Abstract
Organophosphate pesticides (OPs) are environmental toxicants known to inhibit the catalytic activity of acetylcholinesterase (AChE) resulting in hypercholinergic toxicity symptoms. In developing embryos, OPs have been hypothesized to affect both cholinergic and non-cholinergic pathways. In order to understand the neurological pathways affected by OP exposure during embryogenesis, we developed a subacute model of OP developmental exposure in zebrafish by exposing embryos to a dose of the OP metabolite chlorpyrifos oxon (CPO) that is non-lethal and significantly inhibited AChE enzymatic activity compared to control embryos (43% at 1 day post-fertilization (dpf) and 11% at 2 dpf). Phenotypic analysis of CPO-exposed embryos demonstrated that embryonic growth, as analyzed by gross morphology, was normal in 85% of treated embryos. Muscle fiber formation was similar to control embryos as analyzed by birefringence, and nicotinic acetylcholine receptor (nAChR) cluster formation was quantitatively similar to control embryos as analyzed by α-bungarotoxin staining. These results indicate that partial AChE activity during the early days of zebrafish development is sufficient for general development, muscle fiber, and nAChR development. Rohon-Beard (RB) sensory neurons exhibited aberrant peripheral axon extension and gene expression profiling suggests that several genes responsible for RB neurogenesis are down-regulated. Stability of CPO in egg water at 28.5 °C was determined by HPLC-UV-MS analysis which revealed that the CPO concentration used in our studies hydrolyzes in egg water with a half-life of one day. The result that developmental CPO exposure affected RB neurogenesis without affecting muscle fiber or nAChR cluster formation demonstrates that zebrafish are a strong model system for characterizing subtle neurological pathologies resulting from environmental toxicants.
Keywords: Organophosphate, chlorpyrifos, Rohon-Beard sensory neurons, developmental neurotoxicity, biomarkers
1. Introduction
Thirty-seven organophosphate pesticides (OPs) are currently registered for use in the US including chlorpyrifos, malathion, parathion, azinphos, and diazinon. Over 80 million pounds are used each year for crop protection and for the control of vector-borne disease (EPA, 2006). The United States Centers for Disease Control reports approximately 25,000 cases of OP poisoning are made to poison control centers each year, and 40% of those reports involved children under age six. OPs cause excitotoxicity resulting from acetylcholinesterase (AChE) inhibition by phosphorylation of a catalytic serine residue. Inhibition of AChE renders it unable to hydrolyze the neurotransmitter acetylcholine (ACh). ACh reaches toxic concentrations in the cholinergic neural synapse causing hyperstimulation of receptors and a depolarizing block of neuromuscular junction receptors on the post-synaptic neuron (Fukuto, 1990). Acute toxic symptoms of OP exposure include tremors, lacrimation, and bradyarrhythmia, as well as potential fatality (reviewed in (Sultatos, 1994; Costa, 2006)). AChE has non-cholinergic roles in neuron developmental processes including cell adhesion, neurite growth and network formation, and it is thought that OPs may affect these functions as well as enzymatic activity (reviewed in (Paraoanu and Layer, 2008)).
The prevalence of childhood exposures has raised concerns over the long-term effects of developmental exposure (reviewed in (Slotkin, 2004)). Recent studies have associated adverse neurologic and growth outcomes in children exposed to OPs in utero including motor inhibition and verbal learning (Jacobson and Jacobson, 2006). Two associated studies correlated developmental exposure with abnormal reflexes (Young et al., 2005) and diagnosed mental developmental problems (Eskenazi et al., 2007). OPs are thought to cause developmental neurotoxicity and long-term cognitive and behavior effects through routes including cholinergic mechanisms, interference with non-enzymatic functions of AChE (such as neurite outgrowth), and effects on cell signaling pathways involved in neural cell differentiation (reviewed in (Slotkin, 2004)).
Studies on in vitro mammalian neuronal cultures have supported the hypothesis that OPs inhibit neurite outgrowth. Primary embryonic rat dorsal root ganglion preps were exposed to chlorpyrifos-oxon (CPO) and axon extension was inhibited. These authors concluded that this effect of CPO is AChE-dependent because cultures from ache-/- mice did not show this effect unless transfected with an AChE-expression construct (Yang et al., 2008). CPO has also been shown to bind residues in purified tubulin to inhibit polymerization (Grigoryan and Lockridge, 2009) which may result in impaired neurite outgrowth. We predict that the zebrafish model will be useful in identifying not only defects in neurite outgrowth but also susceptible neuron populations which are preferentially affected by developmental OP exposure.
Studies on the developmental neurotoxicity of chlorpyrifos in zebrafish have indicated neurobehavioral defects. In two related studies, developmental exposure to chlorpyrifos-thionate (CPS) has demonstrated effects on spatial discrimination and response latency in adult zebrafish that were exposed during development (Levin et al., 2003), and a slowing of larvae swimming behavior (Levin et al., 2004). These doses of CPS were also shown to cause a latency in inhibition of AChE enzymatic activity. Exposure of embryos to CPS did not cause inhibition of AChE until 2 dpf, correlating with the requirement for metabolic activation of CPS to CPO (Linney et al., 2004).
We are interested in studying the developmental effects of OPs on early neurogenesis in order to understand the cognitive and locomotor defects previously described in human populations (Jacobson and Jacobson, 2006; Eskenazi et al., 2007). In human exposures to the pesticide CPS, CPS undergoes cytochrome P450 metabolism to the bioactive metabolite, CPO. Pharmacokinetic studies in rodent models have shown that CPS is rapidly metabolized in the mother prior to crossing the placenta, and the fetus is primarily exposed to CPO (Abdel-Rahman et al., 2002). The rate of metabolic activation is highly dependent on the route of exposure, and cytochrome P450 enzyme expression levels (Smith et al., 2009). Due to these confounding factors and the fact that we wanted to look at changes in early neurological development, we exposed zebrafish embryos to CPO, which should result in a more consistent exposure.
In this study, a zebrafish model of OP developmental neurotoxicity has been established. A sublethal dose of CPO affected general morphology of a small percentage of zebrafish embryos, but did affect movement and touch response of most of the exposed embryos at 1 day post-fertilization (dpf). Muscle development and neuromuscular junctions were relatively unaffected by the CPO exposure. Due to lack of obvious phenotypes and prevalence of a behavioral phenotype, we hypothesized that development of Rohon-Beard neurons (RB) may be affected. RB neurons are an important subset of early sensory neurons which detect touch stimuli and initiate an escape response in the early zebrafish embryo (Clarke et al., 1984). Development of RB neurons was shown to be affected, and examination of axons projecting from the RB neurons indicated that projections of peripheral axons, but not central axons, were strongly affected. Gene expression analysis of a subset of genes that participate in RB development demonstrated that several were down-regulated. This study demonstrates that zebrafish are an excellent model system to study pesticide developmental neurotoxicity and can lead to more defined studies in rodent models. Zebrafish are also a useful tool to identify biomarkers (i.e. genes, affected pathways) of developmental exposure.
2. Materials and Methods
2.1 Fish stocks and embryo production
Wild-type strain AB zebrafish were maintained, bred, and raised as described (Westerfield, 2000). Egg medium consisted of 60 μg/ml sea salts (S9883, Sigma-Aldrich, St. Louis, MO). All anesthetizations and euthanizations were carried out with 200 mg/L Tricaine. Animal care and experimentation were performed in compliance with IACUC Animal Use Protocol 034-07KGBMED.
2.2 Chlorpyrifos treatment
Diethoxy chlorpyrifos oxon (CPO) was purchased (MET-674B, ChemService, Inc.). For each experiment, CPO was first diluted in absolute ethanol to the appropriate concentration. Either diluted CPO or ethanol alone (vehicle control) was added to fertilized eggs in egg medium at 3 hours post-fertilization (hpf) (final v/v of 0.1%) and embryos were incubated at 28.5°C. This was a single dosing regimen, as CPO was not re-added on subsequent dpf. For all staining and biochemical assays, embryos were euthanized at 24 hour increments after the initial exposure at 3 hpf. To simplify the text and figure legends, we state that 27 hpf embryos are 1 dpf, 51 hpf embryos are 2 dpf, and 75 hpf embryos are 3 dpf.
2.3 Dose-response survival curve
Embryos were placed individually in wells of a 96-well microplate and egg water containing either ethanol or CPO (20 nM to 3 μm) was added. Each day post-fertilization, embryos were inspected under a dissecting microscope and scored for viability by gross morphology and heartbeat. At least twenty-four embryos were analyzed per treatment.
2.4 Gross morphology analysis
Gross morphological analysis was performed on embryos at 1-3 dpf in order to initially characterize the CPO phenotype. Control and CPO-exposed embryos were imaged under an Olympus SZX16 dissecting microscope each day of development. Independent, unbiased observers analyzed the images and recorded normal morphology versus severe morphology phenotypes by scoring embryos with reduced pigmentation, curved backs, and yolk sac defects as severe phenotypes.
2.5 Microscopy of live embryos
Embryo responses to touch with a horsehair needle were recorded with a digital camera (Olympus DP71) attached to an Olympus SZX16 dissecting microscope using DP Controller software (Olympus). Each response was recorded at a rate of 25 frames/s. Image file size was compressed using Prism Video File Format Converter (v. 1.40).
2.6 In situ measurement of AChE enzymatic activity: Karnovsky-Roots stain
Detection of AChE activity in situ was adapted (Karnovsky and Roots, 1964; Downes and Granato, 2004). Embryos were euthanized, washed, and incubated for 4 hours (h) at room temperature (RT) in 60 mM sodium acetate pH 6.4, 5 mM sodium citrate, 4.7 mM cupric sulfate, 0.5 mM potassium ferricyanide, and 1.7 mM acetylthiocholine-iodide (ATCh-I). The intensity of the copper ferrocyanide staining is indicative of AChE activity. Prior to imaging, stained embryos were washed in egg water.
2.7 Spectrophotometric measurement of AChE activity: Ellman assay
Detection of AChE activity in whole embryo lysates was adapted (Behra et al., 2004; Thullbery et al., 2005). Embryos were euthanized and washed in egg water. Embryos (15-25) were suspended in 150-250 μL lysis buffer (20mM Tris/HCl pH 7.0, 5 mM EDTA, 1% Triton X-100) and passed through a 27-gauge syringe 10 times. Extracts were centrifuged at 9300 × g; supernatants were diluted in 100 mM phosphate buffer, pH 7.0, and 0.3 mM 5,5′-Dithiobis(2-nitrobenzoic acid) (DTNB). ATCh-I was added to a final concentration of 0.75 mM. Absorbance was measured at OD 412 nm over a 3-min period at 25°C on a VersaMax microplate reader with SOFTMAX PRO v.3.0 software (Molecular Devices, Menlo, CA). Total protein concentration was determined using a BCA Protein Assay Kit (Pierce Biotechnology, Inc., Rockford, IL). AChE activity is expressed as μmole ATCh-I hydrolyzed/min/mg of protein using ε=1.36×104 M−1cm−1 as the extinction coefficient of the anion generated by DTNB cleavage and 0.6 cm as the length of light path. To account for non-enzymatic hydrolysis of substrate, blanks (with no lysate) were measured and subtracted from test sample values. Ellman assays were performed in triplicate in three independent experiments.
2.8 Birefringence analysis
Birefringence was analyzed as described previously (Behra et al., 2002). Briefly, embryos were embedded in methylcellulose, mounted under an epifluorescent Nikon microscope and illuminated with polarized light. The parallel alignment of normal muscle fibers reflects polarized light. The degree of reflection was translated into a color code to reveal the differences in intensities between control and CPO-exposed embryos (Gradient Map tool in Adobe Photoshop 11.0). The Gradient Map adjustment converts the equivalent grayscale range of an image to the colors of a specified gradient fill. Ten control and CPO-exposed embryos were analyzed for birefringence and all demonstrated equivalent birefringence.
2.9 α-bungarotoxin (α-btx) staining
Embryos were euthanized and fixed in 4% paraformaldehyde for 4 h at RT followed by permeabilization in 1 mg/ml collagenase solution (Sigma-Aldrich) for 30 min at RT. Embryos were incubated for 30 min at RT in 10 μg/ml α-btx-Alexa 594 (Molecular Probes-Invitrogen) diluted in 10% fetal calf serum, 1% DMSO in PBS-T (Downes and Granato, 2004).
2.10 Immunofluorescence
At each timepoint, zebrafish embryos were dechorionated and euthanized. For HNK-1 staining, embryos were fixed in 4% paraformaldehyde for 4 h at RT, permeabilized in 1 mg/ml Proteinase K for 15 min, and blocked in 10% normal goat serum (NGS) in PBS-DT (phosphate-buffered saline, 0.1% Tween-20, 1% DMSO) for 4 h. Embryos were incubated with α-HNK-1 antibody (C0678, Sigma-Aldrich) (1:1000) in blocking buffer overnight (o/n) at 4°C, followed by a secondary antibody α-mouse IgM Alexa Fluor 488 (1:1000, Jackson ImmunoResource, West Grove, PA) o/n at 4 C This antibody recognizes the CD57/HNK-1 human myeloid cell associated surface glycoprotein and has been shown to bind Rohon-Beard and trigeminal neurons at 1 dpf (Kruse et al., 1984; Trevarrow et al., 1990). For acetylated tubulin staining, embryos were fixed in 4% paraformaldehyde for 4 h at RT, permeabilized in cold 100% acetone for 7 min, washed in water for 1 h, and blocked in 4% NGS in PBS-T for 1 h at RT. Embryos were incubated with α-acetylated tubulin antibody (T7451, Sigma-Aldrich) in blocking buffer o/n at 4°C, followed by a secondary antibody goat-α-mouse IgG Alexa Fluor 488 (Molecular Probes – Invitrogen) for 1 h at RT.
2.11 Microscopy and image analysis
Images were taken on a NIKON epifluorescent microscope, an Olympus SZX16 dissecting stereoscope, and an Olympus FV1000 confocal microscope. Each image shown was representative of at least 10 embryos. Image J was used to analyze chevron angles. An independent observer measured chevron angles on twenty control and CPO-exposed embryo images. Image Pro Analyzer 6.3 was used for α-btx quantitation using the IOD tool.
2.12 Quantitative Real-Time PCR
Total RNA was extracted from pools of fifty 1 dpf control and 300 nM CPO-exposed embryos with TRIzol/chloroform, DNaseI treated, and column purified (Omega Bio-Tek, Inc., Norcross, GA). PCR primers were designed using the Roche Universal Probe Library (UPL) for Zebrafish Assay Design Center (Supplemental Table S1). Primers were synthesized by Integrated DNA Technologies (IDT, Coralville, IA). All primer sets spanned an exon–exon junction to avoid errors due to contaminating genomic DNA. Probes were selected from the Roche UPL. Amplification and detection of the fluorescence were measured using a Stratagene Mx3005p (Stratagene, La Jolla, CA). All signals were normalized against β-actin and gapdh. The fold change and p-values in Supplemental Figure 1 were determined by comparing mRNA expression in CPO-exposed to control embryos. Once data had been normalized (ΔCT), the average of 4 replicates in each group was used to calculate the ΔΔCt and fold change (fold-change = 2(ΔΔCT)). p-values were calculated using ΔΔCt values, n=4.
2.13 Stability of chlorpyrifos
CPO was diluted in ethanol as described above. CPO was added to fertilized eggs in egg water at final concentrations of 300 nM or 3 μM (final v/v of 0.1%) and incubated at 28.5 °C. An aliquot was removed each day of incubation, and analyzed by HPLC-MS analysis. A HPLC system consisted of Waters 2790 high-performance liquid chromatograph connected to a Waters 2487 dual absorbance UV detector and Waters Micromass LCT KC290 mass spectrometer. This system was equipped with an Agilent Eclipse XDB-C18 (5 μm, 4.6 × 250 mm) column connected with a guard column. The mobile phase for pump A was 0.02 M ammonium acetate in water/ acetonitrile (80: 20, v/v) and for pump B was 0.02 M ammonium acetate in methanol. The gradient profile was 0–5 min (0% B to 100 % B), 5–13 min (100% B), 13–15 min (100% B to 0% B). The flow rate was 0.7 mL/min, and the injection volume was 100 μL. The wavelength of detection was 293 nm. The method was validated for linearity and precision. The limit of detection of CPO using this method was 150 nM. The retention time (tR) for CPO and its hydrolysis product, 3,5,6-trichloro-2-pyridinol (TCP) was 9.37 and 4.60 min respectively. The area under the curve (AUC) for the CPO peak (tR 9.37 min) and degradation products peaks (tR = 4.60 and 7.6 min) was calculated. The ratio of CPO AUC to that of degradation product AUC was calculated, and % CPO or degradation product was plotted against time. This experiment was performed 3 times and the data represents averages of the three experiments with SEM.
2.14 Statistical analysis
Comparison of control and CPO-exposed values were calculated using Student's unpaired two-tailed t-tests (for Figures 6E, 6F, and 7E) and a two-way ANOVA with Bonferroni post-test (Figure 1 and Figure 4) with GraphPad Prism software (v. 4).
Figure 6.
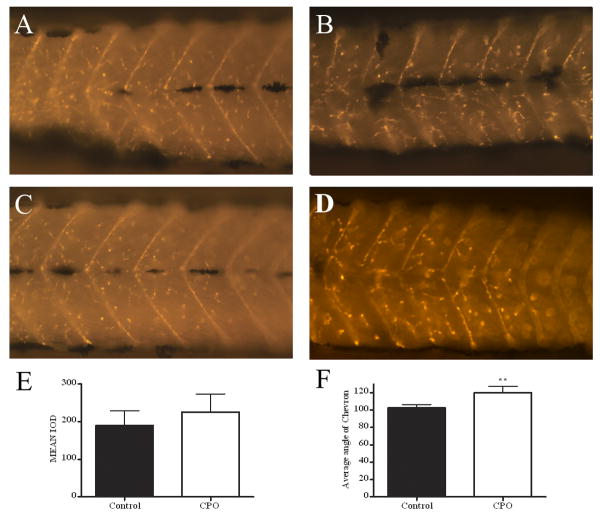
CPO exposure does not affect nAChR cluster formation but does widen the chevron angle. α-bungarotoxin –Alexa 594 staining of nAChRs in control (A and C) and and CPO-exposed (B and D) 3 dpf larvae. (E) Quantification of fluorescent staining for α-btx revealed that the mean IOD was not significant. (F) Measurement of the chevron angle in anterior, middle, and posterior sections of larvae indicated that the CPO-exposed larvae had a significantly wider angle (two-tailed, unpaired t-test, p = 0.002).
Figure 7.
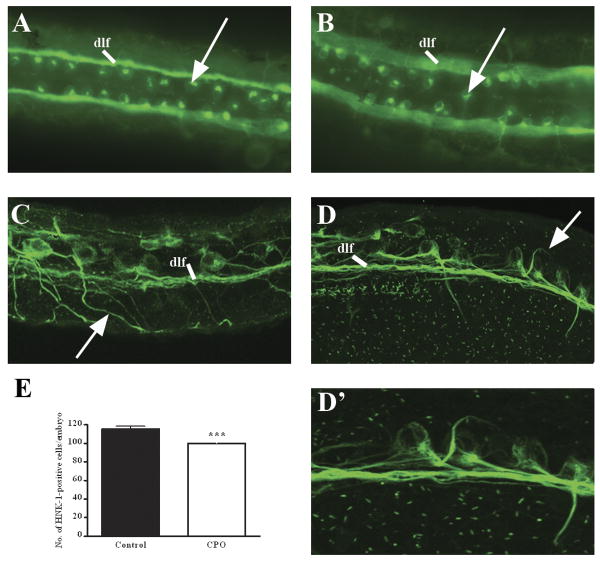
CPO affects RB neurogenesis. Control (A) and CPO-exposed (B) 1 dpf embryos were immunostained with anti-HNK-1. Arrows point to RB neuron bodies. Shown is a dorsal view with anterior to the left. Control (C) and CPO-exposed (D, D′) embryos were immunostained with anti-acetylated tubulin. D′ is a magnification of D. Arrows point to peripheral axon extensions. Shown are lateral views of embryos, anterior left, dorsal up. dlf, dorsal lateral fasiculus. (E) Quantification of HNK-1-positive cells/embryo. Shown is average number of HNK-1-positive cells/embryos with standard deviations. Two-tailed, unpaired t-test ***, p <0.0001.
Figure 1.
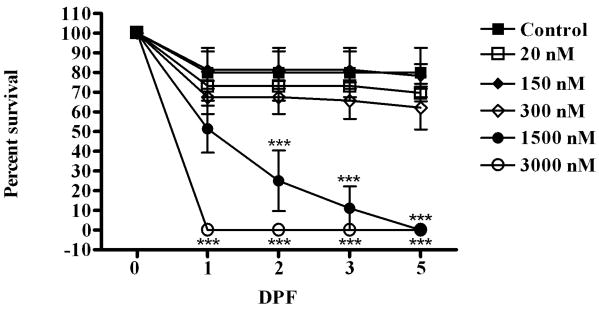
Lethal dose response curve of CPO exposure. Embryos were exposed to varying concentrations of CPO beginning at 3 hpf and analyzed for viability by morphology and heartbeat. Shown is the average values (and standard deviations) from three independent experiments with 24 embryos/treatment analyzed in each experiment. Statistical analysis used two-way ANOVA with Bonferroni post-test ***, p < 0.0001 to compare CPO-treated embryos to control (indicated above the data points for 1500 nM and below data points for 3000 nM).
Figure 4.
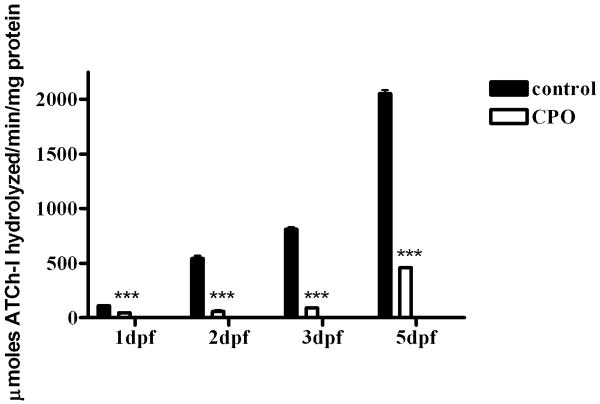
CPO exposure inhibits AChE activity as measured by Ellman assay in pooled whole embryo lysates. Black bars, control embryos; white bars, CPO-exposed embryos. Samples were tested in triplicate in three independent experiment. Averages of the independent experiments are shown with standard deviations. Statistical analysis used two-way ANOVA with Bonferroni post-test ***. p < 0.0001.
3. Results
3.1 Determination of a sublethal dose of CPO
To develop a zebrafish model of subacute developmental CPO exposure, we needed to determine a dose of CPO that is not lethal but affects CNS development. Zebrafish embryos were exposed at 3 hpf to varying concentrations of CPO and effects on survival were quantitated. In this exposure model, CPO was added to egg water at 3 hpf and the water was not changed, nor was additional CPO added during the exposure period. During the first 24 hpf, a percentage of embryos died in all treatments, including controls (Fig. 1). Embryos exposed to the highest concentration of CPO, 300 μM, all died by 1 dpf while other treatments resulted in between 15% and 35% death. By 2 dpf, embryos in the remaining treatment groups leveled off, except the 150 μM treatment group which decreased in survival rate until 3 dpf, when all had died. Kuster measured the effective concentration of paraoxon-methyl to cause 50% zebrafish embryo (EC50) death at 2 dpf as 200 μM (Kuster and Altenburger, 2006). A similar analysis of our data would approximate 1.5 μM as the EC50 at 2 dpf. Our and Kuster's exposure paradigm were very similar and in both studies, the dose response curves showed a non-linear relationship between dose and survival.
From this data, we decided to use 300 nM CPO exposure in the remainder of our experiments to identify neurological effects of OP exposure as this concentration did not result in a significant reduction in survival. We also decided that although this exposure is high, the neurological effects would be relevant in initial establishment of a developmental CPO exposure model.
3.2 Morphology of CPO-exposed zebrafish
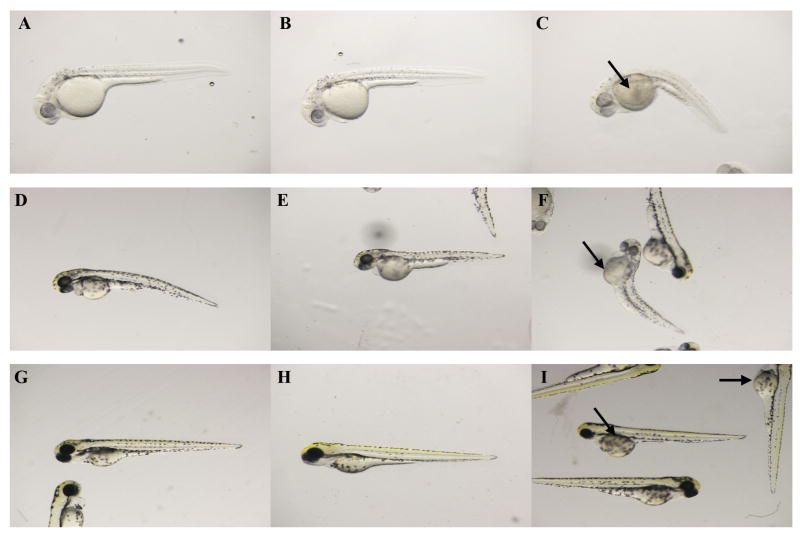
To determine an initial phenotypic effect of CPO exposure on zebrafish embryos, the gross morphology of CPO-exposed embryos was analyzed at 1, 2, and 3 dpf. We observed mild phenotypes (similar to control embryos) as well as severe phenotypes (axial curvature, reduced body size, and reduced pigment). At 1 dpf, a majority of CPO-treated zebrafish appeared similar to control, although 13.5% exhibited the severe phenotype (Fig. 2, A-C). When manually released from their chorions, CPO-exposed embryos did show spontaneous twitching, but did not exhibit the robust movement similar to control embryos or respond to touch (Downes and Granato, 2004) (Supplemental Movies 1 and 2). At 2 dpf, again the majority of CPO-exposed zebrafish appeared similar to controls while 14.6% exhibited the severe phenotype (Fig. 2, D-F). By 3 dpf, most CPO-exposed embryos appeared normal compared to control embryos while 13.3% exhibited the severe phenotype (Fig. 2, H-J). Percentages and phenotypes of control and CPO-treated embryos are summarized in Table 1.
Figure 2.
Gross morphology phenotypes of CPO-exposed embryos. Embryos were imaged at 1 dpf (A-C), 2 dpf (D-F), and 3 dpf (G-I). Control embryos (A, D, G), 300 nM-exposed embryos with mild phenotype (B, E, H), and 300 nM-exposed embryos with severe phenotype (C, F, I). Arrows indicate enlarged yolk sacs with edema.
TABLE 1. Percentage of Control and CPO-exposed Embryos with Normal or Severe Phenotypes.
| Treatment | dpf | Normal | Severe |
|---|---|---|---|
| Control | 1 | 98.0 | 2.0 |
| 2 | 96.0 | 4.0 | |
| 3 | 96.2 | 3.8 | |
| CPO | 1 | 86.5 | 13.5 |
| 2 | 85.4 | 14.6 | |
| 3 | 86.7 | 13.3 |
Note: Embryos were evaluated for gross morphology phenotypes. Severe phenotypes include albino pigmentation, curved backs, and yolk sac defects. Data is represented as percentage of total. N=80-100.
In summary, approximately 87% of CPO-exposed zebrafish embryos appeared morphologically similar to control embryos. The percentage of embryos with severe phenotypes (axial curvature, reduced body size, and reduced pigment) was consistent through the course of the experiment. This indicates that the severe phenotype was established by 1 dpf and did not increase or change with time. In further microscopy experiments, we analyzed zebrafish embryos and larvae that exhibited a normal gross morphology.
3.3 Effect of 300 nM CPO on AChE activity
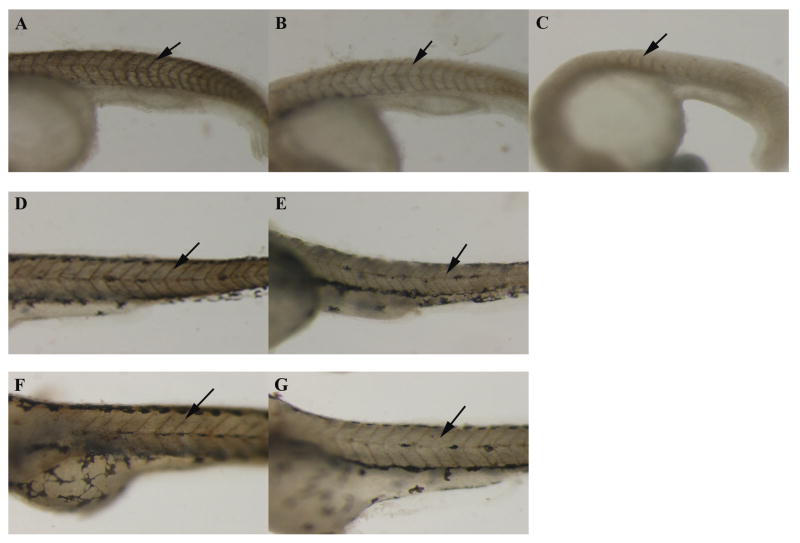
The primary target of OPs is inhibition of the catalytic activity of AChE, so the effect of CPO on AChE activity in zebrafish embryos was measured with the in situ Karnovsky-Roots stain (Karnovsky and Roots, 1964) and the quantitative, biochemical Ellman assay (Ellman et al., 1961). Embryos were exposed to 300 nM or 3 μM CPO at 3 hpf, and AChE activity was detected with Karnovsky-Roots stain at 1, 2, and 3 dpf (Fig. 3). The intensity of the copper ferrocyanide staining is indicative of AChE activity. At 1 dpf, 300 nM exposure resulted in reduced AChE activity compared to controls (Fig. 3, A-B), and 3 μM exposure completely inhibited AChE activity (Fig. 3, C). Note the axial curvature of the 3 μM-exposed embryo indicating severe developmental abnormalities. The 3 μM exposed zebrafish embryos died by 2 dpf, so Karnovsky-Roots staining is shown only for the control and 300 nM CPO-exposed embryos. Similarly, at 2 and 3 dpf, the 300 nM CPO-exposed embryos displayed much less intense Karnovsky-Roots stain, indicating that the CPO exposure reduced AChE activity.
Figure 3.
CPO exposure inhibits in situ AChE activity as measured by Karnovsky-Roots staining. Embryos were imaged at 1 dpf (A-C), 2 dpf (D and E), and 3 dpf (F and G). Control embryos (A, D, F), 300 nM-exposed embryos (B, E, G), 3 μM embryos (C) are shown. Intensity of brown stain indicates activity of AChE (see Materials and Methods section). Staining can be clearly seen along the somite boundaries in the control embryos and slightly in the 300 nM-exposed embryos (arrows).
The Karnovsky-Roots stain is a visual indication of AChE activity, but is not quantitative. To quantitate the amount of AChE activity in the exposed embryos, we employed the Ellman assay, which spectrophotometrically measures AChE activity. In this experiment, AChE activity of whole embryo lysates was analyzed using embryos treated with either 300 nM CPO or ethanol control. At 1 dpf, AChE activity was inhibited to 43% of control, but on subsequent days, the percent of activity dramatically dropped to approximately 11% of control (Fig. 4). We hypothesize that AChE activity was affected more at 2 dpf than at 1 dpf because the chorion protects the embryos during the first day of development. This protective effect of chorions for environmental toxicants was also demonstrated for salmonid eggs (Finn, 2007). The result that the CPO-exposed embryos appear morphologically similar to control indicates that the amount of AChE activity during the first day of development was sufficient to allow most developmental processes to occur.
The amount of AChE activity determined in our Ellman assays on control embryos is consistent with previous results using whole embryo lysates (Kuster and Altenburger, 2006). Paraoxon-methyl exposure of zebrafish embryos demonstrated that significant inhibition of AChE was accomplished with 120 nM paraoxon-methyl at 2 dpf, and our exposure of 300 nM CPO also significantly inhibited AChE activity. In both our and Kuster's exposure paradigms, the lethal dose to zebrafish embryos was significantly higher than the concentration sufficient to inhibit AChE activity.
In summary, 300 nM CPO does not kill zebrafish embryos but does significantly inhibit AChE activity. This indicates that 100% AChE activity is not necessary for embryonic survival. To further examine the developmental phenotypes due to CPO toxicity, we analyzed muscle formation, nAChR synapse formation, and the formation of a prominent neuron sub-population, the Rohon-Beard (RB) neurons.
3.4 Effect of developmental exposure of CPO on muscle development
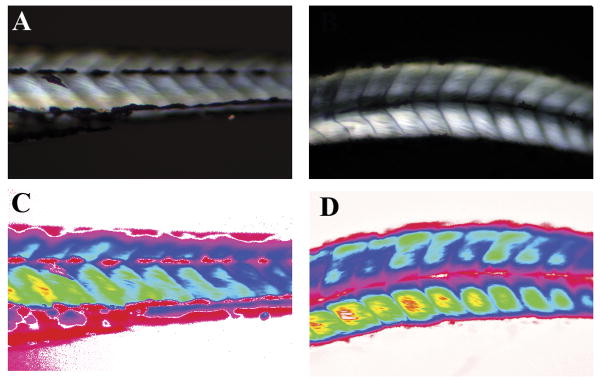
Previous studies have shown that a lack of functional AChE protein caused a progressive myopathy in achesb55 and zim(ache) mutant zebrafish larvae (Behra et al., 2002; Downes and Granato, 2004), and it was of interest to determine whether CPO exposure results in similar defects. CPO-exposed zebrafish were slower to respond to touch once released from chorions, and this behavioral phenotype could be due to defects in muscle development and/or neurological innervations of muscles. We examined whether CPO affected muscle development by observing birefringence intensity on 3 dpf embryos. The overall health of muscle fibers can be analyzed with birefringence; the reflection of polarized light is observed from parallel aligned fibers but not disorganized muscle fibers (Behra et al., 2002). Control and CPO-exposed zebrafish demonstrated similar birefringence (Fig 5, compare A and C with B and D). This indicates that muscle fibers and myofibrils align in parallel properly after CPO exposure, and CPO does not severely affect muscle development. When comparing our treated embryos with the phenotype of ache-/- embryos, it is apparent that the moderate inhibition of AChE catalytic activity during the 1 dpf followed by the almost complete inhibition in the following days still allowed for sufficient muscle formation.
Figure 5.
CPO exposure does not affect birefringence of larvae. Control (A) and CPO-exposed (B) larvae were imaged for birefringence at 3 dpf. Intensity of birefringence is translated to red/yellow colors by spectral representation for control (C) and CPO-exposed (D) larvae. Larvae are in lateral view at the level of the hindgut extension. Anterior left, dorsal up. Larvae imaged were from the normal phenotype group based on gross morphology (Fig.2).
3.5 Neuromuscular junction development in CPO-exposed zebrafish larvae
AChE has been shown to be important in establishing nAChR clusters so it was of interest to determine whether CPO exposure affected nAChR cluster development. Control and CPO-exposed zebrafish larvae were stained with α-bungarotoxin (α-btx) to label nAChR, and were imaged in the anterior, middle, and posterior segments. α-btx staining appeared qualitatively similar in both control and CPO-exposed embryos; however, the angle of the chevron seemed wider (Fig 6, compare A and C with B and D). In order to quantitate the nAChR clusters stained by α-btx, we measured the number and intensity of fluorescent pixels in the images by setting the intensity of fluorescence with a control larvae image and comparing the integrated optical density (IOD) of fluorescence in control and CPO-exposed larvae images in an identical anatomical region (posterior to yolk sac extension) (Fig 6E). The IOD was not statistically different between control and CPO-exposed larvae indicating that the number and density of nAChR clusters is not affected by CPO. The angle of the chevron was measured in control and CPO-exposed larvae and the chevron angle was indeed broader in CPO-exposed larvae (Fig. 6F) indicating that while axial muscle formation and differentiation does not appear to be drastically affected (see birefringence above), some aspect of somite or myotome development was altered by this dose of CPO. The broader angle of the chevron in our exposure has some similarities, but are not identical, to mutations which affect somite formation in zebrafish including fused somites (fss) and you-too (yot) (van Eeden et al., 1996)
3.6 Neurogenesis of RB neurons and axon projections
At 1 dpf, CPO-exposed embryos spontaneously twitched, but did not respond as well to touch as control embryos (Supplemental movies 1 and 2). Based on this behavioral phenotype and the minimal effect of CPO on muscle and nAChR formation, we hypothesized that RB neurogenesis may be affected. RBs are an important subset of early sensory neurons and express high levels of AChE activity (Bertrand et al., 2001). RB cell bodies are large and lie in two bilateral rows within the dorsal spinal cord on either side of the dorsal midline roof plate (Liu and Halloran, 2005), and are easily identified by immunofluorescent staining with anti-HNK-1 antibody. This antibody recognizes the CD57/HNK-1 human myeloid cell associated surface glycoprotein and has been shown to bind Rohon-Beard and trigeminal neurons at 1 dpf (Kruse et al., 1984; Trevarrow et al., 1990). Examination of HNK-1 staining of CPO embryos indicated that the bilateral placement of RB neurons was slightly disorganized (Fig. 7A and B), and CPO-exposed embryos had a significantly less number of RB neurons (Fig. 7E). The reduction of RB neurons, in addition to the disorganization of RBs along the dorsal midline, suggests that CPO may affect the formation of this subset of neurons.
To further examine the axon projections of RB neurons, embryos were stained with anti-acetylated tubulin antibody. RB neurons send out two axon projections: central axons which extend anteriorly and posteriorly to form the dorsal longitudinal fasciculus (DLF), and peripheral axons which exit the spinal cord and branch over the lateral surface of the embryo. In control embryos, the DLF and peripheral axons were clearly visible (Fig. 7C). In CPO-exposed embryos, central axons properly form the DLF, however the peripheral axons do not extend out of the spinal cord, but begin to extend ventrally and then deviate from their defined path (Fig. 7D and 7D′). The multiple effects on RBs in CPO-exposed embryos demonstrates that CPO is highly toxic to this subset of neurons. RBs express high amounts of AChE and our data indicates that the inhibition of AChE in RBs may have directly caused defects in their neurogenesis.
3.7 q-RT-PCR analysis of genes involved in RB neurogenesis
We hypothesized that the defects in RB neurogenesis and peripheral axon projection may be due to the differential expression of genes involved in RB neurogenesis. We identified four candidate genes whose expression may be altered during CPO exposure: agrin, cntn2, ntf3, and sema3d. Agrin has been shown to be required for nAChR cluster size and number as well as formation of RB axons (Kim et al., 2007). Ntf3 (neurotrophin 3) is the neurotrophic ligand for the TrkC1 receptor and has been implicated in survival of RB neurons (Williams et al., 2000). Cntn2 (transient axonal glycoprotein-1, contactin2) has been shown to guide RB central axons and sema3d (semaphorin3D) has been shown to guide RB peripheral axons (Liu and Halloran, 2005). We chose to analyze these genes by q-RT-PCR to determine whether their expression had changed upon exposure to CPO. Primer sequences and UPL probes are listed in Supplemental data, Table 1. q-RT-PCR demonstrated that all 4 genes are down-regulated. agrin and cntn2 were down-regulated 0.5-fold (fold change = 2(ΔΔCT)) or more (p values <0.002) while ntf3 and sema3d were slightly down-regulated less than 0.5 fold (p-values <0.05) (Supplemental data, Fig. 1). This data suggests that CPO exposure affects the expression of genes required for RB neurogenesis, and may be the first biomarkers of developmental OP exposure. Further gene expression studies will enable elucidation of other biomarkers (genes) that alter in expression after developmental CPO exposure.
3.8 CPO hydrolysis during treatment
An important consideration in any laboratory environmental toxicant exposure is the half-life of the toxicant during exposure. OPs are widely used in agriculture due to their rapid degradation under environmental conditions, such as water and heat. Hydrolysis of CPO under aqueous conditions has been extensively studied, and it is clear that pH, modifiers (such as solvents or salts), and initial concentration dramatically affect hydrolysis rates (Meikle and Youngson, 1978; Racke, 1993). For the interpretation of our observations, it was important to determine the extent of CPO hydrolysis during the exposure. Our treatment regimen was to add 300 nM CPO to egg water at 3 hpf with no addition of CPO at later time points throughout exposure. If CPO did not hydrolyze during the exposure period, AChE synthesized in the embryos would be inhibited, and the phenotypes observed may be due to chronic inhibition of AChE, in addition to non-cholinergic toxic mechanisms. Alternatively, if CPO did hydrolyze during treatment, depending on extent of hydrolysis, newly synthesized AChE protein would not be inhibited. CPO hydrolysis results in two products primarily, diethylphosphate and 3,5,6-trichloro-2-pyridinol (TCP). Neither product is capable of inhibiting AChE, although both compounds have been shown to be toxic to fish species (Meier et al., 1979; Wan et al., 1987).
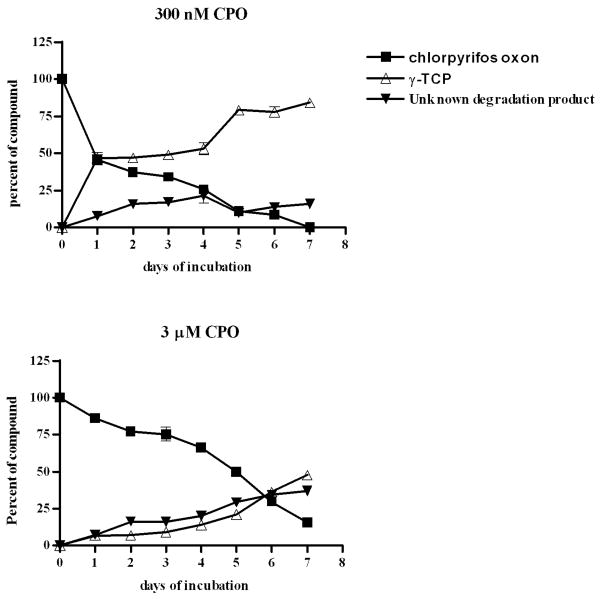
We examined CPO hydrolysis in egg water by monitoring the formation of TCP using HPLC-UV-MS analysis. CPO was added to fertilized eggs in egg water and incubated at 28.5°C for periods of time ranging from 0 hours of incubation to 7 doi (days of incubation). Samples of egg water were analyzed by HPLC-UV-MS for CPO (retention time, tR = 9.39 min) and the hydrolysis product, TCP (tR = 4.6 min). Internal standards were generated by diluting different concentrations of CPO in egg water and in egg water plus NaOH (to generate the hydrolysis products). The half-life of 300 nM CPO in egg water incubated at 28.5°C was found to be 1 doi. After this first half-life, the hydrolysis slowed as the next half-life measured was 5 doi (Fig. 8A). The same method was used to measure the half-life of a higher concentration of CPO (3 μM). The half-life of 3 μM CPO was markedly longer than 300 nM CPO—5 doi (Fig. 8B). HPLC spectra are shown in Supplemental Data Figures 2 and 3. This analysis was also performed with egg water containing no fertilized eggs, and the half-lives of 300 nM and 3 μM CPO were unchanged (data not shown) indicating that development of zebrafish embryos does not affect hydrolysis of CPO.
Figure 8.
CPO hydrolysis rate in egg water. (A) 300 nM CPO; (B) 3 μM CPO. Black squares, CPO oxon; open triangles, TCP; the predominant breakdown product of CPO, and black triangles, unknown degradation product. Shown is average of samples analyzed in three independent experiments with SEM.
Therefore, in our experimental regimen, the concentration of CPO was significantly reduced by 1 dpf resulting in a lower exposure at later time points. The RB phenotype could be due to a reduction in AChE activity in the first 24 hpf. The observation that the muscle development and nAChR cluster development were similar to control at 3 dpf could be due to the reduction in CPO present in the egg water, however, the remaining CPO and reduced AChE activity could be affecting other developmental processes later in larval development.
In summary, developmental CPO exposure is a kinetic complex paradigm. Non-lethal, CPO exposure during development inhibits AChE activity and affects RB neurogenesis. Developmental CPO exposure does not appear to drastically affect gross morphology, muscle development, or nAChR cluster development at 3 dpf indicating that AChE activity is not required for these processes.
4. Discussion
In the field of environmental toxicology, the ability to identify and treat environmental toxicant exposures is a complex matrix due to different toxic symptoms resulting from acute, chronic, and developmental exposures. OPs have well-defined acute toxic symptoms; the molecular events that transpire to and result in those toxic symptoms have been studied in great detail. However, the symptoms and mechanisms resulting from chronic and developmental exposures are more difficult to identify due to underlying genetic, environmental, and exposure level differences. Another level of complexity in OP developmental neurotoxicology is that AChE has both cholinergic and non-cholinergic roles in neurodevelopment, and OPs inhibit AChE as well as other target molecules that lead to disruption of non-cholinergic pathways in neurodevelopment. We have developed a zebrafish model of OP exposure to identify the underlying mechanisms of OP neurotoxicity during embryonic development. As a model vertebrate system, zebrafish have advantages over mammalian model systems in identification and characterization of developmental toxic phenotypes because the embryos develop ex utero, in a clear chorion, and are transparent.
4.1 CPO exposure affects RB neurogenesis
In this study, we have characterized CPO developmental exposure using biochemical, immunohistochemical, and mass spectrometric methods. The concentration of CPO that we used (300 nM) was not lethal during development and allowed relatively normal overall development, as analyzed by gross morphology, muscle development, and nAChR cluster development. CPO exposure significantly inhibited AChE activity and affected RB neurogenesis. The affected RB neurogenesis represents a specific neuron population affected by OP exposure.
4.2 Reduction in AChE enzyme activity resulted in normal gross morphology and the zebrafish chorion may protect the embryos from CPO exposure early in development
We observed relatively normal gross morphology despite the fact that the zebrafish embryos possess relatively little AChE activity. This variance in gross morphology (approximately 14% with affected phenotypes) has been demonstrated for other toxicant exposures (Hen Chow and Cheng, 2003; Egan et al., 2009) and is likely due to variable penetrance and solubility of the organic compound in egg water. AChE enzyme activity was inhibited to a greater extent at 2 and 3 dpf than at 1 dpf, even though the amount of CPO present in egg water was lower as measured by mass spectrometry. We hypothesize that CPO does not penetrate the chorion efficiently the first day of exposure resulting in the gross morphology phenotype. CPO-exposed zebrafish hatch out of their chorions earlier than control zebrafish which is common for many environmental toxicants, indicating that OP disrupts the integrity of the chorion which protects the embryo from exposure (Supplemental data, Figure 4). Mammalian placentas may protect embryos from toxicant exposures as well. In pregnant dams lacking p-glycoprotein, a multidrug efflux transporter, it was shown that embryos exhibited higher sensitivity to the pesticide, avermectin, (Smit et al., 1999). In an in vitro human placenta perfusion model, parathion was shown to penetrate into the placenta at 40% of full dose indicating that the mammalian placenta functions to exclude harmful toxicants during development (Benjaminov et al., 1992).
4.3 CPO exposure phenotypes compared to zebrafish ache mutants
Comparison of our data with reports of two ache mutants in zebrafish (achesb55 and zim(ache)) reveals some similarities and some differences (Behra et al., 2002; Downes and Granato, 2004). ache mutations in zebrafish completely inhibited AChE activity and resulted in death by paralysis at 3 dpf indicating that complete abolition of AChE activity results in a lethal phenotype. Muscle fiber formation was severely disrupted in ache mutants, but only mildly affected in our CPO-exposed larvae, indicated by the broader angle of the Chevron (Fig. 6F). nAChR cluster formation was severely reduced in ache mutant larvae, but not significantly affected in our CPO exposure model.
RB phenotypes differed in the two ache mutants. In achesb55 zebrafish mutants, which express a full length yet catalytically inactive AChE protein, RB dendritic extensions were reduced and RBs underwent apoptosis at an earlier time point than wild-type embryos (Behra et al., 2002). In zim(ache) zebrafish mutants that express a truncated AChE protein (with no catalytic activity), RB neurons developed normally (Downes and Granato, 2004). The CPO-induced RB phenotype we observed was more similar to Behra et al. in that we observed disorganization of the dorsal-lateral placement of RB cell bodies, reduced number of RBs, and inhibited axon extension. Downes et al. noted that the differences in RB formation observed with the two different ache mutants may be a result in background strain (Downes and Granato, 2004). The strain we used in our studies (AB) was also used in Behra et al., which may account for the similar RB phenotype between the two studies.
4.4 Stability of CPO oxon in exposure studies
For environmental toxicants, such as organophosphates, stability in aqueous systems is a large concern. In our laboratory, storage of OP in solvents at low concentrations has often led to lower stability. This was demonstrated in this manuscript by the large difference in half-lives of 300 nM CPO (1 doi) compared to 3 μM CPO (5 doi) and should be taken into account for any organic compound toxicity studies with zebrafish. The HPLC analysis of CPO hydrolysis during zebrafish exposure raises several interesting questions. The concentration of CPO in our exposure dropped by half during the first day of incubation indicating that, later in our exposure paradigm, the embryos were exposed to approximately 150 nM CPO. While this dose was sufficent to further inhibit AChE activity (by Ellman assays), it may have not been high enough to alter muscle development and nAChR cluster formation as analyzed at 3 dpf.
4.5 CPO exposure down-regulates expression of genes involved in RB neurogenesis
Expression of several genes affecting RB development and axonogenesis was reduced. We analyzed agrin, cntn2, ntf3, and sema3d. agrin and cntn2 were down-regulated greater than 0.5 (fold change = 2(ΔΔCT)), and ntf3 and sema3d were down-regulated less than 0.5 fold. The down-regulation of agrin and cntn2 represent the first developmental targets (biomarkers) of gene expression which may result in a definable phenotype. Agrin is a heparin sulfate proteoglycan which was initially described as having a role in AChR aggregation during synaptogenesis (Nitkin et al., 1987; Kim et al., 2007). Agrin morpholinos exhibit similar phenotypes as CPO-exposed embryos, such as fewer RB neurons and truncated RB axons. However, our phenotype is not as severe as the agrin morpholinos, likely correlated with reduced expression in CPO-exposed embryos compared to no expression in agrin morpholinos. Cntn2 (formerly called transient axonal glycoprotein I) has been demonstrated to regulate central axon projections of RB neurons (Liu and Halloran, 2005). Central axons of cntn2 morphants advance more slowly than in control embryos. We did not observe significant differences in central axons between control and CPO-exposed embryos, although a significant reduction and altered guidance of peripheral axons was observed.
The challenge in using a well-defined developmental biology model, such as zebrafish, to study developmental toxicity is to not put as much emphasis on well-defined phenotypes observed when a genetic mutation eliminates expression of a gene in an organism but rather to accept the more subtle phenotypes seen in a model that is exposed to a compound that modifies gene expression. An environmental toxicant will most likely not completely eliminate expression of any gene, but will instead alter (reduce or increase) expression of genes involved in specific cellular pathways as demonstrated in our gene expression analysis. The intellectual hurdle is to identify modulation of a pathway and not elimination of a pathway.
OPs have been hypothesized to affect cholinergic and non-cholinergic pathways in the developing vertebrate embryo. In this study, we show data that supports the hypothesis that inhibition of AChE during the first day of development affects RB neurogenesis. Future studies will aim to discover other biomarkers and developmental pathways that are affected directly or indirectly in order to discover how OPs may alter neuronal development during embryogenesis.
Supplementary Material
Supplemental Movie 1. 1 dpf control zebrafish embryo responds to touch by a horsehair needle with smooth coil-like tail flips.
Supplemental Movie 2. 1 dpf 300 nM CPO-exposed zebrafish embryo does not respond to touch by a horsehair needle.
Acknowledgments
We thank Kristin Artinger and her lab (University of Colorado, Denver) for generously supplying us with zebrafish, reagents, and advice. We also acknowledge Erica Woodahl for assistance in interpretation of pharmacokinetic data; Ray Hamilton for statistical analysis; Lou Herritt in the UM Confocal Microscopy and Image Analysis Laboratory; David Bonislawski (Center for Structural and Functional Neuroscience, UM) for use of and assistance with the confocal microscopes; and Corbin Schwanke in the UM Molecular Biology Core Facility.
Funding. Funding for this work was provided by National Institutes of Health Center for Structural and Functional Neuroscience (P20RR015583), Core Laboratory for Neuromolecular Production (5P30NS055022-02), Center for Environmental Health Sciences (5P20RR017670), and 5U01ES016102 (K.M.G.) and F32 DE018594 (D.A.B.)
Abbreviations
- OP
Organophosphate pesticide
- AChE
acetylcholinesterase
- CPO
chlorpyrifos oxon
- nAChR
nicotinic acetylcholine receptor
- RB
Rohon-Beard
- ACh
acetylcholine
- CPS
chlorpyrifos-thionate
- dpf
day post-fertilization
- hpf
hour post-fertilization
- DTNB
5,5′-Dithiobis(2-nitrobenzoic acid
- ATCh-I
acetylthiocholine-iodide
- α-btx
α-bungarotoxin
- TCP
3,5,6-trichloro-2-pyridinol
- tR
retention time
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Saskia M. Jacobson, Email: saskia1.jacobson@umontana.edu.
Denise A. Birkholz, Email: dabirkholz@gmail.com.
Marcy L. McNamara, Email: marcy1.mcnamara@umontana.edu.
Sandip B. Bharate, Email: sandip.bharate@umontana.edu.
References
- Abdel-Rahman AA, Blumenthal GM, Abou-Donia SA, Ali FA, Abdel-Monem AE, Abou-Donia MB. Pharmacokinetic profile and placental transfer of a single intravenous injection of [(14)C]chlorpyrifos in pregnant rats. Arch Toxicol. 2002;76:452–9. doi: 10.1007/s00204-002-0366-2. [DOI] [PubMed] [Google Scholar]
- Behra M, Cousin X, Bertrand C, Vonesch JL, Biellmann D, Chatonnet A, Strahle U. Acetylcholinesterase is required for neuronal and muscular development in the zebrafish embryo. Nat Neurosci. 2002;5:111–8. doi: 10.1038/nn788. [DOI] [PubMed] [Google Scholar]
- Behra M, Etard C, Cousin X, Strahle U. The use of zebrafish mutants to identify secondary target effects of acetylcholine esterase inhibitors. Toxicol Sci. 2004;77:325–33. doi: 10.1093/toxsci/kfh020. [DOI] [PubMed] [Google Scholar]
- Benjaminov O, Hoffer E, Taitelman U, Urbach J, Brandes JM. Parathion transfer and acetylcholinesterase activity in an in-vitro perfused term human placenta. Vet Hum Toxicol. 1992;34:10–2. [PubMed] [Google Scholar]
- Bertrand C, Chatonnet A, Takke C, Yan YL, Postlethwait J, Toutant JP, Cousin X. Zebrafish acetylcholinesterase is encoded by a single gene localized on linkage group 7. Gene structure and polymorphism; molecular forms and expression pattern during development. J Biol Chem. 2001;276:464–74. doi: 10.1074/jbc.M006308200. [DOI] [PubMed] [Google Scholar]
- Clarke JD, Hayes BP, Hunt SP, Roberts A. Sensory physiology, anatomy and immunohistochemistry of Rohon-Beard neurones in embryos of Xenopus laevis. J Physiol. 1984;348:511–25. doi: 10.1113/jphysiol.1984.sp015122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG. Current issues in organophosphate toxicology. Clin Chim Acta. 2006;366:1–13. doi: 10.1016/j.cca.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Downes GB, Granato M. Acetylcholinesterase function is dispensable for sensory neurite growth but is critical for neuromuscular synapse stability. Dev Biol. 2004;270:232–45. doi: 10.1016/j.ydbio.2004.02.027. [DOI] [PubMed] [Google Scholar]
- Egan RJ, Bergner CL, Hart PC, Cachat JM, Canavello PR, Elegante MF, Elkhayat SI, Bartels BK, Tien AK, Tien DH, Mohnot S, Beeson E, Glasgow E, Amri H, Zukowska Z, Kalueff AV. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav Brain Res. 2009;205:38–44. doi: 10.1016/j.bbr.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellman GL, Courtney KD, Andres V, Jr, Feather-Stone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- EPA, U S. Organophosphorus Cumulative Risk Assessment. O P Programs 2006 [Google Scholar]
- Eskenazi B, Marks AR, Bradman A, Harley K, Barr DB, Johnson C, Morga N, Jewell NP. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environ Health Perspect. 2007;115:792–8. doi: 10.1289/ehp.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn RN. The physiology and toxicology of salmonid eggs and larvae in relation to water quality criteria. Aquat Toxicol. 2007;81:337–54. doi: 10.1016/j.aquatox.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Fukuto TR. Mechanism of action of organophosphorus and carbamate insecticides. Environ Health Perspect. 1990;87:245–54. doi: 10.1289/ehp.9087245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigoryan H, Lockridge O. Nanoimages show disruption of tubulin polymerization by chlorpyrifos oxon: implications for neurotoxicity. Toxicol Appl Pharmacol. 2009;240:143–8. doi: 10.1016/j.taap.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hen Chow ES, Cheng SH. Cadmium affects muscle type development and axon growth in zebrafish embryonic somitogenesis. Toxicol Sci. 2003;73:149–59. doi: 10.1093/toxsci/kfg046. [DOI] [PubMed] [Google Scholar]
- Jacobson SW, Jacobson JL. New evidence of effects of organophosphate pesticides on neurodevelopment in children: commentary on the article by Kofman et al. on page 88. Pediatr Res. 2006;60:22–3. doi: 10.1203/01.pdr.0000220353.05515.d4. [DOI] [PubMed] [Google Scholar]
- Karnovsky MJ, Roots L. A “Direct-Coloring&rdquo Thiocholine Method For Cholinesterases. J Histochem Cytochem. 1964;12:219–21. doi: 10.1177/12.3.219. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Liu IH, Song Y, Lee JA, Halfter W, Balice-Gordon RJ, Linney E, Cole GJ. Agrin is required for posterior development and motor axon outgrowth and branching in embryonic zebrafish. Glycobiology. 2007;17:231–47. doi: 10.1093/glycob/cwl069. [DOI] [PubMed] [Google Scholar]
- Kruse J, Mailhammer R, Wernecke H, Faissner A, Sommer I, Goridis C, Schachner M. Neural cell adhesion molecules and myelin-associated glycoprotein share a common carbohydrate moiety recognized by monoclonal antibodies L2 and HNK-1. Nature. 1984;311:153–5. doi: 10.1038/311153a0. [DOI] [PubMed] [Google Scholar]
- Kuster E, Altenburger R. Comparison of cholin- and carboxylesterase enzyme inhibition and visible effects in the zebra fish embryo bioassay under short-term paraoxon-methyl exposure. Biomarkers. 2006;11:341–54. doi: 10.1080/13547500600742136. [DOI] [PubMed] [Google Scholar]
- Levin ED, Chrysanthis E, Yacisin K, Linney E. Chlorpyrifos exposure of developing zebrafish: effects on survival and long-term effects on response latency and spatial discrimination. Neurotoxicol Teratol. 2003;25:51–7. doi: 10.1016/s0892-0362(02)00322-7. [DOI] [PubMed] [Google Scholar]
- Levin ED, Swain HA, Donerly S, Linney E. Developmental chlorpyrifos effects on hatchling zebrafish swimming behavior. Neurotoxicol Teratol. 2004;26:719–23. doi: 10.1016/j.ntt.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Linney E, Upchurch L, Donerly S. Zebrafish as a neurotoxicological model. Neurotoxicol Teratol. 2004;26:709–18. doi: 10.1016/j.ntt.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Liu Y, Halloran MC. Central and peripheral axon branches from one neuron are guided differentially by Semaphorin3D and transient axonal glycoprotein-1. J Neurosci. 2005;25:10556–63. doi: 10.1523/JNEUROSCI.2710-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier EP, Dennis WH, Rosencrance AB, Randall WF, Cooper WJ, Warner MC. Sulfotepp, a toxic impurity in formulations of diazinon. Bull Environ Contam Toxicol. 1979;23:158–64. doi: 10.1007/BF01769935. [DOI] [PubMed] [Google Scholar]
- Meikle RW, Youngson CR. The hydrolysis rate of chlorpyrifos, O-O-diethyl O-(3,5,6-trichloro-2-pyridyl) phosphorothioate, and its dimethyl analog, chlorpyrifos-methyl, in dilute aqueous solution. Arch Environ Contam Toxicol. 1978;7:13–22. doi: 10.1007/BF02332034. [DOI] [PubMed] [Google Scholar]
- Nitkin RM, Smith MA, Magill C, Fallon JR, Yao YM, Wallace BG, McMahan UJ. Identification of agrin, a synaptic organizing protein from Torpedo electric organ. J Cell Biol. 1987;105:2471–8. doi: 10.1083/jcb.105.6.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paraoanu LE, Layer PG. Acetylcholinesterase in cell adhesion, neurite growth and network formation. Febs J. 2008;275:618–24. doi: 10.1111/j.1742-4658.2007.06237.x. [DOI] [PubMed] [Google Scholar]
- Racke KD. Environmental fate of chlorpyrifos. Rev Environ Contam Toxicol. 1993;131:1–150. doi: 10.1007/978-1-4612-4362-5_1. [DOI] [PubMed] [Google Scholar]
- Slotkin TA. Cholinergic systems in brain development and disruption by neurotoxicants: nicotine, environmental tobacco smoke, organophosphates. Toxicol Appl Pharmacol. 2004;198:132–51. doi: 10.1016/j.taap.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Smit JW, Huisman MT, van Tellingen O, Wiltshire HR, Schinkel AH. Absence or pharmacological blocking of placental P-glycoprotein profoundly increases fetal drug exposure. J Clin Invest. 1999;104:1441–7. doi: 10.1172/JCI7963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JN, Campbell JA, Busby-Hjerpe AL, Lee S, Poet TS, Barr DB, Timchalk C. Comparative chlorpyrifos pharmacokinetics via multiple routes of exposure and vehicles of administration in the adult rat. Toxicology. 2009;261:47–58. doi: 10.1016/j.tox.2009.04.041. [DOI] [PubMed] [Google Scholar]
- Sultatos LG. Mammalian toxicology of organophosphorus pesticides. J Toxicol Environ Health. 1994;43:271–89. doi: 10.1080/15287399409531921. [DOI] [PubMed] [Google Scholar]
- Thullbery MD, Cox HD, Schule T, Thompson CM, George KM. Differential localization of acetylcholinesterase in neuronal and non-neuronal cells. J Cell Biochem. 2005;96:599–610. doi: 10.1002/jcb.20530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevarrow B, Marks DL, Kimmel CB. Organization of hindbrain segments in the zebrafish embryo. Neuron. 1990;4:669–79. doi: 10.1016/0896-6273(90)90194-k. [DOI] [PubMed] [Google Scholar]
- van Eeden FJ, Granato M, Schach U, Brand M, Furutani-Seiki M, Haffter P, Hammerschmidt M, Heisenberg CP, Jiang YJ, Kane DA, Kelsh RN, Mullins MC, Odenthal J, Warga RM, Allende ML, Weinberg ES, Nusslein-Volhard C. Mutations affecting somite formation and patterning in the zebrafish, Danio rerio. Development. 1996;123:153–64. doi: 10.1242/dev.123.1.153. [DOI] [PubMed] [Google Scholar]
- Wan MT, Moul DJ, Watts RG. Acute toxicity to juvenile Pacific salmonids of Garlon 3A, Garlon 4, triclopyr, triclopyr ester, and their transformation products: 3,5,6-trichloro-2-pyridinol and 2-methoxy-3,5,6-trichloropyridine. Bull Environ Contam Toxicol. 1987;39:721–8. doi: 10.1007/BF01698468. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The zebrafish book. A guide for the laboratory use of zebrafish (Danio rerio) Eugene: University of Oregon Press; 2000. [Google Scholar]
- Williams JA, Barrios A, Gatchalian C, Rubin L, Wilson SW, Holder N. Programmed cell death in zebrafish rohon beard neurons is influenced by TrkC1/NT-3 signaling. Dev Biol. 2000;226:220–30. doi: 10.1006/dbio.2000.9860. [DOI] [PubMed] [Google Scholar]
- Yang D, Howard A, Bruun D, Ajua-Alemanj M, Pickart C, Lein PJ. Chlorpyrifos and chlorpyrifos-oxon inhibit axonal growth by interfering with the morphogenic activity of acetylcholinesterase. Toxicol Appl Pharmacol. 2008;228:32–41. doi: 10.1016/j.taap.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JG, Eskenazi B, Gladstone EA, Bradman A, Pedersen L, Johnson C, Barr DB, Furlong CE, Holland NT. Association between in utero organophosphate pesticide exposure and abnormal reflexes in neonates. Neurotoxicology. 2005;26:199–209. doi: 10.1016/j.neuro.2004.10.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Movie 1. 1 dpf control zebrafish embryo responds to touch by a horsehair needle with smooth coil-like tail flips.
Supplemental Movie 2. 1 dpf 300 nM CPO-exposed zebrafish embryo does not respond to touch by a horsehair needle.