Abstract
Metastasis to distant tissues is the chief driver of breast cancer-related mortality, but little is known about the systemic physiologic dynamics that regulate that process. To investigate the role of neuroendocrine activation in cancer progression, we used in vivo bioluminescence imaging to track development of metastasis in an orthotopic mouse model of breast cancer. Stress-induced neuroendocrine activation had a negligible impact on growth of the primary tumor, but induced a 30-fold increase in metastasis to distant tissues including lymph nodes and lung. These effects were mediated by beta-adrenergic signaling, which increased infiltration of CD11b+F4/80+ macrophages into primary tumor parenchyma and thereby induced a pro-metastatic gene expression signature accompanied by indications of M2 macrophage differentiation. Pharmacologic activation of beta-adrenergic signaling induced similar effects, and treatment of stressed animals with the beta-antagonist propranolol reversed stress-induced macrophage infiltration and inhibited tumor spread to distant tissues. Effects of stress on distant metastasis were also inhibited by in vivo macrophage suppression using the CSF1 receptor kinase inhibitor GW2580. These findings identify activation of the sympathetic nervous system as a novel neural regulator of breast cancer metastasis, and suggest new strategies for anti-metastatic therapies that target beta-adrenergic induction of pro-metastatic gene expression in primary breast cancers.
Keywords: metastasis, breast cancer, sympathetic nervous system, macrophage, beta-adrenergic
INTRODUCTION
Metastasis is responsible for much of the morbidity and mortality of breast cancer. Multiple host cell types contribute to metastasis and recent research has focused on the host microenvironment as a target for new anti-metastatic therapies. Neuroendocrine dynamics have the potential to regulate gene expression in many cell types in the tumor microenvironment (1–2), but their contribution to breast cancer metastasis remains largely unexplored. Nerve fibers from the sympathetic nervous system (SNS) are present in organs that serve as key targets for breast cancer metastasis, including lymph nodes, lung and bone (3–4). However, little is known about the effects of SNS neural activation on breast cancer progression and, in particular, its effects on metastasis to distant tissues.
Potential SNS regulation of tumor cell biology is suggested by the presence of beta-adrenergic receptors in archival human breast cancer samples (5) and by clinical relationships between beta-blocker usage and reduced cancer risk (6–7). Physiological stress activates sympathetic nerve fibers to release micromolar concentrations of the neurotransmitter norepinephrine into the local tissue microenvironment and nanomolar concentrations into systemic circulation (2, 8–9). Norepinephrine ligation of cellular beta-adrenergic receptors triggers a G-protein coupled signaling cascade leading to cAMP synthesis, protein kinase A phosphorylation, and transcription factor activation (10–11). In vitro studies have shown that adrenergic signaling can regulate multiple cellular processes involved in cancer progression, including tumor cell proliferation, extracellular matrix invasion, angiogenesis, matrix metalloprotease activation, and expression of inflammatory and chemotactic cytokines (12–14). In a xenograft model of ovarian cancer metastasis, SNS activation by chronic stress increased VEGF-mediated vascularization of intra-peritoneal tumors (2). However, little is known about the effect of chronic stress on the dissemination of metastatic cancer cells from a primary tumor to distant target organs such as the lymph nodes and lung. In the present study, we sought to determine whether experimentally imposed chronic stress can influence the rate of distant metastasis from primary breast tumors, and to define the neuroendocrine, cellular, and molecular mechanisms involved.
METHODS
In vivo metastasis model
Six week old female Balb/c and nu/nu mice (Charles River Laboratories, Wilmington, MA) were housed under BSL2 barrier conditions. 66cl4 mammary adenocarcinoma cells (a gift from Prof Robin Anderson, Peter MacCallum Cancer Centre, Australia) were transduced with the FUhlucW lentiviral vector containing firefly luciferase under control of the ubiquitin-C promoter (15) and were cultured as previously described (16). Tumor cells (1 × 105) were injected into the 4th mammary fat pad for spontaneous metastasis studies or into the tail vein for organ colonization studies. Mammary tumor size was measured using digital calipers and tumor volume was calculated as (length × width2)/2. Frequency and quantity of metastases were tracked in live mice by repeated non-invasive optical imaging of tumor-specific luciferase activity using the IVIS 100 system (Caliper, Hopkinton MA). After anaesthetization with 2% isofluorane and intravenous injection of 150 mg/kg luciferin, mice were photographed under brightfield and images were overlaid with luminescence data gathered over the maximum exposure period without pixel saturation (0.5–60 sec). Metastasis was measured by triplicate determination at each time point of total bioluminescence in a region of interest of constant size located distant from the primary tumor (usually the chest region containing lung, axillary and brachial lymph nodes). Tissue-specific metastasis was measured ex vivo by bioluminescence immediately after sacrifice on day 28, or by microscopic evaluation on day 41 in experiments involving removal of the primary tumor. All procedures were carried out under protocols approved by the Institutional Animal Use and Care, and University Institutional Review Board at the University of California Los Angeles. Each experiment was repeated 2–4 times.
Chronic stress
Mice were randomly assigned to home cage control conditions or 2 hrs per day restraint for 20 days commencing 5 days prior to tumor cell inoculation, or, for 14 days commencing 2 days after surgical removal of the primary tumor. Mice were restrained in a confined space that prevented them from moving freely but did not press on them or induce pain (2). This paradigm has been shown to induce chronic stress as shown by neuroendocrine activation (2, 17), weight loss and anxiety-like behaviors (18–19) but does not cause pain or wounding (20).
Pharmacological studies
For beta-adrenergic agonist studies, mice received 10 mg/kg isoproterenol (Sigma, St. Louis, MO) (a receptor-saturating dose (2)) by daily subcutaneous injection commencing 5 days prior to tumor cell inoculation. For beta-adrenergic antagonist studies, mice were implanted intrascapularly with a 21-day release pellet containing 0.5 mg propranolol or placebo (Innovative Research of America, Sarasota, FL) 2 days prior to commencing stress (or control conditions). This dose has been established to block peripheral beta-adrenergic receptors (21). For macrophage inhibition studies, mice were treated with 160 mg/kg GW2580 (5-(3-Methoxy-4-[4-Methoxybenzyl)oxy]Benzyl)-Pyrimidine-2,4-Diamine) (R.I. Chemical, Orange, CA) or filtered diluent control (0.5% hydroxypropyl methylcellulose, 0.1% Tween20) (Sigma) by daily oral gavage commencing one day after tumor cell inoculation (22). This dose was shown to have maximum bioavailability while maintaining substrate specificity (22).
Determination of cAMP synthesis
66cl4 tumor cells were treated with 0, 0.1, 1.0 or 10 µM norepinephrine ± 10 µM nadolol, and intracellular cAMP concentration was measured by cAMP-Glo Assay (Promega, Madison, WI) according to the manufacturer’s instructions.
Analysis of macrophage infiltration and vascular density
For in situ analysis of macrophage infiltration, 5 µm cryosections were fixed in −20°C acetone then incubated with antibodies against F4/80 (clone BM8 at 10 µg/ml, eBioscience, San Diego CA) and β2-adrenergic receptor (β2AR) (H-20 rabbit polyclonal diluted 1:120, Santa Cruz Biotechnologies, Santa Cruz CA) for 16 hr at 4°C, followed by incubation with fluorescent Alexa-conjugated secondary antibodies (Invitrogen, Carlsbad, CA) and Hoechst 33342 nuclear stain (Sigma). Immunostaining was imaged using an inverted Axio Observer D1 microscope with fluorescence filters (Zeiss, Thornwood NY), coupled to a computer with Axiovision 4.7 software (Zeiss). The relative frequency of F4/80+ and β2AR+ cells was determined by averaging the pixel density of positive cells in 5 randomly selected microscope fields in each of 5 tissue sections from each tumor using ImageJ software (NIH, Bethesda MD). CD31+ vascular density was analyzed in tumor cryosections fixed with 4% paraformaldehyde (Sigma), pre-treated with Proteinase K (Invitrogen) for antigen retrieval and incubated for 16 hr at 4°C with 10 µg/ml anti-CD31 (MEC13.3) (BD Bioscience, Franklin Lakes NJ) followed by anti-rat Alexa-568 (Invitrogen). Relative vascular density was determined by pixel threshold analysis using ImageJ software as described above.
For flow cytometric analysis of immune cell infiltration, single cell suspensions were prepared from tumors harvested 28 days after mammary fat pad inoculation and incubated with directly conjugated antibodies against CD45, CD11b, Gr1, F4/80 (eBiosciences, San Diego, CA) and Ly6C (BD Bioscience, San Jose, CA) as previously described (22). Samples were analyzed using LSRII flow cytometer (Beckman Coulter, Brea, CA) and FlowJo software (TreeStar, Ashland, OR).
Expression of metastasis-related genes
Quantitative real-time RT-PCR was used to quantify Arg1, Csf1, Mmp9, Ptgs2, Tgfb, Vcam1, and Vegf mRNA isolated from 5 mg mammary tumor tissue using RNeasy kit (Qiagen, Valencia CA), or CSF1-differentiated bone marrow-derived macrophages treated with 0, 1, 10 µM NE ± nadolol. mRNA was assayed by real-time RT-PCR reactions using gene-specific Taqman Gene Expression assays (Applied Biosystems, Foster City CA) and Quantitect RT-PCR reagents (Qiagen) with 50 PCR amplification cycles of 15 sec strand separation at 95°C and 60 sec annealing and extension at 60°C. Triplicate determinations were quantified by threshold cycle analysis of FAM fluorescence intensity using iCycler software (BioRad, Hercules CA).
Statistical analysis
Standard power calculations selected a sample size for in vivo experiments that had a power of 0.89 to detect a 1.5 standard deviation effect at p < .05 (23). Data are presented as mean ± standard error, and all statistical analyses were carried out using SAS Version 9.2 (SAS Institute, Cary NC). Student’s t test analyzed the effect of stress on size and frequency of metastasis and differences in gene expression and protein levels. To determine the effect of stress on the longitudinal growth trajectory of tumors, and whether those effects were modified by pharmacological interventions that targeted beta-adrenoreceptors or macrophages, we examined the stress × treatment interaction term in a 2 (control vs stress) × 2 (treatment vs placebo) experimental design in the context of mixed-effects linear model analysis (SAS PROC MIXED). Spearman’s correlation coefficient quantifed relationships between macrophage infiltration and metastastic burden.
RESULTS
Chronic stress enhances metastasis to distant tissues
To assess the effect of chronic physiologic stress on breast cancer progression, we used in vivo optical imaging to track metastasis of luciferase-tagged 66cl4 breast cancer cells from the mammary gland to distant target tissues (Figure 1A). This approach can resolve as few as 3000 cells (Supplementary Figure 1A). Balb/c mice syngenic to 66cl4 cells were randomly assigned to 2 hr/day restraint or home cage control conditions for 20 days, starting 5 days prior to tumor cell inoculation into the 4th mammary fat pad. Physical restraint is a standardized stressor that increases circulation of catecholaminergic neurotransmitters and corticosterone but avoids physical pain or wounding (2, 17–18, 20). We have previously documented a 2.5- to 3.5-fold increase in tissue catecholamine levels in stressed animals (2). Physiological stress response was confirmed by 6.3% weight loss (p = .004) that was rapidly reversed at the conclusion of restraint (Supplementary Figure 1B) (19).
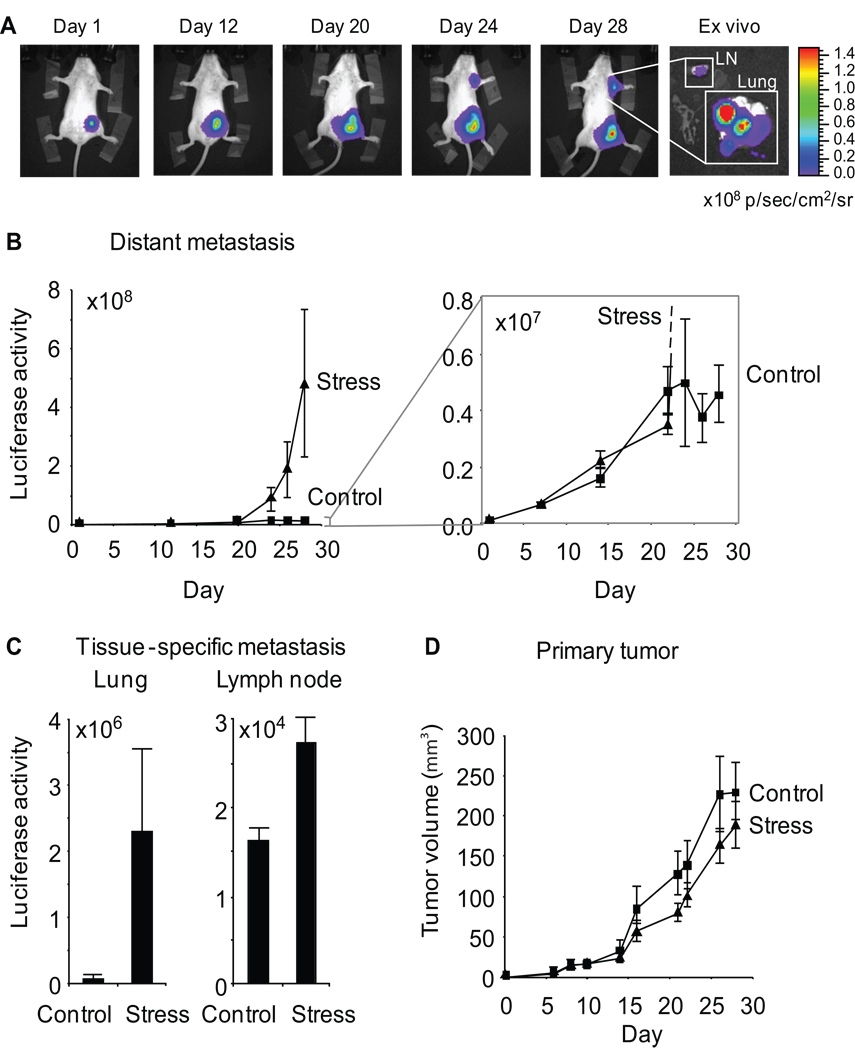
Figure 1. Effect of chronic stress on breast cancer metastasis to distant tissues.
A. Metastasis was quantified by periodic imaging of tumor-specific bioluminescence signal in chest region in Balb/c mice and (B) quantified over multiple mice (5 per group). Inset shows increased resolution of the control condition. Luciferase activity: photons/sec. C. Tissue-specific metastasis was quantified by ex vivo bioluminescent imaging of tumor masses in lung and lymph node (axillary and brachial). D. Primary tumor volume was derived from 2-dimensional caliper measurements. Data: Mean ± S.E.
Chronic stress increased metastasis of primary breast tumor cells to distant tissues by 38-fold vs. controls (p = .04) (Figure 1B). In control mice, luciferase signal from metastatic tumor cells that had disseminated to the chest region (lung and lymph node) increased steadily and then reached a plateau at 21 days after tumor cell inoculation (Figure 1B, inset). Stress increased metastasis in clinically relevant tissues, with a 37-fold increase in lung (p = .034) and a 67% increase in lymph node (p = .009) (Figure 1C). These effects were driven by both increased numbers of metastatic masses (control vs stress: 1.4 ± .74 metastatic foci in lung vs 4.6 ± .75, p = .016, shown by microscopic analysis) and increased size of metastatic masses (9.9-fold increase in metastatic luciferase signal, p = .04). Despite greater rates of metastasis, primary tumor growth rate was not significantly altered by stress (Figure 1D) (p = .41).
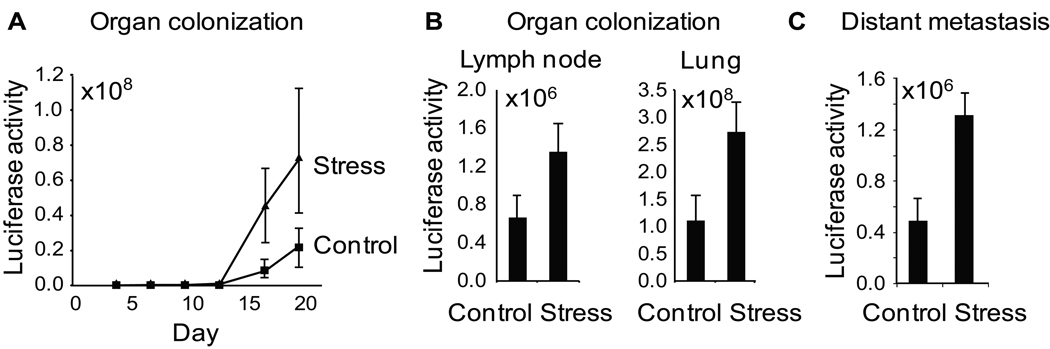
To identify mechanisms of stress-induced metastatic up-regulation, we injected tumor cells into the blood stream and assayed the bioluminescent tumor signal in distant tissues over time. This direct assay of metastatic colonization (which avoids variance in primary tumor escape and intravasation) showed a 3.3-fold increase in colonization rates as a function of stress (Figure 2A). Ex vivo analyses of tissue harvested at 27 days post-injection found that stress increased lung tumor colonization by 2.5-fold (p = .038) and lymph node colonization by 2.1-fold (p = .037) (Figure 2B). To examine the impact of stress on metastatic development independently of effects on the primary tumor, daily restraint was initiated only after surgical removal of the primary tumor. Results showed that stress increased metastasis by 2.7-fold (p = .05) (Figure 2C). However, these 2- to 3-fold increases in colonization efficiency were not sufficient in magnitude to account for the total ~30-fold increase in spontaneous metastasis that occurred from primary tumors (Figure 1B). Additional analyses thus focused on dynamics within the primary tumor microenvironment occurring prior to intravasation.
Figure 2. The effect of stress on colonization of metastatic target tissues.
A. Organ colonization by 1.0 × 105 i.v-injected 66cl4 tumor cells was quantified by repeated imaging of tumor-specific bioluminescence signal in chest region. B. Tissue specific colonization was quantified by ex vivo imaging of lymph node and lung on day 27 after intravenous inoculation of tumor cells. C. Distant metastasis was quantified by bioluminescence imaging at day 41 following surgical removal of primary tumors on day 14. Stress was commenced only after surgical removal of primary tumors. Data: Mean ± S.E.
Sympathetic nervous system regulates distant metastasis
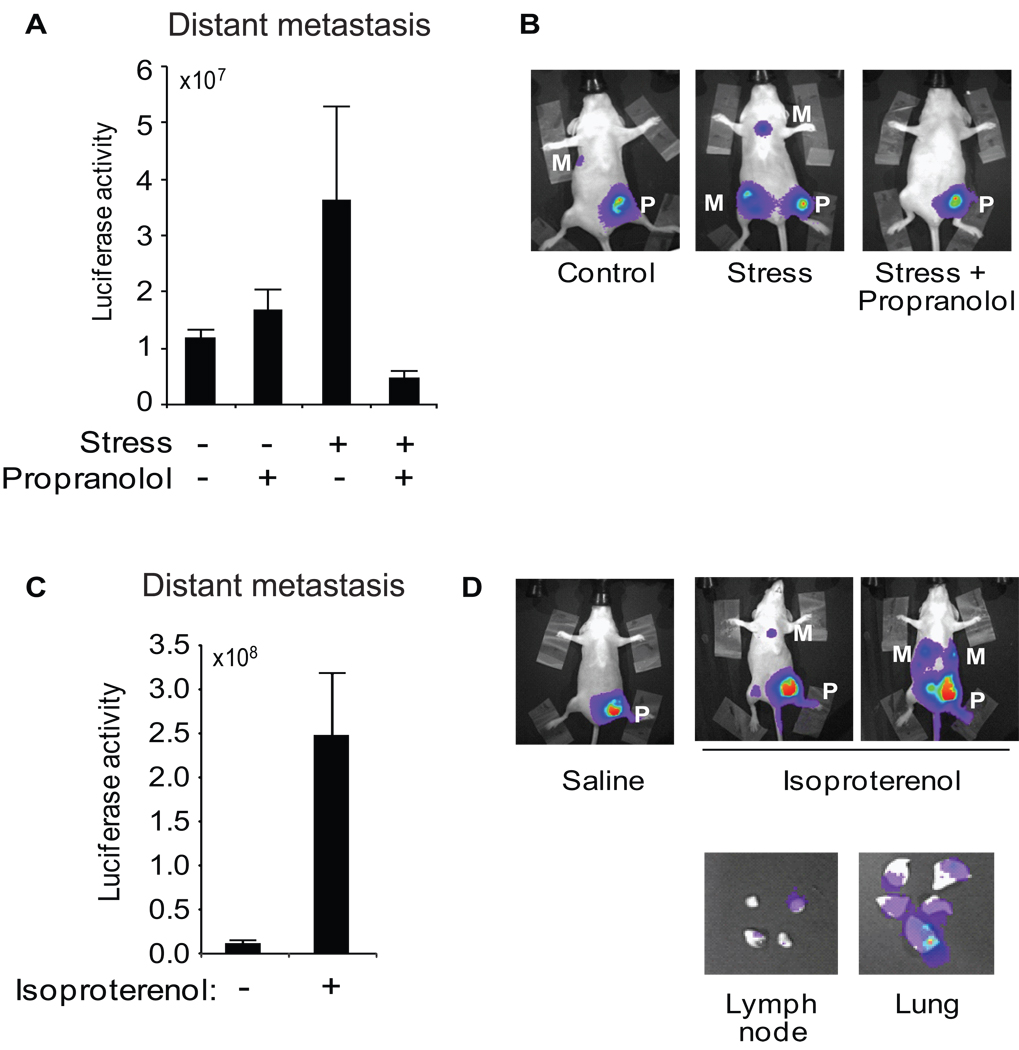
Physiological stressors activate the sympathetic nervous system (SNS) to release NE, both systemically and within common metastatic target organs (3, 9). NE ligation of β-adrenergic receptors activates the associated cAMP/PKA signaling pathway, and β2-adrenergic receptors (β2AR) have been documented on both breast cancer cells and immune cells (e.g., T lymphocytes and macrophages) present in the breast cancer microenvironment (5, 9, 24–25). To investigate the role of SNS activation in stress-enhanced tumor progression, we treated stressed and control mice with the beta-adrenergic antagonist propranolol starting 2 days prior to tumor cell inoculation and continuing throughout the 20-day stress period. Immunostaining against β2AR showed the expected compensatory increase in β2AR cell surface expression in tumors from propranolol-treated animals (placebo vs. propranolol: 1.9 ± 0.1 % tissue area vs 6.7 ± 1.1, p < .001, see also Supplementary Figure 3B), confirming functional penetration of β2AR antagonism throughout the tumor parenchyma (26). Propranolol treatment had no significant effect on metastatic burden in non-stressed control mice (p = .08; Figure 3A). However, propranolol completely blocked stress-enhanced metastasis in animals subject to chronic restraint stress (p < .0001) (Figure 3A, B). Propranolol treatment had no effect on primary tumor growth in the mammary fat pad for either stressed (p = .73) or non-stressed control animals (p = .89).
Figure 3. Role of sympathetic nervous system in stress-induced metastasis.
A. Distant metastasis was quantified in the lung and lymph node region by in vivo imaging at day 28 following tumor cell inoculation into stressed vs control mice treated with propranolol or placebo. B. Representative images of mice in control (+ placebo), stress (+ placebo) and stress + beta-blocker groups. C. Distant metastasis was quantified 28 days after primary tumor inoculation in mice treated with saline vs isoproterenol. D. Representative in vivo images of saline- and isoproterenol-treated mice and (E) ex vivo lung and lymph node showing tumor-specific bioluminescent signal (× 106 p/sec/cm2/sr). P: primary tumor, M: distant metastasis.
To determine whether beta-adrenergic activation without concomitant activation of other receptor types is sufficient to enhance metastasis, unstressed mice were treated with the beta-agonist isoproterenol to pharmacologically activate β2AR. Isoproterenol increased metastasis to distant tissues by 22-fold compared to saline-treated controls (p = .03) (Figure 3C, D). Ex vivo analysis of tissues confirmed increased metastasis (Figure 3D).
T lymphocytes are not essential for stress-enhanced metastasis
To determine whether stress-induced alterations in T cell-mediated immune responses might contribute to the metastatic dynamics observed, we repeated studies in T cell-deficient nu/nu mice. Similar to immune-intact Balb/c mice, chronic stress increased the total metastatic mass by 28-fold in nu/nu mice (p = .05) (Supplementary Figure 2A). Ex vivo analysis confirmed significant increases in metastasis to both lung (p = .03) and lymph node (p < .001) (Supplementary Figure 2B). Again, stress did not alter primary tumor growth (Supplementary Figure 2C). These results indicate that T lymphocytes do not mediate the observed stress effect, and our subsequent studies thus focused on other cellular mediators.
Macrophages are an intratumoral target of SNS signaling
To define the cellular pathway by which beta-adrenergic signaling might regulate pro-metastatic signals in primary tumors, we conducted two-color immunofluorescence analyses of cellular differentiation markers and β2AR in sectioned primary tumors. Weak β2AR staining was observed on primary tumor cells (Figure 4A, panel i). To verify the functional activity of those receptors, we treated 66cl4 cells with NE and assayed activity of the second messenger molecule cAMP. Results showed significant cAMP flux in response to β2AR ligation that was inhibited by the beta-blocker nadolol (Supplementary Figure 3), consistent with other studies documenting catecholamine regulation of breast cancer cell functional activity (14). Within the tumor parenchyma, we also observed strong β2AR staining co-localizing with the macrophage marker F4/80 (Figure 4A, overlay). β2AR activation is known to functionally regulate gene expression in macrophages (9, 27–29), raising the possibility that SNS activity may regulate primary tumor dynamics by recruiting or modifying the activity of tumor-associated macrophages.
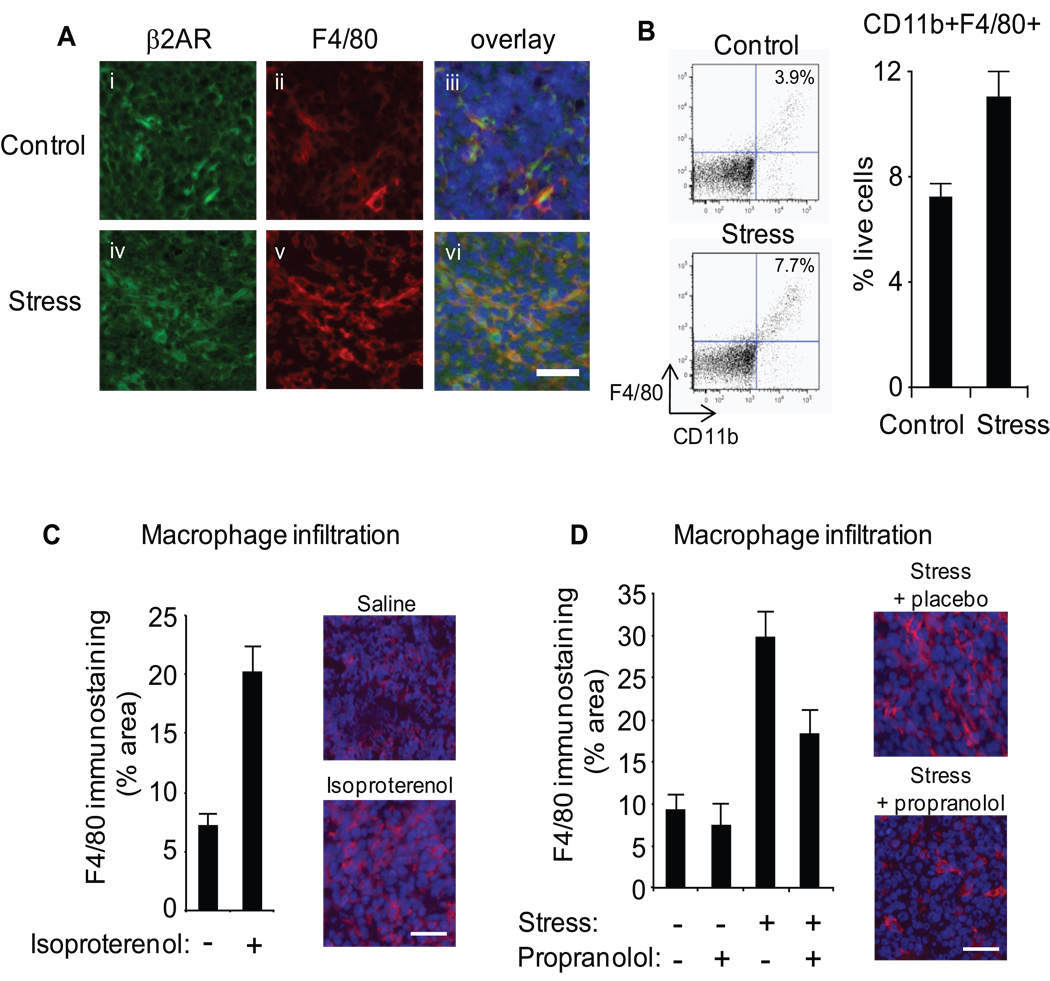
Figure 4. Role of macrophage infiltration.
A. Mammary tumor cryosections from control and stressed mice were immunostained with anti-β2AR (green) (i, iv) and anti-F4/80 (red) (ii, v), and nuclear counterstained with Hoechst 33324 (blue) (iii, vi). B. CD11b+F4/80+ cells were quantified by flow cytometry in disaggregated 66cl4 primary tumors harvested 28 days after inoculation. C,D. Macrophage infiltration of mammary tumors was visualized by immunostaining with anti-F4/80 (red) and nuclei counterstained (blue) in tumors harvested at day 28 from saline- or isoproterenol-treated mice (C) or stressed mice treated with placebo vs. propranolol (D), and quantified across sections from multiple mice (n = 5/gp). Scale bars: 30µm.
Stress increases tumor infiltration by macrophages
To determine whether chronic stress impacts macrophage infiltration into primary mammary tumors, we used flow cytometry to quantify cell composition in digested 66cl4 primary tumors harvested from mammary fat pads (Figure 4B). Stress increased mammary tumor infiltration of CD11b+F4/80+ macrophages by 53% (control vs. stress: 7.23 ± 0.49 % of live cells vs. 11.04 ± 1.22 %, p = .013) (Figure 4B). Stress also marginally increased tumor infiltration of CD11b+ GrloLy6Chi cells (myeloid-derived suppressor cells) (22) (43% increase, p = .073). Stress-induced recruitment of immune cells was specific to macrophages, as no significant increase was observed for other myeloid cell types such as CD11b+ GrhiLy6Clo polymorphonuclear neutrophil-like suppressor cells (p = .76) (22) or for CD45+CD11b- lymphocytes (p = .15).
In situ analysis of F4/80+ immunostaining confirmed a 47% increase in macrophage recruitment within primary tumors from stressed animals (p < .006) (Figure 4A). Isoproterenol stimulation of β-adrenergic signaling similarly increased macrophage infiltration into primary tumor parenchyma (saline vs isoproterenol: 7.12 ± 1.0 % tissue area vs. 20.2 ± 2.2, p < .001) (Figure 4C, Supplementary Figure 4). To confirm that stress-induced infiltration of macrophages was mediated by SNS activation, we mapped the distribution of F4/80+ cells in day 28 tumor parenchyma from stressed animals treated with the beta-adrenergic antagonist propranolol (Figure 4D, Supplementary Figure 4). As shown in Figure 4D, propranolol largely abrogated the stress-induced increase in F4/80+ cells within the primary tumor.
Stress induces expression of macrophage-derived pro-metastatic molecules
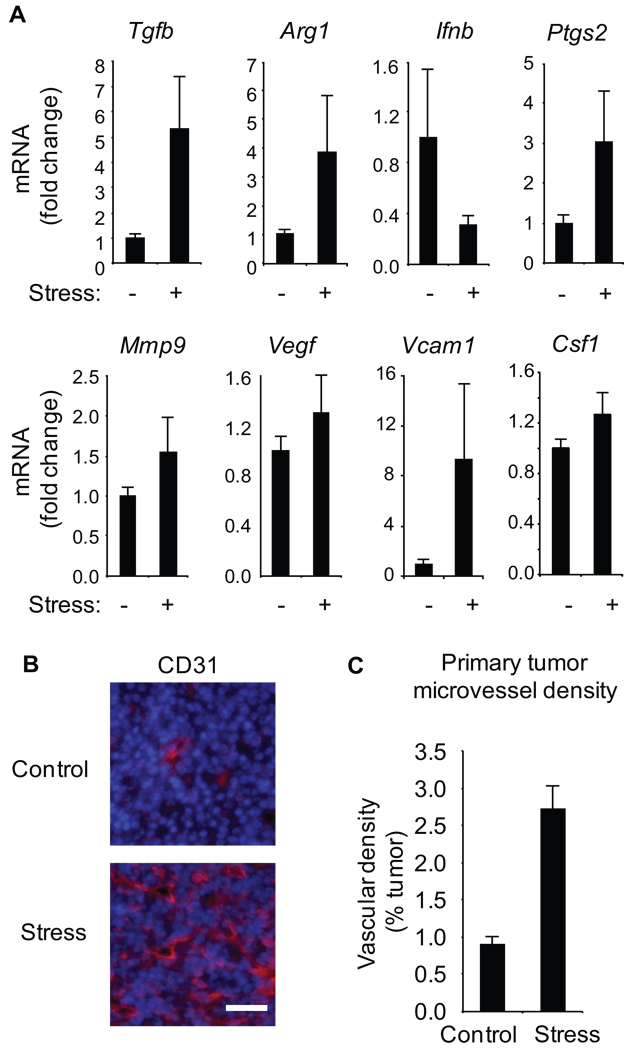
Previous studies indicate that macrophages contributes to breast cancer metastasis by expressing pro-metastatic genes within the tumor microenvironment (30). Myeloid cells including tumor-associated macrophages may also develop an immunosuppressive phenotype, mediated in part through local TGFβ activity and characterized by arginase 1 production (30). Consistent with that dynamic, stress induced a 5.3-fold increase in Tgfb gene expression (p < .001) and 3.9-fold increase in Arg1 expression (p < .001) in primary tumors (Figure 5A). In contrast, stress reduced Ifnb gene expression by 3.3-fold, consistent with a protective role for type I interferons in cancer progression (31). Stress also increased expression of pro-inflammatory and pro-metastatic gene products including Cox2 (Ptgs2) (3.0-fold increase, p < .001), Mmp9 (54% increase, p = .02), Vegf (30% increase, p = .046) and Vcam1 (9.4-fold increase, p < .001) (Figure 5A), as well as macrophage chemoattractant and growth factor CSF-1 (25% increase, p = .02) (Figure 5A). To investigate the impact of adrenergic signaling on macrophage phenotype, bone marrow-derived macrophages were treated with NE and gene expression was assayed by quantitative RT-PCR 6 hrs later. Adrenergic signaling increased expression of genes characteristic of an M2 phenotype (Arg1: 24-fold, p < .001 and Tgfb: 2.5-fold, p = .02) and decreased expression of the key M1 gene Nos2 (44% decrease, p = .05). Consistent with transcriptional alternations in whole mammary tumours (Figure 5A), NE also increased macrophage expression of Vegf (3.2-fold, p = .003) and Mmp9 (3.0-fold, p = .04). To determine if increased Vegf expression was sufficient to modulate angiogenesis in vivo, we mapped primary tumor blood vessel density through CD31 immunostaining and found a 2.8-fold increase in stressed animals (p < .001) (Figure 5B, C). Consistent with SNS regulation of angiogenesis (2), these studies showed that propranolol inhibited the stress-induced increase in vascularization of mammary tumors (p < .001).
Figure 5. Effect of stress on pro-metastatic gene expression and primary tumor vascularization.
A. Gene expression was quantified by RT-PCR in mammary tumors from control vs. stressed mice (n = 5/gp). B. Mammary tumor cryosections were immunostained with anti-CD31 antibody (red) and nuclei counterstained (blue). Scale bar: 30 µm. C. CD31+ blood vessel density was quantified in 5 sections from multiple tumors (n = 5/gp).
Macrophage infiltration mediates stress-enhanced metastasis
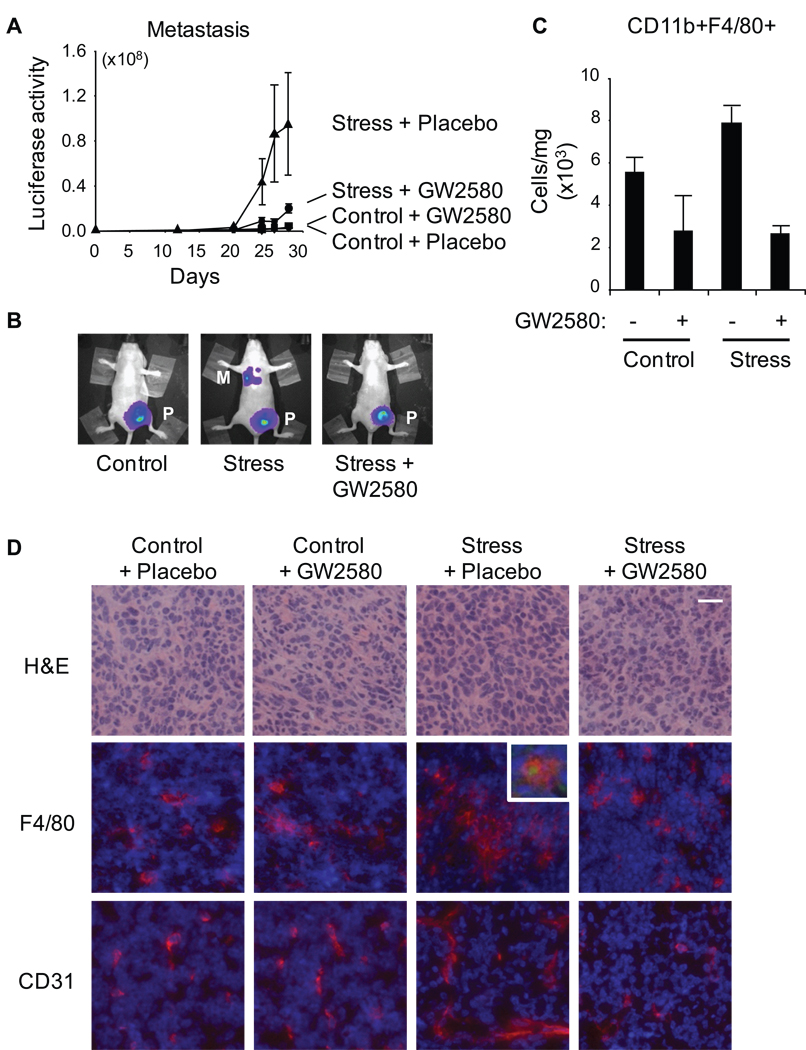
To determine whether SNS-mediated macrophage recruitment might contribute to metastatic spread, we examined the relationship between the extent of F4/80+ macrophage infiltration at 4 weeks and the total cellular burden of distant metastasis at 4 weeks. This correlation was highly significant in stressed mice (Spearman correlation coefficient, r = 0.66, p = .007) but not in controls (r = − .12, p = .67). To confirm that macrophage recruitment functionally mediated stress effects on metastasis, we used a CSF-1 receptor antagonist to suppress macrophage recruitment and function in vivo. After random assignment to control or stress conditions, 50% of mice in each condition received either 160 mg/kg of GW2580 (a small molecule inhibitor of CSF-1 receptor kinase) or placebo (22). Treatment with GW2580 largely reversed stress-enhanced metastasis (Figure 6A). Longitudinal bioluminescent imaging and ex vivo analyses found little colonization of distant tissue in stressed mice treated with GW2580, whereas stressed mice treated with placebo continued to show increased rates of metastasis to distant target tissues (Figure 6A, B). Flow cytometric analysis of primary tumors showed that treatment with GW2580 reduced infiltration of CD11b+F4/80+ macrophages by 2.9-fold (placebo vs. stress: 7.8 × 103 ± .8 × 103 CD11b+F4/80+ cell/mg tissue vs. 2.7 × 103 ± .35 103, p < .001), and thereby blocked the stress-induced increase in macrophage recruitment (Figure 6C, D middle panels). GW2580-treated mice also showed reduced CD11b+F4/80+ cells in bone marrow (45% decrease, p = .05) as expected by CSF-1 receptor inhibition. Consistent with reduced myeloid cell infiltration of mammary tumors, GW2580 largely reversed stress-induced upregulation of Arg1 gene expression (Supplementary Figure 5). Inhibition of macrophage infiltration by GW2580 had no effect on primary tumor growth (mean volume at week 4 = 395 mm3 in stressed animals treated with placebo vs. 397 in stressed animals treated with GW2580, p = .88). However, analyses of CD31 expression showed that GW2580 reversed stress enhancement of blood vessel density (Figure 6D lower panels) which indicates a central role for macrophages in SNS regulation of vascularization.
Figure 6. Effect of macrophage inhibition on stress-enhanced metastasis.
A. Metastasis was quantified using live in vivo imaging in control vs stressed mice treated with the CSF1 receptor inhibitor GW2580 or placebo. (NB: Control + placebo and control + GW2580 curves are indistinguishable.) B. Representative images of mice in control (+ placebo), stress (+ placebo) and stress + GW2580 conditions. C. Frequency of CD11b+F4/80+ cells in 66cl4 primary tumors from control vs stressed mice treated with GW2580 or placebo. D. Mammary tumor cryosections were stained with hematoxylin and eosin to show general morphology or immunostained with anti-F4/80 (red, middle panels) or anti-CD31 (red, lower panels) and nuclei counterstained (blue). Scale bar: 20µm. Inset shows representative F4/80 macrophages positive for β2AR (green). P: primary tumor, M: distant metastasis.
DISCUSSION
These studies identify SNS activation as a novel physiologic regulator of breast cancer metastasis to distant tissue sites including lymph node and lung. SNS effects were mediated through β-adrenergic signaling, which acted to recruit alternatively activated macrophages into the primary tumor parenchyma and thereby induce a pro-metastatic gene expression signature. These effects occurred in syngeneic tumors in immunocompetent mice, as well as in mice lacking a functional T cell compartment. Thus, direct regulation of macrophage biology by the SNS appears to constitute a previously unrecognized pathway by which external conditions affecting the autonomic nervous system can activate a metastatic switch within a growing primary tumor. These findings expand our understanding of the physiological processes that regulate breast cancer metastasis, and they suggest novel strategies for inhibiting metastatic spread through targeted inhibition of SNS-regulated macrophage dynamics.
The current findings confirm previous indications that macrophage infiltration can influence breast cancer metastasis (30), and extend those findings to identify a novel SNS / β-adrenergic signaling pathway that can drive changes in macrophage recruitment and differentiation, and thereby alter gene expression within the primary tumor. SNS regulation of pro-metastatic macrophage dynamics provides an alternative mechanism to Th2 lymphocyte regulation of tumor-associated macrophage activity (32) and suggests that therapeutic strategies that seek to promote a tumoricidal M1 macrophage phenotype by orienting T helper cell response towards Th1 cytokine production (33) may be insufficient to modulate metastasis in the presence of chronic SNS activation. Furthermore, SNS regulation of macrophages in the absence of a T lymphocyte population suggests an antigen-independent mechanism of tumor-associated inflammation that need not invoke exogenous triggers (eg. tumor-associated viruses) (33), but instead occurs through direct neurotransmitter signaling to myeloid cells. SNS regulation of physiological dynamics via altered macrophage / monocyte communication would extend the influence of the peripheral nervous system beyond the distance of neurotransmitter diffusion from primary neural fibers (10–100s µm) (34), and suggest a mechanism for transmitting the effects of autonomic nervous system activation into non- or poorly-innervated tissues such as solid epithelial tumors.
The present studies clearly indicate a role for macrophages in mediating SNS effects on metastasis, and they rule out any requirement for T lymphocytes in such dynamics, but it remains conceivable that other immune cells act upstream of macrophages to regulate their recruitment and polarization (e.g., NK cells might potentially play a role, as suggested in recent studies (35)). Defining the tumor and microenvironmental dynamics that ultimately shape SNS modulation of tumor-associated macrophage biology represents an important topic for future research. Particularly important would be identification of the cell types and signaling pathways that regulate macrophage recruitment and the functional impact of stress-induced M2 macrophage phenotype. The current study shows that SNS signaling modulates both the extent of tumor infiltration by macrophages and associated intratumoral expression of pro-metastatic genes, including Cox2 (Ptgs2), Mmp9, Arg1, Tgfb and Vegf. Consistent with SNS regulation of pro-metastatic macrophage gene expression, SNS activation has been shown to promote peritoneal growth of metastatic ovarian tumors in nude mice through increased VEGF-mediated vascularization (2). The present studies extend those observations into the domain of metastatic dissemination to distant tissues (including vascular-mediated colonization of target organs), and they identify macrophage modulation within the primary tumor as a central molecular mechanism for alterations in primary tumor metastatic seeding.
Neural regulation of macrophage pro-metastatic activity may provide potential cellular and molecular mechanisms for clinical observations linking chronic stress to increased breast cancer progression in humans (1, 36–37). Although these observations remain controversial, recent analyses have begun to define the circumstances in which such relationships are most likely to be observed. Few consistent relationships have been found between stress and the initial incidence of breast cancer (38–41). However, several epidemiological studies and a large meta-analysis of 131 prospective studies have linked chronic stress to increased progression of established breast cancers (36–37, 42–45). Those results are consistent with data from the present experimental model in which stress-induced activation of the SNS showed no significant impact on the growth of primary tumors, but reliably enhanced metastatic spread via effects on both primary tumor seeding of metastasis and tumor cell extravasation / colonization of distant target tissues. The present findings also suggest that other physiologic or pharmacologic influences on SNS activity besides stress might potentially influence cancer progression. Such results would be consistent with epidemiologic findings linking beta-blocker usage to reduced cancer risk (6–7). Two case-control studies of advanced breast cancer did not find a link between beta-blocker use and breast cancer incidence (46–47) but a recent study has linked beta-blockers to reduced metastasis and breast cancer-specific mortality (48).
The present data suggest that pharmacological inhibition of SNS activity (e.g., with beta antagonists or anti-NGF antibodies (49)) could potentially constitute a novel adjunctive strategy for minimizing breast cancer metastasis. Localized targeting of tumor-associated macrophages (e.g., with GW2580) might also block adverse effects of neuroendocrine activation on macrophage recruitment into primary tumors and thereby reduce pro-metastatic gene expression. Macrophage recruitment into the primary tumor might also serve as a biomarker for early detection of disease progression and/or therapeutic impact in clinical and intervention studies.
The present results show that systemic physiologic conditions can significantly shape the primary tumor microenvironment and may alter conditions in distant tissues in ways that facilitate metastasis to distant organs. This raises the intriguing possibility that systemic interventions (e.g., that target neural or immune compartments) may provide new adjunctive strategies to complement existing anti-cancer therapies (50). These findings highlight the importance of considering the patient’s overall physiology in the development of new therapeutic approaches to limit cancer progression and minimize metastatic rates in breast cancer.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Robin Anderson for providing cell lines, Irvin Chen for luciferase expression vectors, David Stout and Waldemar Ladno at the UCLA Crump Molecular Imaging Center, and Patricia Ganz, Susan Lutgendorf, John Sheridan, Michael Irwin, Andrew Cuddihy, Robin Anderson and laboratory, and Dan Welch and laboratory for their thoughtful discussions of this research. This work was supported by CDMRP BCRP W81XWH-08-1-0629, NIH CA138687, CA116778, CA109298, and CA110793, the UCLA Norman Cousins Center and a UCLA Heffron Foundation grant. SJP is supported by CDMRP PCRP W81XWH-09-1-0538 training award.
REFERENCES
- 1.Antoni MH, Lutgendorf SK, Cole SW, Dhabhar FS, Sephton SE, McDonald PG, et al. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat Rev Cancer. 2006;6:240–248. doi: 10.1038/nrc1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thaker PH, Han LY, Kamat AA, Arevalo JM, Takahashi R, Lu C, et al. Chronic stress promotes tumor growth and angiogenesis in a mouse model of ovarian carcinoma. Nat Med. 2006;12:939–944. doi: 10.1038/nm1447. [DOI] [PubMed] [Google Scholar]
- 3.Sloan EK, Capitanio JP, Tarara RP, Mendoza SP, Mason WA, Cole SW. Social stress enhances sympathetic innervation of primate lymph nodes: mechanisms and implications for viral pathogenesis. J Neurosci. 2007;27:8857–8865. doi: 10.1523/JNEUROSCI.1247-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang Y, Shankar R, Gamelli R, Jones S. Dynamic norepinephrine alterations in bone marrow: evidence of functional innervation. J Neuroimmunol. 1999;96:182–189. doi: 10.1016/s0165-5728(99)00032-6. [DOI] [PubMed] [Google Scholar]
- 5.Cakir Y, Plummer HK, 3rd, Tithof PK, Schuller HM. Beta-adrenergic and arachidonic acid-mediated growth regulation of human breast cancer cell lines. Int J Oncol. 2002;21:153–157. [PubMed] [Google Scholar]
- 6.Algazi M, Plu-Bureau G, Flahault A, Dondon MG, Le MG. Could treatments with beta-blockers be associated with a reduction in cancer risk? Rev Epidemiol Sante Publique. 2004;52:53–65. doi: 10.1016/s0398-7620(04)99022-0. [DOI] [PubMed] [Google Scholar]
- 7.Perron L, Bairati I, Harel F, Meyer F. Antihypertensive drug use and the risk of prostate cancer (Canada) Cancer Causes Control. 2004;15:535–541. doi: 10.1023/B:CACO.0000036152.58271.5e. [DOI] [PubMed] [Google Scholar]
- 8.Shimizu N, Hori T, Nakane H. An interleukin-1 beta-induced noradrenaline release in the spleen is mediated by brain corticotropin-releasing factor: an in vivo microdialysis study in conscious rats. Brain Behav Immun. 1994;8:14–23. doi: 10.1006/brbi.1994.1002. [DOI] [PubMed] [Google Scholar]
- 9.Madden KS, Sanders VM, Felten DL. Catecholamine influences and sympathetic neural modulation of immune responsiveness. Annu Rev Pharmacol Toxicol. 1995;35:417–448. doi: 10.1146/annurev.pa.35.040195.002221. [DOI] [PubMed] [Google Scholar]
- 10.Cole SW, Korin YD, Fahey JL, Zack JA. Norepinephrine accelerates HIV replication via protein kinase A-dependent effects on cytokine production. J Immunol. 1998;161:610–616. [PubMed] [Google Scholar]
- 11.Kobilka B. Adrenergic receptors as models for G protein-coupled receptors. Annu Rev Neurosci. 1992;15:87–114. doi: 10.1146/annurev.ne.15.030192.000511. [DOI] [PubMed] [Google Scholar]
- 12.Lutgendorf SK, Cole S, Costanzo E, Bradley S, Coffin J, Jabbari S, et al. Stress-related mediators stimulate vascular endothelial growth factor secretion by two ovarian cancer cell lines. Clin Cancer Res. 2003;9:4514–4521. [PubMed] [Google Scholar]
- 13.Nilsson MB, Armaiz-Pena G, Takahashi R, Lin YG, Trevino J, Li Y, et al. Stress hormones regulate interleukin-6 expression by human ovarian carcinoma cells through a Src-dependent mechanism. J Biol Chem. 2007;282:29919–29926. doi: 10.1074/jbc.M611539200. [DOI] [PubMed] [Google Scholar]
- 14.Lang K, Drell TLt, Lindecke A, Niggemann B, Kaltschmidt C, Zaenker KS, et al. Induction of a metastatogenic tumor cell type by neurotransmitters and its pharmacological inhibition by established drugs. Int J Cancer. 2004;112:231–238. doi: 10.1002/ijc.20410. [DOI] [PubMed] [Google Scholar]
- 15.Morizono K, Xie Y, Ringpis GE, Johnson M, Nassanian H, Lee B, et al. Lentiviral vector retargeting to P-glycoprotein on metastatic melanoma through intravenous injection. Nat Med. 2005;11:346–352. doi: 10.1038/nm1192. [DOI] [PubMed] [Google Scholar]
- 16.Sloan EK, Pouliot N, Stanley KL, Chia J, Moseley JM, Hards DK, et al. Tumor-specific expression of alphavbeta3 integrin promotes spontaneous metastasis of breast cancer to bone. Breast Cancer Res. 2006;8:R20. doi: 10.1186/bcr1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manni L, Di Fausto V, Fiore M, Aloe L. Repeated restraint and nerve growth factor administration in male and female mice: effect on sympathetic and cardiovascular mediators of the stress response. Curr Neurovasc Res. 2008;5:1–12. doi: 10.2174/156720208783565654. [DOI] [PubMed] [Google Scholar]
- 18.Hermann G, Beck FM, Tovar CA, Malarkey WB, Allen C, Sheridan JF. Stress-induced changes attributable to the sympathetic nervous system during experimental influenza viral infection in DBA/2 inbred mouse strain. J Neuroimmunol. 1994;53:173–180. doi: 10.1016/0165-5728(94)90027-2. [DOI] [PubMed] [Google Scholar]
- 19.Smagin GN, Howell LA, Redmann S, Jr, Ryan DH, Harris RB. Prevention of stress-induced weight loss by third ventricle CRF receptor antagonist. Am J Physiol. 1999;276:R1461–R1468. doi: 10.1152/ajpregu.1999.276.5.R1461. [DOI] [PubMed] [Google Scholar]
- 20.Sheridan JF, Padgett DA, Avitsur R, Marucha PT. Experimental models of stress and wound healing. World J Surg. 2004;28:327–330. doi: 10.1007/s00268-003-7404-y. [DOI] [PubMed] [Google Scholar]
- 21.Dobbs CM, Vasquez M, Glaser R, Sheridan JF. Mechanisms of stress-induced modulation of viral pathogenesis and immunity. J Neuroimmunol. 1993;48:151–160. doi: 10.1016/0165-5728(93)90187-4. [DOI] [PubMed] [Google Scholar]
- 22.Priceman SJ, Sung JL, Shaposhnik Z, Burton JB, Torres-Collado AX, Moughon DL, et al. Targeting distinct tumor-infiltrating myeloid cells by inhibiting CSF-1 receptor: combating tumor evasion of anti-angiogenic therapy. Blood. 2009 doi: 10.1182/blood-2009-08-237412. Online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd Edition ed. Hillsdale NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 24.Wahle M, Neumann RP, Moritz F, Krause A, Buttgereit F, Baerwald CG. Beta2-adrenergic receptors mediate the differential effects of catecholamines on cytokine production of PBMC. J Interferon Cytokine Res. 2005;25:384–394. doi: 10.1089/jir.2005.25.384. [DOI] [PubMed] [Google Scholar]
- 25.Shakhar G, Ben-Eliyahu S. In vivo beta-adrenergic stimulation suppresses natural killer activity and compromises resistance to tumor metastasis in rats. J Immunol. 1998;160:3251–3258. [PubMed] [Google Scholar]
- 26.Ferguson SS. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev. 2001;53:1–24. [PubMed] [Google Scholar]
- 27.Hasko G, Nemeth ZH, Szabo C, Zsilla G, Salzman AL, Vizi ES. Isoproterenol inhibits Il-10, TNF-alpha, and nitric oxide production in RAW 264.7 macrophages. Brain Res Bull. 1998;45:183–187. doi: 10.1016/s0361-9230(97)00337-7. [DOI] [PubMed] [Google Scholar]
- 28.Lutgendorf SK, Lamkin DM, Jennings NB, Arevalo JM, Penedo F, DeGeest K, et al. Biobehavioral influences on matrix metalloproteinase expression in ovarian carcinoma. Clin Cancer Res. 2008;14:6839–6846. doi: 10.1158/1078-0432.CCR-08-0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cole SW, Arevalo JM, Takahashi R, Sloan EK, Lutgendorf SK, Sood AK, et al. Computational identification of gene-social environment interaction at the human IL6 locus. Proc Natl Acad Sci U S A. 2010;107:5681–5686. doi: 10.1073/pnas.0911515107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 31.Bielenberg DR, McCarty MF, Bucana CD, Yuspa SH, Morgan D, Arbeit JM, et al. Expression of interferon-beta is associated with growth arrest of murine and epidermal cells. J Invest Dermatol. 1999;112:802–809. doi: 10.1046/j.1523-1747.1999.00566.x. [DOI] [PubMed] [Google Scholar]
- 32.DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, et al. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pardoll D. Metastasis-promoting immunity: when T cells turn to the dark side. Cancer Cell. 2009;16:81–82. doi: 10.1016/j.ccr.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 34.Sloan EK, Tarara RP, Capitanio JP, Cole SW. Enhanced replication of simian immunodeficiency virus adjacent to catecholaminergic varicosities in primate lymph nodes. J Virol. 2006;80:4326–4335. doi: 10.1128/JVI.80.9.4326-4335.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melamed R, Rosenne E, Shakhar K, Schwartz Y, Abudarham N, Ben-Eliyahu S. Marginating pulmonary-NK activity and resistance to experimental tumor metastasis: suppression by surgery and the prophylactic use of a beta-adrenergic antagonist and a prostaglandin synthesis inhibitor. Brain Behav Immun. 2005;19:114–126. doi: 10.1016/j.bbi.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 36.Chida Y, Hamer M, Wardle J, Steptoe A. Do stress-related psychosocial factors contribute to cancer incidence and survival? Nat Clin Pract Oncol. 2008 doi: 10.1038/ncponc1134. [DOI] [PubMed] [Google Scholar]
- 37.Kroenke CH, Kubzansky LD, Schernhammer ES, Holmes MD, Kawachi I. Social networks, social support, and survival after breast cancer diagnosis. J Clin Oncol. 2006;24:1105–1111. doi: 10.1200/JCO.2005.04.2846. [DOI] [PubMed] [Google Scholar]
- 38.Lillberg K, Verkasalo PK, Kaprio J, Teppo L, Helenius H, Koskenvuo M. Stressful life events and risk of breast cancer in 10,808 women: a cohort study. Am J Epidemiol. 2003;157:415–423. doi: 10.1093/aje/kwg002. [DOI] [PubMed] [Google Scholar]
- 39.Li J, Johansen C, Hansen D, Olsen J. Cancer incidence in parents who lost a child: a nationwide study in Denmark. Cancer. 2002;95:2237–2242. doi: 10.1002/cncr.10943. [DOI] [PubMed] [Google Scholar]
- 40.Levav I, Kohn R, Iscovich J, Abramson JH, Tsai WY, Vigdorovich D. Cancer incidence and survival following bereavement. Am J Public Health. 2000;90:1601–1607. doi: 10.2105/ajph.90.10.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kvikstad A, Vatten LJ, Tretli S, Kvinnsland S. Widowhood and divorce related to cancer risk in middle-aged women. A nested case-control study among Norwegian women born between 1935 and 1954. Int J Cancer. 1994;58:512–516. doi: 10.1002/ijc.2910580410. [DOI] [PubMed] [Google Scholar]
- 42.Ell K, Nishimoto R, Mediansky L, Mantell J, Hamovitch M. Social relations, social support and survival among patients with cancer. J Psychosom Res. 1992;36:531–541. doi: 10.1016/0022-3999(92)90038-4. [DOI] [PubMed] [Google Scholar]
- 43.Soler-Vila H, Kasl SV, Jones BA. Prognostic significance of psychosocial factors in African-American and white breast cancer patients: a population-based study. Cancer. 2003;98:1299–1308. doi: 10.1002/cncr.11670. [DOI] [PubMed] [Google Scholar]
- 44.Barraclough J, Pinder P, Cruddas M, Osmond C, Taylor I, Perry M. Life events and breast cancer prognosis. Bmj. 1992;304:1078–1081. doi: 10.1136/bmj.304.6834.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Graham J, Ramirez A, Love S, Richards M, Burgess C. Stressful life experiences and risk of relapse of breast cancer: observational cohort study. Bmj. 2002;324:1420. doi: 10.1136/bmj.324.7351.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li CI, Malone KE, Weiss NS, Boudreau DM, Cushing-Haugen KL, Daling JR. Relation between use of antihypertensive medications and risk of breast carcinoma among women ages 65–79 years. Cancer. 2003;98:1504–1513. doi: 10.1002/cncr.11663. [DOI] [PubMed] [Google Scholar]
- 47.Meier CR, Derby LE, Jick SS, Jick H. Angiotensin-converting enzyme inhibitors, calcium channel blockers, and breast cancer. Arch Intern Med. 2000;160:349–353. doi: 10.1001/archinte.160.3.349. [DOI] [PubMed] [Google Scholar]
- 48.Powe DG, Voss MJ, Habashy HO, Zanker KS, Green AR, Ellis IO, et al. Beta-blocker treatment is associated with a reduction in tumour metastasis and an improvement in specific survival in patients with breast cancer; 7th European Breast Cancer Conference; 2010. Abstracts:#445. [Google Scholar]
- 49.Halvorson KG, Kubota K, Sevcik MA, Lindsay TH, Sotillo JE, Ghilardi JR, et al. A blocking antibody to nerve growth factor attenuates skeletal pain induced by prostate tumor cells growing in bone. Cancer Res. 2005;65:9426–9435. doi: 10.1158/0008-5472.CAN-05-0826. [DOI] [PubMed] [Google Scholar]
- 50.Cole SW. Chronic Inflammation and breast cancer recurrence. J Clin Oncol. 2009;27:3418–3419. doi: 10.1200/JCO.2009.21.9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.