Abstract
Our objectives were to evaluate the time course for atenolol pharmacokinetics in lactating women postpartum and to quantify atenolol plasma concentrations in their 3–4 months old nursing infants. Data were collected over one dosing interval from lactating women treated with atenolol for therapeutic reasons, at 2–4 weeks (n=32), 3–4 months (n=22), and 6–8 months (n=17) postpartum. A single blood sample was collected from 15 nursing infants (3–4 months of age) of the mothers participating in the study. At 2–4 weeks, 3–4 months, and 6–8 months postpartum, atenolol infant dose, relative to the mother’s weight-adjusted dose, were 14.6 ± 7.6%, 8.3 ± 5.2% and 5.9 ± 2.9%, respectively. Over this time, maternal atenolol pharmacokinetics did not change to a clinically significant extent. Atenolol concentrations were below assay quantification limits (< 10 ng/mL) in the plasma of all 3–4 months old nursing infants studied. Our findings support the careful use of atenolol during breastfeeding, since in the vast majority of healthy, term infants, atenolol concentrations will be too low to be clinically relevant. Premature infants and those with kidney disease require further study. Infant exposure depends on maternal dose and decreases during the first 6–8 months postpartum.
Keywords: atenolol, pharmacokinetics, breast milk, postpartum, kidney function
INTRODUCTION
Atenolol is used in some medical centers for the management of hypertension during and after pregnancy. However, the American Academy of Pediatrics cautions against the use of atenolol during breastfeeding 1 and product labeling for atenolol states that “Atenolol is excreted in human breast milk at a ratio of 1.5 to 6.8 when compared to the concentration in plasma. Caution should be exercised when [atenolol] is administered to a nursing woman. Clinically significant bradycardia has been reported in breast-fed infants. Premature infants, or infants with impaired renal function, may be more likely to develop adverse effects.” 2 These warnings are based on a single case report describing bradycardia and cyanosis in a 5 days old nursing infant whose mother was taking atenolol (50 mg twice daily) for the treatment of postpartum hypertension, 3 and on measurements of atenolol concentrations in breast milk in several case reports 3–5 and small studies. 6–8 In two of those studies, the breast milk-to-plasma concentration ratios were determined based on a single time point measurement (2 hours post-dosing in 5 women) 8 or samples obtained on 3 to 7 occasions post-dosing (7 women). 7 Another study assessed the plasma-to-milk ratios based on repeated measurements over the entire dosing interval, but results are based on measurements in only 4 women. 6 In this study, most samples were obtained from the left breast, and differences were found between the right and the left breast milk concentrations of atenolol. Thus, the actual amount of atenolol excreted in the breast milk over a dosing interval has not been previously characterized in detail. In addition, high milk-to-plasma ratios indicate significant infant exposure to the drug only if the amounts ingested by the infant are greater than those required for pharmacological effect. Furthermore, infant exposure to atenolol through breast milk may change if maternal absorption, distribution and elimination of atenolol, including excretion into breast milk, change with time postpartum (PP). We recently reported that atenolol renal clearance was higher during the second and third trimesters of pregnancy as compared to three months PP. 9 The time course for atenolol pharmacokinetics (PK) returning to baseline PP has not been characterized.
Because of the health benefits for infants, exclusive breastfeeding for ≥ 6 months is recommended by the American Academy of Pediatrics and the World Health Organization.10, 11 However, women on medications may avoid either breastfeeding or pharmacotherapy because of concerns about risks to their nursing infant. 12 Thus, the objectives of the current study were to evaluate the time course for atenolol PK in lactating women and quantify atenolol plasma concentrations in their 3–4 months old nursing infants.
METHODS
We examined the time course of steady-state oral atenolol PK in lactating women 2–4 weeks (n=32), 3–4 months (n=22) and 6–8 months PP (n=17). Subjects treated with atenolol for medical reasons were screened and consented at the University of Washington Maternal Infant Care Center between January 2005 and February 2008. All women enrolled in the study had the intent to breastfeed their infant for 6–8 months and complete all 3 study days. However, substantial dropout occurred throughout the study, leading to a larger number of women completing the earlier study days. For each subject that completed the study, the 6–8 months PP study day served as control because the pregnancy changes in atenolol PK were expected to have returned to baseline by that time. The study protocol was approved by the University of Washington Investigational Review Board and written informed consent was obtained from all subjects.
Subject Selection
Based on our data evaluating atenolol PK in pregnancy and PP, 9 our power analysis demonstrated that 13 subjects completing all 3 study days would be necessary to detect a 25% change in renal clearance with 80% power, intra-subject correlation of 0.50 and P < 0.025. Given the extremely high dropout rate and the prolonged duration of the study (6–8 months), we enrolled women until 13 were anticipated to complete all 3 study days. By the time the thirteenth woman completed her third study day, several other women had already started the study and were therefore continued in the study such that a total of 32 women participated in the study and 17 women completed all 3 study days.
Maternal subjects were prescribed atenolol with dosages adjusted based on clinical need, without regard to the study. Women were eligible if they were 18–50 years of age, receiving atenolol for therapeutic reasons, planning to nurse their infant for ≥ 6 months and on a stable atenolol dose for 3 days prior to each study day. Women were excluded for hematocrit < 28%. Infant subjects were eligible if their mothers were taking part in the atenolol PK study and nursing.
Maternal Dosing Regimen
Total atenolol daily dosages (range 25–200 mg/day) were divided in half and given every 12 hours, with tablets (Mylan Pharmaceuticals Inc, Morgantown WV) provided by investigators for the 3 days prior to each study day. Subjects kept dosing calendars for the 3 days prior to each study day and pill counts were conducted. Subjects fasted (except for clear liquids) for 6 hours prior to study drug administration until 1 hour post-dosing and avoided caffeine-containing foods and beverages for 24 hours prior to each study day and throughout sampling.
Sample and Data Collection
Maternal Subjects
Serial blood samples were collected on all study days from an indwelling venous catheter for measurement of atenolol plasma concentrations. On the 2–4 weeks study day, collection times were: 0, 0.5, 1, 2, 3, 4, 6, 8, 10 and 12 hours post-atenolol dosing. During the 3–4 and 6–8 months study days, the protocol was the same except that the 10 hour sample was shifted to 9 hours to correspond with the breast milk sample collection. Urine collections were performed 0–4, 4–8 and 8–12 hours post-dosing. Breast milk collections were performed at 2 hour intervals (2–4 weeks) or 3 hour intervals (3–4 months and 6–8 months) using the Medela ClassicR double electric breast pump, over the 12 hour study period. Both breasts were completely emptied of milk during each collection and nursing was not allowed during study days. A small breast milk aliquot was saved for determination of atenolol concentration and the remaining milk returned to the mother for feeding her infant.
Infants
During the 3–4 months study day, a single blood sample was collected ~9 hours after maternal dosing from 15 nursing infants for measurement of atenolol concentration. Volume of breast milk consumed by the infant over the time interval from maternal atenolol dose to infant blood sample collection was recorded. Resting and crying heart rates were measured prior to and immediately following blood sample collection.
Sample analysis
Atenolol plasma concentrations were determined by high-performance liquid chromatography with ultraviolet detection as previously described. 9 Atenolol urine and breast milk assays were conducted with minor modifications of the previous assays as described below. Urine standards, quality controls, and samples were extracted and assayed as described in the plasma assay, 9 with the following exceptions. Standards were prepared in 50 μL of blank urine and 450 μL of deionized water. One hundred μL of atenolol stock solutions and albuterol (Sigma-Aldrich, St. Louis, MO) solution in methanol were added to tubes and evaporated to create 8 standards at urine concentrations of 0.2–32.0 μg/mL. One hundred μL of albuterol internal standard solution was added prior to 50 μL of urine and 450 μL of distilled water for samples. A few urine samples with concentrations above the standard curve were diluted 50-fold and re-assayed. Atenolol urine concentrations for all three phases for each subject were assayed in duplicate during the same run. Intra-day and inter-day coefficients of variation were below 2.6% and 4.6%, respectively.
Breast milk samples were extracted and assayed as described previously for plasma samples, 9 except extraction was performed on 100 μL breast milk (blank for standards) and 400 μL deionized water. The five point standard curve corresponded to 50–1000 ng/mL breast milk concentrations before dilution. The intra-day and inter-day variability were below 6.6% and 10.7%, respectively.
Data analysis
Non-compartmental PK analysis was performed using Microsoft Office Excel 2002 (Microsoft, Redmond, WA), as previously described. 9 The amount of atenolol excreted in the breast milk for each collection interval was summed over the 12 hour dosing interval (breast milk volume•concentration for each interval). The percent of maternal dose excreted in breast milk was determined by (amount of atenolol excreted in the breast milk over 12 hours/maternal dose)•100. Breast milk:plasma ratio was determined by breast milk area under the concentration-time curve (AUC)/maternal plasma AUC over 1 dosing interval. Atenolol clearance from maternal plasma into breast milk (henceforth referred to as mammary clearance) was calculated by (amount of atenolol excreted in the breast milk over 12 hours/maternal plasma AUC). Actual body weights were used for weight adjusted parameters. Infant daily exposure was calculated by (amount of atenolol excreted in the breast milk over 12 hours•2)/body weight of an age-matched 50th percentile infant girl, 13 conservatively assuming consumption of twice the volume of milk obtained from the mother over one daytime 12-hour dosing interval. Relative infant dose was calculated by [(amount of atenolol excreted in the breast milk over 12 hours•2) / body weight of an age-matched 50th percentile infant girl)] / [(maternal daily dose/actual mother’s body weight)]*100. Maternal creatinine clearance was calculated by CrCl = (Uv•UCr)/(SCr•time), where Uv was urine volume, UCr was urine creatinine, and SCr was serum creatinine.
Statistical analysis
Paired Student’s T-test with Bonferroni’s correction was used to compare the subject characteristics and the estimated maternal PK parameters for the 2–4 weeks and 3–4 months study days to the 6–8 months PP study day as well as resting and crying infant heart rates. Results are reported as mean ± standard deviation unless otherwise indicated, with P < 0.025 considered significant.
RESULTS
Of the 32 women that completed the first study day (Table 1), five women stopped breastfeeding, 4 stopped atenolol treatment, 3 were lost to follow up and 3 stopped the study for technical reasons such that sampling completion was not possible, before completion of all 3 study days. The races/ethnicities were 26 White, 1 Pacific Islander, 1 Pacific Islander/Asian, 1 Hispanic/Latina, 1 Native American, 1 Asian and 1 Black. The women were treated with atenolol for hypertension (n=28), hypertrophic cardiomyopathy (n=2), or cardiac arrhythmias (n=2).
Table 1.
Subject characteristics.
| Characteristics | 2–4 Weeks (n=32) | 3–4 Months (n=22) | 6–8 Months (n=17) |
|---|---|---|---|
| Actual Body Weight (kg) | 93.3 ± 26.1 | 88.6 ± 24.9 | 86.1 ± 24.8 |
| Age (years) | 32.3 ± 6.5 | ||
| Height | 166.1 ± 6.2 | ||
| Serum Creatinine (mg/dL) | 0.8 ± 0.1 | 0.7 ± 0.1 | 0.7 ± 0.1 |
| Creatinine Clearance (mL/min) | 140.1 ± 35.4 | 146.6 ± 40.2 | 158.9 ± 48.2 |
| Number of Subjects Treated with 25 mg/day Atenolol | 8 | 9 | 8 |
| Number of Subjects Treated with 50 mg/day Atenolol | 17 | 9 | 8 |
| Number of Subjects Treated with 100 mg/day Atenolol | 4 | 4 | 1 |
| Number of Subjects Treated with 200 mg/day Atenolol | 3 | 0 | 0 |
Actual body weights, serum creatinine and creatinine clearances did not differ significantly between study stages.
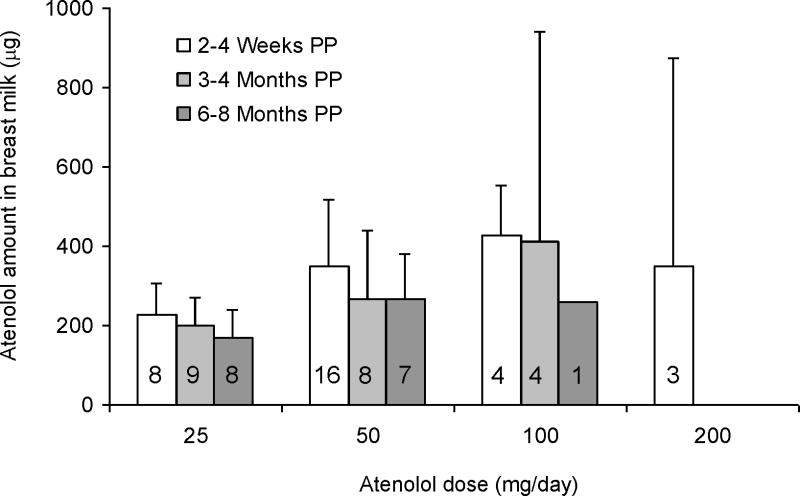
Following administration of atenolol 25, 50, 100 and 200 mg/day, the mean daily excretions of atenolol in breast milk at 2–4 weeks PP were 227 ± 80 μg (range 138–345 μg; n = 8), 350 ± 167μg (range 56–630 μg; n = 16), 429 ± 126 μg (range 307–596 μg; n = 4) and 350 ± 524 μg (range 30–955 μg; n = 3), respectively (Figure 1). At 3–4 months, the amounts of atenolol excreted in the breast milk daily were 198 ± 72 μg (range 72–294 μg; n = 9), 265 ± 175 μg (range 11–462μg; n = 8), and 413 ± 530 μg (range 11–1191 μg; n = 4), at maternal doses of 25, 50 and 100 mg/day, respectively. The amounts of atenolol excreted daily at 6–8 months were 168 ± 71 μg (range 83–273 μg; n = 8), 267 ± 116 μg (range 18–345 μg; n = 7), and 259 μg (n=1) at maternal doses of 25, 50 and 100 mg/day, respectively. The relative infant doses as a percentage of the mother’s weight-adjusted dose were 14.6 ± 7.6% (range 0.4–34.8%), 8.3 ± 5.2% (range 0.3–17.8%) and 5.9 ± 2.9% (range 0.5–14.1%), at 2–4 weeks, 3–4 months, and 6–8 months PP, respectively. One woman during each of the 3 study days had technical difficulties during breast milk collection. Therefore, the amounts excreted in the breast milk are based on a total of 31 women for the 2–4 weeks, 21 women for the 3–4 months, and 16 women for the 6–8 months study days.
Figure 1.
Amount of atenolol excreted daily in breast milk for 2–4 weeks (n=32), 3–4 months (N=22), and 6-8 months (n=15) postpartum. Error bars indicate standard deviations. Numbers in the bars indicate the number of subjects in each dose and study day group.
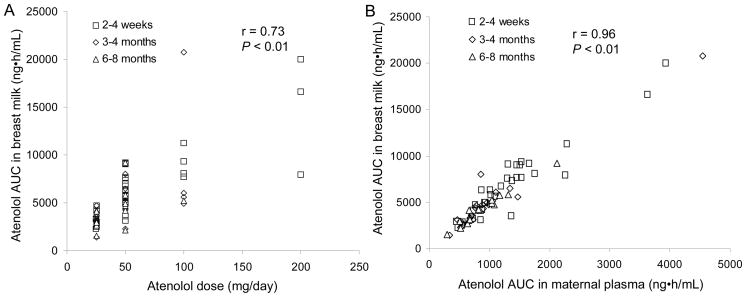
Across all subjects and study days, atenolol AUC in breast milk correlated linearly with maternal dose and maternal plasma AUC (r = 0.73; P < 0.01 and r = 0.96; P < 0.01, respectively; Figure 2). Within study days, the correlation between atenolol AUC in maternal plasma and in breast milk was 0.95 (P < 0.01), 0.97 (P < 0.01) and 0.98 (P < 0.01), for 2–4 weeks, 3–4 months and 6–8 months PP, respectively (data not shown). These correlations indicate that, regardless of the infant’s age, lower maternal doses are generally associated with lesser infant exposure to atenolol through breast milk.
Figure 2.
Correlation between atenolol dose (A) or atenolol maternal plasma AUC (B) and atenolol AUC in breast milk for 2–4 weeks (n=32), 3–4 months (N=22), and 6-8 months (n=15) postpartum.
Paired maternal atenolol PK comparisons were performed with the data from 17 subjects that completed all 3 study days. Two of these 17 subjects experienced difficulties in breast milk collection. Therefore, breast milk results are reported for 15 women completing all 3 study days. In these subjects, average maternal atenolol daily doses at 2–4 weeks, 3–4 months and 6–8 months PP were 52.9 ± 24.8 mg (NS), 47.1 ± 27.8 mg (NS), and 44.1 ± 24.2 mg, respectively.
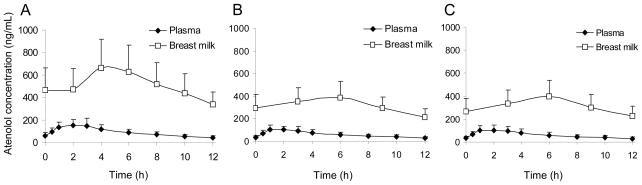
Average atenolol concentration-time profiles (adjusted to 50 mg/day) in breast milk and maternal plasma during all 3 study days are depicted in Figure 3. Atenolol AUC in breast milk, breast milk:plasma AUC ratio, actual and dose-normalized maximum breast milk concentrations, the amount excreted in the breast milk, the fraction of maternal dose excreted in breast milk, and mammary clearance were significantly higher 2–4 weeks PP than 6–8 months PP (Table 2). Infant exposure (absolute and relative) was significantly higher at both 2–4 weeks and 3–4 months as compared to 6–8 months PP (Table 2). Atenolol plasma concentrations in all 3–4 months old study infants were below the limit of assay quantification (10 ng/mL). Crying heart rates were significantly higher than resting heart rates in all study infants, 179 ± 25 beats/minute (range 132–210 beats/minute), vs. 124 ± 15 beats/minute (range 100–160 beats/minute), respectively, P < 0.01). The average increase in heart rate with crying was 46 ± 26%. During the ~9 hour time period between last maternal atenolol dose and blood sampling from the infant, the infants consumed 228 ± 122 mL of breast milk, through which they were exposed to 80 ± 43 μg (range, 5–161 μg; 1–26 μg/kg) of atenolol. This would translate into an average atenolol exposure of 36 ± 19μg/kg/day (range, 2–70 μg/kg/day).
Figure 3.
Average atenolol concentration–time profiles in plasma and breast milk (n=15) for subjects treated with atenolol twice daily (adjusted to 50 mg/day) 2–4 weeks postpartum (A), 3–4 months postpartum (B), and 6-8 months postpartum (C). Error bars represent standard deviations.
Table 2.
Estimated Atenolol Breast Milk Pharmacokinetic Parameters (mean ± SD) 2–4 Weeks, 3–4 Months and 6–8 Months Postpartum.
| Parameter | 2–4 Weeks | 3–4 Months | 6–8 Months |
|---|---|---|---|
| AUC* (ng•hr/mL) | 6199 ± 2248‡ | 3848 ± 1245 | 3833 ± 1306 |
| Dose Normalized AUC (ng•hr/mL at 50 mg/day) | 6810 ± 1744 | 5700 ± 2144 | 5695 ± 2441 |
| Breast Milk:Plasma AUC Ratio | 5.7 ± 0.8‡ | 5.1 ± 0.7 | 4.9 ± 0.6 |
| Cmax* (ng/mL) | 685 ± 254‡ | 404 ± 146 | 406 ± 144 |
| Dose Normalized Cmax (ng/mL at 50 mg/day) | 734 ± 239‡ | 543 ± 220 | 542 ± 208 |
| Tmax (hr) | 4.8 ± 1.7 | 4.4 ± 2.2 | 4.8 ± 2.2 |
| Amount Excreted in Breast Milk Over 12 Hours (μg) | 181 ± 80‡ | 123 ± 60 | 105 ± 50 |
| Fraction of Maternal Dose Excreted in Breast Milk over 12 Hours (%) | 0.8 ± 0.3† | 0.7 ± 0.3 | 0.6 ± 0.3 |
| Atenolol Mammary Clearance (mL/min) | 2.8 ± 0.9† | 2.7 ± 1.2 | 2.2 ± 0.9 |
| Infant Exposure (μg/kg/day)a | 90 ± 38‡ | 42 ± 20‡ | 27 ± 13 |
| Relative infant dose (% of Mother’s Weight-Adjusted Dose) | 9.0 ± 3.4‡ | 5.1 ± 2.4‡ | 3.2 ± 1.6 |
Only subjects completing all breast milk collection over the entire dosing interval on all 3 study days (n = 15) are included.
Atenolol dosage varied between study days and ranged from 25–100 mg/day. AUC = area under the concentration-time curve; Cmax = maximum concentration; Tmax = time to maximum concentration.
Assuming consumption of twice the volume of milk obtained from the mother over one 12-hour dosing interval.
, P < 0.025;
, P < 0.01.
Estimated maternal atenolol plasma PK parameters are reported in Table 3. Of interest is the significantly higher creatinine clearance seen 6–8 months PP as compared to 2–4 weeks and 3–4 months PP within the same subjects (Table 3). There was a strong correlation between creatinine clearance and atenolol renal clearance (r = 0.75, data not shown), although atenolol renal clearance did not change significantly over the 6–8 months PP. Plasma AUC of atenolol was significantly greater (P < 0.01) 2–4 weeks PP than 6–8 months PP. However, when normalized to maternal atenolol dose, this change was not statistically significant (Table 3). No other significant changes were observed in maternal atenolol PK 2–4 weeks or 3–4 months PP as compared to 6–8 months PP.
Table 3.
Estimated Maternal Steady-State Atenolol Plasma Pharmacokinetic Parameters (mean ± SD) 2–4 Weeks, 3–4 Months and 6–8 Months Postpartum
| Parameter | 2–4 Weeks Postpartum | 3–4 Months Postpartum | 6–8 Months Postpartum |
|---|---|---|---|
| Apparent Oral Clearance (L/hr) | 22.1 ± 5.5 | 26.7 ± 11.2 | 26.6 ± 12.2 |
| Renal Clearance (mL/min) | 171 ± 42 | 167 ± 51 | 176 ± 47 |
| AUC (ng•hr/mL) | 1261 ± 715‡ | 1017 ± 955 | 888 ± 415 |
| Dose Normalized AUC (ng •hr/mL at 50 mg/day) | 1201 ± 300 | 1099 ± 452 | 1118 ± 431 |
| Cmax (ng/mL) | 192 ± 124 | 164 ± 155 | 144 ± 67 |
| Dose Normalized Cmax (ng/mL at 50 mg/day) | 182 ± 58 | 175 ± 71 | 182 ± 74 |
| Tmax (hr) | 2.4 ± 0.8 | 1.8 ± 1.0 | 2.0 ± 0.7 |
| Half Life (hr) | 6.2 ± 2.2 | 6.4 ± 1.4 | 6.5 ± 2.4 |
| Apparent Volume of Distribution (L) | 198 ± 81 | 249 ± 126 | 248 ± 151 |
| Fraction Excreted Unchanged in the Urine (%) | 47.7 ± 11.0 | 41.2 ± 13.7 | 44.2 ± 14.6 |
| Creatinine Clearance (mL/min) | 133 ± 23‡ | 139 ± 41‡ | 159 ± 48 |
Only subjects completing all 3 study days (n = 17) are included. Atenolol dose ranged from 25–100 mg/day. AUC = area under the concentration-time curve; Cmax = maximum concentration; Tmax = time to maximum concentration.
, P < 0.01.
DISCUSSION
The American Academy of Pediatrics lists atenolol as one of the drugs that has been “associated with significant effects on some nursing infants” 1 based on a single case report of an infant who appeared to have atenolol side effects from breastfeeding. 3 This case has been debated in the literature, 14, 15 and no other reports of infants developing adverse effects from atenolol as a result of breastfeeding have been found.
Our findings indicate that atenolol concentrates in breast milk, as demonstrated by mean breast milk:plasma AUC ratios greater than 4.9 during all study stages (Table 2). These ratios are in line with the earlier data from 7 patients, 7 but are greater than those reported by others. 5, 6, 8 Nevertheless, the actual amount of atenolol excreted in the breast milk over one dosing interval was on average less than 1% of the maternal dose on all study days. The mean relative infant dose was less than 15% of the mother’s weight-adjusted dose on the 2–4 weeks PP study day. On the other study days, the mean relative infant dose was less than 10%, an exposure level that would be considered safe for most medications. 16 However, for individual outliers, the highest relative infant doses at 2–4 weeks and 3–4 months PP were 34.8% and 17.8%, respectively. In addition, although determination of the relative infant dose is the gold standard for estimating the infant risk, 16 it may be somewhat misleading, as it is reported relative to maternal dose, and actual infant exposure may be more clinically relevant. Therefore, we also reported actual infant exposure as well as the infant exposure as a percentage of atenolol pediatric dosage, using the actual amount of atenolol excreted in the breast milk across all study participants. Although the safety and effectiveness of atenolol in pediatric patients have not been established, it has been reported in a study of 24 children (1.8–18.2 years of age) that dosages less than 800 μg/kg/day are not effective in the treatment of supraventricular tachycardia (SVT). 17 In another study of 22 children (6 weeks to 16.5 years of age) treated with atenolol for SVT, 59% had good control of their tachycardia with therapeutic dosages ranging from 300–1300 μg/kg/day. Only 1 child younger than 1 year of age participated in the study (6 week old infant) and received 2000 μg/kg/day, but the atenolol was discontinued for recurrent tachycardia. Two other children that were less than 2 years of age participated in the study and both were successfully treated with atenolol (800 and 1100 μg/kg/day). 18 Accordingly, the recommended oral initial antiarrhythmic dose of atenolol in children ranges from 300 to 1000 μg/kg/day. 17–19 Based on our estimation, a 2–4 week old infant girl would be exposed to 28 ± 17 % (range 3–86%) of the lowest initial atenolol pediatric dosage (300 μg/kg/day) or 10 ± 6% (1–32%) of the lowest therapeutic atenolol pediatric dosage (800 μg/kg/day) if they consumed the amount of atenolol excreted in the breast milk in our study. The 300 μg/kg/day is approximately equivalent to 25 mg/day for an adult patient. Although for the vast majority of healthy term infants, atenolol exposure via breast milk will have no pharmacologic effect, it is possible that in the case of the extreme outlier, the exposure to 86% of the starting dose or 32% of the therapeutic dose of atenolol could have some pharmacologic effect, particularly if the infant is highly sensitive to beta blockers. Whether this translates into clinically significant effects requiring a change in maternal therapy or discontinuation of breastfeeding would need to be evaluated on a case by case basis. If clinical concerns arise for a nursing infant, monitoring infant heart rate should be helpful. Some have suggested that alternative beta-blockers should be selected during breastfeeding. 16 However, the huge changes in the PK of beta-blockers such as metoprolol 20, 21 during pregnancy and the unknown time course for the changes returning to baseline PP, make this approach somewhat challenging.
In addition to the described exposure to atenolol via breast milk, further evidence of the safety of maternal atenolol administration while breastfeeding in this study is that atenolol was not quantifiable (<10 ng/mL) in any of the 3–4 months old nursing infants’ plasma whose mothers were taking atenolol, despite ingestion of up to 161 μg of atenolol in the 9 hours prior to the blood draw (up to 70 μg/kg/day). This is not surprising, given that in the mothers, 10-fold greater body weight-adjusted dose resulted in average blood concentrations of 118 ± 74 ng/mL and maximal concentrations of 215 ± 138 ng/mL. The atenolol exposure for the infant consuming 70 μg/kg/day was approximately the same or higher than that found in half of the 2–4 weeks infants and that found in all the 3–4 and 6–8 months infants, with the exception of a single 3–4 months outlier. Of the 2–4 weeks infants, only 1 outlier was exposed to > 50% of the 300 μg/kg/day starting atenolol dose. Moreover, the crying heart rates of all the 3–4 months old nursing infants were 46% higher than resting heart rates. The increases were on the same order of magnitude previously reported in normal infants, 22–24 demonstrating that the infants exposed to atenolol via breast milk were not experiencing a pharmacologic effect from atenolol.
The decrease in atenolol excretion into breast milk from 2–4 weeks PP to 6–8 months PP can be explained in part by a concurrent decrease in its maternal blood concentration (Table 3) as well as a decrease in mammary clearance, with corresponding lower breast milk:maternal plasma AUC ratio on the later study days. This suggests alterations in atenolol transfer into breast milk with time PP. Atenolol is a substrate for organic anion transporting polypeptides (OATPs), 25 and several OATPs have been localized to human mammary epithelial cells, 26 but the effect of time postpartum on OATP expression and activity as well as the contribution of these transporters to atenolol transfer into breast milk have not been characterized. Nevertheless, our data are consistent with decreasing mammary OATP activity with time postpartum.
One of the points that the American Academy of Pediatrics suggests considering before prescribing medications to lactating women is “drug exposure to the nursing infant may be minimized by having the mother take the medication just after she has breastfed the infant or just before the infant is due to have a lengthy sleep period.”1 In women taking atenolol, the average time to maximum concentration in the breast milk post-atenolol dosing was greater than 4.4 hours in each of the study stages. Interestingly, 5 of the subjects had at least one study day in which the maximum atenolol concentration in the breast milk occurred in the milk that was pumped just prior to dosing. Therefore, the general suggestion by the American Academy of Pediatrics, which is intended to avoid peak drug concentrations in breast milk will not work and may actually increase atenolol exposure.
Our data show that there is a statistically significant increase in creatinine clearance 6–8 months PP compared with 2–4 weeks and 3–4 months PP, which remains after correcting for body weight (Table 3). Although there was a good correlation (r = 0.75) between creatinine clearance and atenolol renal clearance (data not shown), the change in creatinine clearance did not result in clinically significant changes in maternal atenolol steady-state PK, that would require alterations in medication management. Whereas creatinine clearance has been assumed to return to baseline by 3–4 months PP, 27, 28 we were unable to locate any published studies describing the changes in creatinine clearance in lactating women in the first 6–8 months following delivery. The modest increase in creatinine clearance seen in our study may reflect kidney function returning to baseline after a pregnancy complicated by treated hypertension. Alternatively, it could result from decreasing prolactin concentrations over the first six months PP in lactating women. Between 8 and 60 days PP, basal prolactin concentrations are comparable to those observed in non-nursing women, but increase during breastfeeding. 29, 30 After 60 days, nursing is associated with lower prolactin concentrations, 30 and mean concentrations decrease with time. 31 Prolactin has been associated with decreased renal function in humans and in rodents, 32, 33 possibly via direct effect on renal prolactinergic receptors, alteration in renal blood flow, enhancement of aldosterone secretion, or a combination of these mechanisms. 33 Future studies are needed to investigate the mechanisms for the rise in creatinine clearance 6–8 months PP and its potential implications for drug therapy.
There are limitations of this study that deserve comment. First, we did not study the exposure to atenolol through breast milk for infants younger than 2 weeks or preterm infants, in which renal function is not fully mature. 34 Second, heart rate response and infant atenolol plasma concentrations were evaluated in 3–4 month old infants only. Third, we studied only three mothers treated with the highest daily atenolol dose (200 mg/day). Forth, our estimation of infant atenolol daily exposure were made by doubling the 12 hr exposure which is likely an overestimation of infant exposure because women do not breastfeed their infant as frequently at night. Finally, we conservatively used a 50th percentile female infant as our standard for determining the weight-normalized infant exposure. This normalization may be an overestimation of the exposure to atenolol for male infants.
In conclusion, based on the pharmacokinetic and pharmacodynamic findings in this study, we expect that for the vast majority of healthy, term infants, the exposure to atenolol through breast milk would not result in pharmacologic effects. However, there may be outliers that will be exposed to atenolol amounts close to starting atenolol doses. Therefore, clinicians caring for nursing infants whose mothers are treated with atenolol may be reassured by monitoring infant heart rate. In addition, premature infants that are receiving a substantial portion of their nutrition from breast milk and those with kidney disease require further study. There is a significant increase in creatinine clearance 6–8 months PP relative to 2–4 weeks and 3–4 months PP in lactating women, but this does not translate into clinically significant changes in atenolol pharmacokinetics.
Acknowledgments
Support: This research was supported by the Food and Drug Administration, Office of Women’s Health, NIH/NICHD through support of the Obstetric-Fetal Pharmacology Research Unit grant # U10HD047892 and NIH/NCRR through support of the University of Washington General Clinical Research Unit and Clinical and Translational Science Award grants # M01RR-00037 and UL1RR025014.
We acknowledge Eric Kantor, Ginna Wall, RN, IBCLC and Nancy Estill, RN, IBCLC for their technical assistance and David Blough, PhD, for providing statistical advice.
Abbreviations
- AUC
area under the concentration-time curve
- Cmax
maximal concentration
- tmax
time to maximal concentration
- PK
pharmacokinetics
- PP
postpartum
- NS
not significant
References
- 1.American Academy of Pediatrics, Committee on Drugs. The transfer of drugs and other chemicals into human milk. Pediatrics. 2001;108:776–779. [PubMed] [Google Scholar]
- 2.TenorminR product labeling. AstraZeneca; Wilmington DE: Apr 08, 2008. [Google Scholar]
- 3.Schmimmel MS, Eidelman AJ, Wilschanski MA, Shaw D, Ogilvie RJ, Koren G. Toxic effects of atenolol consumed during breast feeding. J Pediatr. 1989;114:476–478. doi: 10.1016/s0022-3476(89)80578-5. [DOI] [PubMed] [Google Scholar]
- 4.Fowler MB, Brudenell M, Jackson G, Holt DW. Essential hypertension and pregnancy: successful outcome with atenolol. Br J Clin Pract. 1984;38:73–74. [PubMed] [Google Scholar]
- 5.White WB, Andreoli JW, Wong SH, Cohn RD. Atenolol in human plasma and breast milk. Obstet Gynecol. 1984;63(3 Suppl):42S–44S. [PubMed] [Google Scholar]
- 6.Kulas J, Lunell NO, Rosing U, Steen B, Rane A. Atenolol and metoprolol. A comparison of their excretion into human breast milk. Acta Obstet Gynecol Scand Suppl. 1984;118:65–69. doi: 10.3109/00016348409157126. [DOI] [PubMed] [Google Scholar]
- 7.Liedholm H. Transplacental passage and breast milk accumulation of atenolol in humans. Drugs. 1983;25 (Suppl 2):217–218. [Google Scholar]
- 8.Thorley KJ, McAinsh J. Levels of the beta-blockers atenolol and propranolol in the breast milk of women treated for hypertension in pregnancy. Biopharm Drug Dispos. 1983;4:299–301. doi: 10.1002/bdd.2510040310. [DOI] [PubMed] [Google Scholar]
- 9.Hebert MF, Carr DB, Anderson GD, et al. Pharmacokinetics and pharmacodynamics of atenolol during pregnancy and postpartum. J Clin Pharmacol. 2005;45:25–33. doi: 10.1177/0091270004269704. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. Fifty-fifth world health assembly. Infant and young child nutrition. Global strategy on infant and young child feeding. Report by the Secretariat. 2002 April 16;A55(15) [Google Scholar]
- 11.American Academy of Pediatrics Work Group on Breastfeeding. Breastfeeding and the use of human milk. Pediatrics. 1997;100:1035–1039. doi: 10.1542/peds.100.6.1035. [DOI] [PubMed] [Google Scholar]
- 12.Ito S, Lee A. Drug excretion into breast milk--overview. Adv Drug Deliv Rev. 2003;55:617–627. doi: 10.1016/s0169-409x(03)00034-6. [DOI] [PubMed] [Google Scholar]
- 13.National Center for Health Statistics and the National Center for Chronic Disease Prevention and Health Promotion. Birth to 36 months: girls, length-for-age and weight-for-age percentiles. [Accessed February 15, 2009];2000 May 30; http:www.cdc.gov/growthcharts. modified 4/20/01.
- 14.Diamond JM. Toxic effects of atenolol consumed during breast feeding. J Pediatr. 1989;115:336. doi: 10.1016/s0022-3476(89)80110-6. [DOI] [PubMed] [Google Scholar]
- 15.Koren G. Toxic effects of atenolol consumed during breast feeding. J Pediatr. 1989;115:336–337. doi: 10.1016/s0022-3476(89)80110-6. [DOI] [PubMed] [Google Scholar]
- 16.Hale TW. Medications and Mother’s Milk. 13. Amarillo, TX: Hale Publishing; 2008. [Google Scholar]
- 17.Trippel DL, Gillette PC. Atenolol in children with supraventricular tachycardia. Am J Cardiol. 1989;64:233–236. doi: 10.1016/0002-9149(89)90466-9. [DOI] [PubMed] [Google Scholar]
- 18.Mehta AV, Subrahmanyam AB, Anand R. Long-term efficacy and safety of atenolol for supraventricular tachycardia in children. Pediatr Cardiol. 1996;17:231–236. doi: 10.1007/BF02524799. [DOI] [PubMed] [Google Scholar]
- 19.Luedtke SA, Kuhn RJ, McCaffrey FM. Pharmacologic management of supraventricular tachycardias in children. Part 2: atrial flutter, atrial fibrillation, and junctional and atrial ectopic tachycardia. Ann Pharmacother. 1997;31:1347–1359. doi: 10.1177/106002809703101113. [DOI] [PubMed] [Google Scholar]
- 20.Högstedt S, Lindberg B, Peng DR, Regårdh CG, Rane A. Pregnancy-induced increase in metoprolol metabolism. Clin Pharmacol Ther. 1985;37:688–692. doi: 10.1038/clpt.1985.114. [DOI] [PubMed] [Google Scholar]
- 21.Högstedt S, Lindberg B, Rane A. Increased oral clearance of metoprolol in pregnancy. Eur J Clin Pharmacol. 1983;24:217–220. doi: 10.1007/BF00613820. [DOI] [PubMed] [Google Scholar]
- 22.Abdel Razek A, Az El-Dein N. Effect of breast-feeding on pain relief during infant immunization injections. Int J Nurs Pract. 2009;15:99–104. doi: 10.1111/j.1440-172X.2009.01728.x. [DOI] [PubMed] [Google Scholar]
- 23.Efe E, Ozer ZC. The use of breast-feeding for pain relief during neonatal immunization injections. Appl Nurs Res. 2007;20:10–16. doi: 10.1016/j.apnr.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 24.Gray L, Miller LW, Philipp BL, Blass EM. Breastfeeding is analgesic in healthy newborns. Pediatrics. 2002;109:590–593. doi: 10.1542/peds.109.4.590. [DOI] [PubMed] [Google Scholar]
- 25.Kato Y, Miyazaki T, Kano T, Sugiura T, Kubo Y, Tsuji A. Involvement of influx and efflux transport systems in gastrointestinal absorption of celiprolol. J Pharm Sci. 2009;98:2529–2539. doi: 10.1002/jps.21618. [DOI] [PubMed] [Google Scholar]
- 26.Alcorn J, Lu X, Moscow JA, McNamara PJ. Transporter gene expression in lactating and nonlactating human mammary epithelial cells using real-time reverse transcription-polymerase chain reaction. J Pharmacol Exp Ther. 2002;303:487–496. doi: 10.1124/jpet.102.038315. [DOI] [PubMed] [Google Scholar]
- 27.Davison JM, Dunlop W, Ezimokhai M. 24-hour creatinine clearance during the third trimester of normal pregnancy. Br J Obstet Gynaecol. 1980;87:106–109. doi: 10.1111/j.1471-0528.1980.tb04501.x. [DOI] [PubMed] [Google Scholar]
- 28.Ronco C, Brendolan A, Bragantini L, et al. Renal functional reserve in pregnancy. Nephrol Dial Transplant. 1988;2:157–161. [PubMed] [Google Scholar]
- 29.Noel GL, Suh HK, Frantz AG. Prolactin release during nursing and breast stimulation in postpartum and nonpostpartum subjects. J Clin Endocrinol Metab. 1974;38:413–423. doi: 10.1210/jcem-38-3-413. [DOI] [PubMed] [Google Scholar]
- 30.Tyson JE, Friesen HG, Anderson MS. Human lactational and ovarian response to endogenous prolactin release. Science. 1972;177:897–900. doi: 10.1126/science.177.4052.897. [DOI] [PubMed] [Google Scholar]
- 31.Hennart P, Delogne-Desnoeck J, Vis H, Robyn C. Serum levels of prolactin and milk production in women during a lactation period of thirty months. Clin Endocrinol (Oxf) 1981;14:349–353. doi: 10.1111/j.1365-2265.1981.tb00619.x. [DOI] [PubMed] [Google Scholar]
- 32.Buckman MT, Peake GT, Roberson G. Hyperprolactinemia influences renal function in man. Metabolism. 1976;25:509–516. doi: 10.1016/0026-0495(76)90004-4. [DOI] [PubMed] [Google Scholar]
- 33.Morrissey SE, Newth T, Rees R, Barr A, Shora F, Laycock JF. Renal effects of recombinant prolactin in anaesthetized rats. Eur J Endocrinol. 2001;145:65–71. doi: 10.1530/eje.0.1450065. [DOI] [PubMed] [Google Scholar]
- 34.Rubin PC, Butters L, Reynolds B, et al. Atenolol elimination in the neonate. Br J Clin Pharmacol. 1983;16:659–662. doi: 10.1111/j.1365-2125.1983.tb02237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]