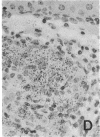
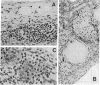
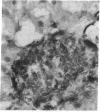
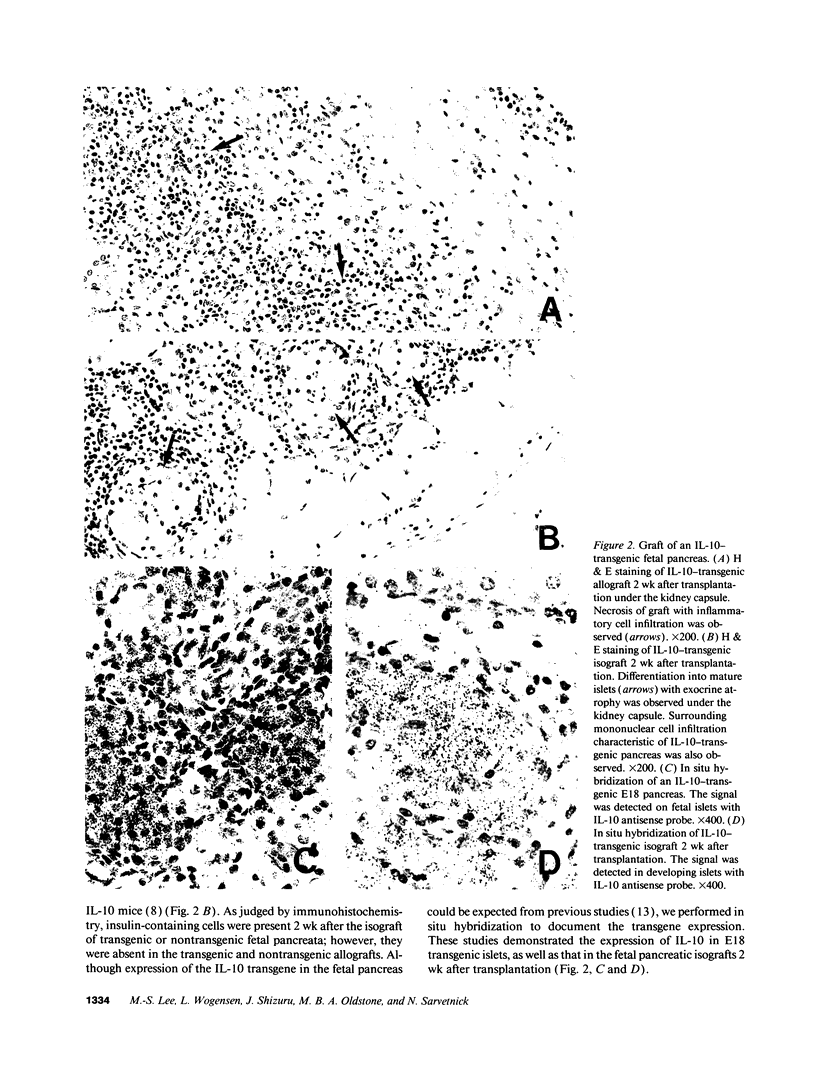
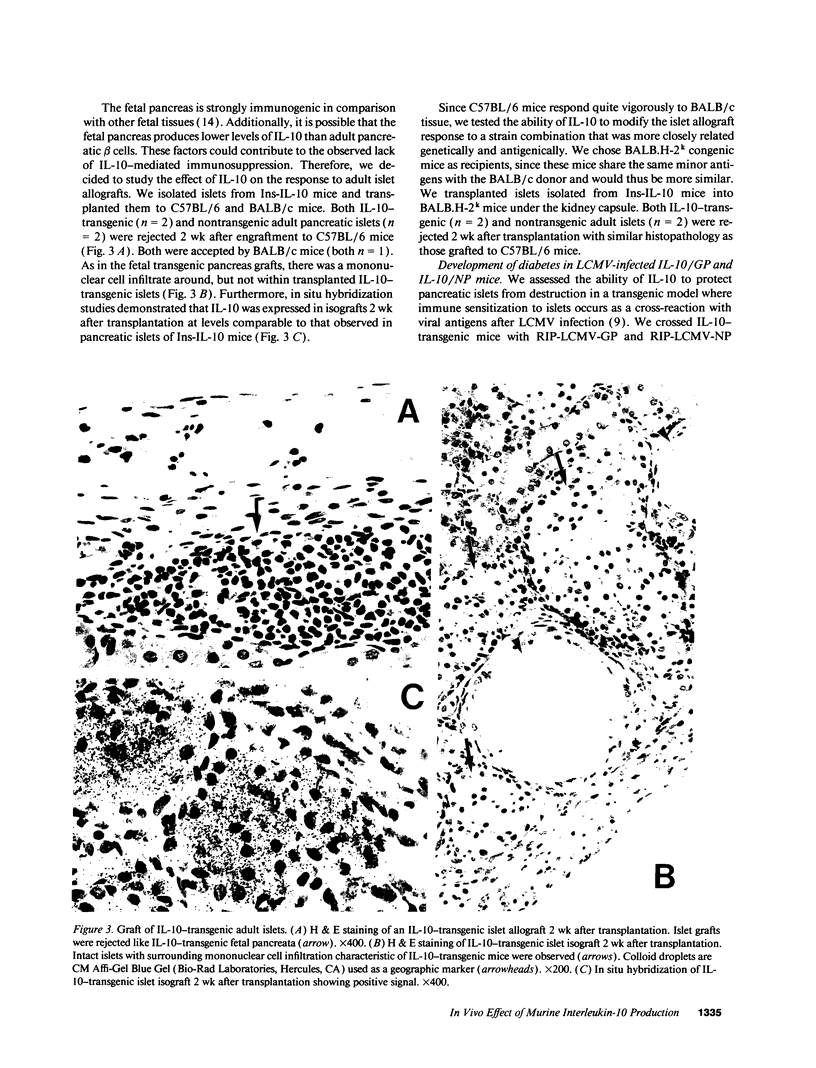
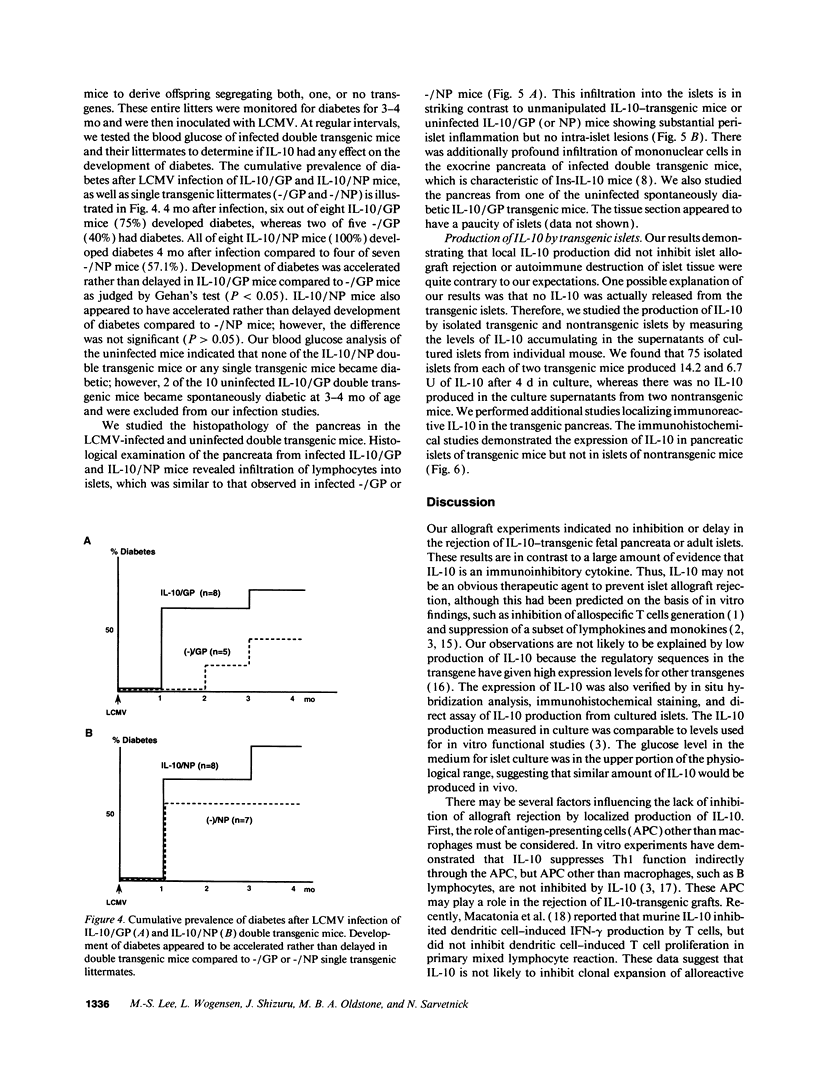
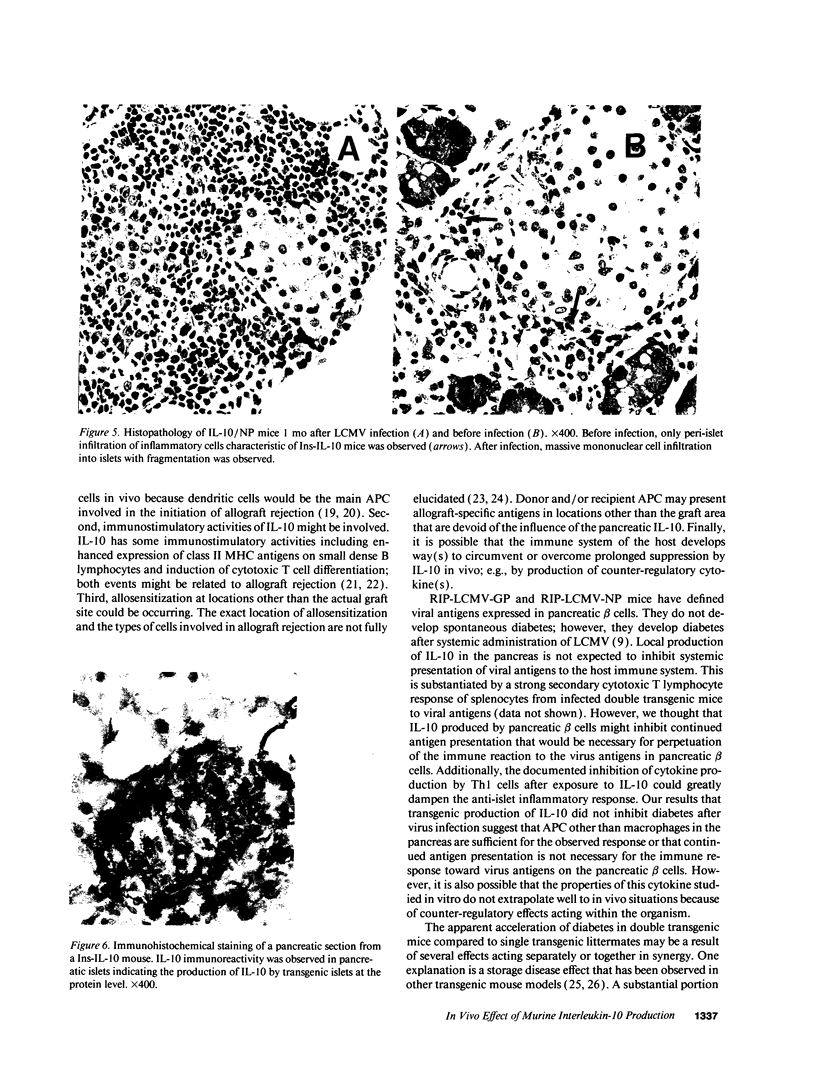
Abstract
IL-10 inhibits macrophage-dependent antigen presentation, cytokine production, and generation of allospecific cells in vitro. These findings have lead to the widespread expectation that IL-10 may be a useful immunosuppressive agent to inhibit allograft rejection or autoimmunity in vivo. We used two experimental paradigms to study effects of murine IL-10 on in vivo immune responses. First, fetal pancreata or adult pancreatic islets from transgenic mice expressing IL-10 in pancreatic beta cells (Ins-IL-10 mice) were grafted across the MHC barrier to examine if IL-10 could inhibit allograft rejection. Second, Ins-IL-10 mice were crossed with transgenic mice expressing lymphocytic choriomeningitis virus (LCMV) antigens in pancreatic beta cells. These mice were infected with LCMV to elicit autoimmune diabetes, allowing us to ask if IL-10 protects islets from autoimmune destruction. We observed that allografts from IL-10-transgenic donors were rejected with comparable kinetics to the rejection of control nontransgenic allografts, indicating that IL-10 does not inhibit allograft rejection. After LCMV infection, IL-10 and LCMV antigen double transgenic mice developed diabetes earlier than LCMV antigen single transgenic littermates, suggesting that IL-10 does not inhibit islet antigen presentation or recognition. Our results contrast to in vitro observations and suggest that IL-10 cannot overcome immune-mediated tissue destruction within the pancreas.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison J., Malcolm L., Culvenor J., Bartholomeusz R. K., Holmberg K., Miller J. F. Overexpression of beta 2-microglobulin in transgenic mouse islet beta cells results in defective insulin secretion. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2070–2074. doi: 10.1073/pnas.88.6.2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpert S., Hanahan D., Teitelman G. Hybrid insulin genes reveal a developmental lineage for pancreatic endocrine cells and imply a relationship with neurons. Cell. 1988 Apr 22;53(2):295–308. doi: 10.1016/0092-8674(88)90391-1. [DOI] [PubMed] [Google Scholar]
- Chen W. F., Zlotnik A. IL-10: a novel cytotoxic T cell differentiation factor. J Immunol. 1991 Jul 15;147(2):528–534. [PubMed] [Google Scholar]
- Fiorentino D. F., Bond M. W., Mosmann T. R. Two types of mouse T helper cell. IV. Th2 clones secrete a factor that inhibits cytokine production by Th1 clones. J Exp Med. 1989 Dec 1;170(6):2081–2095. doi: 10.1084/jem.170.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorentino D. F., Zlotnik A., Vieira P., Mosmann T. R., Howard M., Moore K. W., O'Garra A. IL-10 acts on the antigen-presenting cell to inhibit cytokine production by Th1 cells. J Immunol. 1991 May 15;146(10):3444–3451. [PubMed] [Google Scholar]
- GEHAN E. A. A GENERALIZED WILCOXON TEST FOR COMPARING ARBITRARILY SINGLY-CENSORED SAMPLES. Biometrika. 1965 Jun;52:203–223. [PubMed] [Google Scholar]
- Garvey J. F., Morris P. J., Millard P. R. Early rejection of allogeneic foetal rat pancreas. Transplantation. 1979 May;27(5):342–344. doi: 10.1097/00007890-197905000-00011. [DOI] [PubMed] [Google Scholar]
- Go N. F., Castle B. E., Barrett R., Kastelein R., Dang W., Mosmann T. R., Moore K. W., Howard M. Interleukin 10, a novel B cell stimulatory factor: unresponsiveness of X chromosome-linked immunodeficiency B cells. J Exp Med. 1990 Dec 1;172(6):1625–1631. doi: 10.1084/jem.172.6.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodsky G. M., Ma Y. H., Cullen B., Sarvetnick N. Effect on insulin production sorting and secretion by major histocompatibility complex class II gene expression in the pancreatic beta-cell of transgenic mice. Endocrinology. 1992 Aug;131(2):933–938. doi: 10.1210/endo.131.2.1639031. [DOI] [PubMed] [Google Scholar]
- Gérard C., Bruyns C., Marchant A., Abramowicz D., Vandenabeele P., Delvaux A., Fiers W., Goldman M., Velu T. Interleukin 10 reduces the release of tumor necrosis factor and prevents lethality in experimental endotoxemia. J Exp Med. 1993 Feb 1;177(2):547–550. doi: 10.1084/jem.177.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi Y., Herrera P., Muniesa P., Huarte J., Belin D., Ohashi P., Aichele P., Orci L., Vassalli J. D., Vassalli P. Expression of a tumor necrosis factor alpha transgene in murine pancreatic beta cells results in severe and permanent insulitis without evolution towards diabetes. J Exp Med. 1992 Dec 1;176(6):1719–1731. doi: 10.1084/jem.176.6.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard M., Muchamuel T., Andrade S., Menon S. Interleukin 10 protects mice from lethal endotoxemia. J Exp Med. 1993 Apr 1;177(4):1205–1208. doi: 10.1084/jem.177.4.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard M., O'Garra A. Biological properties of interleukin 10. Immunol Today. 1992 Jun;13(6):198–200. doi: 10.1016/0167-5699(92)90153-X. [DOI] [PubMed] [Google Scholar]
- Ishida H., Hastings R., Kearney J., Howard M. Continuous anti-interleukin 10 antibody administration depletes mice of Ly-1 B cells but not conventional B cells. J Exp Med. 1992 May 1;175(5):1213–1220. doi: 10.1084/jem.175.5.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob C. O., Aiso S., Michie S. A., McDevitt H. O., Acha-Orbea H. Prevention of diabetes in nonobese diabetic mice by tumor necrosis factor (TNF): similarities between TNF-alpha and interleukin 1. Proc Natl Acad Sci U S A. 1990 Feb;87(3):968–972. doi: 10.1073/pnas.87.3.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas P. J., Shearer G. M., Neudorf S., Gress R. E. The human antimurine xenogeneic cytotoxic response. I. Dependence on responder antigen-presenting cells. J Immunol. 1990 Jun 15;144(12):4548–4554. [PubMed] [Google Scholar]
- Macatonia S. E., Doherty T. M., Knight S. C., O'Garra A. Differential effect of IL-10 on dendritic cell-induced T cell proliferation and IFN-gamma production. J Immunol. 1993 May 1;150(9):3755–3765. [PubMed] [Google Scholar]
- Mandel T. E., Higginbotham L. Organ culture and transplantation of fetal mouse pancreatic islets. Transplant Proc. 1979 Jun;11(2):1505–1506. [PubMed] [Google Scholar]
- Metlay J. P., Puré E., Steinman R. M. Control of the immune response at the level of antigen-presenting cells: a comparison of the function of dendritic cells and B lymphocytes. Adv Immunol. 1989;47:45–116. doi: 10.1016/s0065-2776(08)60662-8. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Nerenberg M., Southern P., Price J., Lewicki H. Virus infection triggers insulin-dependent diabetes mellitus in a transgenic model: role of anti-self (virus) immune response. Cell. 1991 Apr 19;65(2):319–331. doi: 10.1016/0092-8674(91)90165-u. [DOI] [PubMed] [Google Scholar]
- Picarella D. E., Kratz A., Li C. B., Ruddle N. H., Flavell R. A. Transgenic tumor necrosis factor (TNF)-alpha production in pancreatic islets leads to insulitis, not diabetes. Distinct patterns of inflammation in TNF-alpha and TNF-beta transgenic mice. J Immunol. 1993 May 1;150(9):4136–4150. [PubMed] [Google Scholar]
- Sarvetnick N. Transgenic mouse models for growth factor studies. Methods Enzymol. 1991;198:519–525. doi: 10.1016/0076-6879(91)98051-7. [DOI] [PubMed] [Google Scholar]
- Starzl T. E., Demetris A. J., Murase N., Ildstad S., Ricordi C., Trucco M. Cell migration, chimerism, and graft acceptance. Lancet. 1992 Jun 27;339(8809):1579–1582. doi: 10.1016/0140-6736(92)91840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wogensen L., Huang X., Sarvetnick N. Leukocyte extravasation into the pancreatic tissue in transgenic mice expressing interleukin 10 in the islets of Langerhans. J Exp Med. 1993 Jul 1;178(1):175–185. doi: 10.1084/jem.178.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal Malefyt R., Abrams J., Bennett B., Figdor C. G., de Vries J. E. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991 Nov 1;174(5):1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal Malefyt R., Haanen J., Spits H., Roncarolo M. G., te Velde A., Figdor C., Johnson K., Kastelein R., Yssel H., de Vries J. E. Interleukin 10 (IL-10) and viral IL-10 strongly reduce antigen-specific human T cell proliferation by diminishing the antigen-presenting capacity of monocytes via downregulation of class II major histocompatibility complex expression. J Exp Med. 1991 Oct 1;174(4):915–924. doi: 10.1084/jem.174.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Waal Malefyt R., Yssel H., Roncarolo M. G., Spits H., de Vries J. E. Interleukin-10. Curr Opin Immunol. 1992 Jun;4(3):314–320. doi: 10.1016/0952-7915(92)90082-p. [DOI] [PubMed] [Google Scholar]