Abstract
Importance of the field
A concerted effort by the pharmaceutical industry over the last decade has led to the successful clinical development of protein kinase inhibitors as effective targeted therapies for certain cancers.
Areas covered in this review
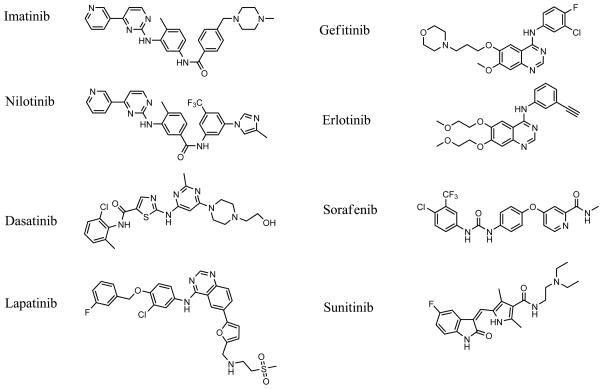
This review details the eight small molecule kinase inhibitors that have been approved for the treatment of cancer in either the United States or Europe as of March 2010: imatinib, sorafenib, gefitinib, erlotinib, dasatinib, lapatinib, sunitinib and nilotinib. These eight compounds vary from the relatively specific inhibitor lapatinib, to the more promiscuous kinase inhibitors dasatinib and sunitinib.
What the reader will gain
A brief discussion on the biology of each inhibitor, selectivity over other kinases, and toxicity are provided. More detailed discussion on metabolism, drug transporters, drug-drug interactions, and the possible roles of metabolism in compound toxicity is provided for each compound.
Take home message
The majority of the currently approved kinase inhibitors are heavily influenced by drug transporters and significantly affected by CYP3A4 inhibitors/inducers. At least three, gefitinib, erlotinib, and dasatinib, are metabolized to form reactive metabolites capable of covalently binding biomolecules.
Keywords: Tyrosine kinase, imatinib, sorafenib, gefitinib, erlotinib, dasatinib, lapatinib, sunitinib, nilotinib, drug metabolism, drug toxicity, bioactivation
1. Introduction
Protein phosphorylation is an essential post-translational signaling mechanism that controls a myriad of cellular processes. It is catalyzed by protein kinases and reversed by protein phosphatases forming complex tightly regulated signaling networks. Aberrant regulation of kinase signaling cascades is known to cause a variety of human diseases. For decades kinases have been recognized as possible drug targets but wide acceptance was hampered by the idea that potency and selectivity between the 518 human kinases would not be possible by targeting the ATP binding pocket. Although selectivity remains a challenge for medicinal chemists, the clinical use of the eight kinase inhibitors reviewed herein is testament to the original concept that inhibition of kinase signaling by small molecule drugs can be an effective anti-cancer therapy.
Each of the eight approved small molecule kinase inhibitors prevent ATP binding to the ATP-binding site. To this end, both the active and inactive conformations of protein kinases have been used in strategies to produce potent and selective compounds. While none of the molecules are selective to a single kinase, they range from lapatinib and imatinib, which bind to the inactive conformation of their target kinase and are reasonably selective, to dasatinib and sunitinib, which bind to the active conformation and inhibit a broad spectrum of kinases. The requirement of kinase inhibitor selectivity has been hotly debated, with the proposal that anti-cancer therapy requires a delicate balance between selectivity, efficacy and toxicity. On one hand, although non-selective inhibitors increase the likelihood of disruption of normal cellular function, thus increasing treatment related toxicity; most cancers are driven by multiple aberrantly regulated kinases each of which may require inhibition for effective therapy. Although all of the kinase inhibitors have significant toxicity and side effect profiles, as a whole they are better tolerated than the alternative, chiefly cytotoxic chemotherapeutics.
In addition to the small molecule kinase inhibitors, there are four antibodies that target receptor tyrosine kinases approved for cancer treatment. They are not specifically addressed in this review, but are referenced to evaluate toxicities one might expect to observe in human patients following inhibitor of specific kinases. Rrastuzumab (Herceptin®) targets the RTK ErbB2 (also called HER2) and is associated with cardiac dysfunction, 4- to 6-fold over control. Bevacizumab (Avastin®) targets VEGF and is associated with several indications indicative of vascular integrity/regulation including: decreased wound healing, gastrointestinal perforations, hemorrhage, and hypertension. Panitumumab (Vectibix®), and cetuximab (Erbitux®) both target EGFR and are associated with significant dermatologic toxicity (90% in panitumumab-treated patients vs. 9% in control and 89% in cetuximab-treated patients vs. 16% in control). Both EGFR inhibitory antibodies are also strongly associated with hypomagnesemia (decreases in magnesium levels).
Two further compounds that function through the inhibition of kinase activity are the rapamycin analogs, everolimus and temsirolimus. Both are approved for renal cell carcinoma (RCC) and in addition temsirolimus is also approved for the treatment of mantle-cell lymphoma. These compounds are not focused on in this review as they are indirect kinase inhibitors. Both everolimus and temsirolimus form a complex with the intracellular receptor FK506 binding protein 12 (FKBP12). Upon everolimus or temsirolimus binding, FKBP12 binds to and indirectly inhibits the kinase activity of the mammalian target of rapamycin complex (mTORC1). Due to the success of everolimus and temsirolimus, small molecule inhibitors of the kinase domain of mTOR itself are currently under development and are in early stage clinical trials.
In this review we focus on the molecular properties influencing bioavailability and metabolism and discuss the significance of influx and efflux drug transporters. The efflux transporter permeability glycoprotein (P-gp), an ABC-transporter (ABCB1) commonly referred to as multi-drug resistance protein (MDR1) is well appreciated in the oncology field. P-gp is an inducible efflux transporter and interacts with a number of substrates, including some of the kinase inhibitors reviewed herein. The related efflux transporter protein, ABCG2, is commonly referred to as breast cancer resistance protein (BCRP), and has been shown to be upregulated in tumors and its expression correlates with decreased efficacy of chemotherapy treatment.
2. Imatinib
Imatinib (GleevecR) was the first FDA approved kinase inhibitor (May 2001) and was an instant breakthrough in the treatment of chronic myelogenous leukemia (CML). CML is a unique indication for the application of kinase inhibitors. CML is the result of the genetic translocation of the c-abl oncogene from chromosome 9 to the breakpoint cluster region (bcr) on chromosome 22 (1, 2), yielding a bcr/abl fusion with constitutive ABL kinase activity referred to as BCR-ABL. This activity subsequently drives increased proliferation and survival of myeloid progenitor cells (3). The high effectiveness of imatinib in CML is attributed to the fact that aberrant signaling by BCR-ABL is the driving force for the disease, a concept often referred to as oncogenic addiction.
The earliest patients treated with imatinib participated in phase 1 trials beginning in mid-1998. Remarkably, hematologic response was observed in 53 out of 54 patients treated with daily doses of 300 mg or higher (4). Five year follow-up data of eleven hundred six patients with newly diagnosed CML showed that 89% of imatinib patients were alive and 83% had experienced no disease-related events five years after initiation of treatment (5). All patients who achieved complete cytogenetic responses during the treatment survived at 5 years. These efficacy data resulted in approval of imatinib for front line therapy in CML.
Despite the achievable remission rates, resistance to imatinib is an important issue for therapy. A minority of CML patients in chronic phase and a substantial proportion in advanced disease phases display either resistance to imatinib or lose imatinib sensitivity over time and experience relapse. Approximately 30% of patients with CML receiving imatinib as first-line therapy will discontinue treatment by 5 years because of disease resistance or drug toxicity (5). Resistant cell lines and patient samples have identified several mechanisms for reduced imatinib efficacy ranging from inherent genetic alterations of BCR-ABL to non-specific multi-drug resistance. The most frequently identified mechanisms of acquired imatinib resistance is point mutations in the BCR-ABL kinase domain or altered splice variants that result in loss of treatment efficacy (6). Therapeutic strategies to overcome imatinib resistance include the use of the second generation tyrosine kinase inhibitors, nilotinib and dasatinib, which are approved in cases of imatinib resistance and are discussed in later sections.
Structural analysis revealed that imatinib binds to the inactive form of BCR-ABL near the ATP-binding site causing a large conformational change of the ABL active site motif Asp-Phe-Gly (DFG), inhibiting BCR-ABL kinase activity and decreasing proliferation. The mechanics of imatinib inhibition are likely responsible for its excellent selectivity. However, imatinib does inhibit at least two other kinases, c-KIT and PDGFR. These additional activities led to the successful use of imatinib to treat myelodysplastic/myeloproliferative diseases associated with PDGFR gene re-arrangements and KIT+ gastrointestinal stromal tumors.
2.1 Toxicity
Imatinib is less toxic than interferon or traditional cytotoxics that were previously used for CML. However, like all drugs, imatinib does have toxicity issues. Most imatinib studies were compared to alternative treatments and did not include placebo groups, so distinguishing toxicity due to imatinib from toxicities due to disease state is not straightforward. Reports of anemia, neutropenia, and thrombocytopenia are commonly associated with imatinib therapy, but all of these are typical of CML and may not be related to imatinib. When compared to alternative treatments, imatinib is often associated with fluid retention and edema. Blood chemistry has also shown elevated levels of proteins used as markers of hepatotoxicity (7). Current prescribing information calls for monthly evaluation of liver function via blood levels of transaminases, bilirubin, and alkaline phosphatase. The imatinib analog nilotinib has significant cardiac indications because of interaction with Human Ether-a-go-go (hERG), a cardiac potassium channel which can lead to life-threatening cardiac arrhythmia by prolonging the hearts QT interval. Retrospective analysis indicates that changes in QT interval by imatinib are detectable, but appear to be small.
2.2 Metabolism considerations
Imatinib has excellent oral absorption in human (>95%) and individual variability is minimal (8, 9). Metabolism of imatinib occurs primarily in the liver via several cytochrome P450s with CYP3A4 being the major enzyme involved and CYP1A2, CYP2D6, CYP2C9 and CYP2C19 playing lesser roles. Alteration of CYP3A4 levels by co-administration of the CYP3A4 inducer rifampicin, decreased the Cmax and imatinib half-life by approximately 50% (9). Concurrent administration with the CYP3A4 inhibitor ketoconazole resulted in a 40% increase in imatinib AUC (10). Selected pharmacokinetic information is provided in Table 1.
Table 1.
Pharmacokinetic parameters.
| Drug | Dose (mg) | %F | Cmax (μM) | AUC (μg*h/ml) | T1/2 (hr) | ref |
|---|---|---|---|---|---|---|
| Imatinib | 200 | 1.9 | 9.6 | 2.5 | (10, 109) | |
| 400 | 98 | 2.9 | 43 | |||
| nilotinib | 400 | 1.2 | 5 | (110) | ||
| 400 BID | 4.1§ | 18 | ||||
| 200 | 0.8 | 10 | 16 | (21) | ||
| dasatinib | 100 | 0.2 | (111) | |||
| 70 BID | 0.15 | 0.14 | 3 | (112) | ||
| 70 BID | 0.24§ | 0.2–0.5 | 5–7 | |||
| gefitinib | 250 | 57 | 0.3 | 3.9 | 27 | (62, 113, 114) |
| 400 | 1.8§ | |||||
| erlotinib | 300 | 5.3 | 43.6 | 12 | (82) | |
| 150 | 6–8§ | (68) | ||||
| lapatinib | 100 | 0.2 | 1.4 | 10 | (92) | |
| 1000 | 3.2§ | 20.6 | (88) | |||
| 1500 | 4.3§ | 32 | (115) | |||
| sorafenib | 50 | 1.0 | 11 | 29 | (100) | |
| sunitinib | 50 | 0.06 | 0.36 | (116) | ||
| 50 | 0.23§ | 1.6 | ||||
%F, fraction absorbed after oral dosing; Cmax, maximum concentration;
AUC, area under the curve; T1/2, half-life
Multiple day dosing
The major circulating imatinib metabolite is generated through the N-demethylation of the piprazine. This metabolite is also active and has a longer biological half-life than the parent, but the metabolite represents only 10–15% of the total drug level under steady state conditions.
Pharmacokinetic drug-drug interactions have been demonstrated with imatinib, which increased the AUC of the CYP3A4 substrate simvastatin by 3.5-fold (11). This observation is somewhat unusual as the KI for CYP3A4 inhibition by imatinib (8 μM) is two to three times above the imatinib Cmax under typical dosing regimens. However, plasma concentration may not reflect the concentration in the liver. The majority of P450 that metabolize imatinib are in the intestine and the liver. Local intestinal concentrations immediately after ingesting imatinib are likely to be high as are concentrations in the portal vein leading to the liver. Human tissue distribution data is not available for imatinib, but positron emission tomography (PET) studies in baboons using carbon-11-labeled imatinib provide some information (12). Drug accumulation was significant in the gall bladder and liver when compared to plasma or other tissues. This may explain why significant clinical 3A4-mediated drug-drug interactions were observed despite plasma levels below the KI. The limitation of this method is that PET does not distinguish between imatinib and imatinib metabolites.
Imatinib is a substrate for multiple drug efflux transport proteins that act to limit the exposure of imatinib to the brain and may play a significant role in imatinib pharmacokinetics. Imatinib is a substrate for both P-glycoprotein (P-gp; ABCB1) and breast cancer resistance protein (BCRP; ABCG2) (13). After IV injection, plasma imatinib levels were increased in P-gp and BCRP1 knockout mice. It is difficult to determine if this reflects reduced tissue exposure or if it is due to decreasing compound efflux from the liver into the bile. Inhibition of P-gp and BCRP1 by elacridar and pantoprazole, respectively, increased the brain to plasma ratio of imatinib.
While drug efflux transporters alter the pharmacokinetic properties of imatinib and decrease the likelihood of successful treatment of tumors in the brain or tumors that overexpress efflux transporters, influx transporters may be of more significance for imatinib treatment. Imatinib is a substrate for multiple solute carrier family transporters (hOATP1A2, hOCT1, and hOCTN2), and cellular imatinib concentrations are decreased in cells when these influx transporters are inhibited resulting in increases in the observed imatinib EC50 (14). This has been shown to be clinically relevant for hOCT1. Patients taking imatinib who have low hOCT1 levels (measured as mRNA levels) have a significantly worse prognosis for disease progression and survival (15, 16). Structural modification to increase passive permeability would be expected to decrease the importance of influx transporters.
While discussion of imatinib resistance in CML is dominated by BCR-ABL mutations, not all imatinib-resistance patients exhibit such mutations (27% of chronic-phase imatinib resistant patients lacked detectable mutations). Resistant patients with more advanced disease progression were even less likely to have detectable BCR-ABL mutations (52% of accelerated-phase patients and 75% of blast crisis patients testing negative for BCR-ABL mutations) (17).
3. Nilotinib
Nilotinib (Tasigna®) received FDA approved in October 2007, and is an analog of imatinib in which the orientation of the amide is reversed and the methylpiperazine is replaced with a trifluoromethyl and a methyl-imidazole (Figure 1). Nilotinib is intended for patients that have become resistant to imatinib and it effectively inhibits most of the BCR-ABL mutations that can decrease imatinib efficacy. Additionally, nilotinib efficacy is less likely to be influenced by transporters because it is more hydrophobic than imatinib and has increased cell permeability (18). In a study of imatinib resistant or unresponsive patients, 92% achieved normal white blood cell counts after 5 months of nilotinib treatment. Similar to imatinib, nilotinib binds to and stabilizes the inactive conformation of the kinase domain of ABL and also inhibits PDGFR and KIT.
Figure 1.
Chemical structures.
3.1 Toxicity
The most significant toxicity associated with nilotinib is the inhibition of hERG (0.13 μM (19) compared to 14 μM for dasatinib (19)). While approved, nilotinib carries two black box warnings for heart arrhythmia. Electrocardiograms are recommended to monitor the QT interval at baseline, seven days after initiation of nilotinib treatment and periodically thereafter. Additional monitoring is recommended following any dose adjustments. Because of the cardiac issues with nilotinib, pharmacokinetic drug-drug interactions that result in increased nilotinib concentration are of concern. There were five sudden deaths reported in patients receiving nilotinib in an on-going study (n=867; 0.6%). A similar incidence was reported in the expanded access program (20). The relative early occurrence of some of these deaths relative to the initiation of nilotinib suggests the possibility that ventricular repolarization abnormalities may have contributed to their occurrence. Thrombocytopenia, neutropenia and anemia, additional to the underlying leukemia, can usually be treated by decreasing dose or treatment interruption. Elevated hepatic enzymes (grade 3 or 4) are seen in approximately 10–15% of patients but rarely progress to hepatitis (20).
3.2 Metabolism considerations
Despite the structural similarity, the pharmacokinetic disposition of nilotinib is significantly different than imatinib. Oral absorption of nilotinib is much lower, about 30% which is increased to about 50% when taken with a high fat meal. After dosing radiolabeled nilotinib to healthy subjects, elimination of unchanged drug accounted for 69% of the dose, primarily in feces, compared to 25% with imatinib. Similar to imatinib, drug accumulation was observed in multiple dose pharmacokinetic studies with maximal concentrations two to three fold higher after seven days of dosing. Nilotinib is greater than 99% protein bound to plasma proteins (21). CYP3A4 is the primary enzyme capable of nilotinib metabolism, but none of the known metabolites contribute significantly to the pharmacological activity of nilotinib. In rats, nilotinib concentration in liver is approximately 9–11 fold higher than plasma but CNS exposure is limited with brain and spinal cord concentrations being only 6 and 5% of plasma levels (19).
While nilotinib is structurally very similar to imatinib, it is significantly more hydrophobic (log D of 0.8 for imatinib and 2.4 nilotinib). Nilotinib rapidly enters cells in culture with maximal concentration reached within 2 minutes (18). In contrast to imatinib, expression of the transporter hOCT1 did not increase the rate of nilotinib influx into cells (18). Nilotinib was however found to inhibit the transfer of known hOCT1 and P-gp substrates. It has been suggested that this is due to nilotinib binding to the transporter non-productively (18). However, it is possible that nilotinib acts as a competitive inhibitor because it is transported by hOCT1 and/or P-gp, but discernable differences in the rate of cellular nilotinib accumulation may be masked by the high rate of nilotinib passive diffusion into cells.
When taken in parallel with the CYP3A4 inhibitor ketoconazole (400 mg once daily for 6 days), the systemic exposure of nilotinib was increased approximately 3-fold. This is greater than would be expected considering only about 30% of a given nilotinib dose is metabolized with the majority excreted unchanged in the bile. The discrepancy may be due to a combined effect of inhibition of nilotinib metabolism by CYP3A4 and inhibition of biliary excretion by P-gp. While ketoconazole is commonly referred to as a selective CYP3A4 inhibitor, this selectivity refers to other cytochrome P450s. Ketoconazole is also a known inhibitor of P-gp, thus inhibition of P-gp by ketoconazole would be expected to increase nilotinib plasma concentration because less of the nilotinib dose is transported into the bile.
Healthy subjects receiving the CYP3A4 inducer rifampicin (600 mg once daily for 12 days), had decreased systemic nilotinib exposure of approximately 80%. This may also require further clarification. Rifampicin increases the levels of CYP3A4 by activation of the nuclear hormone receptor PXR. Rifampicin has also been shown to increase the expression of P-gp through PXR activation of the DR-4 regulatory sequence of the P-gp gene (22). It is possible that both enzymes are upregulated and the decrease in nilotinib exposure is from a summation of both of these elimination pathways.
4. Dasatinib
Dasatinib (Sprycel®), a second generation BCR-ABL inhibitor of novel chemical structure was approved by the FDA in June 2006, for the treatment of imatinib resistant CML. Patients who failed to respond to imatinib treatment or where imatinib efficacy was lost during treatment had no attractive treatment options until the development of second generation BCR-ABL inhibitors. Dasatinib was designed to inhibit BCR-ABL and SRC (a tyrosine kinase involved in cell growth) and has biochemical potency between 0.1 nM and 3 nM for BCR-ABL and most of the common BCR-ABL mutations (23). Dasatinib is a highly promiscuous kinase inhibitor as demonstrated by a recently published selectivity screen of 287 human protein kinases. Reported dasatinib binding affinity was below 100 nM for 16% of tested kinases, however these were heavily weighted towards tyrosine kinases (47% of all the tyrosine kinases had binding affinity below 100 nM, but only 4.8% of serine-threonine kinases did) (24).
Dasatinib proved to be highly effective in imatinib resistant or unresponsive patients, with greater than 90% achieving normal white blood cell counts after 6 month of treatment. In a small study of patients who were non-responsive to both imatinib and nilotinib, treatment with dasatinib was effective in 13/23 patients and complete hematologic response was seen in 10/23 (25). It is unclear if this is due to the addition of SRC inhibition, dasatinib inhibiting a wider number of BRC-ABL mutations, the 50+ fold greater biochemical potency of dasatinib, or the inhibition of additional unappreciated kinases (23). Dasatinib, imatinib and nilotinib are all ineffective at inhibiting the T315I mutant of BRC-ABL (26) and it is unclear if the nonresponders had this mutation.
4.1 Toxicity
Both dasatinib and imatinib are associated with fluid retention, but dasatinib has higher rates of pleural effusion 17/101 vs 0/49 and dyspnea (shortness of breath) (27). Most other commonly observed toxicities are similar or lower in occurrence to imatinib. Dasatinib has the potential to prolong cardiac ventricular repolarization (QT interval), but occurrences appear to be low and dasatinib is a much weaker inhibiter of hERG than nilotinib. In a single-arm clinical study of leukemia patients, the mean QTc interval changes from baseline were 3–6 msec. There are also reports of dasatinib-induced acute hepatitis (28) and dasatinib-induced lupus (29) in the literature. The prevalence and cause of these are unknown.
4.2 Metabolism considerations
Elimination is primarily via the feces (85%) with unchanged dasatinib accounting for 19% of the administered dose. Dasatinib is extensively metabolized by CYP3A4 with contribution from flavin-containing monooxygenase 3 (FMO-3) and uridine diphosphate-glucuronosyltransferase (UGT). CYP3A4 generates an active metabolite, which is equipotent to dasatinib, but represents only about 5% of the dasatinib AUC. The mean Cmax and AUC of dasatinib were decreased by 81% and 82% respectively, when administered following 8 days dosing with 600 mg rifampin, a potent CYP3A4 inducer. Co-administration of 20 mg dasatinib with ketoconazole (200 mg twice daily) resulted in a 385% increase in dasatinib AUC (30). In human liver microsomes, dasatinib was a time-dependent inhibitor of CYP3A4 (31). At clinically relevant concentrations, dasatinib does not inhibit CYP1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6, or 2E1.
In contrast to imatinib, uptake of dasatinib into mononuclear cells of CML patients does not appear to be mediated by OCT1 or OCT3 (32). However, the cellular efflux transporters P-gp and BCRP have been shown to decrease intracellular dasatinib concentration in cell-based studies. This manifested in an EC50 shift of approximately 10 and 2.5 fold for P-gp and BCRP over-expressing cell lines, respectively (32). Efflux transporter activity is proposed to be the reason that dasatinib has poor brain exposure. Studies in which a pregnant rat was given a single oral dose of radiolabeled dasatinib showed extensive tissue distribution with levels in the kidney, liver, lung and placenta greater than five-fold higher than plasma (33). Dasatinib levels were below detection in the brain, spinal cord and bone. However, dasatinib was not excluded from the brain of the rat pups, possibly due to low levels of transporters or lack of tight junctions in the developing brain. Dasatinib levels in rat breast milk were found to be approximately twentyfold higher than in plasma (33).
Dasatinib inactivates CYP3A4 in a time-dependent manner (31). The KI (6 μM) is approximately thirtyfold the Cmax of a single 100 mg oral dose, but upon multiple dosing dasatinib levels increase 2–3 fold. In rat, dasatinib levels were found to be 8 times higher in liver than in plasma (33). If the same is true in humans, dasatinib may initiate pharmacokinetic drug-drug interactions with other CYP3A4 substrates. The prescribing information of Sprycel/dasatinib note a clinical drug-drug interaction study where 54 healthy subjects where co-dosed with 100 mg dasatinib and the cholesterol lowering medication simvastatin, resulting in mean simvastatin Cmax and AUC increases of 37% and 20%, respectively. Full experimental conditions are not published so it is not clear what effect dasatinib had on the concentration of CYP3A4 catalyzed simvastatin metabolites and how the simvastatin hydroxy acid metabolite was accounted for, as its formation is not catalyzed by CYP3A4. It is also not possible to make conclusions about dasatinib’s potential for irreversible mechanism-based CYP3A4 inhibition without pre-dosing experiments.
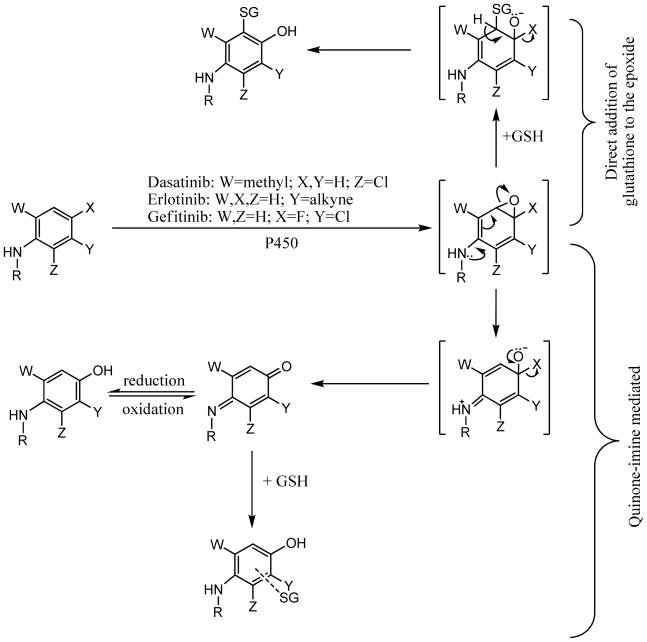
Dasatinib has been demonstrated to be oxidized by CYP3A4 to form two reactive intermediates capable of covalently binding biomolecules (31). Oxidation of dasatinib by CYP3A4 can directly generate a methyl-imine and the CYP3A4 catalyzed para-hydroxy metabolite can be oxidized to form a quinone-imine. Quinone-imine formation could not be prevented by analogs that blocked the para position with either fluorine or chlorine (31). Figure 2 details the mechanism of bioactivation. Similar reactive intermediates were generated from gefitinib and erlotinib. A proposed epoxide intermediate is depicted for the oxidative dehalogenation of dasatinib analogs and gefitinib, but it is not required when the para substituent is hydrogen. Glutathione is used as a generalized nucleophile, but reactive intermediates capable of formation of a covalent bond with the sulfhyral of glutathione would be expected to react with other cellular nucleophiles such as the sulfhydral groups of cysteine. The importance of potential dasatinib-protein adduct formation in vivo is unknown. Idiosyncratic hepatic toxicity has been associated with reactive intermediate formation (diclofenac (34), carbamazepine (35, 36), tienilic acid (37), or dihydralazine (38, 39)), but not all drugs that generate reactive intermediate in vitro have observable clinical hepatic toxicities.
Figure 2.
Proposed mechanism for P450-mediated bioactivation of dasatinib, erlotinib, and gefitinib.
5. Gefitinib
Gefitinib (Iressa®) is a reversible inhibitor of the epidermal growth factor receptor tyrosine kinase (HER1/EGFR) and was approved in May 2003, for patients with non-small cell lung cancer who were refractory to established cancer treatments (40). Deregulation of EGFR signaling is implicated in the proliferation and maintenance of multiple cancers. Gefitinib was initially approved based on data demonstrating gefitinib treatment increased the delay in tumor growth. Subsequent studies have shown that survival times do not track with tumor growth. Factors influencing responder vs. non-responder are complicated and appear to have ethnic and tumor specific factors. While still approved, the FDA limited the use of gefitinib, which has largely been supplanted by erlotinib.
5.1 Toxicity
Gefitinib is associated with diarrhea and rash in over 40% of patients (41, 42). These are not dissimilar to the effects of the EGFR antibodies panitumumab and cetuximab, suggesting that these are indicative of the target and not drug specific. Gefitinib specific toxicities include drug induced liver injury (43, 44) and pulmonary toxicity, such as interstitial lung disease (ILD), which occurs in low incidence but can be serious and sometimes fatal (45–52). The incidence of ILD in patients receiving gefitinib has been estimated at 2% in Japan and 0.3% in the USA (53). A report of 112 Japanese patients receiving gefitinib therapy reported a higher ILD occurrence rate of 5.4%, leading to four deaths (52). Observations in patients that were removed from gefitinib due to ILD and later re-introduced to the drug revealed ILD recurrence was more rapid and severe than the first episode (51, 54) indicating a possible immune system role rather than direct drug cytotoxicity. All reported deaths due to gefitinib-induced ILD occurred in current or former smokers and may be related to reactive metabolite formation discussed in the metabolism section (52).
5.2 Metabolism considerations
Bioavailability of gefitinib after oral dosing is approximately 60% (55), but Cmax in a 24 patient single oral dose clinical trial reported individual variation up to 15-fold (56). In humans, gefitinib was shown to be metabolized extensively by CYP3A4 and to a lesser degree by CYP3A5, 1A1, and 2D6. Clinical evaluation of the effect of rifampin, a potent CYP3A4 inducer, caused decreases in gefitinib concentration and the CYP3A4 inhibitor itraconazole increased gefitinib plasma levels. The major metabolic pathways included morpholine ring-opening, step-wise removal of the morpholine ring, and oxidative defluorination to form a hydroxyaniline metabolite (57–60). The para-hydroxyaniline metabolite may undergo P450-mediated two-electron oxidation, forming a reactive quinone-imine metabolite (61). In experiments using lung microsomes, gefitinib can be activated to a reactive electrophile capable of covalently labeling biomolecules. This is primarily catalyzed in the liver by CYP3A4 and in the lung by CYP1A1, an enzyme which is upregulated in smokers. The rate of reactive gefitinib metabolite formation was 12-fold higher in pulmonary microsomes from smokers when compared to non-smokers (61).
Gefitinib extensively distributes in tissue. A PET study in mice utilizing the isotope 18F found gefitinib concentrations at least five times higher than plasma levels in the liver, colon, and kidney at two hours. High levels were observed in the lung during early timepoints, but these decreased disproportionately with time (62). A second study, using LC-MS/MS quantitation of gefitinib after oral dosing of gefitinib in mouse, found modest gefitinib brain concentration (15% of plasma levels at the two-hour time point), but high concentrations in the liver and lung (10-fold higher than plasma) (61).
There are large differences in the cellular level of gefitinib in BCRP expressing vs. non-expressing cell lines, but these differences diminish or disappear at high gefitinib concentrations. This is likely because the rate of passive diffusion is greater than the capacity for BCRP mediated efflux. An alternative hypothesis suggests that the decreased efficiency of BCRP at excluding high concentrations of gefitinib is because EGFR inhibition disrupts kinase signaling proposed to go through PI3K and AKT, leading to decreased expression of BCRP at the cell surface (63). If this is the case, similar disruption might be expected for other EGFR inhibitors such as erlotinib and lapatinib, but we are unaware of either supporting or contradictory data on this topic.
Gefitinib is an inhibitor of BCRP and P-gp at clinically relevant concentrations. Resistance to the topoisomerase inhibitor topotecan is strongly correlated to the expression level of BCRP in small cell lung tumors. Co-dosing of topotecan and gefitinib increases the potency of topotecan in BCRP expressing tumors in a dose related fashion, but has minimal effect on topotecan potency in tumors that do not express BCRP (64). A similar study using vincristine demonstrated the role of gefitinib inhibition of P-gp (65).
6. Erlotinib
Erlotinib (Tarceva®) is a reversible inhibitor of the epidermal growth factor receptor tyrosine kinase (HER1/EGFR), and was approved for second- and third-line treatment of non-small cell lung cancer (NSCL) in November, 2005 (40). Compared to traditional cytotoxic therapies, erlotinib provides a survival benefit after failure of first line or second line chemotherapy in NSCL and in the treatment of advanced pancreatic adenocarcinomas together with chemotherapy (66, 67). Erlotinib inhibits purified human EGFR and blocks autophosphorylation in cellular assays with IC50 values of 2 and 20 nM, respectively. In binding assays, erlotinib Kd was 0.3–1.6 nM for EGFR and common EGFR mutations (24). Erlotinib was reasonably selective against a panel of 317 tested kinases with Kd below 100 nM for only 1.4% of the kinases. However, 15.2% had Kd below 3 μM, and erlotinib has a Cmax of 6–8 μM under repeat dosing conditions (68).
6.1 Toxicity
Treatment with erlotinib has been associated with similar adverse effects to its structural analog gefitinib such as skin rash and diarrhea (69–71). Reports of adverse hepatic reactions with erlotinib are accumulating (72–77), revealing its potential to cause liver toxicity. In September 2008, OSI Pharmaceuticals and Genentech reported that in a pharmacokinetic study of 15 patients with advanced solid tumors complicated by moderate liver impairment, 1 patient died from hepatorenal syndrome and 1 patient died because of progressive liver failure (78). Clinical evidence confirmed the diagnosis of acute hepatitis and attributed the liver toxicity to erlotinib.
Interestingly, correlation between the severity of erlotinib associated skin rash and efficacy of the drug in non-small cell lung cancer patients from two large phase III trials was strong enough to lead to the conclusion: “Physicians and patients should view rash development as a positive event indicative of greater likelihood of clinical benefit” (79). While there was a trend between increased rash and increased plasma concentration of erlotinib, concentration alone appeared to be a poorer predictor of efficacy than rash severity (79). Gefitinib and EGFR inhibitory antibodies are also associated with rash, leading to a probable mechanistic association of rash and EGFR inhibition.
6.2 Metabolism considerations
Erlotinib is about 60% absorbed after oral administration under fasting conditions and almost 100% when taken with food. Erlotinib solubility decreases at higher pH and co-administration with the proton pump inhibitor omeprazole, decreased erlotinib AUC by 46% (80). In humans, erlotinib is extensively metabolized by CYP3A4 and to a lesser extent by CYP1A2 and the inducible isoform CYP1A1 (60, 81) with metabolites primarily excreted in the bile. Only 1% of the dose is excreted as intact erlotinib. There are three primary routes of erlotinib metabolism: O-demethylation of the side chain with/or without further oxidation to the carboxylic acid, oxidation of the acetylene moiety to the aryl carboxylic acid, and 4-hydroxylation of the phenyl-acetylene moiety (81).
The pharmacokinetic profile of erlotinib is greatly affected by cigarette smoking. While the Cmax was minimally altered in single dose studies between smokers and non-smokers, the AUC in smokers was 2- to 3-times lower and median half-life decreased from 9–12 hours in non-smokers to 4–5 hours in smokers (82). This is likely due to induction of CYP1A1 and CYP1A2 which are known to be upregulated in smokers. Co-treatment with the potent CYP3A4 inhibitor ketoconazole increases erlotinib AUC by approximately 70%. Pre-treatment with the CYP3A4 inducer rifampicin for 7 days decreased erlotinib AUC by 60 to 80%. Even tripling the erlotinib dose to 450 mg resulted in a mean erlotinib AUC of only 58% of that observed following a single 150 mg in patients not previously pretreated with rifampicin (80).
Oxidation of erlotinib by CYP3A4 and CYP1A1 generates a para-hydroxyaniline metabolite through hydroxylation of the phenyl ring (60). Further P450-mediated two-electron oxidation of the para-hydroxyaniline results in the formation of a quinine-imine capable of covalent binding to cellular protein (83). It is not known if the observed erlotinib-associated hepatotoxicity is related to reactive metabolites or due to inhibition of an individual or a combination of kinases.
Erlotinib inactivates CYP3A4 and CYP3A5 in a time- and concentration-dependent manner whereas CYP1A1 is not inactivated despite forming the glutathione-reactive metabolites. The aniline group was proposed to be responsible for P450 inactivation and not the alkyne group, based on the synthesis of analogs were the alkyne of erlotinib was replaced with an ethyl or cyano group. In Human liver microsomes, the inactivation of CYP3A had kinetic constants of kinact = 0.10 min−1 and KI = 9 μM (83). With standard therapy (150 mg daily oral dose) the clinical erlotinib Cmax is 6 to 8 μM (68) implying that erlotinib concentrations may be sufficient to inactivate CYP3A in vivo. Additionally, in mice, erlotinib concentrations were found to be three-fold higher in the liver than in plasma. Similar partitioning in human would increase the likelihood of CYP3A4 inactivation.
Erlotinib is a substrate for P-gp and BCRP and an inhibitor of both enzymes. AUC after oral dosing in Bcrp1−/−/Mdr1a/1b−/− (triple-knockout) mice was 50% higher than in control (84). Erlotinib resistance in non-small cell lung cancer mouse xenografts did not correlate with BCRP or P-gp expression; however, only 6 of 26 tumors were responsive to erlotinib and EGFR over expression or constitutive activation was not evaluated (85).
7. Lapatinib
Lapatinib (Tykerb®, Tyverb®) was approved in March 2007 for the treatment of herceptin refractory HER2 positive breast cancers, inhibits EGFR (ERB1) and HER2 (ERB2). Lapatinib is highly selective when compared to other kinase inhibitors and is commonly used in concert with capecitabine (Xeloda®). A randomized clinical breast cancer trial demonstrated that lapatinib in combination with capecitabine increased time for detectable tumor growth compared to capecitabine alone and the risk of disease progression was reduced by 51% (86). Structural analysis indicates that lapatinib binds in the ATP binding site of EGFR and HER2, inducing a structural rearrangement of the proteins tertiary structure resulting in very long off rates (hours-days) (87). The slow off-rate of lapatinib correlates with prolonged down-regulation of receptor tyrosine phosphorylation in tumor cells.
7.1 Toxicity
Lapatinib treatment is commonly associated with diarrhea, fatigue, nausea and rashes (88). Some of the toxicities noted in lapatinib trials are likely due to it being used in concert with capecitabine. Over 50% of patients developed hand-foot cutanious conditions (Palmar-plantar erythrodysesthesia), but a similar level developed this disorder when taking capecitabine alone.
Lapatinib has been shown to cause an increase in liver enzymes and in a small subset of patients more severe drug-induced hepatotoxicity (approximately 1% of patients), this appears to be reversible when treatment is stopped, but in some cases has been fatal. Increases in bilirubin observed in a fraction of lapatinib patients is a likely indicator of hepatic damage given the clinically observed hepatotoxicity, but increased bilirubin levels may also be influenced by inhibition of transporters such as OATP1B1 which would decrease hepatic bilirubin uptake or by inhibition of Pgp and BCRP which would decrease bilirubin excretion into the bile.
The QT prolongation potential of lapatinib was assessed as part of an uncontrolled, open-label dose escalation study in advanced cancer patients where eighty-one patients received daily doses of lapatinib ranging from 175 mg/day to 1,800 mg/day. Thirteen of the 81 subjects were found to have an increase in QTcF >60 msec (89). Analysis of the data suggested a relationship between lapatinib concentration and the QTc interval. Furthermore, lapatinib is also associated with decreases in the left ventricular ejection fraction in approximately 1% of patients. Similar to other small molecule EGFR inhibitors erlotinib and gefitinib, lapatinib has been associated with interstitial lung disease.
Interestingly, the percentage of patients developing a rash with capecitabine (14%) increased only modestly with the addition of 1250 mg lapatinib (28%). Lapatinib is primarily believed to function through dual inhibition of ERB-2 and EGFR. The other EGFR inhibitors erlotinib and gefitinib are associated with rash in 50–80% of patients, and the two EGFR antibodies panitumumab and cetuximab are associated with significant dermatologic toxicity in 90% and 89% of patients, respectively. Because the severity of the skin rash was found to be a good indicator of clinical efficacy and patient survival with the EGFR inhibitor erlotinib in non-small cell lung cancer (79), there was initial worry in the first lapatinib trials when patients were not exhibiting the expected rash. The reason for this discrepancy is unclear. The therapeutic anti-EGFR antibodies block EGF binding and prevent activation of the kinase domain and EGFR homo- and heterodimer formation. This may disrupt signaling in a kinase independent manner and lead to the associated dermatologic toxicity. Perhaps lapatinib binding results in changes in dimer formation or scaffolding that differs from erlotinib and gefitinib.
7.2 Metabolism considerations
Lapatinib is poorly absorbed and high doses are prescribed (1250 mg taken once daily). A single fasting dose is recommended, but dividing the dose to twice a day results in 2-fold higher exposure and taking lapatinib with a high fat meal increases AUC by 3-fold. This large food effect has started a debate on the economics of lapatinib treatment. The cost of a one-month supply of lapatinib is approximately $2500–3000 and switching from taking 5 × 250 mg pills under fasted conditions to 2 × 250 mg with a high fat meal could provide individual patients with significant savings (90). This suggestion is countered with the potential for greater variability in patient lapatinib concentrations depending on what they had for breakfast (91).
Lapatinib pharmacokinetics was significantly affected by modifying CYP3A4 activity. Pretreatment with ketoconazole, 200 mg twice daily for 7 days, caused a 260% increase in AUC compared to control. Similarly, three-week pretreatment with the CYP3A4 inducer carbamazepine caused approximately 70% decrease in lapatinib AUC (92). In rats, lapatinib concentration in the liver was shown to be 5- to 8-fold higher than the circulating blood concentration (93). It is not known if the same is true in human, but the elevated hepatic concentration would need to be considered in simulations of drug-drug interactions and in any hepatic toxicokinetic interpretation.
In vitro studies indicate that lapatinib is a substrate for the transporters BCRP (ABCG2) and P-gp (ABCB1). Lapatinib is also an inhibitor of these efflux transporters and the hepatic uptake transporter OATP1B1 at clinically relevant concentrations. Inhibition of P-gp and OATP1B1 are reported to have IC50 values of 4 μM and BCRP is 0.025 μM (93). Additionally, the transporter OAT3, which is primarily associated with the kidney but appears to have minor roles in elimination from the brain and import into the liver, was inhibited at higher concentrations. The interaction of lapatinib with cellular transport proteins has the potential to exclude lapatinib from certain tissues and tumors and may lead to deleterious drug-drug interactions. However, useful interactions have been reported such as the increased efficacy of two traditional cytotoxic drugs, doxorubicin and mitoxantrone. When co-dosed with lapatinib in BCRP and P-gp-overexpressing tumor cells, transport of doxorubicin and mitoxantrone out of the tumor cell is inhibited (94).
Brain metastasis has been traditionally observed in 10 to 15% of breast cancer patients, but may be higher in Her2-overexpressing breast cancers. Up to 35% of HER2-positive advanced breast cancer patients have a relapse because of intracranial metastasis despite control of the peripheral tumor (95). The 1-year survival rate is only 20% after brain metastases. Initial data suggests that lapatinib may potentially be beneficial at decreasing brain metastasis, but the results were not statistically significant (86). A study using female BALB/c nude mice showed approximately 50% fewer large metastases in the brain when treated with either 30 or 100 mg/kg lapatinib, in addition autophosphorylation of EGFR was reduced (96). This is tempered by the results of Polli et al, who published a whole-body autoradiogram of a male rat 4 h after oral administration of 10 mg/kg 14C-labeled lapatinib (93). Lapatinib concentrations in the brain and cerebrospinal fluid were extremely low compared to the blood and the rest of the body’s tissues. Brain/plasma ratio in mice was shown to vary with dose, with a ratio of approximately 0.05 with a 1 mg/kg dose and 0.25 with a 10 mg/kg dose. Brain concentrations of lapatinib were increased 3- to 6-fold in mdr1a/1b(−/−) knockout mice (93) when compared to control. It is possible the reported decrease in metastases in mice with lapatinib treatment is dependent upon saturation of brain efflux transporters and thus driving passive diffusion with high drug plasma levels.
The metabolite profile of lapatinib has not been published, but the prescribing information indicates formation of O-dealkylated metabolites. O-dealkylation of lapatinib results in the formation of a para-hydroxyaniline. Formation of para-hydroxyaniline metabolites during the metabolism of dasatinib, erlotinib, and gefitinib were all demonstrated to be further oxidized to a quinone-imine which could covalently modify biomolecules. With the very high lapatinib dose, 1250 mg/day, if a similar reactive intermediate is formed during the metabolism of lapatinib, this could play a role in the observed risk of hepatotoxicity.
8. Sorafenib
Sorafenib (Nexavar®) was approved in December, 2005 for the treatment of advanced renal cell carcinoma and later approved for unresectable hepatocellular carcinoma. Sorafenib is a multiple kinase inhibitor and inhibits multiple intracellular (CRAF, BRAF and mutant BRAF) and receptor cell surface kinases (KIT, FLT-3, RET, VEGFR-1, VEGFR-2, VEGFR-3, and PDGFR-β). Several of these kinases are involved in tumor cell signaling, angiogenesis, and apoptosis. Results of a double-blind, placebo-controlled trial in patients with unresectable hepatocellular carcinoma showed an improvement in overall survival, with a median survival time of 10.7 vs. 7.9 months; p= 0.00058.
8.1 Toxicity
Sorafenib is associated with a range of side effects and toxicities. In separate trials, the incidence of cardiac ischemia/infarction was 2.7% in sorafenib patients compared with 1.3% in the placebo group and in a second trial the rates were 2.9% in the sorafenib group compared with 0.4% with the placebo group (97). Incidence of hypertension was also noted to increase, 9%, compared to 4% for placebo, hence weekly monitoring of patient blood pressure is recommended. Sorafenib did not alter liver function tests and appears to have minimal hepatic toxicity.
VEGF inhibition appears to be associated with adverse effects particularly with function and homeostasis of the cardiovascular and renal systems, plus wound healing and tissue repair. A recent review details the clinical incidence of adverse effects associated with the small molecule inhibitors of VEGF, sorafenib and sunitinib, along with bevacizumab, a VEGF inhibitory monoclonal antibody (98). While it is difficult to make direct comparisons because of differences in patient population and co-medications, the overall cardiovascular and renal system toxicities imply that much of the observed toxicity is target based and not specific to the individual agent.
8.2 Metabolism considerations
Sorafenib is typically dosed at 400 mg, without food twice per day. Bioavailability is decreased if taken with a high-fat meal. With repeat dosing a 2.5- to 7-fold accumulation of sorafenib is observed. An active metabolite (pyridine N-oxide) is 9–16% the concentration of sorafenib at steady-state. Sorafenib is primarily excreted unchanged in the feces, but 15–20% of the dose is excreted in urine as glucuronidated metabolites and approximately 5% of the dose is metabolized through oxidative metabolism. For the percentage of the dose that is metabolized, the major routes of metabolism are CYP3A4 catalyzed oxidations and UGT1A9 catalyzed glucuronidation. In circulation, sorafenib is highly bound (99.5%) to human plasma proteins (99).
Sorafenib inhibits numerous drug metabolizing enzymes; however, most were not significant when tested in clinical drug-drug interaction studies. Sorafenib was a competative inhibitor of CYP2C19, CYP2D6 and CYP3A4 with Ki values of 17 μM, 22 μM and 29 μM respectively; however, when 400 mg sorafenib was concomitantly dosed with midazolam (CYP3A4 substrate), dextromethorphan (CYP2D6 substrate) and omeprazole (CYP2C19 substrate) there was not a significant increase in the AUC of any of the drugs. No clinical assessment was done for the inhibition of CYP2B6 (Ki = 6 μM) or CYP2C8 (Ki = 1 μM) substrates (97).
Sorafenib did cause clinical drug-drug interactions when co-dosed with substrates of UDP-glucuronosyltransferases. Sorafenib inhibits glucuronidation by UGT1A1 (Ki = 1 μM) and UGT1A9 (Ki = 2 μM). When concomitantly dosed with irinotecan, whose active metabolite SN-38 is further conjugated by UGT1A1, there was a 67–120% increase in the AUC of SN-38 and a 26–42% increase in the AUC of irinotecan (97).
Ketoconazole (400 mg) did not alter the mean AUC of sorafenib after a single 50 mg oral dose. This is not unexpected given the relatively small percentage of the drug that is degraded by oxidative metabolism. The level of the sorafenib N-oxide metabolite, which is produced by CYP3A4, was decreased from approximately 80 ng/ml in the sorafenib-alone group to below the level of detection in the sorafenib-ketoconazole group (level of detection was 10 ng/ml) (100).
To our knowledge, studies with CYP3A4 inducers have not been reported, but considering 3A4 is only responsible for the metabolism of approximately 5% of the sorafenib dose, significant alterations in the overall pharmacokinetic profile would not be expected. Inducers of UGT1A9 which is responsible for the metabolism of 15–20% of the sorafenib dose have greater potential for decreasing the sorafenib AUC. UGT1A9 expression is controlled by the aryl hydrocarbon receptor and is induced by agents such as omeprazole and cigarette smoking.
Solute carriers did not appear to significantly increase the transport of sorafenib into cells that over expressed individual transporters: OATP1A2, OATP1B2, OATP1B3, OCT1, OAT2, OAT3, OCTN1, or OCTN2. Sorafenib was transported by the efflux transporter P-gp, and was a low micromolar inhibitor of P-gp and related ABC transporters (101). The authors report in vivo data with P-gp knockout mice and report a modest P-gp effect on minimizing drug levels of sorafenib in the brain.
9. Sunitinib
Sunitinib (Sutent®) is a multi-target receptor tyrosine kinase (RTK) inhibitor, approved by the FDA in January 2006 for the treatment of renal cell carcinoma (RCC) and imatinib-resistant gastrointestinal stromal tumor (GIST). Sunitinib inhibits the kinase domain of all the isoforms of PDGF-R and VEGFR. Interferon alpha (IFNα) or Interleukin 2 (IL-2) were standard RCC care prior to sunitinib, despite their poor rates of efficacy (5–20%). In two Phase II studies, sunitinib demonstrated response rates of approximately 40% in patients who had previously failed IFNα or IL-2 therapy. The FDA approved sunitinib for first-line use in RCC prior to Phase III data, which later confirmed the earlier results that demonstrated sunitinib increased progression-free survival to 11 months compared to 5 months for IFNα (P<.000001) (102, 103).
Sunitinib also inhibits KIT, and is used for KIT-positive gastrointestinal stromal cell tumors (GIST). About 85–90% of imatinib-resistant KIT-positive GIST are associated with a gain-of-function mutation in KIT. Mutations in the ATP binding site of KIT result in loss of imatinib efficacy; whereas sunitinib retains activity with the most common ATP binding site mutations. Sunitinib resistance appears with additional mutations in the activation loop of KIT (104).
9.1 Toxicity
Sunitinib has a significant side-effect profile, likely due to target based toxicity from inhibition of VEGFR and the combination of additionally inhibited kinases. Common side effects include fatigue, diarrhea, nausea, anorexia, hypertension, hand-foot syndrome, yellowing of the skin (due to the yellow color of sunitinib and its metabolites) and other dermatologic and haematological toxicities. While reported to be well tolerated, it should be noted that sunitinib is dosed in cycles of 50 mg daily for 4 weeks followed by two weeks washout for the patient to recover from treatment-associated toxicities. Dose reduction is required in approximately 50% of RCC patients with daily doses of 37.5 or 25 mg recommended if drug toxicity is too great. Despite the severity of the diseases treated with sunitinib, the percentage of patients that discontinue sunitinib due to adverse events have been reported to be as high as 19% (105). Recently published data suggests that the incidence of severe toxicity may be higher among the general RCC patient population than earlier reported due to trial exclusion criteria, with incidence of severe toxicity being higher in females and much higher in the elderly (106).
9.2 Metabolism considerations
Because the toxicity profile of sunitinib is largely concentration dependent, pharmacokinetic drug-drug interactions are of particular concern. Interactions that cause an increase in sunitinib concentration are likely to be associated with increases in the already significant side effects. Metabolism is primarily via CYP3A4 generating an active metabolite (active metabolite levels ≈ 30% of parent) (107). Concomitant dosing with ketoconazole resulted in a 51% increase in sunitinib AUC and rifampicin caused a 46% decrease in AUC. Sunitinib accumulates 3–4-fold with repeat dosing. Brain exposure in human is unknown. In our hands, we found moderate brain exposure of sunitinib in mice and limited exposure in rats. It is not known if this is exclusively due to transporters or a combination of factors.
Brain exposure was increased three-fold in P-gp knockout mice, compared to control (101). Other factors such as plasma protein binding may also be involved. The percentage of bound sunitinib in plasma is approximately 95% in mouse and 99% in rat (human is approximately 95%). While not yet demonstrated in clinical tumor samples, the decrease in brain exposure in P-gp expressing mice compared to P-gp null mice indicates that sunitinib can be transported out of tissues in vivo. This suggests that tumor expression of efflux transporters may influence sunitinib efficacy by decreasing the level of drug within the tumor.
Care needs to be taken when working with sunitinib in vitro because sunitinib readily isomerizes when exposed to light through rotation of the double bond between the indoline and pyrole rings. The most stable form is the Z isoform (as drawn in figure 1). The higher energy E isomer (trans configuration) converts back to the Z isomer over time in the dark. The E isoform is over 100-fold less active against VEGFR.
10. Conclusions
Similarities in metabolism between the eight approved small molecule kinase inhibitors that bind to the ATP site are not unexpected. All contain multiple aromatic rings, have similar mass (393–580 AMU) and are relatively hydrophobic with calculated octanol/water partition coefficients (logP) above 3 (sunitinib is between 2 and 3 depending on the method used). On average, the predominate enzyme involved in the metabolism of these kinase inhibitors is CYP3A4. CYP3A4 is an inducible enzyme and numerous compounds that agonize the nuclear receptor PXR have been shown to increase CYP3A4 expression. While the hepatic levels of the corresponding rat and mouse P450 enzyme are relatively consistent, presumably due to their controlled diet and environment, CYP3A4 levels are highly variable in humans. Differences of five- to ten-fold between individuals are not uncommon (108). This is likely to play a role in the large interindividual differences observed with some of the drugs.
Due to the cellular location of the kinase domain of the targets, each inhibitor must be capable of entering the tumor cell at appropriate concentrations. Drug transport proteins are of particular importance as the presence of influx transporters allows appropriate intracellular levels of imatinib within tumors (14). Decreased levels of these influx transporters led to a resistant phenotype. Similarly, the potency of dasatinib was decreased two- to ten-fold in cells that expressed efflux transporters (32). In addition to the drug level within the tumor, transporters can have large effects on concentration within individual tissues. Most of the kinase inhibitors are actively excluded from the brain and may not be suitable for the treatment of primary brain tumors or tumors that have metastasized to the brain. Because of the dim prognosis of Her2-positive breast cancer patients after brain metastasis, there may be a need for additional Her-2 targeted kinase inhibitors that have improved CNS penetration.
Several compounds were found to accumulate in the liver at concentrations several fold higher than plasma levels. This is likely to increase the rate of compound metabolism and the extent of P450 inhibition is likely to be higher than would otherwise be expected based on plasma levels. However, hepatic accumulation may offer benefit as the liver is a common site of metastasis for several cancers. The high hepatic drug concentrations may make an increasingly hostile environment, decreasing hepatic tumor metastasis.
11. Expert Opinion
The potential of pharmacokinetic drug-drug interactions are commonly evaluated based upon P450 inhibition studies. Of the eight kinase inhibitors reviewed, several are moderate to strong inhibitors of cytochrome P450, leading to the potential for drug-drug interactions. Cancer patients are likely to be on numerous medications due to their disease, age and for pain management. Additionally, treatment-related side effects may require additional medications, e.g. blood pressure lowering drugs for sunitinib or sorafenib. While the reviewed kinase inhibitors may cause clinically relevant pharmacokinetic drug-drug interactions with concomitantly administered drugs due to P450 inhibition, in most cases the bigger concern would be other compounds altering the concentration of the kinase inhibitor due to their more narrow safety index. All of the kinase inhibitors, except sorafenib, had significantly altered concentration when co-dosed with a CYP3A4 inhibitor or inducer. In most cases, inhibitors caused increases in AUC of over 50% and inducers caused decreases greater than 50%. While it may require some level of guessing, it would be nice to have clarification if the AUC increase or decrease would be expected to increase toxicity or lead to a lack of efficacy.
Ketoconazole has been used as the inhibitor of choice to evaluate clinical pharmacokinetic drug-drug interactions via CYP3A4 inhibition. For compounds that are substrates for efflux transporters such as P-gp, results are likely to be complicated by ketoconazole inhibiting metabolism through CYP3A4 and CYP3A5 and by decreased transport of compound from the liver into the bile/gall bladder. The roles of each pathway could be better evaluated using P450 or transporter specific compounds. This is illustrated by nilotinib which is primarily excreted unchanged with only 30% of the dose excreted as metabolites; however, when codosed with ketoconazole, the AUC of nilotinib is increased 3-fold. Inhibition of CYP3A4 alone should not cause such a pronounced effect independently.
The challenges in selectively targeting individual kinases through competitive binding to the kinases ATP site have in some cases resulted in the development of relatively non-specific inhibitors. However, when compared to the available monoclonal antibody inhibitors, it is not clear that limited non-selectivity actually increases toxicity. The most important determination of overall toxicity is likely to correlate with inhibition of select kinases and not necessarily to the total number inhibited. Further data will be required to build a list of kinases that are important to avoid inhibiting. Trying to distinguish acute and chronic toxicity due to inhibition of the intended target and off target effects is a daunting task, particularly considering the numerous kinases most of the current drugs inhibit, the limited number of compounds with which to make meaningful correlations, and toxicities from the underlying disease.
Three of the compounds, dasatinib, gefitinib and erlotinib have been demonstrated to form reactive intermediates which are capable of covalently modifying biomolecules. Similar reactive intermediates might be predicted for lapatinib and sorafenib based on structural similarity. Chemical toxicity due to reactive intermediate formation can be acute such as that seen with acetaminophen overdose, which is usually dose dependent, happens with most individuals, and often can be predicted based on animal models. Idiosyncratic drug-induced toxicity is believed to be immune mediated and has been seen with many compounds that are metabolized to reactive intermediates in the liver, e.g. halothane, tienilic acid, dihydralazine, diclofenac, phenytoin, felbamate and carbamazepine. Idiosyncratic toxicity is usually seen in only a small percentage of drug-treated individuals, is not strictly dose dependent, is usually observed after a few weeks or months of initiating treatment and is poorly predicted by animal models. Because of the severity of the diseases being treated and the high background toxicity of the kinase inhibitors, it may be nearly impossible to determine the extent, if any, reactive intermediate formation has on toxicity of the compounds. While higher associated drug toxicities may be acceptable due to the severity of the disease being treated, if it is possible through slight structural modifications to decrease the potential for toxicity without decreasing efficacy, this should be strived for.
An increased understanding of the importance of cellular transporters has highlighted an addressable limitation in the kinase inhibitors presented here. Efflux transporters are likely to limit the utility of these inhibitors for individual cancers or after metastasis to different tissues. Most of the compounds described were exported by P-gp or BCRP. Tissues that express high concentrations of P-gp such as the brain, adrenal cortex, or adrenal gland or those that express high BCRP levels such as the brain and uterus are unlikely to be effectively treated by the current inhibitors. Development of compounds that are not substrates for these efflux transporters may broaden the utility of the inhibitors and decrease the chance of resistance due to up regulation of drug transporters.
Article highlights.
The primary enzymes influencing the pharmacokinetics and tissue distribution of the reviewed kinase inhibitors are cytochrome P450 3A4 and the ATP-binding cassette transporters P-gp (ABCB1) and BCRP (ABCG2). Despite the importance of transporters, tests are not currently utilized in a clinical setting to match patients and therapies.
Clinical drug-drug interactions are observed for all of the reviewed compounds except sorafenib when simultaneously dosed with CYP3A4 inhibitors or inducers.
Under repeat-dosing conditions, maximum drug concentrations for most of the reviewed kinases accumulate two- to three-fold when compared to single dose conditions.
While better tolerated than traditional cytotoxics, all of the compounds have significant associated toxicity. This is likely a combination of mechanistic toxicity due to inhibition of the intended target, off target toxicity, and possibly for a subset of the inhibitors due to formation of reactive intermediates during metabolism.
Table 2.
Cellular and biochemical potency.
| Drug | Target | Potency | notes | ref |
|---|---|---|---|---|
| Imatinib | BCR-ABL | 188 nM (B) | Decreased activity vs select ABL and KIT mutations | (117) |
| KIT | 413 nM (B) | |||
| PDGFR | 386 nM (B) | |||
| nilotinib | BCR-ABL | 4–11 nM (C) | (118) | |
| dasatinib | BCR-ABL | 0.1–3 nM (B) | Not T315I | (23) |
| 0.6–11 nM (C) | ||||
| gefitinib | EGFR | 0.5–2 nM (B) | (87) | |
| erlotinib | EGFR | 0.4–1.6 nM (B) | (87) | |
| lapatinib | EGFR (ERB1) | 1–8 nM (B) | Dissociation half-life ≥ 5 hr | (87) |
| 3 nM (B) | ||||
| ERB2 (HER2) | 13 nM (B) | |||
| sorafenib | VEGFR2 | 4 nM (B), 10 nM (C) | (119) | |
| FLT-3 | 22 nM (B) | |||
| KIT | 15 nM (B), 29 nM (C) | |||
| PDGFR | 2–5 nM (B), 7 nM (C) | |||
| sunitinib | KIT | .45 nM (B), .3 nM (C) | Not D816V | (119) |
| VEGFR-2 | 5 nM (C) | |||
| FLT3 | .6 nM (B), 1 nM (C) | |||
(B) – biochemical based, (C) – cell based assay
Table 3.
Clinical pharmacokinetic drug-drug interactions.
| Drug | Metabolized by | Change in AUC by P450 inhibitors | Change in AUC by P450 inducers | Active metabolite | Tissue Accumulation |
|---|---|---|---|---|---|
| Imatinib | CYP3A4/5 (Minor –1A2, 2D6, 2C9, 2C19) | + 40% with Ket (9) | − 50% with Rif (10) | Yes (15%) | Liver, GB |
| nilotinib | CYP3A4/5 | + 300% with Ket (19) | − 80% with Rif (19) | No | |
| dasatinib | CYP3A4/5, 1A1 (120) (minor – FMO3, UGT) | + 480% with Ket (30) | − 80% with Rif (121) | Yes (5%) | |
| gefitinib | CYP3A4/5 (minor – 1A1, 2D6) (59) | + 60–80% with itraconazole (122) | −80% with Rif (122) | Liver, Lung GB | |
| erlotinib | CYP3A4/5, CYP1A1 (60) | + 70% with Ket (80) | − 60–80% with Rif (80) − 50–70% in smokers (82) |
Liver | |
| lapatinib | CYP3A4/5 | + 360% with Ket (92) | − 70% with CBZ | ||
| sorafenib | UGT1A9 (100) (minor CYP3A4/5) | None with Ket (100) | Yes (9–16%) | ||
| sunitinib | CYP3A4/5 | + 50% with Ket (123) | − 50% with Rif (123) | Yes (30%) |
(Ket) – ketoconazole, (Rif) – rifampicin, (CBZ) – carbamazepine, (GB) – gall bladder
Footnotes
Declaration of Interest
This work is funded through an NIH grant (1U01AA018665-01(MC) as well as by the Scripps Research Institute.
References
- 1.de Klein A, van Kessel AG, Grosveld G, et al. A cellular oncogene is translocated to the Philadelphia chromosome in chronic myelocytic leukaemia. Nature. 1982;300:765–767. doi: 10.1038/300765a0. [DOI] [PubMed] [Google Scholar]
- 2.Shtivelman E, Lifshitz B, Gale RP, et al. Fused transcript of abl and bcr genes in chronic myelogenous leukaemia. Nature. 1985;315:550–554. doi: 10.1038/315550a0. [DOI] [PubMed] [Google Scholar]
- 3.Calabretta B, Perrotti D. The biology of CML blast crisis. Blood. 2004;103:4010–4022. doi: 10.1182/blood-2003-12-4111. [DOI] [PubMed] [Google Scholar]
- 4.Druker BJ, Talpaz M, Resta DJ, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344:1031–1037. doi: 10.1056/NEJM200104053441401. [DOI] [PubMed] [Google Scholar]
- 5.Druker BJ, Guilhot F, O’Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 6.Deininger M, Buchdunger E, Druker BJ. The development of imatinib as a therapeutic agent for chronic myeloid leukemia. Blood. 2005;105:2640–2653. doi: 10.1182/blood-2004-08-3097. [DOI] [PubMed] [Google Scholar]
- 7.O’Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 8.Peng B, Dutreix C, Mehring G, et al. Absolute bioavailability of imatinib (Glivec) orally versus intravenous infusion. J Clin Pharmacol. 2004;44:158–162. doi: 10.1177/0091270003262101. [DOI] [PubMed] [Google Scholar]
- 9.Bolton AE, Peng B, Hubert M, et al. Effect of rifampicin on the pharmacokinetics of imatinib mesylate (Gleevec, STI571) in healthy subjects. Cancer Chemother Pharmacol. 2004;53:102–106. doi: 10.1007/s00280-003-0722-9. [DOI] [PubMed] [Google Scholar]
- 10.Dutreix C, Peng B, Mehring G, et al. Pharmacokinetic interaction between ketoconazole and imatinib mesylate (Glivec) in healthy subjects. Cancer Chemother Pharmacol. 2004;54:290–294. doi: 10.1007/s00280-004-0832-z. [DOI] [PubMed] [Google Scholar]
- 11.O’Brien SG, Meinhardt P, Bond E, et al. Effects of imatinib mesylate (STI571, Glivec) on the pharmacokinetics of simvastatin, a cytochrome p450 3A4 substrate, in patients with chronic myeloid leukaemia. Br J Cancer. 2003;89:1855–1859. doi: 10.1038/sj.bjc.6601152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kil KE, Ding YS, Lin KS, et al. Synthesis and positron emission tomography studies of carbon-11-labeled imatinib (Gleevec) Nucl Med Biol. 2007;34:153–163. doi: 10.1016/j.nucmedbio.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13•.Breedveld P, Pluim D, Cipriani G, et al. The effect of Bcrp1 (Abcg2) on the in vivo pharmacokinetics and brain penetration of imatinib mesylate (Gleevec): implications for the use of breast cancer resistance protein and P-glycoprotein inhibitors to enable the brain penetration of imatinib in patients. Cancer Res. 2005;65:2577–2582. doi: 10.1158/0008-5472.CAN-04-2416. Demonstrates the role of mouse P-gp and BCRP in influencing pharmacokinetics and brain penetration of imatinib using transfected cells, knockout mice, and selective chemical inhibitors. [DOI] [PubMed] [Google Scholar]
- 14••.White DL, Saunders VA, Dang P, et al. OCT-1-mediated influx is a key determinant of the intracellular uptake of imatinib but not nilotinib (AMN107): reduced OCT-1 activity is the cause of low in vitro sensitivity to imatinib. Blood. 2006;108:697–704. doi: 10.1182/blood-2005-11-4687. In cells from 25 untreated CML patients, imatinib potency strongly correlated with intracellular drug uptake and retention, but nilotinib did not. The imatinib influx by the transporter OCT-1 was shown to be the determining factor in interpatient variability. [DOI] [PubMed] [Google Scholar]
- 15.White DL, Saunders VA, Dang P, et al. Most CML patients who have a suboptimal response to imatinib have low OCT-1 activity: higher doses of imatinib may overcome the negative impact of low OCT-1 activity. Blood. 2007;110:4064–4072. doi: 10.1182/blood-2007-06-093617. [DOI] [PubMed] [Google Scholar]
- 16.Crossman LC, Druker BJ, Deininger MW, et al. hOCT 1 and resistance to imatinib. Blood. 2005;106:1133–1134. doi: 10.1182/blood-2005-02-0694. author reply 1134. [DOI] [PubMed] [Google Scholar]
- 17••.Soverini S, Colarossi S, Gnani A, et al. Contribution of ABL kinase domain mutations to imatinib resistance in different subsets of Philadelphia-positive patients: by the GIMEMA Working Party on Chronic Myeloid Leukemia. Clin Cancer Res. 2006;12:7374–7379. doi: 10.1158/1078-0432.CCR-06-1516. Mutations in the ABL kinase domain resulted in imatinib resistance. It was noteworthy how many imatinib-resistant patients did not have ABL mutations, indicating additional mechanisms for resistance. [DOI] [PubMed] [Google Scholar]
- 18•.Davies A, Jordanides NE, Giannoudis A, et al. Nilotinib concentration in cell lines and primary CD34(+) chronic myeloid leukemia cells is not mediated by active uptake or efflux by major drug transporters. Leukemia. 2009 doi: 10.1038/leu.2009.166. In contrast to imatinib, cellular nilotinib concentrations are not altered by the efflux transporters MDR1 (ABCB1), MRP1 (ABCC1), ABCG2 (BCRP) or the influx transporter hOCT1. The report concluded that nilotinib is not effluxed through the ATP-binding cassette transporters and that nilotinib was an inhibitor of hOCT1, MDR1 and ABCG2. It is unclear if nilotinib inhibits the transporters by binding non-productively, or if nilotinib is transported, but the transporters cannot establish a gradient due to the high passive permeability of nilotinib. [DOI] [PubMed] [Google Scholar]
- 19.European Medicines Agency. Tasigna, INN-nilotinib, report H-798-en6. 2007. ( http://www.ema.europa.eu/humandocs/PDFs/EPAR/tasigna/H-798-en6.pdf, Ed.)
- 20.Hazarika M, Jiang X, Liu Q, et al. Tasigna for chronic and accelerated phase Philadelphia chromosome--positive chronic myelogenous leukemia resistant to or intolerant of imatinib. Clin Cancer Res. 2008;14:5325–5331. doi: 10.1158/1078-0432.CCR-08-0308. [DOI] [PubMed] [Google Scholar]
- 21.Yin OQ, Gallagher N, Tanaka C, et al. Effects of hepatic impairment on the pharmacokinetics of nilotinib: an open-label, single-dose, parallel-group study. Clin Ther. 2009;31(Pt 2):2459–2469. doi: 10.1016/j.clinthera.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 22.Owen A, Goldring C, Morgan P, et al. Induction of P-glycoprotein in lymphocytes by carbamazepine and rifampicin: the role of nuclear hormone response elements. Br J Clin Pharmacol. 2006;62:237–242. doi: 10.1111/j.1365-2125.2006.02587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Hare T, Walters DK, Stoffregen EP, et al. In vitro activity of Bcr-Abl inhibitors AMN107 and BMS-354825 against clinically relevant imatinib-resistant Abl kinase domain mutants. Cancer Res. 2005;65:4500–4505. doi: 10.1158/0008-5472.CAN-05-0259. [DOI] [PubMed] [Google Scholar]
- 24.Karaman MW, Herrgard S, Treiber DK, et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26:127–132. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- 25.Quintas-Cardama A, Kantarjian H, Jones D, et al. Dasatinib (BMS-354825) is active in Philadelphia chromosome-positive chronic myelogenous leukemia after imatinib and nilotinib (AMN107) therapy failure. Blood. 2007;109:497–499. doi: 10.1182/blood-2006-07-035493. [DOI] [PubMed] [Google Scholar]
- 26.Gambacorti-Passerini C, Gasser M, Ahmed S, et al. Abl inhibitor BMS354825 binding mode in Abelson kinase revealed by molecular docking studies. Leukemia. 2005;19:1267–1269. doi: 10.1038/sj.leu.2403775. [DOI] [PubMed] [Google Scholar]
- 27.Kantarjian H, Pasquini R, Hamerschlak N, et al. Dasatinib or high-dose imatinib for chronic-phase chronic myeloid leukemia after failure of first-line imatinib: a randomized phase 2 trial. Blood. 2007;109:5143–5150. doi: 10.1182/blood-2006-11-056028. [DOI] [PubMed] [Google Scholar]
- 28.Bonvin A, Mesnil A, Nicolini FE, et al. Dasatinib-induced acute hepatitis. Leuk Lymphoma. 2008:1–3. doi: 10.1080/10428190802136384. [DOI] [PubMed] [Google Scholar]
- 29.Rea D, Bergeron A, Fieschi C, et al. Dasatinib-induced lupus. Lancet. 2008;372:713–714. doi: 10.1016/S0140-6736(08)61295-7. [DOI] [PubMed] [Google Scholar]
- 30.Johnson FM, Agrawal S, Burris H, et al. Phase 1 pharmacokinetic and drug-interaction study of dasatinib in patients with advanced solid tumors. Cancer. 116:1582–1591. doi: 10.1002/cncr.24927. [DOI] [PubMed] [Google Scholar]
- 31•.Li X, He Y, Ruiz CH, et al. Characterization of dasatinib and its structural analogs as CYP3A4 mechanism-based inactivators and the proposed bioactivation pathways. Drug Metab Dispos. 2009;37:1242–1250. doi: 10.1124/dmd.108.025932. Reactive metabolites are generated during the oxidative metabolism of dasatinib. Several dasatinib analogs were synthesized to elucidate what modifications could prevent reactive metabolite formation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hiwase DK, Saunders V, Hewett D, et al. Dasatinib cellular uptake and efflux in chronic myeloid leukemia cells: therapeutic implications. Clin Cancer Res. 2008;14:3881–3888. doi: 10.1158/1078-0432.CCR-07-5095. [DOI] [PubMed] [Google Scholar]
- 33••.He K, Lago MW, Iyer RA, et al. Lacteal secretion, fetal and maternal tissue distribution of dasatinib in rats. Drug Metab Dispos. 2008;36:2564–2570. doi: 10.1124/dmd.108.022764. In pregnant rats, dasatinib was excreted at high concentrations in the breast milk. Ther was also a large difference in the brain/blood dasatinib ratio between the mother and the fetal rats. This may be due to altered expression of transporters in the fetal brain, or lack of tight junctions in the vasculature of the developing brain. [DOI] [PubMed] [Google Scholar]
- 34.Schapira D, Bassan L, Nahir AM, et al. Diclofenac-induced hepatotoxicity. Postgrad Med J. 1986;62:63–65. doi: 10.1136/pgmj.62.723.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu Y, Sanderson JP, Farrell J, et al. Activation of T cells by carbamazepine and carbamazepine metabolites. J Allergy Clin Immunol. 2006;118:233–241. doi: 10.1016/j.jaci.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 36.Moore NC, Lerer B, Meyendorff E, et al. Three cases of carbamazepine toxicity. Am J Psychiatry. 1985;142:974–975. doi: 10.1176/ajp.142.8.974. [DOI] [PubMed] [Google Scholar]
- 37.Homberg JC, Andre C, Abuaf N. A new anti-liver-kidney microsome antibody (anti-LKM2) in tienilic acid-induced hepatitis. Clin Exp Immunol. 1984;55:561–570. [PMC free article] [PubMed] [Google Scholar]
- 38.Beaune PH, Lecoeur S, Bourdi M, et al. Anti-cytochrome P450 autoantibodies in drug-induced disease. Eur J Haematol Suppl. 1996;60:89–92. doi: 10.1111/j.1600-0609.1996.tb01652.x. [DOI] [PubMed] [Google Scholar]
- 39.Masubuchi Y, Horie T. Toxicological significance of mechanism-based inactivation of cytochrome p450 enzymes by drugs. Crit Rev Toxicol. 2007;37:389–412. doi: 10.1080/10408440701215233. [DOI] [PubMed] [Google Scholar]
- 40.Siegel-Lakhai WS, Beijnen JH, Schellens JH. Current knowledge and future directions of the selective epidermal growth factor receptor inhibitors erlotinib (Tarceva) and gefitinib (Iressa) Oncologist. 2005;10:579–589. doi: 10.1634/theoncologist.10-8-579. [DOI] [PubMed] [Google Scholar]
- 41.Dudek AZ, Kmak KL, Koopmeiners J, et al. Skin rash and bronchoalveolar histology correlates with clinical benefit in patients treated with gefitinib as a therapy for previously treated advanced or metastatic non-small cell lung cancer. Lung Cancer. 2006;51:89–96. doi: 10.1016/j.lungcan.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 42.Pascual JC, Belinchon I, Sivera F, et al. Severe cutaneous toxicity following treatment with gefitinib (ZD1839) Br J Dermatol. 2005;153:1222–1223. doi: 10.1111/j.1365-2133.2005.06885.x. [DOI] [PubMed] [Google Scholar]
- 43.Carlini P, Papaldo P, Fabi A, et al. Liver toxicity after treatment with gefitinib and anastrozole: drug-drug interactions through cytochrome p450? J Clin Oncol. 2006;24:e60–61. doi: 10.1200/JCO.2006.07.8261. [DOI] [PubMed] [Google Scholar]
- 44.Yoshimoto A, Kasahara K, Kimura H, et al. [Transient liver injury caused by gefitinib] Nihon Kokyuki Gakkai Zasshi. 2004;42:56–61. [PubMed] [Google Scholar]
- 45.Cohen MH, Williams GA, Sridhara R, et al. FDA drug approval summary: gefitinib (ZD1839) (Iressa) tablets. Oncologist. 2003;8:303–306. doi: 10.1634/theoncologist.8-4-303. [DOI] [PubMed] [Google Scholar]
- 46.Ieki R, Saitoh E, Shibuya M. Acute lung injury as a possible adverse drug reaction related to gefitinib. Eur Respir J. 2003;22:179–181. doi: 10.1183/09031936.03.00098503. [DOI] [PubMed] [Google Scholar]
- 47.Inomata S, Takahashi H, Nagata M, et al. Acute lung injury as an adverse event of gefitinib. Anticancer Drugs. 2004;15:461–467. doi: 10.1097/01.cad.0000127666.12215.7b. [DOI] [PubMed] [Google Scholar]
- 48.Kitajima H, Takahashi H, Harada K, et al. Gefitinib-induced interstitial lung disease showing improvement after cessation: disassociation of serum markers. Respirology. 2006;11:217–220. doi: 10.1111/j.1440-1843.2006.00835.x. [DOI] [PubMed] [Google Scholar]
- 49.Ohyanagi F, Ando Y, Nagashima F, et al. Acute gefitinib-induced pneumonitis. Int J Clin Oncol. 2004;9:406–409. doi: 10.1007/s10147-004-0418-0. [DOI] [PubMed] [Google Scholar]
- 50.Shih YN, Chiu CH, Tsai CM, et al. Interstitial pneumonia during gefitinib treatment of non-small-cell lung cancer. J Chin Med Assoc. 2005;68:183–186. doi: 10.1016/S1726-4901(09)70246-1. [DOI] [PubMed] [Google Scholar]
- 51.Suzuki M, Asahina H, Konishi J, et al. Recurrent gefitinib-induced interstitial lung disease. Intern Med. 2008;47:533–536. doi: 10.2169/internalmedicine.47.0737. [DOI] [PubMed] [Google Scholar]
- 52.Takano T, Ohe Y, Kusumoto M, et al. Risk factors for interstitial lung disease and predictive factors for tumor response in patients with advanced non-small cell lung cancer treated with gefitinib. Lung Cancer. 2004;45:93–104. doi: 10.1016/j.lungcan.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 53.Liu V, White DA, Zakowski MF, et al. Pulmonary toxicity associated with erlotinib. Chest. 2007;132:1042–1044. doi: 10.1378/chest.07-0050. [DOI] [PubMed] [Google Scholar]
- 54.Inoue A, Saijo Y, Maemondo M, et al. Severe acute interstitial pneumonia and gefitinib. Lancet. 2003;361:137–139. doi: 10.1016/S0140-6736(03)12190-3. [DOI] [PubMed] [Google Scholar]
- 55.Ranson M, Hammond LA, Ferry D, et al. ZD1839, a selective oral epidermal growth factor receptor-tyrosine kinase inhibitor, is well tolerated and active in patients with solid, malignant tumors: results of a phase I trial. J Clin Oncol. 2002;20:2240–2250. doi: 10.1200/JCO.2002.10.112. [DOI] [PubMed] [Google Scholar]
- 56.Swaisland HC, Smith RP, Laight A, et al. Single-dose clinical pharmacokinetic studies of gefitinib. Clin Pharmacokinet. 2005;44:1165–1177. doi: 10.2165/00003088-200544110-00004. [DOI] [PubMed] [Google Scholar]
- 57.McKillop D, Hutchison M, Partridge EA, et al. Metabolic disposition of gefitinib, an epidermal growth factor receptor tyrosine kinase inhibitor, in rat, dog and man. Xenobiotica. 2004;34:917–934. doi: 10.1080/00498250400009171. [DOI] [PubMed] [Google Scholar]
- 58.McKillop D, McCormick AD, Miles GS, et al. In vitro metabolism of gefitinib in human liver microsomes. Xenobiotica. 2004;34:983–1000. doi: 10.1080/02772240400015222. [DOI] [PubMed] [Google Scholar]
- 59.McKillop D, McCormick AD, Millar A, et al. Cytochrome P450-dependent metabolism of gefitinib. Xenobiotica. 2005;35:39–50. doi: 10.1080/00498250400026464. [DOI] [PubMed] [Google Scholar]
- 60.Li J, Zhao M, He P, et al. Differential metabolism of gefitinib and erlotinib by human cytochrome P450 enzymes. Clin Cancer Res. 2007;13:3731–3737. doi: 10.1158/1078-0432.CCR-07-0088. [DOI] [PubMed] [Google Scholar]
- 61•.Li X, Kamenecka TM, Cameron MD. Bioactivation of the epidermal growth factor receptor inhibitor gefitinib: implications for pulmonary and hepatic toxicities. Chem Res Toxicol. 2009;22:1736–1742. doi: 10.1021/tx900256y. Gefitinib was found to be bioactivated by CYP3A4 and CYP1A1. Reactive metabolites were catalyzed by microsomes from the liver, intestine and lung. Lung microsomes from smokers generated 12-times more reactive metabolite vs. non-smokers. [DOI] [PubMed] [Google Scholar]
- 62.Su H, Seimbille Y, Ferl GZ, et al. Evaluation of [(18)F]gefitinib as a molecular imaging probe for the assessment of the epidermal growth factor receptor status in malignant tumors. Eur J Nucl Med Mol Imaging. 2008;35:1089–1099. doi: 10.1007/s00259-007-0636-6. [DOI] [PubMed] [Google Scholar]
- 63.Mogi M, Yang J, Lambert JF, et al. Akt signaling regulates side population cell phenotype via Bcrp1 translocation. J Biol Chem. 2003;278:39068–39075. doi: 10.1074/jbc.M306362200. [DOI] [PubMed] [Google Scholar]
- 64.Nagashima S, Soda H, Oka M, et al. BCRP/ABCG2 levels account for the resistance to topoisomerase I inhibitors and reversal effects by gefitinib in non-small cell lung cancer. Cancer Chemother Pharmacol. 2006;58:594–600. doi: 10.1007/s00280-006-0212-y. [DOI] [PubMed] [Google Scholar]
- 65.Leggas M, Panetta JC, Zhuang Y, et al. Gefitinib modulates the function of multiple ATP-binding cassette transporters in vivo. Cancer Res. 2006;66:4802–4807. doi: 10.1158/0008-5472.CAN-05-2915. [DOI] [PubMed] [Google Scholar]
- 66.Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2007;25:1960–1966. doi: 10.1200/JCO.2006.07.9525. [DOI] [PubMed] [Google Scholar]
- 67.Tang PA, Tsao MS, Moore MJ. A review of erlotinib and its clinical use. Expert Opin Pharmacother. 2006;7:177–193. doi: 10.1517/14656566.7.2.177. [DOI] [PubMed] [Google Scholar]
- 68.Rudin CM, Liu W, Desai A, et al. Pharmacogenomic and pharmacokinetic determinants of erlotinib toxicity. J Clin Oncol. 2008;26:1119–1127. doi: 10.1200/JCO.2007.13.1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bovenschen HJ, Alkemade JA. Erlotinib-induced dermatologic side-effects. Int J Dermatol. 2009;48:326–328. doi: 10.1111/j.1365-4632.2009.03835.x. [DOI] [PubMed] [Google Scholar]
- 70.Chou LS, Garey J, Oishi K, et al. Managing dermatologic toxicities of epidermal growth factor receptor inhibitors. Clin Lung Cancer. 2006;8(Suppl 1):S15–22. doi: 10.3816/clc.2006.s.009. [DOI] [PubMed] [Google Scholar]
- 71.Lubbe J, Masouye I, Dietrich PY. Generalized xerotic dermatitis with neutrophilic spongiosis induced by erlotinib (Tarceva) Dermatology. 2008;216:247–249. doi: 10.1159/000112936. [DOI] [PubMed] [Google Scholar]
- 72.Liu W, Makrauer FL, Qamar AA, et al. Fulminant hepatic failure secondary to erlotinib. Clin Gastroenterol Hepatol. 2007;5:917–920. doi: 10.1016/j.cgh.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 73.Pellegrinotti M, Fimognari FL, Franco A, et al. Erlotinib-induced hepatitis complicated by fatal lactic acidosis in an elderly man with lung cancer. Ann Pharmacother. 2009;43:542–545. doi: 10.1345/aph.1L468. [DOI] [PubMed] [Google Scholar]
- 74.Ramanarayanan J, Krishnan GS. Review: hepatotoxicity and EGFR inhibition. Clin Adv Hematol Oncol. 2008;6:200–201. [PubMed] [Google Scholar]
- 75.Ramanarayanan J, Scarpace SL. Acute drug induced hepatitis due to erlotinib. JOP. 2007;8:39–43. [PubMed] [Google Scholar]
- 76.Saif MW. Hepatic failure and hepatorenal syndrome secondary to erlotinib. Safety reminder. JOP. 2008;9:748–752. [PubMed] [Google Scholar]
- 77.Saif MW. Erlotinib-induced acute hepatitis in a patient with pancreatic cancer. Clin Adv Hematol Oncol. 2008;6:191–199. [PubMed] [Google Scholar]
- 78•.OSI Pharmaceuticals, and Genentech. Dear Healthcare Professional. 2008. http://www.fda.gov/downloads/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/UCM135238.pdf.
- 79•.Wacker B, Nagrani T, Weinberg J, et al. Correlation between development of rash and efficacy in patients treated with the epidermal growth factor receptor tyrosine kinase inhibitor erlotinib in two large phase III studies. Clin Cancer Res. 2007;13:3913–3921. doi: 10.1158/1078-0432.CCR-06-2610. Data from two phase III erlotinib studies showed a p=.0001 correlation between rash during treatment and overall survival. [DOI] [PubMed] [Google Scholar]
- 80.OSI Pharmaceuticals, and Genentech. Tarceva perscribing information 2009 [Google Scholar]
- 81.Ling J, Johnson KA, Miao Z, et al. Metabolism and excretion of erlotinib, a small molecule inhibitor of epidermal growth factor receptor tyrosine kinase, in healthy male volunteers. Drug Metab Dispos. 2006;34:420–426. doi: 10.1124/dmd.105.007765. [DOI] [PubMed] [Google Scholar]
- 82•.Hamilton M, Wolf JL, Rusk J, et al. Effects of smoking on the pharmacokinetics of erlotinib. Clin Cancer Res. 2006;12:2166–2171. doi: 10.1158/1078-0432.CCR-05-2235. The pharmacokinetic profile of erlotinib was significantly changed by smoking. Induction of CYP1A1/2 is the likely cause. [DOI] [PubMed] [Google Scholar]
- 83•.Li X, Kamenecka TM, Cameron MD. P450-Mediated Bioactivation of the EGFR Inhibitor Erlotinib to a Reactive Electrophile. Drug Metab Dispos. doi: 10.1124/dmd.109.030361. Erlotinib is metabolized by CYP3A4 and CYP1A1 to generate a reactive quinone-imine metabolite. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84•.Marchetti S, de Vries NA, Buckle T, et al. Effect of the ATP-binding cassette drug transporters ABCB1, ABCG2, and ABCC2 on erlotinib hydrochloride (Tarceva) disposition in in vitro and in vivo pharmacokinetic studies employing Bcrp1−/−/Mdr1a/1b−/−(triple-knockout) and wild-type mice. Mol Cancer Ther. 2008;7:2280–2287. doi: 10.1158/1535-7163.MCT-07-2250. Systemic exposure and bioavailability of erlotinib was increased in Bcrp1/Mdr1a/1b(−/−) knockout mice. [DOI] [PubMed] [Google Scholar]
- 85.Rolff J, Dorn C, Merk J, et al. Response of Patient-Derived Non-Small Cell Lung Cancer Xenografts to Classical and Targeted Therapies Is Not Related to Multidrug Resistance Markers. J Oncol. 2009:814140. doi: 10.1155/2009/814140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–2743. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 87••.Wood ER, Truesdale AT, McDonald OB, et al. A unique structure for epidermal growth factor receptor bound to GW572016 (Lapatinib): relationships among protein conformation, inhibitor off-rate, and receptor activity in tumor cells. Cancer Res. 2004;64:6652–6659. doi: 10.1158/0008-5472.CAN-04-1168. Lapatinib was shown to binds to an inactive-like conformation of EGFR that is very different from the active-like structure bound by the selective EGFR inhibitor erlotinib. Lapatinib was also found to have a very slow off-rate from EGFR and ErbB-2 which correlated with a prolonged down-regulation ofreceptor tyrosine phosphorylation in tumor cells after the free drug was washed away. [DOI] [PubMed] [Google Scholar]
- 88.Burris HA, 3rd, Hurwitz HI, Dees EC, et al. Phase I safety, pharmacokinetics, and clinical activity study of lapatinib (GW572016), a reversible dual inhibitor of epidermal growth factor receptor tyrosine kinases, in heavily pretreated patients with metastatic carcinomas. J Clin Oncol. 2005;23:5305–5313. doi: 10.1200/JCO.2005.16.584. [DOI] [PubMed] [Google Scholar]
- 89.European Medicines Agency. Assessment report for Tyverb. 2008. Doc.Ref.: EMEA/302222/2008, http://www.emea.europa.eu.
- 90•.Ratain MJ, Cohen EE. The value meal: how to save $1,700 per month or more on lapatinib. J Clin Oncol. 2007;25:3397–3398. doi: 10.1200/JCO.2007.12.0758. This is an interesting commentary on health care economics of lapatinib treatment, but the assertion that reformulation to lower doses would reduce costs are unlikely. [DOI] [PubMed] [Google Scholar]
- 91.Koch KM, Beelen AP, Ho PT, et al. The value of label recommendations: how to dose lapatinib. J Clin Oncol. 2007;25:5331–5332. doi: 10.1200/JCO.2007.13.8644. author reply 5334–5335. [DOI] [PubMed] [Google Scholar]
- 92.Smith DA, Koch KM, Arya N, et al. Effects of ketoconazole and carbamazepine on lapatinib pharmacokinetics in healthy subjects. Br J Clin Pharmacol. 2009;67:421–426. doi: 10.1111/j.1365-2125.2009.03370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93••.Polli JW, Humphreys JE, Harmon KA, et al. The role of efflux and uptake transporters in [N-{3-chloro-4-[(3-fluorobenzyl)oxy]phenyl}-6-[5-({[2-(methylsulfonyl)ethy l]amino}methyl)-2-furyl]-4-quinazolinamine (GW572016, lapatinib) disposition and drug interactions. Drug Metab Dispos. 2008;36:695–701. doi: 10.1124/dmd.107.018374. The authors evaluate lapatinib as a substrate and inhibitor for a large set of drug transporters including both in vitro and in vivo data and a whole body autoradiograph. [DOI] [PubMed] [Google Scholar]
- 94•.Dai CL, Tiwari AK, Wu CP, et al. Lapatinib (Tykerb, GW572016) reverses multidrug resistance in cancer cells by inhibiting the activity of ATP-binding cassette subfamily B member 1 and G member 2. Cancer Res. 2008;68:7905–7914. doi: 10.1158/0008-5472.CAN-08-0499. This manuscript evaluates selected chemotherapeutics that may have increased efficacy due to inhibition of P-gp and BCRP by lapatinib. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Weil RJ, Palmieri DC, Bronder JL, et al. Breast cancer metastasis to the central nervous system. Am J Pathol. 2005;167:913–920. doi: 10.1016/S0002-9440(10)61180-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96•.Gril B, Palmieri D, Bronder JL, et al. Effect of lapatinib on the outgrowth of metastatic breast cancer cells to the brain. J Natl Cancer Inst. 2008;100:1092–1103. doi: 10.1093/jnci/djn216. Demonstrate that lapatinib can reduce tumor growth and phosphorylation of HER2 in a mouse model for metastasis of breast cancer cells to the brain. A large dose (100 mg/kg, oral) was required, but human dose is also high (10–20 mg/kg, oral) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bayer HealthCare Pharmaceuticals Inc. NEXAVAR Prescribing Information 2009 [Google Scholar]
- 98•.Chen HX, Cleck JN. Adverse effects of anticancer agents that target the VEGF pathway. Nat Rev Clin Oncol. 2009;6:465–477. doi: 10.1038/nrclinonc.2009.94. This reviews covers small-molecule and antibody inhibitors of VEGF detailing adverse effects in the cardiovascular and renal systems, as well as wound healing and tissue repair. It also discusses possible mechanism. [DOI] [PubMed] [Google Scholar]
- 99.European Medicines Agency. Nexavar , report H-690-en6. 2006. http://www.ema.europa.eu/humandocs/PDFs/EPAR/nexavar/H-690-en6.pdf.
- 100.Lathia C, Lettieri J, Cihon F, et al. Lack of effect of ketoconazole-mediated CYP3A inhibition on sorafenib clinical pharmacokinetics. Cancer Chemother Pharmacol. 2006;57:685–692. doi: 10.1007/s00280-005-0068-6. [DOI] [PubMed] [Google Scholar]
- 101••.Hu S, Chen Z, Franke R, et al. Interaction of the multikinase inhibitors sorafenib and sunitinib with solute carriers and ATP-binding cassette transporters. Clin Cancer Res. 2009;15:6062–6069. doi: 10.1158/1078-0432.CCR-09-0048. Tritiated sorafenib and sunitinib are used to evaluate substrate and inhibitor profiles for a wide range of transporters. transporters were found to limit brain exposure of both sorafenib and sunitinib. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 103.Motzer RJ, Michaelson MD, Rosenberg J, et al. Sunitinib efficacy against advanced renal cell carcinoma. J Urol. 2007;178:1883–1887. doi: 10.1016/j.juro.2007.07.030. [DOI] [PubMed] [Google Scholar]
- 104.Nishida T, Takahashi T, Nishitani A, et al. Sunitinib-resistant gastrointestinal stromal tumors harbor cis-mutations in the activation loop of the KIT gene. Int J Clin Oncol. 2009;14:143–149. doi: 10.1007/s10147-008-0822-y. [DOI] [PubMed] [Google Scholar]
- 105.Motzer RJ, Hutson TE, Tomczak P, et al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:3584–3590. doi: 10.1200/JCO.2008.20.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106••.van der Veldt AA, Boven E, Helgason HH, et al. Predictive factors for severe toxicity of sunitinib in unselected patients with advancedrenal cell cancer. Br J Cancer. 2008;99:259–265. doi: 10.1038/sj.bjc.6604456. The incidence of severe toxicity, defined as dose reduction or permanent discontinuation, was highly correlated with low body surface area, high age and female gender. A model was developed that could predict the probability of severe toxicity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Haznedar JO, Patyna S, Bello CL, et al. Single-and multiple-dose disposition kinetics of sunitinib malate, a multitargeted receptor tyrosine kinase inhibitor: comparative plasma kinetics in non-clinical species. Cancer Chemother Pharmacol. 2009;64:691–706. doi: 10.1007/s00280-008-0917-1. [DOI] [PubMed] [Google Scholar]
- 108•.Parkinson A, Mudra DR, Johnson C, et al. The effects of gender, age, ethnicity, and liver cirrhosis on cytochrome P450 enzyme activity in human liver microsomes and inducibility in cultured human hepatocytes. Toxicol Appl Pharmacol. 2004;199:193–209. doi: 10.1016/j.taap.2004.01.010. A compilation of data from donated human liver samples collected by the company Xenotech llc. [DOI] [PubMed] [Google Scholar]
- 109.van Erp NP, Gelderblom H, Karlsson MO, et al. Influence of CYP3A4 inhibition on the steady-state pharmacokinetics of imatinib. Clin Cancer Res. 2007;13:7394–7400. doi: 10.1158/1078-0432.CCR-07-0346. [DOI] [PubMed] [Google Scholar]
- 110.Zhou L, Meng F, Yin O, et al. Nilotinib for imatinib-resistant or -intolerant chronic myeloid leukemia in chronic phase, accelerated phase, or blast crisis: a single- and multiple-dose, open-label pharmacokinetic study in Chinese patients. Clin Ther. 2009;31:1568–1575. doi: 10.1016/j.clinthera.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 111.Christopher LJ, Cui D, Wu C, et al. Metabolism and disposition of dasatinib after oral administration to humans. Drug Metab Dispos. 2008;36:1357–1364. doi: 10.1124/dmd.107.018267. [DOI] [PubMed] [Google Scholar]
- 112.Kim DW, Goh YT, Hsiao HH, et al. Clinical profile of dasatinib in Asian and non-Asian patients with chronic myeloid leukemia. Int J Hematol. 2009;89:664–672. doi: 10.1007/s12185-009-0326-1. [DOI] [PubMed] [Google Scholar]
- 113.Bergman E, Forsell P, Persson EM, et al. Pharmacokinetics of gefitinib in humans: the influence of gastrointestinal factors. Int J Pharm. 2007;341:134–142. doi: 10.1016/j.ijpharm.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 114.Nakagawa K, Tamura T, Negoro S, et al. Phase I pharmacokinetic trial of the selective oral epidermal growth factor receptor tyrosine kinase inhibitor gefitinib (‘Iressa’, ZD1839) in Japanese patients with solid malignant tumors. Ann Oncol. 2003;14:922–930. doi: 10.1093/annonc/mdg250. [DOI] [PubMed] [Google Scholar]
- 115.Chu QS, Cianfrocca ME, Goldstein LJ, et al. A phase I and pharmacokinetic study of lapatinib in combination with letrozole in patients with advanced cancer. Clin Cancer Res. 2008;14:4484–4490. doi: 10.1158/1078-0432.CCR-07-4417. [DOI] [PubMed] [Google Scholar]
- 116.Britten CD, Kabbinavar F, Hecht JR, et al. A phase I and pharmacokinetic study of sunitinib administered daily for 2 weeks, followed by a 1-week off period. Cancer Chemother Pharmacol. 2008;61:515–524. doi: 10.1007/s00280-007-0498-4. [DOI] [PubMed] [Google Scholar]
- 117.Manley PW, Cowan-Jacob SW, Buchdunger E, et al. Imatinib: a selective tyrosine kinase inhibitor. Eur J Cancer. 2002;38(Suppl 5):S19–27. doi: 10.1016/s0959-8049(02)80599-8. [DOI] [PubMed] [Google Scholar]
- 118.Golemovic M, Verstovsek S, Giles F, et al. AMN107, a novel aminopyrimidine inhibitor of Bcr-Abl, has in vitro activity against imatinib-resistant chronic myeloid leukemia. Clin Cancer Res. 2005;11:4941–4947. doi: 10.1158/1078-0432.CCR-04-2601. [DOI] [PubMed] [Google Scholar]
- 119.Kumar R, Crouthamel MC, Rominger DH, et al. Myelosuppression and kinase selectivity of multikinase angiogenesis inhibitors. Br J Cancer. 2009;101:1717–1723. doi: 10.1038/sj.bjc.6605366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang L, Christopher LJ, Cui D, et al. Identification of the human enzymes involvedin the oxidative metabolism of dasatinib: an effective approach for determining metabolite formation kinetics. Drug Metab Dispos. 2008;36:1828–1839. doi: 10.1124/dmd.107.020255. [DOI] [PubMed] [Google Scholar]
- 121.FDA. DASATINIB (BMS-354825) ONCOLOGIC DRUG ADVISORY COMMITTEE BRIEFING DOCUMENT -NDA 21-986 2006 [Google Scholar]
- 122.Swaisland HC, Ranson M, Smith RP, et al. Pharmacokinetic drug interactions of gefitinib with rifampicin, itraconazole and metoprolol. Clin Pharmacokinet. 2005;44:1067–1081. doi: 10.2165/00003088-200544100-00005. [DOI] [PubMed] [Google Scholar]
- 123.European Medicines Agency. Sutent , report H-687en. http://www.ema.europa.eu/humandocs/PDFs/EPAR/sutent/emea-combined-h687en.pdf.