years).
In this study, we compared the ability of sMRI and fMRI to predict cognitive decline over 18 months in a sample of cognitively intact older adults with varying degrees of AD risk, based on family history of dementia and APOE ε4 allele carrier status. The sMRI technique involved measurement of hippocampal volumes. The fMRI task required the discrimination of famous from unfamiliar names. Our previous studies using this task reported activation of a semantic memory system, including bilateral hippocampi, posterior cingulate, middle frontal gyrus, and lateral temporoparietal junction [51–53]. The task can be performed with a high degree of accuracy (> 90% correct) even in symptomatic amnestic MCI patients [54]. In a cross-sectional study [55], we demonstrated that the brain activation patterns of healthy elders at risk for developing AD (APOE ε4, family history) could be differentiated using this task. The current longitudinal prospective study used logistic regression to compare the relative efficacy of sMRI and fMRI, alone and in combination, for predicting cognitive decline after an 18-month retest interval. Because a greater potential exists for accelerated cognitive decline among APOE ε4 carriers [33–35], we examined APOE genotype as an additional predictor of decline.
MATERIALS AND METHODS
Participants
Participants were 78 healthy older adults (73% female; Mage = 73 years, SD = 4.9 years; Meducation = 14.9 years, SD = 2.7 years). The participants were drawn from a larger sample of 459 community-dwelling adults who were recruited via newspaper advertisements. Following telephone screening, 92 participants met study inclusion and exclusion criteria, and 81 persons agreed to undergo ApoE genotyping from blood samples, a neuropsychological evaluation, and an fMRI scanning session. MRI data were not able to be obtained for three participants. Family history was defined as a report of a clear clinical diagnosis of AD or a reported history of gradual decline in memory and other cognitive functions, confusion, or judgment problems without a formal diagnosis of AD prior to death in a first-degree relative. One participant reported a diagnosis of AD in a second degree relative, with some mild cognitive changes noted in a parent prior to the parent’s death. Because our study examined the influence of AD risk factors on prediction of cognitive decline, half of the participants were purposely selected because they had a positive family history of AD. We expected that enrichment of our sample with persons with a positive family history of AD would also increase the number of persons who were APOE ε4 positive, because APOE ε4 tends to be more common among individuals with a positive AD family history than among those with a negative AD family history [56,57].
Family history of dementia was present in 51.3% of participants, and 33.3% of the sample carried the APOE ε4 allele. All participants underwent neuropsychological evaluation (see below) and were cognitively intact when entering the study. Informed consent was obtained consistent with the Declaration of Helsinki and institutional guidelines established by the Medical College of Wisconsin Human Subjects Review Committee; all participants received financial compensation.
Neuropsychological assessment and APOE genotyping
All participants underwent baseline neuropsychological testing, fMRI scanning, and APOE genotyping. The neuropsychological battery included the Mini-Mental State Examination [58], Mattis Dementia Rating Scale-2 (DRS-2) [59,60], Rey Auditory Verbal Learning Test (RAVLT) [61], Geriatric Depression Scale [62], and Lawton Instrumental Activities of Daily Living Scale (ADL) [63]. Alternate forms of the DRS-2 [64,65] and RAVLT [66] were used. APOE genotype was determined using a PCR method [67]. DNA was isolated with Gentra Systems Autopure LS for Large Sample Nucleic Acid Purification. All participants underwent a follow-up neuropsychological examination after approximately 18 months.
Definition of cognitive decline
We defined cognitive decline as a reduction from baseline performance of at least one SD on at least one of the three principal outcome indices (DRS-2, RAVLT Sum of Trials 1–5 [T1–5], RAVLT Delayed Recall [DR]). Residualized change scores were computed for each cognitive measure by predicting T2 scores using T1 scores; this procedure adjusts for baseline performance, practice effects, and regression to the mean [68–70]. Participants with standardized residuals of −1.0 or lower were assigned to the cognitively declining group; the remaining participants were classified as cognitively stable.
fMRI task
For the fame discrimination task [53], stimuli consisted of 30 famous and 30 unfamiliar names randomly interspersed with 20 presentations of a centrally placed crosshair in order to introduce “jitter” into the fMRI time series (interstimulus interval = 4 sec). Participants made a right index or right middle finger key press for famous or unfamiliar names, respectively. Accuracy and reaction time were recorded, and nonparametric signal detection indices were calculated [71]. The imaging run began and ended with 12 sec of fixation and was 5 min and 44 sec in duration.
Image acquisition
Whole-brain, event-related fMRI was conducted on a General Electric (Waukesha, WI) Signa Excite 3.0 Tesla short bore scanner equipped with a quad split quadrature transmit/receive head coil. Echoplanar images were collected using an echoplanar pulse sequence (TE = 25 ms; flip angle = 77 degrees; field of view (FOV) = 24 cm; matrix size = 64 × 64; TR = 2 s). Thirty-six contiguous axial 4-mm-thick slices provided coverage of the entire brain (voxel size = 3.75 × 3.75 × 4 mm). High-resolution, three-dimensional spoiled gradient-recalled at steady-state (SPGR) anatomic images were acquired (TE = 3.9 ms; TR = 9.5 ms; inversion recovery (IR) preparation time = 450 ms; flip angle = 12 degrees; number of excitations (NEX) = 2; slice thickness = 1.0 mm; FOV = 24 cm; resolution = 256 × 224). Foam padding was used to reduce head movement within the coil.
Image analysis
Functional images were generated with the Analysis of Functional NeuroImages (AFNI) software package [72]. Each image series was time shifted to the beginning of the TR and spatially registered to reduce head motion effects using a rigid body iterative linear least squares method. A deconvolution analysis was used to extract separate hemodynamic response functions (HRFs) for correctly recognized famous and unfamiliar names. HRFs were modeled for the 0–16 second period post-stimulus onset. Motion parameters and incorrect trials were incorporated into the model as nuisance regressors. Area under the curve (AUC) was calculated by summing the hemodynamic responses at time points 4, 6, and 8 seconds post trial onset, a measure of the curve peak yielding maximum signal-to-noise. Anatomical and functional scans were transformed into standard stereotaxic space [73]. To compensate for anatomical variation, functional images were blurred using a 6 mm Gaussian full-width half-maximum filter.
Spatial extent of activation for cognitively stable and declining groups
Voxelwise t-tests were used to generate separate statistical parametric maps for the stable and declining groups. These maps indicate regions where the AUCs for famous and unfamiliar names were significantly different. The statistical threshold was based on an individual voxel probability (p = 0.005) coupled with a minimum cluster volume (0.73 ml). These values were derived from 3,000 Monte Carlo simulations [74] and correspond to a whole brain family-wise error threshold of p < 0.05.
Functional ROI analysis
A separate voxelwise t-test, comparing famous and unfamiliar names, was conducted on all 78 participants using the identical statistical threshold. This method identified significant cluster volumes, which we refer to as functional regions of interest (fROIs). For each participant, an “average AUC” was calculated for all voxels within each fROI. These data were then subjected to a principal components analysis (PCA) to further reduce the number of regions that would serve as predictors in the logistic regression analysis (see below).
Hippocampal volume measurement
Left and right hippocampal volumes were created using Freesurfer [75,76] and manually edited on T1-weighted SPGR images by two raters blinded to participant group membership. Using coronal views, the mask was further refined by excluding the fimbria and alveus and retaining the hippocampus (uncal apex, cornu ammonis, subiculum, gyrus of retzius, and fasciola cinerea). Hippocampal volumes were normalized by dividing by the total intracranial volume. Intraclass correlation for the two raters was 0.87. The left and right hippocampal volumes were then summed to create a single score.
Data analysis
Statistical analyses were performed using R, version 2.9.0. Group differences on demographics, total hippocampal volume, and neuropsychological and fMRI task performance were compared using t-tests and r2 effect size measures or Fisher’s Exact tests, as appropriate. Logistic regression tested the ability of specific baseline variables to discriminate between stable and declining participants. To avoid overfitting the data and to maintain a reasonable subjects-to-variables ratio for each model, we restricted the set of predictors to no more than four variables. Our models tested the effects of age, education, and gender (Model 1); APOE ε4 status and dementia family history (Model 2); hippocampal volume (Model 3); fMRI activation (Model 4). Models 5 and 6 examined the additive effect of APOE ε4 status with either hippocampal volume or fMRI activation, respectively. Model 7 combined APOE ε4 status with both imaging predictors. The ability of these models to differentiate between stable and declining participants was assessed using the Nagelkerke R2 and the concordance or C index (related to the area under the receiver operating characteristic curve [77]. The Nagelkerke R2 assesses the importance of the predictors in a given model relative to a “perfectly fitting” null model [78]. The C index reflects the proportion of all possible pairs of declining and stable subjects in which the declining participant in the pair had a higher predicted probability of decline than the stable participant [77]. Values of R2 and C for each logistic regression model were validated with a bootstrapping analysis using 5000 resamples in order to assess each model’s accuracy of prediction of decline across the entire range of probabilities [77]. This approach yielded bootstrap-corrected values for R2 and C. Bootstrapping is the most efficient model validation procedure, as it does not require holding out any data for cross-validation, and each phase of model development (including assessment of the degree of overfitting the data) is revalidated using repeated resampling from the entire sample [77].
RESULTS
Identification of cognitive decline
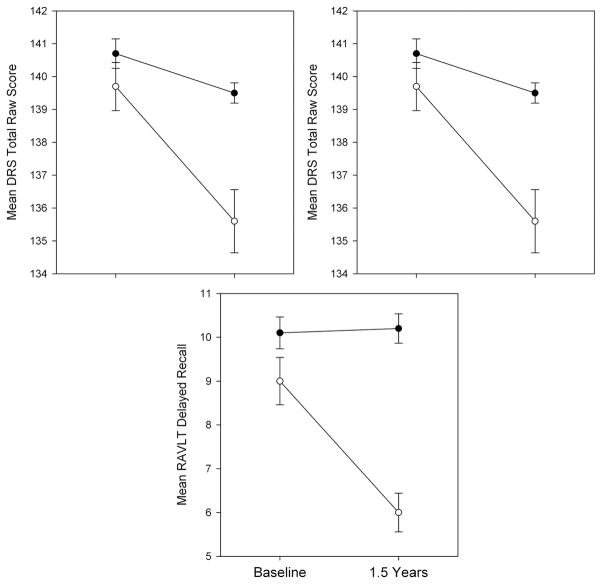
A total of 27/78 (34.6%) participants showed a one SD decline on at least one of the three neuropsychological indices (DRS-2, RAVLT Trials 1–5, and RAVLT Delayed Recall). These participants constituted the cognitively declining group and the remaining participants formed the stable group. Figure 1 illustrates performance changes on the neuropsychological outcome measures for the stable and declining groups. As expected, the stable group showed no significant neuropsychological change after 18 months, while the declining group demonstrated significant reductions on each of the three neuropsychological indices.
Fig. 1.
Mean baseline and follow-up performance (with standard errors) on principal neuropsychological outcome measures for cognitively stable and declining participants. There were no significant (p < 0.05) group differences at baseline. The 18-month follow-up shows expected group differences in cognitive functioning, validating the group selection criteria.
Subjective memory complaints were present in 33.3% of the declining group. Of the declining participants, 2 (7.6%) satisfied criteria for MCI [79]. No participant demonstrated impaired ADL skills at follow-up. Declining participants did not differ from stable participants on age, education, gender, or neuropsychological retest interval (Table 1). However, the APOE ε4 allele was over twice as prevalent (51.9% versus 23.6%) among declining (3 ε2/ε3, 10 ε3/ε3, 14 ε3/ε4) than stable (5 ε2/ε3, 34 ε3/ε3, 11 ε3/ε4, 1 ε4/ε4) participants.
Table 1.
Sample characteristics, neuropsychological performance and fMRI behavioral data for stable and declining groups
| Stable (n = 51) |
Declining (n = 27) |
p | r2 | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | |||
| Sample Characteristics | ||||||||
| Age (years) | 72.7 | 5.1 | 65–88 | 73.7 | 4.7 | 65–84 | 0.41 | 0.01 |
| Education (years) | 15.1 | 2.5 | 12.0–23.0 | 14.6 | 3.2 | 11.0–23 | 0.40 | 0.01 |
| Gender | 13M (25%), 38F (75%) | 8M (30%) 19F (70%) | 0.79 | – | ||||
| Family History Status | 27− (53%), 24+ (47%) | 11− (41%), 16+ (59%) | 0.35 | – | ||||
| APOE e4 Status | 39− (76%), 12+ (24%) | 13− (48%), 14+ (52%) | 0.02 | – | ||||
| Retest Interval (days) | 551.7 | 43.5 | 489–763 | 560.6 | 47 | 489–691 | 0.41 | 0.01 |
| Total Hippocampal Volume | 4.5 | 0.55 | 3.2–5.9 | 4.2 | 0.6 | 3.1–5.6 | 0.01 | 0.06 |
| Neuropsychological Testing | ||||||||
| MMSE | 29.4 | 0.8 | 27–30 | 28.9 | 1.2 | 25–30 | 0.04 | 0.06 |
| DRS-2 Total | 140.7 | 3.2 | 127–144 | 139.7 | 3.8 | 130–144 | 0.25 | 0.02 |
| RAVLT Trials 1–5 | 50.6 | 8.8 | 33–66 | 46.8 | 8.1 | 29–59 | 0.07 | 0.05 |
| RAVLT DR | 10.1 | 2.6 | 4.0–15.0 | 9 | 2.8 | 4.0–13 | 0.08 | 0.04 |
| Lawton IADL | 5 | 0 | 5–5 | 5 | 0 | 5–5 | * | – |
| GDS | 2.7 | 2.5 | 0–8 | 1.4 | 1.8 | 0–8 | 0.02 | 0.07 |
| fMRI Task Performance | ||||||||
| % Correct Famous | 93.1 | 6.8 | 73–100 | 90.4 | 7.4 | 73–100 | 0.10 | 0.04 |
| % Correct Unfamiliar | 96.9 | 4.6 | 77–100 | 95.2 | 8.8 | 63–100 | 0.27 | 0.02 |
| d′ | 5.8 | 1.2 | 3.4–7.6 | 5.4 | 1.2 | 2.5–7.6 | 0.15 | 0.03 |
| RT Famous | 1266 | 222 | 790–1886 | 1270 | 180 | 873–1679 | 0.94 | 0.00 |
| RT Unfamiliar | 1670 | 354 | 912–2602 | 1611 | 385 | 984–2306 | 0.50 | 0.00 |
Note: MMSE = Mini-Mental State Examination; DRS-2 = Mattis Dementia Rating Scale-2; RAVLT = Rey Auditory Verbal Learning Test; DR = delayed recall; IADL = Instrumental Activities of Daily Living; GDS = Geriatric Depression Scale; d′ = signal detection discrimination; RT = Reaction Time.
data were constant.
Baseline neuropsychological testing and fMRI task performance
On baseline measures (Table 1), no significant differences were observed on the MMSE, DRS-2, RAVLT Trials 1–5, and RAVLT Delayed Recall between the stable and declining groups after controlling for multiple comparisons (Bonferroni adjusted alpha level = 0.0125; 0.05/4 tests). The stable group reported significantly more depressive symptoms on the GDS, but no participant in either group was in the clinically depressed range. None of the participants reported ADL impairments at baseline.
On the fMRI fame discrimination task, no differences were observed in accuracy, RT, or on a signal detection measure of discriminability (d′) between the stable and declining groups. For both groups, mean accuracy exceeded 90% for identification of famous names and rejection of unfamiliar names.
Baseline sMRI
Declining participants had a significantly smaller total hippocampal volume at baseline than cognitively stable participants (Table 1).
Baseline fMRI
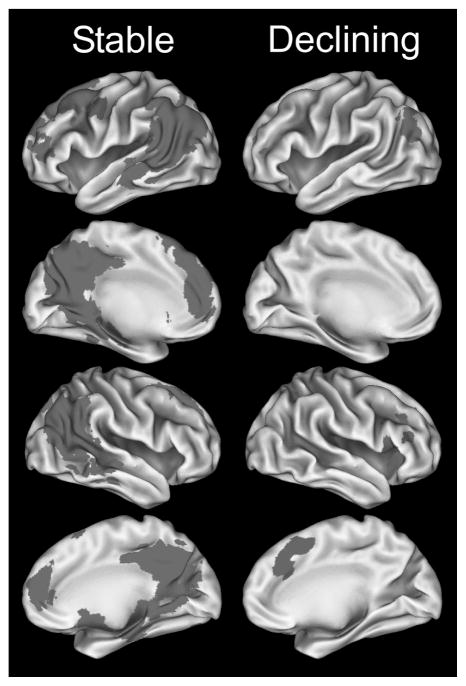
Figure 2 presents significant clusters based on a voxelwise analysis performed separately for the stable and declining groups. The spatial extent of activated voxels is greater in the stable than declining group, with most of the differences reflecting more activation during recognition of famous names relative to unfamiliar names. The declining group showed a smaller amount of activated tissue, with some regions showing the opposite pattern.
Fig. 2.
Group differences in activation derived from the comparison of the famous versus unfamiliar names condition: Famous > Unfamiliar represented in red; Unfamiliar > Famous in blue. Note the greater spatial extent of activation in the Famous > Unfamiliar names comparison in the stable group. (Colours are visible in the electronic version of the article at www.iospress.nl.)
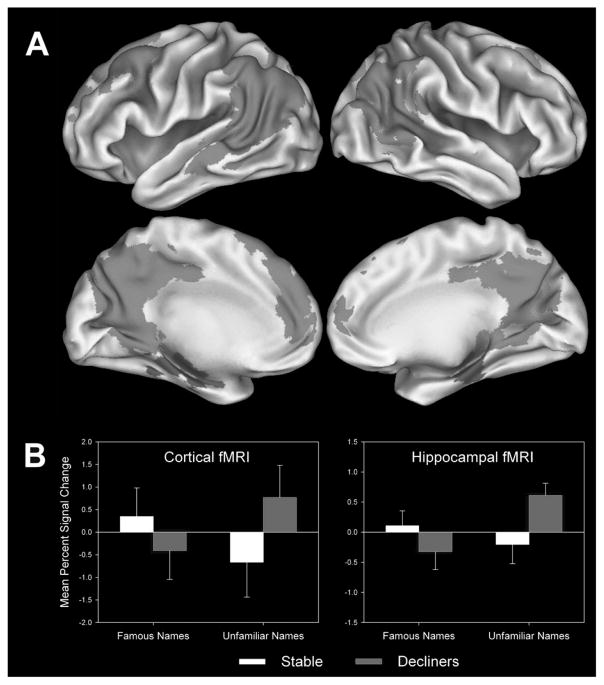
Figure 3A represents the results of the voxelwise analysis performed on the entire sample. This analysis, restricted to the famous > unfamiliar name comparison, yielded eight fROIs (Table 2). A PCA was conducted on the average AUCs of these fROIs, yielding two components accounting for 73% of total variance (Table 2). Five fROIs loaded significantly on a “Cortical” component, shown in green in Fig. 3A, whereas two regions loaded on the “Hippocampal” component (purple regions in Fig. 3A). The right cerebellum did not demonstrate significant loadings [80] on either component and was dropped from the analysis. The unfamiliar > famous name comparison resulted in four fROIs and a single PCA component accounting for 63.3% of the total variance. This component did not predict cognitive decline and is not discussed further.
Fig. 3.
A) Regions comprising the Cortical (green) and Hippocampal (purple) fMRI activation principal components for the Famous > Unfamiliar names comparison. B) Cortical and Hippocampal fMRI signals (areas under the curve) contrasting famous name recognition versus fixation and unfamiliar name identification versus fixation for cognitively stable and declining participants. (Colours are visible in the electronic version of the article at www.iospress.nl.)
Table 2.
Activation foci for famous versus unfamiliar name subtraction (Famous > Unfamiliar)*
| Region # | Region | Cortical Component Loadings | Hippocampal Component Loadings | x | y | z | Stable Group Volume | Declining Group Volume |
|---|---|---|---|---|---|---|---|---|
| 1 | Bilateral Posterior Cingulate Cortex, Precuneus | 0.884 | 0.257 | −0.9 | −52.4 | 24.6 | 37.05 | |
| 2 | Left Angular Gyrus | 0.889 | 0.266 | −45.4 | −55.9 | 23.7 | 26.14 | 1.44 |
| 3 | Left Superior Frontal Gyrus | 0.805 | 0.018 | −17.8 | 29.5 | 41 | 30.84 | |
| 4 | Right Angular Gyrus | 0.815 | 0.275 | 46.2 | −59.6 | 27.1 | 18.68 | |
| 6 | Right Superior, Middle Frontal Gyrus | 0.839 | 0.009 | 22.5 | 16.5 | 46.8 | 3.2 | |
| 5 | Left Parahippocampal Gyrus, Hippocampus | 0.269 | 0.898 | −21.9 | −21 | −10.7 | 2.29 | |
| 7 | Right Parahippocampal Gyrus, Hippocampus | 0.053 | 0.946 | 23.9 | −23.2 | −11.6 | 0.86 | |
| 8 | Right Cerebellum (Crus 1) | 0.425 | 0.268 | 11.3 | −74.8 | −21.9 | 1.03 |
Note: Critical value (2*rcrit,p = .01) used to identify significant component loadings was 0.560 [80].
PCA conducted on four negative activation (Unfamiliar > Familiar) clusters (Left Precentral Gyrus; Bilateral Supplementary Motor Area; Right Insula; Left Middle Occipital Gyrus) revealed one component that did not predict decline; this component was dropped from subsequent analyses.
Figure 3B presents a graph of the fMRI signal response to famous and unfamiliar names compared to fixation (rather than just the comparison of these conditions) to address the question of whether the effect is driven primarily by activation to famous names or the response to novel names. For cognitively stable participants, both the Cortical and Hippocampal fMRI signal demonstrated positive changes in the AUC in response to famous names and a decreased AUC in response to unfamiliar names. In contrast, the cognitively declining participants showed the opposite pattern, with greater AUC in response to unfamiliar names and reduced AUC when presented with unfamiliar names. Using a mixed-design ANOVA that tested the effects of group (stable vs. declining) and stimulus type (famous vs. unfamiliar), significant group by stimulus type interactions were observed for the Cortical (F(1,76) = 8.88, p < 0.004) and Hippocampal (F(1,76) = 8.11, p = 0.006) fMRI components.
Logistic regression analyses
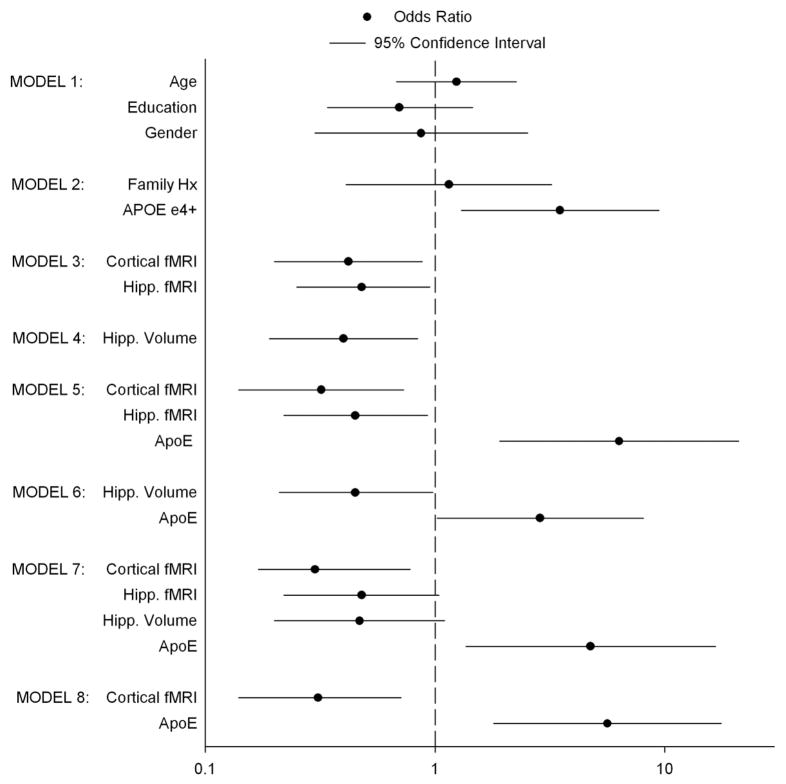
Seven logistic regression models were evaluated. For each model, bootstrap-corrected R2 and C values are presented in Table 3. For each predictor within a model, coefficients, standard errors, and significance levels are shown in Table 3, and odds ratios with 95% confidence intervals are presented in Fig. 4. Models 1 and 2 indicate that age, education, gender and family history of AD were not significant predictors of future cognitive decline. For Models 2–7, APOE status, cortical and hippocampal fMRI activation, and hippocampal volume each contributed significantly to the prediction of cognitive decline. Although Model 7 demonstrates the largest R2 (0.293) and C index (0.789), only two of the four predictors were statistically significant (cortical fMRI activation and APOE status), whereas the remaining two predictors (hippocampal fMRI and hippocampal volume) were not. Model 5 (R2 = 0.285; C = 0.787) was the second best model, with APOE genotype and both cortical and hippocampal fMRI activation each contributing significantly to the prediction of future cognitive decline.
Table 3.
Results of logistic regressions
| Likelihood Ratio | Adequacy Index | R2 | C Index | Variables | Coeff | SE | p | |
|---|---|---|---|---|---|---|---|---|
| Model 1 | 1.69 | - - - | 0 | 0.585 | Age | 0.036 | 0.051 | 0.644 |
| Education | −0.088 | 0.093 | 0.347 | |||||
| Gender | −0.142 | 0.546 | 0.795 | |||||
| Model 2 | 6.33 | 0.252 | 0.063 | 0.615 | Family Hx | 0.143 | 0.524 | 0.785 |
| ApoE | 1.207 | 0.533 | 0.014 | |||||
| Model 3 | 11.53 | 0.46 | 0.155 | 0.713 | Cortical fMRI | −0.67 | 0.293 | 0.022 |
| Hipp. fMRI | −0.642 | 0.302 | 0.034 | |||||
| Model 4 | 6.76 | 0.27 | 0.098 | 0.687 | Hipp. Volume | −1.16 | 0.48 | 0.016 |
| Model 5 | 21.77 | 0.869 | 0.285 | 0.787 | Cortical fMRI | −0.874 | 0.309 | 0.013 |
| Hipp. fMRI | −0.699 | 0.323 | 0.031 | |||||
| ApoE | 1.846 | 0.612 | 0.003 | |||||
| Model 6 | 10.8 | 0.431 | 0.132 | 0.702 | Hipp. Volume | −0.991 | 0.49 | 0.043 |
| ApoE | 1.056 | 0.528 | 0.045 | |||||
| Model 7 | 25.06 | 1 | 0.293 | 0.789 | Cortical fMRI | −0.929 | 0.336 | 0.007 |
| Hipp. fMRI | −0.644 | 0.348 | 0.064 | |||||
| Hipp. Volume | −0.945 | 0.545 | 0.083 | |||||
| ApoE | 1.558 | 0.64 | 0.015 |
Note: Adequacy index reflects the total explanatory power of a subset of predictors relative to a model containing the total set of predictors (Model 7) using the ratio of the likelihood ratio of the model of interest to the likelihood ratio of the model containing the total set of predictors.
Fig. 4.
Odds ratios and 95% confidence intervals for seven logistic regression models. Odds ratios whose 95% confidence intervals overlap with 1.0 (represented by vertical dashed line) are not statistically significant. Odds ratios > 1 indicate greater probability of decline with increasing value of predictor; odds ratios < 1 indicate reduced probability with increasing predictor values.
The Adequacy Index [77] is a recommended way of comparing the adequacy of a set of predictors across models. It is unitless and is represented by ratio of the −2 log likelihood statistic for testing a subset of predictors for the model of interest to the −2 log likelihood ratio statistic for testing the joint significance of the full set of predictors. It ranges between 0 (no predictive information for the subset of predictors) to 1 (complete predictive information for the subset of predictors). Using the full set of predictors in Model 7, the Adequacy Indexes for Models 2–6 are presented in Table 3. Model 1 was not included as there was no significant predictor of decline using demographic variables. The fMRI measures alone (Model 3) account for 46% of the total explanatory power for the set of variables, compared to hippocampal volume alone (Model 4), which accounts for only 27% of the total explanatory power. Perhaps more dramatically, Model 5, which uses the fMRI measures plus APOE genotype status accounts for 87% of the explanatory power compared to Model 6 (hippocampal volume plus APOE genotype status), which accounts for only 43% of the explanatory power.
DISCUSSION
Clinical trials involving pharmacological and lifestyle (exercise, cognitive enrichment, diet) interventions are being considered to prevent or delay the onset of AD, even before symptoms emerge. For clinical trials to be maximally successful, enrichment of the sample with elders at the greatest risk for experiencing cognitive decline over the course of a typical clinical trial (2–3 years) is essential. Results of our prospective study indicate that combining genetic risk and MRI biomarkers can effectively identify such individuals, even after a relatively brief 18-month retest interval. Specifically, we were able to correctly order 78.9% of possible pairs of stable and declining participants using a combination of APOE genotype, cortical and hippocampal fMRI, and hippocampal volumes. APOE genotype and fMRI (cortical and hippocampal) predictors alone correctly ordered 78.7% of possible pairs. In contrast, hippocampal volume, alone or combined with APOE status, correctly ordered only 68.7% and 70.2% of pairs, respectively. Without the benefit of imaging data, family history of dementia and APOE status correctly ordered only 61.5% of possible pairs (chance prediction = 50%). Overall, our findings suggest that the combination of fMRI and APOE genotype status holds promise for successfully screening at-risk, but asymptomatic, participants for prevention trials.
Our results would appear to be at odds with a similar prospective fMRI study [48], in which increased brain activation was associated with lower scores on episodic memory tasks after a two year retest interval. It is important to note two important methodological differences between the two studies. First, the number of participants who underwent follow-up neuropsychological testing in the earlier study (n = 14) was considerably smaller than those in the current study (n = 78). Second, the previous study used an effortful episodic learning and recall task and did not report task performance during fMRI scanning. It is conceivable that declining participants performed more poorly at baseline on the fMRI task than those who were stable over the retest interval. Such differences in task performance, if present, could have a meaningful impact on the pattern of brain activation, especially since error trials could not be eliminated from the blocked design trial format used in the previous fMRI study. In contrast, the current event-related study used a low effort, high accuracy (> 90% correct) semantic memory task in which the few error trials that did occur were excluded from the final image analyses.
In a previous study [55], we reported greater semantic memory activation in cognitively intact, APOE ε4 carriers relative to non-carriers. As in the current study, we defined semantic memory activation by a greater BOLD response to famous than unfamiliar name stimuli. Based solely on the cross-sectional results reported in our previous study, one might predict that greater semantic activation would be a predictor of future cognitive decline. However, in our prior study, we did not segregate declining from stable participants within each of the two risk groups. The current longitudinal results suggest that having increased semantic memory activation may paradoxically afford a protective effect against future cognitive decline in both high and low risk individuals. This effect is illustrated in Fig. 5. The 12 APOE ε4 carriers in the stable group demonstrated greater cortical activation in response to familiar than unfamiliar names; in contrast, the 14 declining APOE ε4 carriers exhibited greater activation to unfamiliar than to familiar names. Among the non-carriers, a similar pattern was observed, albeit with less overall semantic memory activation for the group as a whole. Among the 39 stable non-carriers, the degree of cortical activation was comparable for famous and unfamiliar names, whereas the 13 declining non-carriers demonstrated greater activation for unfamiliar than famous names.
Fig. 5.
Percent MR signal intensity (± SEM) for stable and declining APOE ε4 carriers (ε4+) and non-carriers (ε4−). Positive values reflect greater BOLD response aggregated across activated cortical regions in response to famous relative to unfamiliar names; negative values reflect greater BOLD response to unfamiliar relative to familiar names.
Our finding that increased baseline fMRI activation is protective against future cognitive decline in cognitively intact elders is consistent with prior studies reporting increased task-related BOLD signal in parietal cortex in cognitively stable participants after five years [50,81]. Increased activation may reflect greater cognitive reserve in asymptomatic persons, particularly in regions subserved by the cholinergic system. Increased brain activation in these regions has been observed following administration of cholinesterase inhibitors in MCI and AD patients [82–87]. We speculate that improved cognitive reserve, possibly manifested by increased neuronal firing rate or recruitment of additional supportive neuronal regions, permits continued functioning at a higher level in the face of early neurodegenerative changes. Persons who have lost this propensity for functional compensation are at increased risk of future cognitive deterioration.
Our famous name recognition task activates brain regions (posterior cingulate gyrus, posterior inferior parietal cortex, middle temporal gyrus, fusiform and parahippocampal gyri, hippocampus, and medial superior frontal gyrus) commonly associated with the “default mode network” (DMN) [88,89]. The DMN is frequently correlated with uncontrolled semantic processing resulting from task-unrelated thoughts that occur during resting scan conditions. Prior work by Binder and colleagues [90,91] has demonstrated considerable overlap between brain systems associated with the resting state DMN and those activated by controlled semantic memory processing tasks. Recent studies [92–94] have suggested that disruption of the DMN can occur in early AD. Not surprisingly, our results have shown that participants who have experienced cognitive decline after 18 months also demonstrate reduced baseline semantic memory activation in cortical regions that overlap with the DMN (see Fig. 5). Moreover, the AUCs corresponding to the fMRI signal in both cortical and hippocampal regions were reduced in response to famous names and increased in response to unfamiliar names for cognitively declining participants (Fig. 3B). Cognitively stable participants showed the opposite pattern. Future studies are required to determine the relative sensitivity of baseline measurements of the resting state DMN versus task-activated semantic memory processes in predicting future cognitive decline among asymptomatic persons.
Baseline hippocampal volume, corrected for intracranial volume, significantly predicted future cognitive decline, both alone and combined with APOE genotype. However, its predictive accuracy was not as strong as the combination of fMRI and APOE genotype. Considerable inter-individual variability in hippocampal volumes occurs in cross-sectional studies of cognitively intact elders, and hippocampal volume may sometimes be inversely related to cognitive abilities [95]. A recent meta-analysis concluded that the relationship between hippocampal size and episodic memory performance across the lifespan was weak [96]. While rate of hippocampal atrophy has provided more compelling evidence of a relationship with cognitive decline in healthy older adults [97–99], the requirement of two measurement periods separated by up to two or more years makes this biomarker impractical for widespread use for enriching prevention trials.
This study adds to the growing body of literature showing that combinations of biomarkers show greater predictive accuracy compared to individual biomarkers [16,100]. A stepwise combination of biomarkers might be considered for balancing invasiveness, cost-containment, and predictive accuracy when used in the context of identifying at-risk, but otherwise healthy, participants for prevention trials. For instance, a prevention trial screening process might include APOE genotyping as a first step, followed by task-related fM-RI activation performed in APOE ε4 carriers. More invasive tests, such as CSF biomarkers and PET imaging (FDG or amyloid), could then be administered as further selection criteria for enriching study samples. However, because we did not perform these additional tests, we cannot state conclusively whether these procedures provide incremental predictive accuracy beyond the combination of APOE genotyping and task-activated fMRI.
It is important to acknowledge other limitations of this study. Our neuropsychological battery focused on cognitive abilities most likely to be affected in early AD and may have missed significant changes in other cognitive domains among our stable participants. Our study also defined cognitive decline based on change in neuropsychological test scores rather than on a change in diagnostic category, i.e., conversion to MCI or early AD. In our opinion, the rate of conversion from intact cognition to MCI/AD is too low to be used as a meaningful outcome variable in prevention trials. Nevertheless, the extent to which increased baseline fM-RI activation is specific to predicting early AD-related changes or more general age-related cognitive decline will await long-term follow-up studies. Finally, despite the fact that all participants performed within normal limits at baseline on all cognitive measures, baseline neuropsychological performance demonstrated non-significant trends for lower performance in the declining group on the two RAVLT measures and on the MMSE. Thus, it is conceivable that several participants in the declining group were actively undergoing cognitive decline. However, our outcome measure was based on the degree of cognitive decline from baseline performance (1 SD or more) rather than absolute levels of performance. Furthermore, our regression-based approach to defining cognitive change controlled each participant’s follow-up level of performance for his or her baseline level of performance. Small baseline differences would be unlikely to account for the dramatic cognitive change in the declining group relative to the stable group as depicted in Fig. 1.
In summary, our study provides evidence of the ability of task-related fMRI, in combination with APOE genotype, to predict future cognitive change in healthy older adults. This combination of static genetic propensity to develop AD and an fMRI approach that measures brain activity during a low-effort, high accuracy task, can be valuable for enriching a prevention trial with healthy persons at high risk of impending cognitive decline. Biomarker combinations tapping different aspects of pathological changes associated with AD that are widely available, easily implemented, minimally invasive, and relatively inexpensive will likely assume increasing importance in future clinical trials designed to prevent or slow AD progression.
Acknowledgments
This study was supported by grants from the National Institutes on Aging (AG022304), Medical College of Wisconsin General Clinical Research Center (RR00058), and the W.M. Keck Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging or the National Institutes of Health.
Footnotes
Authors’ disclosures available online (http://www.j-alz.com/disclosures/view.php?id=431).
References
- 1.Kok E, Haikonen S, Luoto T, Huhtala H, Goebeler S, Haapasalo H, Karhunen PJ. Apolipoprotein E-dependent accumulation of Alzheimer disease-related lesions begins in middle age. Ann Neurol. 2009;65:650–657. doi: 10.1002/ana.21696. [DOI] [PubMed] [Google Scholar]
- 2.Albert MS, Moss MB, Tanzi R, Jones K. Preclinical prediction of AD using neuropsychological tests. J Int Neuropsychol Soc. 2001;7:631–639. doi: 10.1017/s1355617701755105. [DOI] [PubMed] [Google Scholar]
- 3.DeCarli C, Mungas D, Harvey D, Reed B, Weiner M, Chui H, Jagust W. Memory impairment, but not cerebrovascular disease, predicts progression of MCI to dementia. Neurology. 2004;63:220–227. doi: 10.1212/01.wnl.0000130531.90205.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Jager CA, Hogervorst E, Combrinck M, Budge MM. Sensitivity and specificity of neuropsychological tests for mild cognitive impairment, vascular cognitive impairment and Alzheimer’s disease. Psychol Med. 2003;33:1039–1050. doi: 10.1017/s0033291703008031. [DOI] [PubMed] [Google Scholar]
- 5.Nestor PJ, Scheltens P, Hodges JR. Advances in the early detection of Alzheimer’s disease. Nat Med. 2004;10(Suppl):S34–41. doi: 10.1038/nrn1433. [DOI] [PubMed] [Google Scholar]
- 6.de Leon MJ, George AE, Stylopoulos LA, Smith G, Miller DC. Early marker for Alzheimer’s disease: the atrophic hippocampus. Lancet. 1989;2:672–673. doi: 10.1016/s0140-6736(89)90911-2. [DOI] [PubMed] [Google Scholar]
- 7.Jack CR, Petersen RC, Xu YC, O’Brien PC, Smith GE, Ivnik RJ, et al. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52:1397–1403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wolf H, Jelic V, Gertz HJ, Nordberg A, Julin P, Wahlund LO. A critical discussion of the role of neuroimaging in mild cognitive impairment. Acta Neurol Scand Suppl. 2003;179:52–76. doi: 10.1034/j.1600-0404.107.s179.10.x. [DOI] [PubMed] [Google Scholar]
- 9.Henneman WJ, Sluimer JD, Barnes J, van der Flier WM, Sluimer IC, Fox NC, Scheltens P, Vrenken H, Barkhof F. Hippocampal atrophy rates in Alzheimer disease: added value over whole brain volume measures. Neurology. 2009;72:999–1007. doi: 10.1212/01.wnl.0000344568.09360.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morra JH, Tu Z, Apostolova LG, Green AE, Avedissian C, Madsen SK, Parikshak N, Hua X, Toga AW, Jack CR, Jr, Schuff N, Weiner MW, Thompson PM. Automated 3D mapping of hippocampal atrophy and its clinical correlates in 400 subjects with Alzheimer’s disease, mild cognitive impairment, and elderly controls. Hum Brain Mapp. 2009;30:2766–2788. doi: 10.1002/hbm.20708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stoub TR, Rogalski EJ, Leurgans S, Bennett DA, Detoledo-Morrell L. Rate of entorhinal and hippocampal atrophy in incipient and mild AD: Relation to memory function. Neurobiol Aging. 2010;31:1089–1098. doi: 10.1016/j.neurobiolaging.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cardenas VA, Du AT, Hardin D, Ezekiel F, Weber P, Jagust WJ, Chui HC, Schuff N, Weiner MW. Comparison of methods for measuring longitudinal brain change in cognitive impairment and dementia. Neurobiol Aging. 2003;24:537–544. doi: 10.1016/s0197-4580(02)00130-6. [DOI] [PubMed] [Google Scholar]
- 13.Devanand DP, Pradhaban G, Liu X, Khandji A, De Santi S, Segal S, Rusinek H, Pelton GH, Honig LS, Mayeux R, Stern Y, Tabert MH, de Leon MJ. Hippocampal and entorhinal atrophy in mild cognitive impairment: prediction of Alzheimer disease. Neurology. 2007;68:828–836. doi: 10.1212/01.wnl.0000256697.20968.d7. [DOI] [PubMed] [Google Scholar]
- 14.Juottonen K, Lehtovirta M, Helisalmi S, Riekkinen PJ, Sr, Soininen H. Major decrease in the volume of the entorhinal cortex in patients with Alzheimer’s disease carrying the apolipoprotein E epsilon4 allele. J Neurol Neurosurg Psychiatry. 1998;65:322–327. doi: 10.1136/jnnp.65.3.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brys M, Pirraglia E, Rich K, Rolstad S, Mosconi L, Switalski R, Glodzik-Sobanska L, De Santi S, Zinkowski R, Mehta P, Pratico D, Saint Louis LA, Wallin A, Blennow K, de Leon MJ. Prediction and longitudinal study of CSF biomarkers in mild cognitive impairment. Neurobiol Aging. 2009;30:682–690. doi: 10.1016/j.neurobiolaging.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Leon MJ, DeSanti S, Zinkowski R, Mehta PD, Pratico D, Segal S, Rusinek H, Li J, Tsui W, Saint Louis LA, Clark CM, Tarshish C, Li Y, Lair L, Javier E, Rich K, Lesbre P, Mosconi L, Reisberg B, Sadowski M, DeBernadis JF, Kerkman DJ, Hampel H, Wahlund LO, Davies P. Longitudinal CSF and MRI biomarkers improve the diagnosis of mild cognitive impairment. Neurobiol Aging. 2006;27:394–401. doi: 10.1016/j.neurobiolaging.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 17.de Leon MJ, Mosconi L, Li J, De Santi S, Yao Y, Tsui WH, Pirraglia E, Rich K, Javier E, Brys M, Glodzik L, Switalski R, Saint Louis LA, Pratico D. Longitudinal CSF isoprostane and MRI atrophy in the progression to AD. J Neurol. 2007;254:1666–1675. doi: 10.1007/s00415-007-0610-z. [DOI] [PubMed] [Google Scholar]
- 18.Buerger K, Teipel SJ, Zinkowski R, Blennow K, Arai H, Engel R, Hofmann-Kiefer K, McCulloch C, Ptok U, Heun R, Andreasen N, DeBernardis J, Kerkman D, Moeller H, Davies P, Hampel H. CSF tau protein phosphorylated at threonine 231 correlates with cognitive decline in MCI subjects. Neurology. 2002;59:627–629. doi: 10.1212/wnl.59.4.627. [DOI] [PubMed] [Google Scholar]
- 19.Buerger K, Zinkowski R, Teipel SJ, Tapiola T, Arai H, Blennow K, Andreasen N, Hofmann-Kiefer K, DeBernardis J, Kerkman D, McCulloch C, Kohnken R, Padberg F, Pirttila T, Schapiro MB, Rapoport SI, Moller HJ, Davies P, Hampel H. Differential diagnosis of Alzheimer disease with cerebrospinal fluid levels of tau protein phosphorylated at threonine 231. Arch Neurol. 2002;59:1267–1272. doi: 10.1001/archneur.59.8.1267. [DOI] [PubMed] [Google Scholar]
- 20.Hampel H, Buerger K, Zinkowski R, Teipel SJ, Goernitz A, Andreasen N, Sjoegren M, DeBernardis J, Kerkman D, Ishiguro K, Ohno H, Vanmechelen E, Vanderstichele H, Mc-Culloch C, Moller HJ, Davies P, Blennow K. Measurement of phosphorylated tau epitopes in the differential diagnosis of Alzheimer disease: a comparative cerebrospinal fluid study. Arch Gen Psychiatry. 2004;61:95–102. doi: 10.1001/archpsyc.61.1.95. [DOI] [PubMed] [Google Scholar]
- 21.Blennow K, Hampel H. CSF markers for incipient Alzheimer’s disease. Lancet Neurol. 2003;2:605–613. doi: 10.1016/s1474-4422(03)00530-1. [DOI] [PubMed] [Google Scholar]
- 22.Hampel H, Teipel SJ, Fuchsberger T, Andreasen N, Wiltfang J, Otto M, Shen Y, Dodel R, Du Y, Farlow M, Moller HJ, Blennow K, Buerger K. Value of CSF beta-amyloid1–42 and tau as predictors of Alzheimer’s disease in patients with mild cognitive impairment. Mol Psychiatry. 2004;9:705–710. doi: 10.1038/sj.mp.4001473. [DOI] [PubMed] [Google Scholar]
- 23.Hansson O, Zetterberg H, Buchhave P, Londos E, Blennow K, Minthon L. Association between CSF biomarkers and incipient Alzheimer’s disease in patients with mild cognitive impairment: a follow-up study. Lancet Neurol. 2006;5:228–234. doi: 10.1016/S1474-4422(06)70355-6. [DOI] [PubMed] [Google Scholar]
- 24.Chetelat G, Desgranges B, De La Sayette V, Viader F, Eustache F, Baron JC. Mild cognitive impairment: Can FDG-PET predict who is to rapidly convert to Alzheimer’s disease? Neurology. 2003;60:1374–1377. doi: 10.1212/01.wnl.0000055847.17752.e6. [DOI] [PubMed] [Google Scholar]
- 25.Chetelat G, Eustache F, Viader F, De la Sayette V, Pelerin A, Mezenge F, Hannequin D, Dupuy B, Baron JD, Desgranges B. FDG-PET measurement is more accurate than neuropsychological assessments to predict global cognitive deterioration in patients with mild cognitive impairment. Neurocase. 2005;11:14–25. doi: 10.1080/13554790490896938. [DOI] [PubMed] [Google Scholar]
- 26.Rowe CC, Ng S, Ackermann U, Gong SJ, Pike K, Savage G, Cowie TF, Dickinson KL, Maruff P, Darby D, Smith C, Woodward M, Merory J, Tochon-Danguy H, O’Keefe G, Klunk WE, Mathis CA, Price JC, Masters CL, Villemagne VL. Imaging beta-amyloid burden in aging and dementia. Neurology. 2007;68:1718–1725. doi: 10.1212/01.wnl.0000261919.22630.ea. [DOI] [PubMed] [Google Scholar]
- 27.Wolk DA, Klunk W. Update on amyloid imaging: from healthy aging to Alzheimer’s disease. Curr Neurol Neurosci Rep. 2009;9:345–352. doi: 10.1007/s11910-009-0051-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolk DA, Price JC, Saxton JA, Snitz BE, James JA, Lopez OL, Aizenstein HJ, Cohen AD, Weissfeld LA, Mathis CA, Klunk WE, De-Kosky ST. Amyloid imaging in mild cognitive impairment subtypes. Ann Neurol. 2009;65:557–568. doi: 10.1002/ana.21598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller SL, Fenstermacher E, Bates J, Blacker D, Sperling RA, Dickerson BC. Hippocampal activation in adults with mild cognitive impairment predicts subsequent cognitive decline. J Neurol Neurosurg Psychiatry. 2008;79:630–635. doi: 10.1136/jnnp.2007.124149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vannini P, Almkvist O, Dierks T, Lehmann C, Wahlund LO. Reduced neuronal efficacy in progressive mild cognitive impairment: a prospective fMRI study on visuospatial processing. Psychiatry Res. 2007;156:43–57. doi: 10.1016/j.pscychresns.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 31.Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium [see comments] JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]
- 32.Yip AG, McKee AC, Green RC, Wells J, Young H, Cupples LA, Farrer LA. APOE, vascular pathology, and the AD brain. Neurology. 2005;65:259–265. doi: 10.1212/01.wnl.0000168863.49053.4d. [DOI] [PubMed] [Google Scholar]
- 33.Caselli RJ, Reiman EM, Locke DE, Hutton ML, Hentz JG, Hoffman-Snyder C, Woodruff BK, Alexander GE, Osborne D. Cognitive domain decline in healthy apolipoprotein E epsilon4 homozygotes before the diagnosis of mild cognitive impairment. Arch Neurol. 2007;64:1306–1311. doi: 10.1001/archneur.64.9.1306. [DOI] [PubMed] [Google Scholar]
- 34.de Leon MJ, Convit A, Wolf OT, Tarshish CY, DeSanti S, Rusinek H, Tsui W, Kandil E, Scherer AJ, Roche A, Imossi A, Thorn E, Bobinski M, Caraos C, Lesbre P, Schlyer D, Poirier J, Reisberg B, Fowler J. Prediction of cognitive decline in normal elderly subjects with 2-[(18)F]fluoro-2-deoxy-D-glucose/positron-emission tomography (FDG/PET) Proc Natl Acad Sci U S A. 2001;98:10966–10971. doi: 10.1073/pnas.191044198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Swan GE, Lessov-Schlaggar CN, Carmelli D, Schellenberg GD, La Rue A. Apolipoprotein E epsilon4 and change in cognitive functioning in community-dwelling older adults. J Geriatr Psychiatry Neurol. 2005;18:196–201. doi: 10.1177/0891988705281864. [DOI] [PubMed] [Google Scholar]
- 36.Saunders AM, Strittmatter WJ, Schmechel D, St George-Hyslop PH, Pericak-Vance MA, Joo SH, Rosi BL, Gusella JF, Crapper-MacLachlan DR, Alberts MJ, Hulette C, Crain B, Goldgaber D, Roses AD. Association of apolipoprotein E allele e4 with late-onset familial and sporadic Alzheimer’s disease. Neurology. 1993;43:1467–1472. doi: 10.1212/wnl.43.8.1467. [DOI] [PubMed] [Google Scholar]
- 37.Traykov L, Bayle AC, Latour F, Lenoir H, Seux ML, Hanon O, Pequignot R, Bert P, Moulin F, Cantegreil I, Wenisch E, Batouche F, Mehrabian S, Rotrou J, Rigaud AS. Apolipoprotein E epsilon4 allele frequency in elderly depressed patients with and without cerebrovascular disease. J Neurol Sci. 2007;257:280–283. doi: 10.1016/j.jns.2007.01.038. [DOI] [PubMed] [Google Scholar]
- 38.Breitner JC. APOE genotyping and Alzheimer’s disease. Lancet. 1996;347:1184–1185. doi: 10.1016/s0140-6736(96)90642-x. [DOI] [PubMed] [Google Scholar]
- 39.Devanand DP, Pelton GH, Zamora D, Liu X, Tabert MH, Goodkind M, Scarmeas N, Braun I, Stern Y, Mayeux R. Predictive utility of apolipoprotein E genotype for Alzheimer disease in outpatients with mild cognitive impairment. Arch Neurol. 2005;62:975–980. doi: 10.1001/archneur.62.6.975. [DOI] [PubMed] [Google Scholar]
- 40.Slooter AJ, Breteler MB, Ott A, Van Broeckhoven C, van Duijn CM. APOE genotyping in differential diagnosis of Alzheimer’s disease. Lancet. 1996;348:334. doi: 10.1016/s0140-6736(05)64501-1. [DOI] [PubMed] [Google Scholar]
- 41.Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. Cerebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol. 2007;64:343–349. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- 42.Cardenas VA, Chao LL, Studholme C, Yaffe K, Miller BL, Madison C, Buckley ST, Mungas D, Schuff N, Weiner MW. Brain atrophy associated with baseline and longitudinal measures of cognition. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.04.011. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.den Heijer T, Geerlings MI, Hoebeek FE, Hofman A, Koudstaal PJ, Breteler MM. Use of hippocampal and amygdalar volumes on magnetic resonance imaging to predict dementia in cognitively intact elderly people. Arch Gen Psychiatry. 2006;63:57–62. doi: 10.1001/archpsyc.63.1.57. [DOI] [PubMed] [Google Scholar]
- 44.Gluck MA, Myers CE, Nicolle MM, Johnson S. Computational models of the hippocampal region: implications for prediction of risk for Alzheimer’s disease in non-demented elderly. Curr Alzheimer Res. 2006;3:247–257. doi: 10.2174/156720506777632826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Golomb J, Kluger A, de Leon MJ, Ferris SH, Mittelman M, Cohen J, George AE. Hippocampal formation size predicts declining memory performance in normal aging. Neurology. 1996;47:810–813. doi: 10.1212/wnl.47.3.810. [DOI] [PubMed] [Google Scholar]
- 46.Marquis S, Moore MM, Howieson DB, Sexton G, Payami H, Kaye JA, Camicioli R. Independent predictors of cognitive decline in healthy elderly persons. Arch Neurol. 2002;59:601–606. doi: 10.1001/archneur.59.4.601. [DOI] [PubMed] [Google Scholar]
- 47.Mungas D, Harvey D, Reed BR, Jagust WJ, DeCarli C, Beckett L, Mack WJ, Kramer JH, Weiner MW, Schuff N, Chui HC. Longitudinal volumetric MRI change and rate of cognitive decline. Neurology. 2005;65:565–571. doi: 10.1212/01.wnl.0000172913.88973.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, Small GW. Patterns of brain activation in people at risk for Alzheimer’s Disease. N Engl J Med. 2000;343:450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smith CD, Kryscio RJ, Schmitt FA, Lovell MA, Blonder LX, Rayens WS, Andersen AH. Longitudinal functional alterations in asymptomatic women at risk for Alzheimer’s disease. J Neuroimaging. 2005;15:271–277. doi: 10.1177/1051228405277340. [DOI] [PubMed] [Google Scholar]
- 50.Lind J, Persson J, Ingvar M, Larsson A, Cruts M, Van Broeckhoven C, Adolfsson R, Backman L, Nilsson LG, Petersson KM, Nyberg L. Reduced functional brain activity response in cognitively intact apolipoprotein E epsilon4 carriers. Brain. 2006;129:1240–1248. doi: 10.1093/brain/awl054. [DOI] [PubMed] [Google Scholar]
- 51.Nielson KA, Douville KL, Seidenberg M, Woodard JL, Miller SK, Franczak M, Antuono P, Rao SM. Age-related functional recruitment for famous name recognition: an event-related fMRI study. Neurobiol Aging. 2006;27:1494–1504. doi: 10.1016/j.neurobiolaging.2005.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Woodard JL, Seidenberg M, Nielson KA, Miller SK, Franczak M, Antuono P, Douville KL, Rao SM. Temporally graded activation of neocortical regions in response to memories of different ages. J Cogn Neurosci. 2007;19:1113–1124. doi: 10.1162/jocn.2007.19.7.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Douville K, Woodard JL, Seidenberg M, Miller SK, Leveroni CL, Nielson KA, Franczak M, Antuono P, Rao SM. Medial temporal lobe activity for recognition of recent and remote famous names: an event-related fMRI study. Neuropsychologia. 2005;43:693–703. doi: 10.1016/j.neuropsychologia.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 54.Woodard JL, Seidenberg M, Nielson KA, Antuono P, Guidotti L, Durgerian S, Zhang Q, Lancaster M, Hantke N, Butts A, Rao SM. Semantic memory activation in amnestic mild cognitive impairment. Brain. 2009;132:2068–2078. doi: 10.1093/brain/awp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Seidenberg M, Guidotti L, Nielson KA, Woodard JL, Durgerian S, Antuono P, Zhang Q, Rao SM. Semantic memory activation in individuals at risk for developing Alzheimer disease. Neurology. 2009;73:612–620. doi: 10.1212/WNL.0b013e3181b389ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Johnson SC, Schmitz TW, Trivedi MA, Ries ML, Torgerson BM, Carlsson CM, Asthana S, Hermann BP, Sager MA. The influence of Alzheimer disease family history and apolipoprotein E epsilon4 on mesial temporal lobe activation. J Neurosci. 2006;26:6069–6076. doi: 10.1523/JNEUROSCI.0959-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sager MA, Hermann B, La Rue A. Middle-aged children of persons with Alzheimer’s disease: APOE genotypes and cognitive function in the Wisconsin Registry for Alzheimer’s Prevention. J Geriatr Psychiatry Neurol. 2005;18:245–249. doi: 10.1177/0891988705281882. [DOI] [PubMed] [Google Scholar]
- 58.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State”: A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 59.Jurica PJ, Leitten CL, Mattis S. Dementia Rating Scale-2 professional manual. Psychological Assessment Resources; Lutz, FL: 2001. [Google Scholar]
- 60.Mattis S. Dementia Rating Scale professional manual. Psychological Assessment Resources; Odessa, Florida: 1988. [Google Scholar]
- 61.Rey A. L’examen clinique en psychologie. Presses Universitaires; de France, Paris: 1958. [Google Scholar]
- 62.Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO. Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res. 1983;17:37–49. doi: 10.1016/0022-3956(82)90033-4. [DOI] [PubMed] [Google Scholar]
- 63.Lawton MP, Brody EM. Assessment of older people: Self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 64.Schmidt KS. DRS-2: Alternate form professional manual. Psychological Assessment Resources; Lutz, FL: 2004. [Google Scholar]
- 65.Schmidt KS, Mattis PJ, Adams J, Nestor P. Alternate-form reliability of the Dementia Rating Scale-2. Arch Clin Neuropsychol. 2005;20:435–441. doi: 10.1016/j.acn.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 66.Schmidt M. Rey Auditory and Verbal Learning Test: A handbook. Western Psychological Services; Los Angeles: 1996. [Google Scholar]
- 67.Saunders AM, Hulette O, Welsh-Bohmer KA, Schmechel DE, Crain B, Burke JR, Alberts MJ, Strittmatter WJ, Breitner JC, Rosenberg C. Specificity, sensitivity, and predictive value of apolipoprotein-E genotyping for sporadic Alzheimer’s disease. Lancet. 1996;348:90–93. doi: 10.1016/s0140-6736(96)01251-2. [DOI] [PubMed] [Google Scholar]
- 68.Frerichs RJ, Tuokko HA. A comparison of methods for measuring cognitive change in older adults. Arch Clin Neuropsychol. 2005;20:321–333. doi: 10.1016/j.acn.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 69.McSweeny AJ, Naugle RI, Chelune GJ, Luders H. “T scores for change”: An illustration of a regression approach to depicting change in clinical neuropsychology. Clin Neuropsychol. 1993;7:300–312. [Google Scholar]
- 70.Temkin NR, Heaton RK, Grant I, Dikmen SS. Detecting significant change in neuropsychological test performance: a comparison of four models. J Int Neuropsychol Soc. 1999;5:357–369. doi: 10.1017/s1355617799544068. [DOI] [PubMed] [Google Scholar]
- 71.Grier JB. Nonparametric indexes for sensitivity and bias: Computing formulas. Psychol Bull. 1971;75:424–429. doi: 10.1037/h0031246. [DOI] [PubMed] [Google Scholar]
- 72.Cox R. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 73.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. Thieme; New York: 1988. [Google Scholar]
- 74.Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33:636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- 75.Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 76.Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, Dale AM. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23(Suppl 1):S69–84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 77.Harrell FE. Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. Springer; New York: 2001. [Google Scholar]
- 78.Nagelkerke NJD. A note on a general definition of the coefficient of determination. Biometrika. 1991;78:691–692. [Google Scholar]
- 79.Petersen RC. Mild cognitive impairment: transition between aging and Alzheimer’s disease. Neurologia. 2000;15:93–101. [PubMed] [Google Scholar]
- 80.Stevens JP. Applied multivariate statistics for the social sciences. Lawrence Erlbaum Associates; Mahwah, NJ: 2002. [Google Scholar]
- 81.Lind J, Ingvar M, Persson J, Sleegers K, Van Broeckhoven C, Adolfsson R, Nilsson LG, Nyberg L. Parietal cortex activation predicts memory decline in apolipoprotein E-epsilon4 carriers. Neuroreport. 2006;17:1683–1686. doi: 10.1097/01.wnr.0000239954.60695.c6. [DOI] [PubMed] [Google Scholar]
- 82.Bentley P, Driver J, Dolan RJ. Cholinesterase inhibition modulates visual and attentional brain responses in Alzheimer’s disease and health. Brain. 2008;131:409–424. doi: 10.1093/brain/awm299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bentley P, Driver J, Dolan RJ. Modulation of fusiform cortex activity by cholinesterase inhibition predicts effects on subsequent memory. Brain. 2009;132:2356–2371. doi: 10.1093/brain/awp176. [DOI] [PubMed] [Google Scholar]
- 84.Bentley P, Vuilleumier P, Thiel CM, Driver J, Dolan RJ. Cholinergic enhancement modulates neural correlates of selective attention and emotional processing. Neuroimage. 2003;20:58–70. doi: 10.1016/s1053-8119(03)00302-1. [DOI] [PubMed] [Google Scholar]
- 85.Kaasinen V, Nagren K, Jarvenpaa T, Roivainen A, Yu M, Oikonen V, Kurki T, Rinne JO. Regional effects of donepezil and rivastigmine on cortical acetylcholinesterase activity in Alzheimer’s disease. J Clin Psychopharmacol. 2002;22:615–620. doi: 10.1097/00004714-200212000-00012. [DOI] [PubMed] [Google Scholar]
- 86.Rombouts SA, Barkhof F, Van Meel CS, Scheltens P. Alterations in brain activation during cholinergic enhancement with rivastigmine in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2002;73:665–671. doi: 10.1136/jnnp.73.6.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Saykin AJ, Wishart HA, Rabin LA, Flashman LA, McHugh TL, Mamourian AC, Santulli RB. Cholinergic enhancement of frontal lobe activity in mild cognitive impairment. Brain. 2004;127:1574–1583. doi: 10.1093/brain/awh177. [DOI] [PubMed] [Google Scholar]
- 88.Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Raichle ME, Snyder AZ. A default mode of brain function: a brief history of an evolving idea. Neuroimage. 2007;37:1083–1090. doi: 10.1016/j.neuroimage.2007.02.041. discussion 1097–1089. [DOI] [PubMed] [Google Scholar]
- 90.Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Rao SM, Cox RW. Conceptual processing during the conscious resting state. A functional MRI study. J Cogn Neurosci. 1999;11:80–95. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- 91.Binder JR, Desai RH, Graves WW, Conant LL. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cereb Cortex. 2009;19:2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 93.Pihlajamaki M, Sperling RA. Functional MRI assessment of task-induced deactivation of the default mode network in Alzheimer’s disease and at-risk older individuals. Behav Neurol. 2009;21:77–91. doi: 10.3233/BEN-2009-0231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sperling RA, Dickerson BC, Pihlajamaki M, Vannini P, Laviolette PS, Vitolo OV, Hedden T, Becker JA, Rentz DM, Selkoe DJ, Johnson KA. Functional alterations in memory networks in early Alzheimer’s disease. Neuromolecular Med. 2010;12:27–43. doi: 10.1007/s12017-009-8109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jack CR, Jr, Lowe VJ, Senjem ML, Weigand SD, Kemp BJ, Shiung MM, Knopman DS, Boeve BF, Klunk WE, Mathis CA, Petersen RC. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain. 2008;131:665–680. doi: 10.1093/brain/awm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Van Petten C. Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: review and meta-analysis. Neuropsychologia. 2004;42:1394–1413. doi: 10.1016/j.neuropsychologia.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 97.Jack CR, Jr, Petersen RC, Xu Y, O’Brien PC, Smith GE, Ivnik RJ, Boeve BF, Tangalos EG, Kokmen E. Rates of hippocampal atrophy correlate with change in clinical status in aging and AD. Neurology. 2000;55:484–489. doi: 10.1212/wnl.55.4.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kramer JH, Mungas D, Reed BR, Wetzel ME, Burnett MM, Miller BL, Weiner MW, Chui HC. Longitudinal MRI and cognitive change in healthy elderly. Neuropsychology. 2007;21:412–418. doi: 10.1037/0894-4105.21.4.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rusinek H, De Santi S, Frid D, Tsui WH, Tarshish CY, Convit A, de Leon MJ. Regional brain atrophy rate predicts future cognitive decline: 6-year longitudinal MR imaging study of normal aging. Radiology. 2003;229:691–696. doi: 10.1148/radiol.2293021299. [DOI] [PubMed] [Google Scholar]
- 100.Sunderland T, Hampel H, Takeda M, Putnam KT, Cohen RM. Biomarkers in the diagnosis of Alzheimer’s disease: are we ready? J Geriatr Psychiatry Neurol. 2006;19:172–179. doi: 10.1177/0891988706291088. [DOI] [PubMed] [Google Scholar]