Abstract
The heat shock transcription factor HSF1 was recently demonstrated to play a key role in the development of tumors associated with activation of Ras or inactivation of p53. Here we show that HSF1 is required for cell transformation and tumorigenesis induced by HER2 oncogene responsible for aggressive breast tumors. Upon expression of HER2, untransformed human mammary epithelial cells MCF-10A underwent neoplastic transformation, formed foci in culture and tumors in nude mouse xenografts. However, expression of HER2 in MCF-10A cells with knockdown of HSF1 did not cause either foci formation or tumor growth in xenografts. The anti-tumorigenic effect of downregulation of HSF1 was associated with HER2-induced accumulation of the CDK inhibitor p21 and decrease of the mitotic regulator survivin, which resulted in growth inhibition and cell senescence. In fact, either knockout of p21 or overexpression of survivin alleviated these effects of HSF1 knockdown. Proliferation of certain human HER2-postitive breast cancer lines also requires HSF1, since its knockdown led to upregulation of p21 and/or drop of survivin, precipitating growth arrest. Similar effects were observed with a small molecular weight inhibitor of the heat shock response NZ28. Effects of HSF1 knockdown on growth arrest and senescence of HER2-expressing cells were associated with downregulation of Hsp72 and Hsp27. Therefore, HSF1 is critical for proliferation of HER2-expressing cells, most likely since it maintains levels of HSPs, which in turn control regulators of senescence p21 and survivin.
Keywords: HSF1, HER2, growth arrest, senescence, p21, survivin
Introduction
Induction of Hsp72, Hsp27 and other heat shock proteins represents a highly conserved protective mechanism against environmental insults, which is predominantly regulated by the heat shock transcription factor HSF1 (Lindquist and Craig, 1988; Morimoto et al., 1992; Sorger, 1991). Recent reports demonstrated that HSF1 plays an essential role in development of lymphomas in p53-deficienct mice and development of carcinomas in Ras tumor model (Dai et al., 2007; Min et al., 2007). Moreover, HSF1 was shown to support proliferation of several cancer cell lines (Dai et al., 2007), but specific mechanisms of these effects have not been explored.
Normal tissues or non-transformed cells usually express relatively low levels of Hsp72 and Hsp27, and these proteins are strongly up-regulated following stressful treatments in a HSF1-dependent manner (Jolly and Morimoto, 2000). On the other hand, many human tumors and cancer cell lines express Hsp72 or/and Hsp27 at elevated levels constitutively (Calderwood et al., 2006; Jaattela, 1999; Jolly and Morimoto, 2000; Mosser and Morimoto, 2004). High levels of Hsp72 and Hsp27 correlate with metastasis, resistance to anticancer drugs, and poor prognosis in many human cancers (Ciocca and Calderwood, 2005). Previous results from this laboratory indicated that certain cancer cells become “addicted” to high level of Hsp72, since specific depletion of Hsp72 rapidly suppressed cell proliferation and precipitated senescence in several cancer lines but not in untransformed epithelial cells (Gabai et al., 2009; Yaglom et al., 2007). Interestingly, in different cancer cells distinct senescence signaling pathways were activated upon depletion of Hsp72 depending on the presence of either PIK3CA or Ras oncogenes (Gabai et al., 2009).
Constitutively elevated expression of Hsp72 and/or Hsp27 in cancers is often due to the HSF1-dependent mechanisms (though sometimes it is HSF1-independent) (Cai and Zhu, 2003; Wu et al., 2005). However, the role of HSF1 in cancers may not be limited to regulating the expression of HSPs, since this transcription factor also controls expression of many other proteins (Cahill et al., 1997; Cahill et al., 1996; Singh et al., 2002; Singh et al., 2000; Xie et al., 2002a; Xie et al., 2002b).
Human epidermal growth factor receptor-2 (HER2) is a member of the EGFR tyrosine kinase family, which is overexpressed in 25–30% of human breast and ovarian cancers (Moasser, 2007). Besides activating mitogenic pathways, HER2 can upregulate growth inhibitory/senescence signaling. (Trost et al., 2005), suggesting that malignant transformation not only needs oncogene activation but also a shutdown of senescence pathways. Here, in addressing the role of HSF1 in HER2-induced tumorigenesis, we uncovered that HSF1 controls senescence signaling initiated by HER2.
Results
Depletion of HSF1 inhibits the transformation ability of HER2
To investigate the role of HSF1 in neoplastic transformation, we depleted HSF1 from breast epithelial cells MCF-10A, and then introduced HER2 oncogene. Briefly, late passage MCF-10A cells were infected with control retrovirus or retrovirus expressing the shRNA against HSF1 (shHSF1), followed by puromycin selection, which resulted in more than 95% depletion of HSF1 (Fig. 1A). These treatments did not affect cell viability, and only slightly diminished growth (see below in Fig. 2A), further indicating that HSF1 is dispensable for growth of untransformed cells. Then cells were infected with retrovirus expressing the constitutively active HER2 mutant V664E, which led to increased expression of vimentin and formation of foci (Fig. 1B and C), indicating that cells underwent neoplastic transformation. In contrast, expression of HER2 in shHSF1 cells led to merely mild upregulation of vimentin, and foci formation was inhibited (Fig. 1B and C), indicating that HSF1 is required for HER2-induced transformation in vitro. Of note, in contrast to late passage cells, infection with HER2-expressing retrovirus of early passage MCF-10A cells did not cause transformation and triggered senescence even without HSF1 depletion (not shown).
Figure 1.
HER2-induced MCF-10A transformation requires HSF1. (A) HSF1 depletion by shHSF1 virus. MCF-10A cells were infected with control (Cont.) or shHSF1 retroviruses, and following 3 days selection HSF1 levels were measured by immunoblotting. (B) Effect of HSF1 depletion on the HER2-induced vimentin expression. Control (Cont.) and shHSF1 MCF-10A cells were infected with control empty vector (EV) or HER2-overexpressing (HER2) retroviruses, and vimentin levels were measured on day 4 post-infection. (C) Effect of HSF1 depletion on the HER2-induced foci formation. Cells described in Fig. 1B were stained with hematoxylin to visualize foci. Dark masses in the bottom left panel represent foci. (D) HSF1 depletion suppresses tumor emergence in xenografts. Growth curves of individual tumor in xenografts of HER2-expressing control (cont.) and shHSF1 MCF-10A cells.
Figure 2.
HER2 triggers senescence in HSF1-deficient MCF-10A cells. (A) Growth curves of control (Cont.) and shHSF1 MCF-10A cells with (HER2) or without (empty vector, EV) HER2 overexpression. (B) Representative morphology of MCF-10A cultures described in (A). Please note that the mass seen in the bottom left panel represents the focus. (C) β-galactosidase activity staining of MCF-10A populations described in (A) and its quantification.
If infection with shHSF1 retrovirus followed infection with HER2 retrovirus, downregulation of HSF1 still triggered senescence even in fully transformed MCF-10A cells (Fig. S1), indicating that HSF1 is necessary for the maintenance of growth of HER2-transformed cells.
To address the role of HSF1 in tumorigenesis in vivo, HER2-expressing control and shHSF1 cells were tested for the ability to form tumors in xenografts. Control HER2-expressing cells formed noticeable tumors at the injection site by day 3 post-injection (Fig. 1D). However, HER2-expressing cells depleted of HSF1 could not form tumors at least until day 25. Two tumors appeared at days 26 and 35 post-injection, and these tumors were much smaller. Importantly, in both tumors control of HSF1 expression by shRNA was lost, and levels of HSF1 partially restored (Fig. S2), further supporting that in the absence of HSF1, HER2-induced tumors cannot be formed.
HER2 triggers growth arrest and senescence in cells with knockdown of HSF1
We investigated effects of HSF1 on growth rates of HER2-expressing cells. Control and shHSF1 cells were infected with HER2-expressing retrovirus or control retrovirus. On day 2 post-infection cells were plated, and the cell number was counted every 48 hours. Knockdown of HSF1 caused slightly slower growth in normal MCF-10A cells (Fig. 2A). Similarly minor reduction of growth rate was seen upon expression of HER2. Importantly, when we expressed HER2 in HSF1-depleted cells, a dramatic growth inhibition was observed (Fig. 2A), associated with a dramatic change in cells’ appearance, including enlarged, flattened morphology, and extensive vacuolization reminiscent of senescence (Fig. 2B). To assess senescence, cells were stained for the acidic β-galactosidase (β-gal) activity (Fig. 2C). In control population there was only 1% of β-gal-positive cells, and in shHSF1 cells this fraction raised to 4%. HER2 expression in control MCF-10A cells led to a significant increase in β-gal-positive population (~30%). Importantly, expression of HER2 in shHSF1 MCF10A cells resulted in about 70% of β-gal-positive cells (Fig. 2C). Therefore, HSF1 appears to become critical for growth upon expression of HER2 because it keeps HER2-induced growth arrest and senescence under control.
p21 is a mediator in HER2-induced senescence upon depletion of HSF1
p21Cip is the major regulator of senescence in cancer cells (Roninson, 2003). Expression of HER2 leads to upregulation of p21 in MCF7 cells (Trost et al., 2005). Therefore, we tested for the role of p21 in senescence caused by expression of HER2 in shHSF1 cells. p21 was mildly up-regulated by HER2 expression in control cells, and knockdown of HSF1 on its own also led to a similar minor increase in p21 levels (Fig. 3A). However, there was a dramatic induction of p21 upon expression of HER2 in shHSF1 cells (Fig. 3A).
Figure 3.
p21 is involved in HER2-induced senescence in HSF1-deficient cells. (A) p21 levels in control and shHSF1 MCF-10A cells with (HER2) or without (EV) HER2 overexpression. (B) Cell morphology in wildtype (WT) and p21 knockout (p21KO) MCF-10A cells with same retrovirus infections as in (A). Pictures were taken at day 4 post-infection. Whitish masses represent foci. (C) Wildtype (WT) and p21 knockout (KO) MCF-10A cells were treated with control (Cont.) or shHSF1 retroviruses, followed by HER2 overexpression. Growth curves of xenograft tumors from these HER2-expressing cells were shown.
To investigate the role of p21 in the onset of senescence under these conditions, wild type (WT) and p21 knockout (KO) MCF-10A cells (kind gift of Dr. Park (Bachman et al., 2004)) were infected with shHSF1 and control retroviruses, followed by brief puromycin selection. Then cells were infected with virus expressing HER2, and plated to monitor senescence. In contrast to WT cells, in p21 KO cells combination of shHSF1 and HER2 expression did not cause significant growth inhibition or senescence, as judged by cell morphology (Fig 3B).
Further, we tested whether suppression of senescence in the p21 KO cells can restore tumor development in HSF1 knockdown cells. Control and p21 KO cells were depleted of HSF1, then infected with HER2 retrovirus and injected into nude mice to establish xenograft tumors. As seen in Fig. 3C, in contrast to WT cells, depletion of HSF1 had only mild inhibitory effect on tumor development by p21 KO cells. Therefore, suppression of senescence by p21 KO was sufficient to restore tumor growth, indicating that HER2-triggered senescence of HSF1-depleted cells represents the major barrier for tumor development.
Survivin is involved in HER2-induced senescence upon depletion of HSF1
We noticed a decrease in survivin levels upon expression of HER2 in shHSF1 cells (see below). Survivin, a member of inhibitors of apoptosis (IAPs) family is a critical regulator of proliferation (Altieri, 2003a). Upon HER2 expression survivin levels were dramatically decreased in shHSF1 cells, but not in control cells (Fig. 4A). Knockout of p21 suppressed the drop of survivin after expression of HER2 in shHSF1 cells, indicating that downregulation of survivin is at least partially p21-dependent (Fig. 4A). On the other hand, in other cell lines the decrease in survivin levels was p21-independent (see below), indicating an alternative mechanism of the survivin control.
Figure 4.
Survivin is another mediator in HER2-induced senescence in HSF1-deficient cells. (A) Survivin levels in control or shHSF1 WT (upper panel) and p21KO (lower panel) MCF-10A cells with (HER2) or without (EV) HER2 overexpression (The numbers below each panel are the relative percentage of survivin levels). (B) Control and shHSF1 cells were infected with retrovirus expressing survivin (surv), and two days later these cells were infected with HER2-expressing (HER2) retrovirus. EV1 – control empty vector for survivin. EV2 – control empty vector for HER2. Cells were stained for β-galactosidase activity, and (C) quantification of β-gal staining was performed.
To test the role of survivin in the HER2-induced senescence, we infected control and shHSF1 cells with retrovirus expressing survivin, and following brief selection infected cells with HER2-expressing virus. Survivin overexpression restored growth and rescued senescence phenotype in HSF1-knockdown cells upon HER2 expression, as judged by cell morphology and β-galactosidase activity staining (Fig. 4B, C), indicating that survivin is important for regulation of growth inhibition and senescence under these conditions.
HSF1 is critical for proliferation of HER2-positive human cancer cells
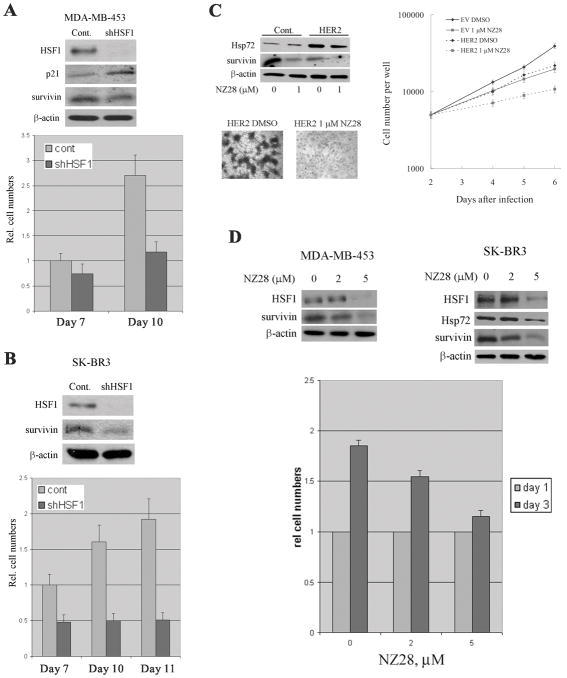
We further investigated effects of HSF1 depletion on HER2-positive human cancer cell lines SK-BR-3 (having mutant p53) and MDA-MB-453 (having WT p53). HSF1 was depleted in these cell lines using retroviral vector, as described above. In both cell lines, infection with shHSF1 retrovirus led to more than 80% depletion of HSF1 (Fig. 5A and B). As with MCF-10A cells, HSF1 depletion in MDA-MB-453 which has normal p53 (Lacroix et al., 2006), led to induction of p21, as well as slight downregulation of survivin (Fig. 5A).
Figure 5.
HSF1 is critical for proliferation of HER2-positive human cancer cells. MDA-MB-453 (A) and SK-BR3 (B) cells were infected with shHSF1 retrovirus, and selected with puromycin. The levels of HSF1, p21 and survivin were assessed by immunoblotting on day 10 post-infection (A and B upper panels). Cell number was assessed starting on day 7 post-infection (A and B lower panels). (C) MCF-10A cells were infected with HER2-expressing retrovirus, selected for 2 days and incubated with NZ28 for additional 2 days. Then the levels of Hsp72 and survivin, and foci formation ability were assessed. Dark masses represent foci stained with hematoxylin. Effects of NZ28 on growth rate of MCF-10A cells were also determined (right panel). (D) MDA-MB-453 and SK-BR3 cells were incubated with 2 and 5 μM NZ28 for 3 days and the levels of HSF1, Hsp72 and survivin were measured by immunoblotting (upper panels). Effect of NZ28 on growth of SK-BR3 cells was determined by cell counting with hemocytometer (lower panel).
Knockdown of HSF1 in SK-BR-3 cells led to strong downregulation of survivin (Fig. 5B) but no upregulation of p21 (not shown). Since SK-BR-3 cells have mutant p53 (Lacroix et al., 2006), these data indicate that effect of HSF1 on survivin could be independent of p53, while its effect on p21 appears to be p53-dependent. As expected, in both MDA-MB-453 and SK-BR-3 cells alterations of survivin and/or p21 upon HSF1 depletion were associated with growth inhibition (Fig. 5A and 5B, lower panels). Of note, these cell lines did not demonstrate signs of senescence in spite of growth arrest. This observation suggests that in certain cancer lines at least some components of the senescence program are defective. Overall, these data indicate that in HER2-positive human cell lines HSF1 serves an important function in supporting cell proliferation via regulation of p21 and/or survivin.
Heat shock response inhibitor NZ28 suppresses HER2-induced transformation
NZ28, a small molecular weight inhibitor of heat shock response that we recently developed (Zaarur et al., 2006), was used to further address the role of HSF1 in HER2-induced cell transformation. MCF-10A cells were infected with HER2-expressing retrovirus, and NZ28 was administered to cells two days after infection. Incubation with 1 μM of NZ28 for three days led to reduction of the Hsp72 levels in HER2-expressing cells (Fig. 5C), which were associated with growth inhibition (see below). NZ28 treatment also dramatically suppressed foci formation in HER2-overexpressing MCF-10A cells (Fig. 5C). In fact, in control untransformed cells 1 μM of NZ28 caused only minor growth inhibition, while in HER2-expressing cells the inhibition was more than 70% (Fig. 5C right panel). Importantly, treatment with NZ28 led to a strong downregulation of survivin (Fig. 5C), while the p21 levels were not significantly changed (not shown), also pointing towards additional effects of this drug.
In HER2-positive human cancer lines, effects of incubation with NZ28 (5 μM) for three days in general were similar to the effects of HSF1 depletion. Indeed, we observed strong downregulation of Hsp72, a decrease of HSF1 levels, downregulation of survivin and growth inhibition (Fig. 5D). Overall, these results indicate that inhibition of HSF1 or its knockdown can prevent HER2-induced cell transformation and suppress proliferation of HER2-expressing tumor cells.
Effects of HSF1 on HER2-induced transformation may be related to expression of Hsps
Hsp90, one of the major HSF1 targets, plays an important role in activity and stability of cancer-related signaling proteins (Pratt and Toft, 2003). Therefore, effects of HSF1 on tumorigenesis could be related to the control of Hsp90. However, we did not observe downregulation of major Hsp90 client proteins Akt, α-MET and GSK3β, and activation of ERK1/2 was not reduced in shHSF1 cells (Fig. 6B). Therefore effects of HSF1 knockdown on HER2-induced growth inhibition and senescence are unrelated to downregulation of Hsp90.
Figure 6.
Effects of HSF1 on HER2-induced transformation may be related to expression of heat shock proteins. (A) Control and shHSF1 MCF-10A cells were infected with control (EV) or HER2-expressing retroviruses. The protein level of Hsp90 was measured. (B) Hsp90 client proteins and pERK levels were monitored in cells described in (A). (C) The levels of Hsp72 and Hsp27 were examined by immunoblotting in cells described in (A). (D) Efficiency of knockdown of Hsp27 or Hsp72 by shHsp27 or shHsp72 retroviruses in MCF-10A cells; and (E) the development of senescence in shHsp27 or shHsp72 cells following HER2 overexpression was assessed by β-galactosidase activity staining.
Control of senescence could be specifically linked to expression of Hsp72, since this heat shock protein regulates senescence signaling pathways (Gabai et al., 2009; Yaglom et al., 2007). Indeed, we observed significant upregulation of both Hsp72 and Hsp27 following HER2 expression, which was fully dependent on HSF1 (Fig. 6C). Notably, there is no change in levels of Hsp70-2 (Fig. S3).
We tested whether prevention of induction of Hsp72 and/or Hsp27 is involved in the HER2-triggered senescence. Infection with shHsp72 or shHsp27 retroviruses brought about depletion of these chaperones by more than 70% (Fig. 6D). No significant effect on either cell morphology or growth was seen under these conditions in untransformed MCF-10A cells (Fig. 6E). However, subsequent expression of HER2 rapidly triggered senescence (Fig. 6E). Therefore, lack of Hsp72 or Hsp27 is sufficient for senescence triggered by HER2, which may explain effects of depletion of HSF1 on HER2-triggered senescence.
Discussion
Here we uncovered that HSF1 plays a critical role in HER2-induced tumorigenesis in mammary epithelial cells. In fact, depletion of HSF1 strongly suppressed both transformation judged by foci formation in culture and tumor development in xenografts. The suppression of transformation was associated with a paradoxical effect of HER2 on cells that lacked HSF1. Upon expression of HER2, instead of transformation these cells stopped proliferating and underwent senescence. These data strongly suggest that HER2 activates both mitogenic and senescence signals, and HSF1 serves in suppression of senescence. Accordingly, in cells that lack HSF1, senescence signals prevail and proliferation stops.
In part, growth inhibition signaling was due to the cell cycle inhibitor p21. HER2 was able to mildly upregulate p21 even in naïve cells. However, p21 induction was much stronger in cells with depleted of HSF1. Furthermore, p21 knockout suppressed the senescence phenotype triggered by HER2.
Interestingly, in addition to p21, which is a known player in the oncogene-induced senescence, we uncovered a novel role of survivin in this process. Survivin is homologous to IAPs, and originally was found to suppress apoptosis (Altieri, 2003a). In addition, survivin regulates mitosis via modulation of Aurora B kinase (Altieri, 2008). Here we found that survivin was downregulated in cells with HSF1 knockdown upon expression of HER2, and, importantly, ectopic expression of survivin suppressed senescence under these conditions. Expression of survivin was previously shown to be regulated by p21(Altieri, 2003a), and indeed the survivin decrease was partially suppressed in p21 knockout cells. On the other hand, the survivin decrease was partially p21-independent, indicating that a distinct signaling pathway controlled by HSF1 regulates survivin levels. Analyses of HER2-positive human cancer cells suggested that even in SK-BR-3 line with mutant p53 that has undetectable levels of p21, HSF1 depletion led to survivin decrease and precipitated senescence, indicating a p21-independent pathway of survivin regulation. At least in part, HSF1-mediated control of cancer cell senescence was due to the regulation of expression of Hsp72 and Hsp27. In fact, if either Hsp72 or Hsp27 was depleted, expression of HER2 also brought about senescence instead of transformation, indicating that downregulation of any of these HSPs is sufficient for the paradoxical effects of HER2. In line with this notion, overexpression of Hsp72 alone was not sufficient for reversing the effect of depletion of Hsf1 on senescence. Furthermore, analyses of various human cancer cell lines indicated that there was an association between the senescence (or growth inhibition) in response to HSF1 and depletion of Hsp72. There is a possibility that a general decline of protein homeostasis contributes to the oncogene-induced senescence. On the other hand, it is currently clear that HSF1 may have other effects on tumorigenesis unrelated to expression of Hsp72. In fact, HSF1 was found to regulate pro-inflammatory cytokines (Cahill et al., 1997; Xie et al., 2002b), translational machinery, glucose metabolism (Dai et al., 2007), and cooperate with the invasion-associated protein MTA (Khaleque et al., 2008), which obviously may contribute to tumor development.
Very importantly, we observed that HER2 expression triggers growth arrest in MCF-10A cells treated with a small molecular weight inhibitor of heat shock response, NZ28. Originally, we developed this inhibitor in order to enhance anti-cancer activities of the proteasome and Hsp90 inhibitors (Zaarur et al., 2006). Here we found that it inhibits growth of MCF-10A cells that express HER2, as well as HER2-positive cancer lines. These findings support the idea that HSF1 could be an interesting drug target for treatment HER2-positive herceptin-resistant cancers.
Materials and Methods
Cell cultures and reagents
MCF-10A wildtype and p21−/− cells were cultured in DMEM/F12 medium supplemented with 5% horse serum, 20 ng/mL epidermal growth factor, 0.5 μg/mL hydrocortisone, 10μg/mL human insulin, and 100 ng/mL cholera toxin. HEK293T, SK-BR3, MDA-MB-453 cells were from ATCC. HEK293T cells were cultivated in Dulbecco’s modified Eagle’s medium supplemented with 10% heat inactivated FBS. All other cells were cultivated in Dulbecco’s modified Eagle’s medium supplemented with 10% FBS.
Retroviral vectors and infection
RNAi-Ready pSIREN-RetroQ vector from BD Biosciences retroviral delivery system was used for knockdown of HSF1. The sequence of human HSF1 gene was selected as reported before (Zaarur et al., 2006): 5′-TATGGACTCCAACCTGGATAA-3′. HER2 and control (pBABE) retroviral vectors were a kind gift of C. Spangenberg (Trost et al., 2005). This version of HER2 carries the activating V664E mutation (NeuT). Retroviruses were produced as reported before (Gabai et al., 2009; Yaglom et al., 2007). Briefly, HEK293T cells were co-transfected with plasmids expressing retroviral proteins Gag-Pol, VSV-G pseudotype, and enhanced green fluorescent protein (EGFP) or our constructs using lipofectamine 2000 (Invitrogen). 48 hours after transfection, supernatants containing the retroviral particles were collected and frozen at −70 until use. MCF-10A cells were infected with diluted supernatant in the presence of 10 μg/ml Polybrene overnight, and were selected with puromycin (0.75 μg/ml) 48 hours after infection. Retroviral vectors expressing EGFP was used as infection efficiency indicator: usually ~90% of cells were fluorescent 2 days after infection.
Mouse xenografts
Animal maintenance and experiments were conducted in compliance with the guidelines of IACUC. Briefly, HER2-infected MCF-10A cells with or without shHSF1 were were trypsinized, mixed at 1:1 ratio with matrigel, and 0.25 million cells of each culture were injected subcutaneously into 6-week-old female NCR nude mice (Taconic). Tumor growth was monitored weekly.
Immunoblot analysis
Cells were washed twice with PBS and lysed in lysis buffer (40 mM HEPES [pH 7.5], 50 mM KCl, 1% Triton X-100, 1mM Na3VO4, 50 mM glycerophosphate, 50 mM NaF, 5 mM EDTA, 5 mM EGTA, 1 mM phenylmethylsulfonyl fluoride, 1 mM benzamidine, and 5 μg/ml each leupeptin, pepstatin A, aprotinin). Protein concentration of the lysates was measured with the Bio-Rad protein assay reagent, after which they were diluted with lysis buffer to achieve equal protein concentrations. Antibodies used for this study were listed as follow: anti-AKT, anti-pERK, anti-ERK, anti-αMET, and anti-GSK3β from cell signaling; anti-HSF1, anti-Hsp90, anti-Hsp72, and anti-Hsp27 from Stressgen; anti-p21 and anti-PARP from BD PharMingen; anti-vimentin from LabVision (Thermo Scientific); anti-survivin from Santa Cruz; anti-β-actin from Sigma.
β-galactosidase assay
β-galactosidase assay was carried out using X-gal (pH 6.0) as described previously (Gabai et al., 2009; Yaglom et al., 2007). Cells were plated at a low density and fixed with 2% formaldehyde and 0.2% glutaraldehyde. The β-galactosidase activity was determined by incubation with 1 mg/ml of solution of X-gal (5-bromo-4-chloro-3-indolyl β-D-galactopyranoside) in 40 mM sodium citrate, 5 mM K3FeCN6, 5 mM K4FeCN6, 150 mM NaCl, 2 mM MgCl2 diluted in phosphate-buffered saline (pH 6.0). The number of stained cells was counted under microscope from five different fields and the proportion of stained cells were calculated. The results were expressed as mean value ± S.D. on three independent experiments.
Cell growth and foci formation
Cell growth curve was performed by seeding 5000 cells per well in 12-well plate and cell number was monitored every two days. To study foci formation, cells were seeded at 3×105 per well in 6-well plate and were let grow for 2 days until foci can be observed under microscope. Then cells were washed twice with PBS, fixed with 2% formaldehyde/0.2% glutaraldehyde in PBS for 10 min, and stained with hematoxylin.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institute of Health CA081244. We thank Dr. B. Park and Dr. C. Spangenberg for their kind supply of MCF-10A cells and HER2-overexpressing retroviral vector.
References
- Altieri DC. Survivin, versatile modulation of cell division and apoptosis in cancer. Oncogene. 2003a;22:8581–9. doi: 10.1038/sj.onc.1207113. [DOI] [PubMed] [Google Scholar]
- Altieri DC. Validating survivin as a cancer therapeutic target. Nat Rev Cancer. 2003b;3:46–54. doi: 10.1038/nrc968. [DOI] [PubMed] [Google Scholar]
- Altieri DC. Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer. 2008;8:61–70. doi: 10.1038/nrc2293. [DOI] [PubMed] [Google Scholar]
- Bachman KE, Blair BG, Brenner K, Bardelli A, Arena S, Zhou S, et al. p21(WAF1/CIP1) mediates the growth response to TGF-beta in human epithelial cells. Cancer Biol Ther. 2004;3:221–5. doi: 10.4161/cbt.3.2.666. [DOI] [PubMed] [Google Scholar]
- Cahill CM, Lin HS, Price BD, Bruce JL, Calderwood SK. Potential role of heat shock transcription factor in the expression of inflammatory cytokines. Adv Exp Med Biol. 1997;400B:625–30. [PubMed] [Google Scholar]
- Cahill CM, Waterman WR, Xie Y, Auron PE, Calderwood SK. Transcriptional repression of the prointerleukin 1beta gene by heat shock factor 1. J Biol Chem. 1996;271:24874–9. [PubMed] [Google Scholar]
- Cai L, Zhu JD. The tumor-selective over-expression of the human Hsp70 gene is attributed to the aberrant controls at both initiation and elongation levels of transcription. Cell Res. 2003;13:93–109. doi: 10.1038/sj.cr.7290154. [DOI] [PubMed] [Google Scholar]
- Calderwood SK, Khaleque MA, Sawyer DB, Ciocca DR. Heat shock proteins in cancer: chaperones of tumorigenesis. Trends Biochem Sci. 2006;31:164–72. doi: 10.1016/j.tibs.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Ciocca DR, Calderwood SK. Heat shock proteins in cancer: diagnostic, prognostic, predictive, and treatment implications. Cell Stress Chaperones. 2005;10:86–103. doi: 10.1379/CSC-99r.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai C, Whitesell L, Rogers AB, Lindquist S. Heat shock factor 1 is a powerful multifaceted modifier of carcinogenesis. Cell. 2007;130:1005–18. doi: 10.1016/j.cell.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabai VL, Yaglom JA, Waldman T, Sherman MY. Heat shock protein Hsp72 controls oncogene-induced senescence pathways in cancer cells. Mol Cell Biol. 2009;29:559–69. doi: 10.1128/MCB.01041-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn JS, Hu Z, Thiele DJ, Iyer VR. Genome-wide analysis of the biology of stress responses through heat shock transcription factor. Mol Cell Biol. 2004;24:5249–56. doi: 10.1128/MCB.24.12.5249-5256.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaattela M. Escaping cell death: survival proteins in cancer. Exp Cell Res. 1999;248:30–43. doi: 10.1006/excr.1999.4455. [DOI] [PubMed] [Google Scholar]
- Jolly C, Morimoto RI. Role of the heat shock response and molecular chaperones in oncogenesis and cell death. J Natl Cancer Inst. 2000;92:1564–72. doi: 10.1093/jnci/92.19.1564. [DOI] [PubMed] [Google Scholar]
- Khaleque MA, Bharti A, Gong J, Gray PJ, Sachdev V, Ciocca DR, et al. Heat shock factor 1 represses estrogen-dependent transcription through association with MTA1. Oncogene. 2008;27:1886–93. doi: 10.1038/sj.onc.1210834. [DOI] [PubMed] [Google Scholar]
- Khaleque MA, Bharti A, Sawyer D, Gong J, Benjamin IJ, Stevenson MA, et al. Induction of heat shock proteins by heregulin beta1 leads to protection from apoptosis and anchorage-independent growth. Oncogene. 2005;24:6564–73. doi: 10.1038/sj.onc.1208798. [DOI] [PubMed] [Google Scholar]
- Lacroix M, Toillon RA, Leclercq G. p53 and breast cancer, an update. Endocr Relat Cancer. 2006;13:293–325. doi: 10.1677/erc.1.01172. [DOI] [PubMed] [Google Scholar]
- Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–77. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Min JN, Huang L, Zimonjic DB, Moskophidis D, Mivechi NF. Selective suppression of lymphomas by functional loss of Hsf1 in a p53-deficient mouse model for spontaneous tumors. Oncogene. 2007;26:5086–97. doi: 10.1038/sj.onc.1210317. [DOI] [PubMed] [Google Scholar]
- Moasser MM. The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene. 2007;26:6469–87. doi: 10.1038/sj.onc.1210477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto RI, Sarge KD, Abravaya K. Transcriptional regulation of heat shock genes. A paradigm for inducible genomic responses. J Biol Chem. 1992;267:21987–90. [PubMed] [Google Scholar]
- Mosser DD, Morimoto RI. Molecular chaperones and the stress of oncogenesis. Oncogene. 2004;23:2907–18. doi: 10.1038/sj.onc.1207529. [DOI] [PubMed] [Google Scholar]
- Pratt WB, Toft DO. Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery. Exp Biol Med (Maywood) 2003;228:111–33. doi: 10.1177/153537020322800201. [DOI] [PubMed] [Google Scholar]
- Roninson IB. Tumor cell senescence in cancer treatment. Cancer Res. 2003;63:2705–15. [PubMed] [Google Scholar]
- Singh IS, He JR, Calderwood S, Hasday JD. A high affinity HSF-1 binding site in the 5′-untranslated region of the murine tumor necrosis factor-alpha gene is a transcriptional repressor. J Biol Chem. 2002;277:4981–8. doi: 10.1074/jbc.M108154200. [DOI] [PubMed] [Google Scholar]
- Singh IS, Viscardi RM, Kalvakolanu I, Calderwood S, Hasday JD. Inhibition of tumor necrosis factor-alpha transcription in macrophages exposed to febrile range temperature. A possible role for heat shock factor-1 as a negative transcriptional regulator. J Biol Chem. 2000;275:9841–8. doi: 10.1074/jbc.275.13.9841. [DOI] [PubMed] [Google Scholar]
- Sorger PK. Heat shock factor and the heat shock response. Cell. 1991;65:363–6. doi: 10.1016/0092-8674(91)90452-5. [DOI] [PubMed] [Google Scholar]
- Trost TM, Lausch EU, Fees SA, Schmitt S, Enklaar T, Reutzel D, et al. Premature senescence is a primary fail-safe mechanism of ERBB2-driven tumorigenesis in breast carcinoma cells. Cancer Res. 2005;65:840–9. [PubMed] [Google Scholar]
- Wu G, Osada M, Guo Z, Fomenkov A, Begum S, Zhao M, et al. DeltaNp63alpha up-regulates the Hsp70 gene in human cancer. Cancer Res. 2005;65:758–66. [PubMed] [Google Scholar]
- Xie Y, Chen C, Stevenson MA, Auron PE, Calderwood SK. Heat shock factor 1 represses transcription of the IL-1beta gene through physical interaction with the nuclear factor of interleukin 6. J Biol Chem. 2002a;277:11802–10. doi: 10.1074/jbc.M109296200. [DOI] [PubMed] [Google Scholar]
- Xie Y, Chen C, Stevenson MA, Hume DA, Auron PE, Calderwood SK. NF-IL6 and HSF1 have mutually antagonistic effects on transcription in monocytic cells. Biochem Biophys Res Commun. 2002b;291:1071–80. doi: 10.1006/bbrc.2002.6562. [DOI] [PubMed] [Google Scholar]
- Yaglom JA, Gabai VL, Sherman MY. High levels of heat shock protein Hsp72 in cancer cells suppress default senescence pathways. Cancer Res. 2007;67:2373–81. doi: 10.1158/0008-5472.CAN-06-3796. [DOI] [PubMed] [Google Scholar]
- Zaarur N, Gabai VL, Porco JA, Jr, Calderwood S, Sherman MY. Targeting heat shock response to sensitize cancer cells to proteasome and Hsp90 inhibitors. Cancer Res. 2006;66:1783–91. doi: 10.1158/0008-5472.CAN-05-3692. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.