Summary
Although vaccinia virus infection results in induction of a robust immunizing response, many closely related poxviruses such as variola (smallpox) and ectromelia (mousepox) are highly pathogenic in their natural hosts. We developed a strategy to map the activation of key signaling networks in vivo and applied this approach to define and compare the earliest signaling events elicited by poxvirus infections in mice. Vaccinia induced rapid TLR2-dependent responses leading to IL-6 production, which then initiated STAT3 signaling in dendritic cells and T cells. In contrast, ectromelia did not induce TLR2 activation and profound mouse strain-dependent responses were observed. In resistant C57BL/6 mice, the STAT1 and STAT3 pathways were rapidly activated, whereas in susceptible BALB/c mice, IL-6-dependent STAT3 activation did not occur. These results indicate that vaccination with vaccinia is dependent on rapid TLR2 and IL-6 driven responses and link the earliest immune signaling events to the outcome of infection.
Highlights.
Vaccinia infection induces rapid STAT1 and STAT3 activation in DCs and T cells.
STAT3 activation is dependent on TLR2 recognition of vaccinia and IL-6 secretion.
Both TLR2 and IL-6 deficient mice exhibit defects in vaccinia clearance.
Ectromelia (mousepox) does not activate TLR2 but IL-6 is necessary for survival.
Introduction
Successful antiviral immune responses rely on the induction of a complex cytokine network that activates gene regulatory programs across numerous cell populations (Ramshaw et al., 1997). Many of these immune system-specific programs are critically dependent on the STAT family of transcription factors, which is regulated by phosphorylation status (Kisseleva et al., 2002). In this study we quantified the in vivo immune response at the single-cell level using phosphorylation-specific STAT monoclonal antibodies (mAbs) and flow cytometry (Krutzik et al., 2005; Krutzik and Nolan, 2003). We applied this approach to identify the earliest in vivo immune responses to immunizing or lethal poxvirus infections, and then used mouse genetics and bioluminescence imaging of viral gene expression to interrogate the roles of these events in disease outcome.
Poxviruses are double-stranded DNA viruses (dsDNA) that usually exhibit strict species tropisms typically making these viruses highly pathogenic only in their adapted hosts (Buller and Palumbo, 1991). Although members of the genus Orthopoxvirus, including human smallpox (variola), mousepox (ectromelia), and vaccinia, are all capable of infecting most mammalian cells, these closely related viruses demonstrate broad differences in their pathogenicity at the organismal level (McFadden, 2005). It is likely that the outcome of infection with these viruses is dependent both on the kinetics and type of response induced within cells of the host immune system, as well as by the ability of the viruses to suppress these responses. Variola virus, one of the most dangerous pathogens in human history, was eradicated in the wild through immunization with vaccinia. The success of this vaccination strategy raises important biological and evolutionary questions. Is the evolutionary consequence of adaptation of a strain to its host one of the pathogen evolving to evade rapid immune recognition or one of diversion of the immune system into inappropriate responses? Does the immune system recognize vaccinia through qualitatively different mechanisms than variola? Attenuated vaccinia strains have been developed as recombinant vaccine vehicles (Manrique et al., 2009; Rodriguez et al., 2009) and thus the mechanics by which the immune system initially responds to non-host adapted immunizing versus host-adapted pathogenic poxvirus infections are of relevance for future vaccine development.
Similar to the case with variola, vaccinia virus immunization also cross- protects against lethal ectromelia infection in mice (Fenner, 1949; 1981). Furthermore, ectromelia has increased pathogenicity in certain mouse strains due to qualitative differences in cytokine production among strains (Chaudhri et al., 2004). To better define the molecular basis for poxvirus species and strain specificity we focused on how two poxviruses, vaccinia and ectromelia, activate the phylogenetically conserved microbial pattern recognition receptors of the innate immune system. Toll-like receptors (TLRs) are the best characterized family of pattern recognition receptors and are expressed primarily in dendritic cells and macrophages (Beutler et al., 2007; Koyama et al., 2008). Numerous TLRs have been discovered and each has specificity for particular microbe-associated molecular patterns. TLR specificities for lipopeptides (TLR2), double-stranded RNA (dsRNA, TLR3), lipopolysaccharide (LPS, TLR4), single-stranded RNA (ssRNA, TLR7), and unmethylated CpG-containing dsDNA (TLR9) have all been well characterized (Akira et al., 2006). Recent studies have implicated various TLRs as necessary for innate recognition of poxviruses (Delaloye et al., 2009; Samuelsson et al., 2008; Zhu et al., 2007); however, a systematic comparison of how the immune system initially responds in vivo to vaccinia versus ectromelia virus infection has not been performed.
Here, we show that the STAT-signaling networks induced early during infection by these related poxviruses varied extensively depending on the virus and/or mouse strain used. Overall, the kinetics and potency of STAT3 activation directly correlated with enhanced humoral responses, reduced viral burden, and survival from lethal infection. Mice susceptible to infection demonstrated little to no early activation of the STAT networks whereas resistant mice experienced potent and rapid STAT induction. Vaccinia infection induced rapid STAT3 activation, which was dependent on TLR2 and IL-6. Surprisingly, despite the important role of these molecules in the earliest immune response, their genetic absence did not affect viral burden during initial infection, but instead led to a delayed clearance of vaccinia and reduction in neutralizing antibody levels. In contrast to vaccinia, ectromelia detection was independent of TLR2; however, IL-6 production was essential for surviving mousepox infection. These data suggest that an important component in the efficacy of vaccinia in eliciting protective immune responses relative to the pathogenic nature of smallpox could be how these viruses activate different pattern recognition receptors early after infection. These results could be useful in the design of improved vaccines or vaccine adjuvants, and may lead to therapeutic approaches to help treat viral infections soon after exposure.
Results
Examination of the early STAT response network
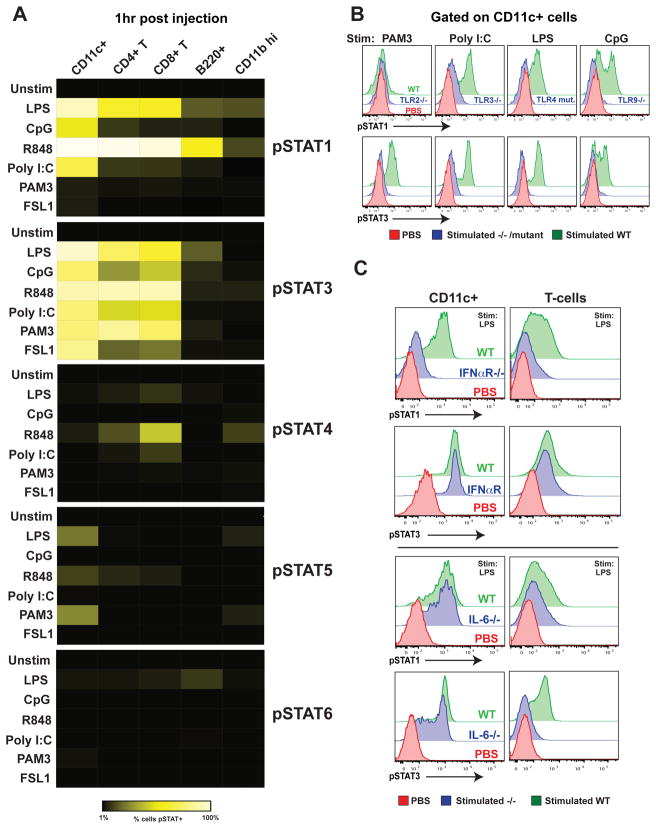
To evaluate the very earliest STAT signaling networks induced during an in vivo immune response, we used single-cell biochemical analysis of cell populations critical for immunity (Krutzik et al., 2005; Krutzik and Nolan, 2003). Activation (tyrosine phosphorylation) of STATs 1, 3, 4, 5, and 6 was monitored in conventional dendritic cells (cDCs), CD4+ T cells, CD8+ T cells, B cells, and granulocytes using phosphorylation-specific monoclonal antibodies and flow cytometry. Prior to evaluating the complexity of an intact pathogen we first focused on reductionist ligands known to activate specific TLRs. Natural and synthetic ligands were injected into wild-type C57BL/6 mice to initiate an immune response. At various time points (30 minutes to 24 hours) after injection, splenic single-cell suspensions were prepared, immediately fixed in paraformaldehyde, and subjected to intracellular analysis. Using this method it was possible to examine the signaling events induced upon stimulation as they occurred within different cell subsets within the living animal. The initial peak of STAT phosphorylation occurred 1 hour following ligand injection. STAT1 and STAT3 pathway activation was observed in a large proportion, often greater than half, of splenic conventional dendritic cells (cDCs) and T cells (Figure 1A) and at much lower levels in B cells (B220+ CD11c−) and granulocytes (CD11bhi). TLR3, 4, 7, and 9 ligands elicited both pSTAT1 and pSTAT3 activation whereas the TLR2 ligands, the lipoproteins PAM3CSK4 (Pam3CysSerLys4) and FSL1 (Pam2CGDPKHPKSF), activated only the pSTAT3 pathway. Early pSTAT5 responses were apparent at low frequencies in conventional dendritic cells in response to TLR 1/2, 4, and 7 stimulation. pSTAT4 activation at one hour was potently induced by TLR7 stimulation in a subset of CD8+ T cells. STAT6 phosphorylation was observed at very low levels in B cells in response to LPS. These experiments revealed that STAT activation occurs rapidly and only in particular cell populations in response to specific stimuli during the initiation of an in vivo immune response. Next, we sought to define the molecular basis for these signals both at the receptor and cytokine level.
Figure 1.
Early in vivo response to TLR ligands is characterized by ligand-specific Type I IFN and IL-6 dependent pSTAT1 and pSTAT3 activation in multiple cell types.
(A) Relative response to various TLR ligands in different cells as determined by levels of STAT phosphorylation. The indicated natural and synthetic microbial ligands specific for TLR4 (LPS), TLR9 (CpG), TLR7 (R848), TLR3 (poly I:C), TLR1/2 (PAM3CSK4), TLR2/6 (FSL1), and phosphate-buffered saline (PBS) controls were intravenously injected into C57BL/6 mice (20 μg/mouse). One hour after injection, spleens were harvested and immediately fixed and prepared for surface marker and pSTAT 1, 3, 4, 5, and 6 staining. Cell populations were identified as CD11c+ B220− (cDCs); CD4+ T cells; CD8+ T cells; B220+ CD11c− (B cell), or CD11bhi (granulocytes). The percentage of cells showing greater than basal STAT phosphorylation is shown as a heat map (black, 0%; bright yellow, 100%).
(B) STAT1 and 3 phosphorylation is TLR dependent. Wild-type (green) or TLR2−/−, TLR3−/−, TLR4 mutant, and TLR9−/− mice (all in blue) were challenged with PAM3CSK4, poly (I:C), LPS, and CpG, respectively. Control PBS treatment is shown in red. Spleens were prepared for intracellular staining after 1 hour. Levels of pSTAT1 and pSTAT3 in CD11c+ cells are shown.
(C) STAT1 and STAT3 phosphorylation are type I IFN and IL-6 dependent respectively. IFNAR−/− and IL6−/− mice were injected with 20 μg LPS; 1 hour later cells were harvested and prepared as before. All experiments were performed in at least three separate mice; data from one representative experiment is shown.
Receptor and cytokine dependencies of early STAT activation
Although TLRs are accepted as critical for microbial recognition, distinct mechanisms of pattern recognition exist (Hornung et al., 2006; Kato et al., 2006). To verify that TLRs were necessary for the early STAT inductions in vivo, mutant mice lacking specific functional TLR gene products were injected with ligands known to activate TLRs 1/2, 3, 4, and 9. Injection of PAM3CSK4, LPS, and CpG DNA failed to elicit early STAT activation in TLR2−/−, TLR4 mutant, and TLR9−/− mice, respectively (Figure 1B). In contrast, early STAT phosphorylation was reduced but not totally eliminated after poly (I:C) treatment of TLR3−/− mice. It is likely that other dsRNA specific receptors, such as MDA-5 are responsible for the rapid poly (I:C) responses induced in the absence of TLR3 (Gitlin et al., 2006).
The recognition of microbe-associated molecular patterns by the innate immune system induces the release of a complex set of cytokines, potentially containing numerous redundant elicitors of STAT phosphorylation (Adamson et al., 2009; Beutler et al., 2007). Mice with targeted gene deletions were used to identify cytokines necessary for early pSTAT1 and pSTAT3 inductions since these pathways dominated the early immune response. Mice lacking the type I interferon (IFN) receptor (IFNAR−/−), interferon-γ (IFN-γ −/−), interleukin-6 (IL6−/−), or interleukin-10 (IL10−/−) were injected with LPS and spleens were harvested 1 hour after intravenous injection. Early pSTAT1 activation in CD11c+ cells and T cells was found to be primarily dependent upon type I IFN and to a lesser extent IFN-γ (Figures 1C and S1A). pSTAT3 responses in the same cell populations were not affected by the absence of type I IFN or IFN-γ signaling.
In IL6−/− mice injected with LPS, pSTAT3 induction was reduced in cDCs and eliminated in T cells (Figure 1C). The pattern of pSTAT3 induction in cDCs suggests that other cytokines capable of inducing pSTAT3 are rapidly produced and sensed specifically by a sub-population of cDCs but not by T cells. Like IL-6, IL-10 also potently induces STAT3 phosphorylation in immune cells (Finbloom and Winestock et al., 1995); however, under conditions used here, we found IL-10 to be dispensable for early STAT3 pathway activation (Figure S1B). Similar results were obtained when IL6−/− and IFNAR−/− mice were treated with PAM3CSK4, poly (I:C), and CpG DNA (data not shown).
Vaccinia infection rapidly induces TLR2 and IL-6 dependent STAT3 activation
Having established the receptor and cytokine basis for the major early STAT activation events in response to singular TLR activation, we next examined a complex microbial stimulus capable of initiating an antiviral immune response. Vaccinia virus (Western Reserve strain) was injected intravenously into C57BL/6 mice and spleens were harvested at various time points after infection. Intravenous injection was used to initiate a synchronous response and to simulate the viremia that occurs during the natural life cycle of many poxviruses (Moss, 2001).
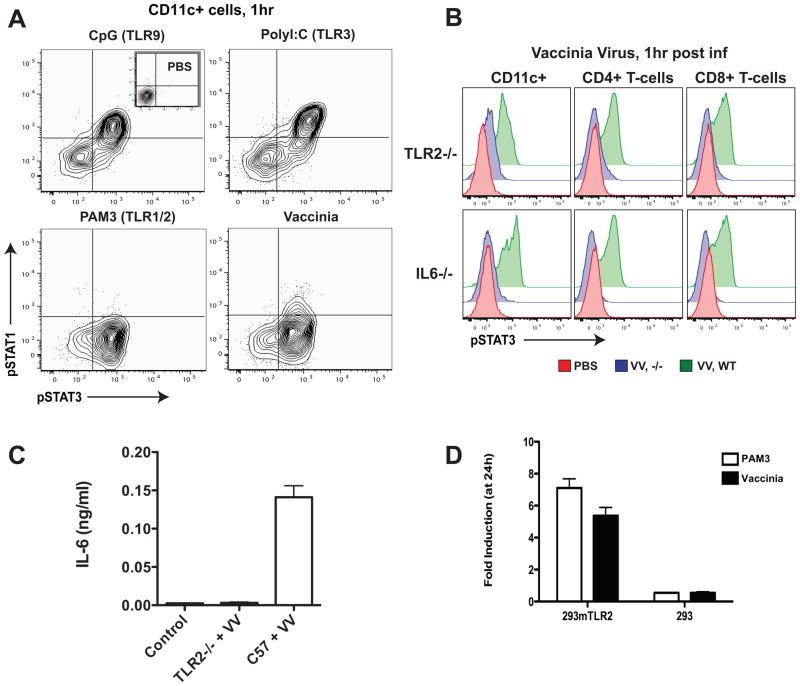
Since vaccinia is a DNA virus particular attention was paid to the patterns and timing of STAT activation elicited by stimulation of the nucleic acid sensors TLR3 and TLR9 that recognize ssRNA and dsDNA, respectively (Koyama et al., 2008). Stimulation of these receptors in vivo with synthetic agonists potently induced both STAT1 and STAT3 phosphorylation in cDCs (Figure 2A). In contrast, stimulation with the TLR2 agonist, PAM3CSK4, elicited only pSTAT3 activation. Surprisingly, systemic vaccinia infection did not typically cause synchronous pSTAT1 and pSTAT3 activation in cDCs as would have been observed if a single nucleic acid sensing TLR was triggered. Instead, initial experiments revealed that only pSTAT3 was induced at 1 hour after vaccinia infection resulting in a STAT activation profile resembling TLR2 activation (Figure 2A). Additional experiments with extended kinetics showed that pSTAT1 responses were induced early but with variability suggesting that the elicitors of STAT1 and STAT3 activation were most likely not produced by a single nucleic acid sensing TLR. In further experiments, we evaluated the effects of deletion of B18R, a vaccinia virulence gene that binds extracellular type I IFN (Symons et al., 1995), to determine if viral virulence genes were mediating the unexpectedly low pSTAT1 response to vaccinia infection. Loss of B18R had minimal effects on pSTAT1 induction (data not shown). In contrast to our findings with synthetic TLR ligands, we observed the STAT1 response to vaccinia to be more dependent on IFN-γ than on type I IFNs (Figure S3). Nevertheless, in accordance with a previous study (Muller et al., 1994), we found both IFNα R−/− and IFNγ −/− mice to be lethally sensitive to viral dosages that are sublethal in wild-type C57BL/6 mice (data not shown).
Figure 2.
Systemic vaccinia infection elicits TLR2 and IL-6-dependent responses that promote viral clearance and neutralizing antibody production.
(A) Early in vivo STAT response to vaccinia virus infection resembles the PAM3CSK4 (TLR1/2 ligand) response. pSTAT1 and pSTAT3 activation in CD11c+ cells at 1 hour after intravenous (i.v.) injection with PBS control, CpG, poly (I:C), or PAM3CSK4, compared to vaccinia virus infection (1 × 107 pfu; Western Reserve strain).
(B) Vaccinia induction of pSTAT3 is TLR2 and IL-6 dependent. Wild-type (green), TLR2−/−, or IL6−/− (both in blue) mice were infected with vaccinia virus (VV) as before, and, after one hour, spleens were excised, dissociated, and prepared for intracellular analysis. pSTAT3 levels were determined in CD11c+, CD4+, and CD8+ cells.
(C) IL-6 production in response to vaccinia infection is TLR2 dependent. Wild-type and TLR2−/− mice were infected i.v. with 1 × 107 pfu of vaccinia virus; after 1 hour serum was harvested for detection of secreted IL-6 by ELISA. Serum from uninfected mice was used as control.
(D) Vaccinia is recognized by TLR2 in vitro. HEK-293 cells transfected with mouse TLR2 and an NFκ B-driven luciferase reporter and untransfected control cells with the NFκ B-driven luciferase reporter alone were exposed to UV-inactivated vaccinia virus (5 viral particles per cell). NFkB-driven luciferase expression was evaluated by bioluminescence signal (after addition of luciferase) 24 hours later (data represented as mean ± SD).
The observed pattern of early pSTAT inductions in vivo led us to hypothesize that TLR2 activation could be the critical early signaling event induced upon vaccinia infection, with cytosolic pattern recognition occurring only as a secondary event. To test this hypothesis vaccinia virus was injected into TLR2−/− mice (Figure 2B). In TLR2−/− mice, activation of the STAT3 pathway was dramatically reduced (by 6-fold in dendritic cells) and peaked 6 hours later than in wild-type mice (Figure S2). Thus, TLR2 is necessary for robust early pSTAT3 responses in cDCs and T cells following vaccinia infection but later responses still elicit some STAT3 activation.
Since we previously observed that IL-6 was the predominant cytokine causing STAT3 signaling downstream of TLR2 activation with lipopeptides, we hypothesized that IL-6 was also the major driver of STAT3 phosphorylation upon vaccinia infection. Indeed, injection of vaccinia into IL6−/− mice did not produce a pSTAT3 response at early time points, demonstrating that early responses in the STAT3 pathway following infection are dependent on IL-6 (Figure 2B). As an independent method of demonstrating that TLR2 engagement is necessary for IL-6 production following infection, serum levels of IL-6 were monitored by ELISA in wild-type and TLR2−/− mice (Figure 2C). Serum IL-6 increased dramatically 1 hour following vaccinia injection and, as previously described (Zhu et al., 2007), TLR2 is required for IL-6 secretion in response to vaccinia infection in vivo.
To determine whether TLR2 is necessary for vaccinia recognition in vitro, we employed a cellular assay in which TLR2 was ectopically expressed in HEK-293 cells. Cells were co-transfected with a luciferase reporter construct driven by an NFκ B-dependent promoter to enable luminescent quantitation of TLR2 activity. Cells that did not express TLR2 were unable to respond to viral infection or the synthetic TLR2 agonist, PAM3CSK4. However, in cells expressing TLR2, vaccinia exposure caused an increase in NFkB activity similar to that resulting from PAM3CSK4 treatment (Figure 2D). TLR2 is thus required for activation and may dimerize with itself or other TLRs to mediate vaccinia recognition. Taken together, these results are consistent with a model in which TLR2 mediates vaccinia virus recognition, which is necessary for IL-6 release and STAT3 pathway activation across a variety of immune cells.
Delayed vaccinia clearance in TLR2- and IL-6-deficient mice
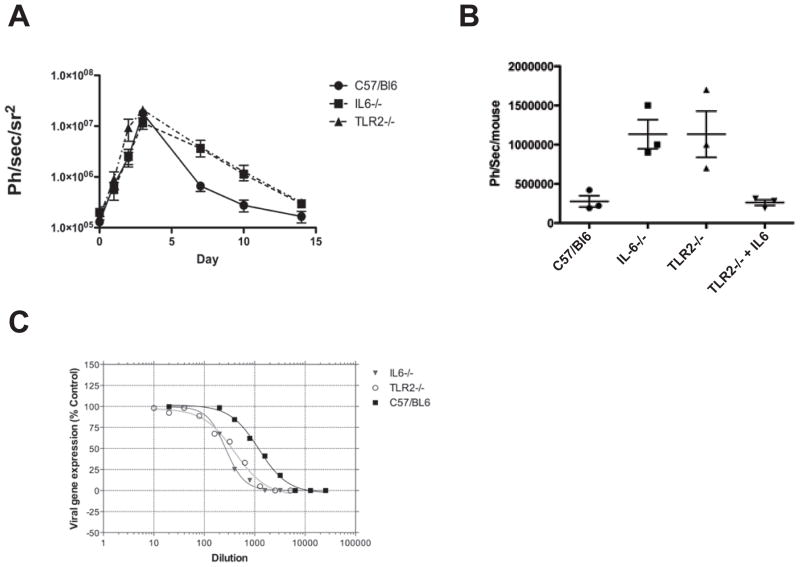
Our pathway analyses clearly demonstrated the molecular basis for STAT3 activation during an early antiviral immune response. Nevertheless, it was still possible that TLR2 and IL-6 responses were not physiologically important and were dispensable for the control of virus replication. Using in vivo bioluminescence imaging of mice infected with a luciferase-expressing vaccinia virus, we quantified viral gene expression levels, which are known to correlate directly with viral burden (Luker et al., 2005), daily for 15 days following infection. Surprisingly, viral burden was increased in TLR2−/− and IL6−/− mice relative to that in wild-type mice only during the later stages of infection (Figure 3A). This suggests that although IL-6 is produced within the first hour of infection it may primarily promote later adaptive anti-viral immune responses. Importantly, injection of recombinant IL-6 alone was sufficient to induce the pSTAT3 pathway in vivo (data not shown), and a single dose of IL-6 rescued antiviral immunity in TLR2−/− mice as measured by viral gene expression 7 days after infection (Figure 3B).
Figure 3.
Robust long-term immune response to vaccinia is dependent on early TLR2 and IL-6 signaling.
(A) TLR2−/− or IL6−/− mice display delayed clearance of vaccinia virus. Mice (wild-type, TLR2−/−, or IL6−/− of the C57BL/6 background) were infected i.v. with 1 × 107 pfu of Western Reserve strain vaccinia expressing luciferase. Overall viral load was determined at different times by bioluminescence imaging (BLI) after luciferin substrate delivery by intraperitoneal injection in an IVIS200 (Xenogen). No differences were seen in initial overall viral gene expression (correlating with viral replication) or biodistribution (except that an early spleen signal seen in C57/BL6 mice was absent from the gene knock-out strains). Significant differences were not seen until 6 days after infection, when the gene knock-out strains displayed delayed viral clearance (data represented as mean ± SD, with n = at least 3 mice per group).
(B) Addition of exogenous IL-6 can substitute for loss of TLR2. C57BL/6, TLR2−/−, and IL6−/− mice were infected with vaccinia expressing luciferase as before; in one group, TLR2−/− mice were additionally treated with 500ng IL-6 delivered intraperitoneally. Viral luciferase gene expression is shown for whole animals as determined 7 days post infection (data represented as mean ± SD, with n = at least 3 mice per group)
(C) Loss of TLR2-IL6-pSTAT3 early signaling pathway leads to reduced levels of anti-vaccinia neutralizing antibody. Mice treated as above were sacrificed 21 days after initial viral infection, and levels of vaccinia specific antibody in the serum were determined with a viral neutralization assay. The TLR2−/− and IL6−/− strains both displayed reduced levels of neutralizing antibody.
The ability of IL-6 to promote antibody production is well-established (Kopf et al., 1994, 1998; Suematsu et al., 1989). IL-6 has been previously shown to enhance humoral immune responses against vaccinia (Ramsay et al., 1994); however, it has not previously been shown that early TLR2-dependent recognition can influence antibody production. We therefore monitored circulating anti-vaccinia antibody levels using a virus neutralization assay. Serum from infected IL6−/− and TLR2−/− mice had approximately five-fold less neutralizing activity than wild-type controls (Figure 3C), indicating that early TLR2-dependent IL-6 production enhanced vaccinia specific humoral responses and clearance of the viral infection. This is in agreement with a recent study demonstrating that recombinant IL-6 injected early during influenza infection causes increased influenza-specific IgM and IgG1 production (Dienz et al., 2009).
Differences in early immune recognition between poxviruses
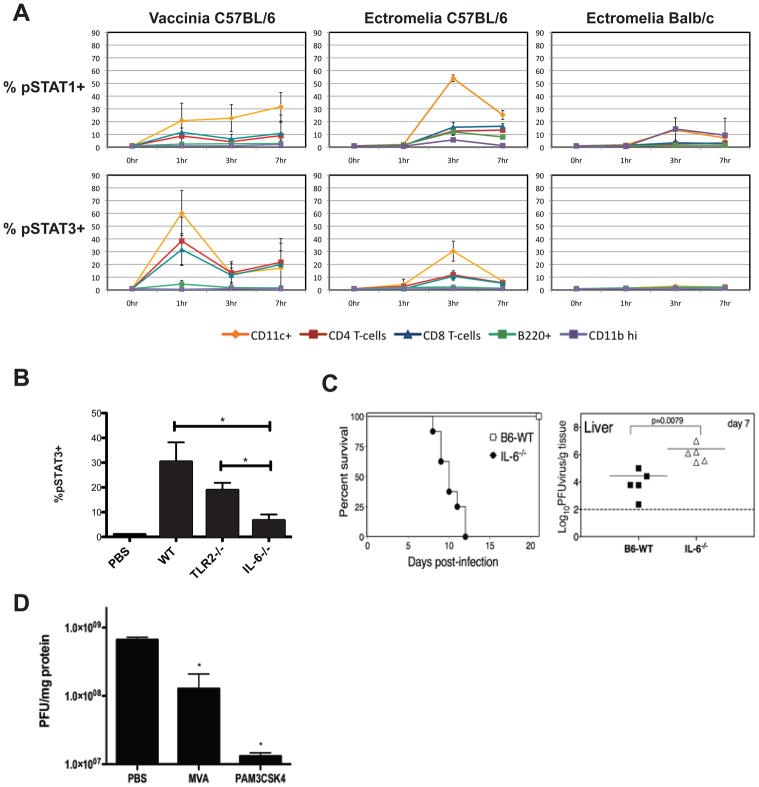
Orthopoxviruses are often capable of infecting cells from numerous mammalian species in vitro but display strict species specificity on the organismal level (McFadden, 2005). Vaccinia is capable of infecting many cell types in vitro and in vivo and induces a robust immunization in mice without causing disease; in contrast, ectromelia, a closely related virus, is highly pathogenic in mice, its cognate host (Buller and Palumbo, 1991). Using our single-cell approach, differences in immune recognition of related poxviruses were monitored. In C57BL/6 mice, vaccinia infection induced pSTAT1 and pSTAT3 activation in cDCs and T cells more rapidly than did ectromelia infection (Figure 4A). Furthermore, the percentage of cells displaying STAT3 activation after vaccinia infection was approximately twice that observed after ectromelia infection in multiple cell types. However, at three hours the percentage of cells with phosphorylated STAT1 was comparable between the two viruses. The pSTAT3 activation induced by ectromelia infection was not significantly dependent on TLR2 but was IL-6 dependent (Figure 4B) indicating that vaccinia and ectromelia engage different pattern recognition receptors early during infection. In further support of this notion, ectromelia had far less stimulatory activity than vaccinia in in vitro HEK-293 based TLR2 reporter assays (Figure S4A). As expected based on the results of this in vitro assay, wild-type and TLR2−/− mice were equally susceptible to ectromelia infection in survival studies and TLR2−/− mice did not exhibit a significant increase in viral burden (Figure S4B and data not shown). These results suggest that TLR2 is dispensable for resistance to lethal ectromelia infection.
Figure 4.
Resistance to poxvirus infection correlates with early and potent STAT network activation in dendritic cells and T cells.
(A) Ectromelia virus infection produces delayed and reduced pSTAT1 and pSTAT3 induction, especially in sensitive BALB/c mice. In vivo time course of the early immune responses in C57BL/6 and BALB/c mice infected intravenously with 1 × 107 pfu of either vaccinia virus or ectromelia virus (Moscow strain) was determined as in Figure 1. Percentage of cells demonstrating pSTAT1 or pSTAT3 activation at different times after infection is plotted for conventional dendritic cells (CD11c+), T cells (CD4+ or CD8+), B cells (B220+), and granulocytes (CD11bhi) (data represented as mean ± SD).
(B) Ectromelia virus induction of pSTAT3 is IL-6- but not TLR2-dependent. Wild-type (C57BL/6), TLR2−/−, and IL6−/− mice were infected with 1 × 107 pfu ectromelia virus. After 1 hour spleens were excised, dissociated, and prepared for intracellular analysis. Levels of pSTAT3 in CD11c+ cells are shown (data represented as mean +/− SD; two-tailed, unpaired t-test: * p<0.02 for IL6−/− compared to WT and TLR2−/−, WT compared to TLR2−/− was not significant).
(C) IL-6 induction is necessary for resistance to ectromelia virus infection in C57BL/6 mice. Wild-type and IL6−/− mice of the C57BL/6 background were subcutaneously infected with 1 × 103 pfu of ectromelia virus. Survival was monitored for 20 days following infection. Livers were extracted from separate groups of infected mice on day 7 and a plaque-forming assay was used to monitor viral burden.
(D) TLR2 activation prior to ectromelia infection reduces viral burden. PBS, 1 × 107 pfu of MVA (modified vaccinia Ankara), or 20 μg of PAM3CSK4 was intravenously injected into C57BL/6 mice one hour before systemic infection with 1 × 107 pfu of ectromelia. Spleens were excised 72 hours later and ectromelia burden was quantified using a plaque-forming assay (data represented as mean ± SD; two-tailed, unpaired t-test: * p< 0.01 MVA compared to PBS, p< 0.007 PAM3CSK4 compared to PBS).
We sought to compare serum ELISA levels of IL-6 between vaccinia and ectromelia infected mice; however, increased levels of IL-6 could not be detected in the serum of ectromelia infected mice (data not shown). It is possible that pSTAT3 monitoring in dendritic cells may be a more sensitive method than serum ELISA for detecting minor increases in IL-6 production in vivo.
IL-6 is an essential resistance factor in the pathogenesis of ectromelia
Ectromelia virus demonstrates increased pathogenicity and lethality in specific strains of mice; the C57BL/6 strain is relatively resistant and the BALB/c strain is extremely sensitive (Wallace et al., 1985). We hypothesized that differences in early STAT activation could underlie mechanisms of host resistance to ectromelia in C57BL/6 mice. Strikingly, ectromelia infection of BALB/c mice yielded only slight activation of pSTAT1 and no pSTAT3 response in the cell populations we analyzed (Figure 4A). Importantly, no differences were observed in the activation of these pathways between C57BL/6 mice and BALB/c mice after vaccinia infection (data not shown).
IL-6 dependent STAT3 activation occurs within three hours of systemic ectromelia infection in C57BL/6 mice. To establish whether C57BL/6 mice genetically lacking IL-6 demonstrate increased susceptibility to infection, wild-type and IL6−/− mice were infected with 1 × 103 plaque-forming units (pfu) of ectromelia and survival was monitored for twenty days following infection. All IL6−/− mice succumbed to infection by 13 days post-infection, whereas all wild-type mice survived (Figure 4C). In addition, IL6−/− mice carried a higher liver viral burden compared to wild-type controls (Figure 4C). These experiments demonstrate that IL-6 is an important resistance factor essential for surviving ectromelia infection.
TLR2 activation prior to ectromelia infection reduces viral burden
Therapeutic vaccination with an attenuated vaccinia strain (modified vaccinia Ankara, MVA) protects ectromelia-challenged mice from an otherwise lethal infection even if administered days after ectromelia inoculation (Samuelsson et al., 2008). Surprisingly, TLR9 stimulation by MVA DNA is dispensable for protection since therapeutic vaccination is also observed in lethally infected TLR9−/− mice. We considered the possibility that the therapeutic effects of MVA pre-treatment are mediated by TLR2 activation. Similar to vaccinia infection, we found that MVA immunization induces potent pSTAT3 activation in dendritic cells and T cells (data not shown). Therefore, to evaluate whether TLR2 activation alone could reduce viral burden, 20 μg of PAM3CSK4 was injected into C57BL/6 mice 1 hour prior to ectromelia infection. Both MVA and PAM3CSK4 pre-treatment significantly reduced ectromelia burden in the spleen at 72 hours following infection (Figure 4D). In contrast, ectromelia burden was not reduced by PAM3CSK4 pretreatment in IL6−/− mice (Figure S5) further evidencing the importance of IL-6 in mediating resistance to ectromelia. Unexpectedly, TLR2 activation with the purified ligand PAM3CSK4 was more effective than immunization with attenuated virus in lowering ectromelia viral burden in both spleen and liver, suggesting that therapeutic vaccination of poxvirus infection could be performed with TLR agonists instead of intact viral particles.
Discussion
The recognition of pathogen-associated molecular patterns (PAMPs) occurs during the earliest steps of an innate immune response. Pattern recognition causes the release of numerous cytokines capable of initiating gene regulatory programs through the activation of transcription factor networks. Until now it has been impossible to examine how this transcription factor network is activated in response to a microbial infection in the context of an intact immune system, within a living host. Direct measurement of serum cytokines in vivo demonstrates the presence of specific interleukins but does not reveal which immune cells actually respond to a particular cytokine. Because of this, our current understanding of cytokine responsiveness is based largely on in vitro experiments in which bone marrow-derived or sorted primary cells are treated with recombinant cytokines and then subjected to western blot analysis. In vivo cytokine responsiveness may differ drastically from the in vitro responsiveness of cellular monocultures due to competition between different cell types for available cytokines (Pandiyan et al., 2007), as well as biophysical constraints imposed by the stromal microarchitecture of secondary lymphoid organs (Gretz et al., 2000). Here we have developed a novel strategy to study the kinetics and levels of activation of different signaling pathways within diverse immune cell populations at times after exposure to a pathogen. This approach allowed us to define the earliest STAT signaling pathways induced by different (immunizing or pathogenic) poxviruses in the context of a living organism, and to correlate network activation with different immune outcomes.
Vaccinia was key to what is possibly mankind's greatest medical achievement, the eradication of smallpox. Attenuated and wild-type vaccinia strains continue to be evaluated clinically as recombinant vaccine vehicles for a number of infectious agents, including HIV (Manrique et al., 2009; Rodriguez et al., 2009). Deeper comprehension of the immune response to vaccinia may produce strategies to enhance vaccine efficacy and predict potentially harmful side effects, while an understanding of how the early immune response differs in vaccinating and pathogenic infections may lead to alternative therapeutic approaches or means to predict the outcome of infection.
A number of innate and adaptive cell populations have been linked to protective responses against orthopoxviruses (Xu et al., 2004). Innate immunity is thought to be highly dependent upon type I IFN-activated natural killer cells (Martinez et al., 2008) and γ δ T cells (Selin et al., 2001). Adaptive CD8+ T cell responses are often critical for destruction of virally infected cells; however, only a minor role for CD8+ T cell mediated cellular immunity has been described for resistance against vaccinia infection (Spriggs et al., 1992; Xu et al., 2004). In contrast, humoral immune responses are known to be important in controlling vaccinia in both humans (Lane et al., 1969) and mice (Galmiche et al., 1999). In mice depleted of either CD4+ T cells or B cells, severe defects in neutralizing antibody production are associated with enhanced viral replication. These studies revealed the CD4+ T cell dependence of a large portion of the anti-vaccinia neutralizing antibody response.
Here, we demonstrate how vaccinia infection induces immune network signals that promote humoral immunity. Early IL-6 production, preferential activation of the STAT3 pathway, and potent neutralizing antibody responses all depended on viral recognition via TLR2. We found that IL-6 was necessary for robust activation of the STAT3 pathway, although numerous cytokines can potentially activate STAT3 in T cells (including IL-10 and IL-21). IL-6 is thus a non-redundant regulator of STAT3 programming with reported effects ranging from increasing T cell viability (Takeda et al., 1998), to the deactivation of regulatory T cells (Korn et al., 2007), and, prominently, the promotion of antibody responses.
Surprisingly, although IL-6 was first discovered as a B cell hybridoma factor (Hirano et al., 1985), in our experiments, naïve B cells were only minimally sensitive to IL-6 in vivo at times early after exposure to vaccinia (Figure 4A). Our observation is in agreement with a recent study that suggests B cells are indirectly affected by IL-6 through the IL-6-dependent generation of IL-21 producing T follicular-helper (TFH) cells (Dienz et al., 2009). Alternatively, B cell receptor stimulation increases IL-6Rα expression (Burdin et al., 1996), thus B cells most likely achieve robust STAT3 activation indirectly through IL-21 produced by TFH cells or via IL-6 directly only after antigen recognition.
In the ectromelia model, at least two lines of evidence indicate that the early induction of IL-6-dependent pSTAT3 can significantly impact on the outcome of infection. First, pSTAT3 is induced rapidly in resistant C57BL/6 mice whereas in the susceptible strain it is not. Second, C57BL/6 mice deficient in IL-6 are highly susceptible to infection. Although the precise molecular mechanism(s) through which IL-6-dependent pSTAT3 induction restricts ectromelia replication is not yet known, our data suggests it acts early and may likely have a role in the innate and inflammatory responses. This is because the absence of IL-6 does not affect generation of a robust antiviral cytotoxic T cell response (data not shown) and IL6−/− mice succumb to mousepox significantly earlier than B cell-deficient mice (Chaudhri et al., 2006). Nevertheless, it is likely that IL-6 plays an important role early during the innate response and later in generation of anti-ectromelia antibody responses.
A recent study has shown that TLR9 recognition of ectromelia is necessary for IL-6 production by dendritic cells in vitro (Samuelsson et al., 2008). It is therefore probable that TLR9 sensing is also responsible for IL-6 production and STAT activation in our in vivo model. Additionally, cytosolic recognition of vaccinia DNA is most likely responsible for the IL-6 production observed later in infection in the absence of TLR2 (Figure S2). These results suggest that a fundamental difference exists in the initial immune response to these closely related poxviruses: vaccinia immediately triggers TLR2 and eventually activates microbial nucleic acid sensing receptors, whereas ectromelia evades TLR2 recognition and is only recognized later by nucleic acid sensors such as TLR9. If this initial IL-6 response to ectromelia in vivo is purely TLR9-dependent, then the mechanism for TLR9 evasion by ectromelia in BALB/c mice demands further investigation. This fundamental difference in the early host response to vaccinia versus ectromelia may be a key determinant in the maintenance of host-species specificity and could underlie the unique and potent immunogenicity of vaccinia virus.
The single-cell approach to determining the status of signaling pathways utilized in this study facilitated the in vivo deconstruction of the antiviral cytokine response network subsequent to poxvirus infection. Furthermore, we found early and robust STAT1 and STAT3 pathway activation was directly correlated with positive long-term immune outcomes. As single-cell biochemical analysis advances, it will be possible to simultaneously interrogate the activation states of all critical immune signaling pathways across the entire spectrum of hematopoietic cell populations. Definitive in vivo network maps revealing how cytokines program the immune system during homeostasis and disease are the likely byproduct of evaluating the immune system cell-by-cell and may provide means to develop novel therapeutic approaches, to closely assess an ongoing immune response, and to predict immune outcome.
Experimental Procedures
Mice
C57BL/6J, BALB/c, B6; 129, TLR2−/−, TLR3−/−, TLR4lps-del , IFNγ −/−, IL6−/−, and IL-10−/− mice were purchased from Jackson Laboratories (Bar Harbor, Maine). IFNAR receptor−/− mice were a gift from Denise Monack (Stanford University) and Hideho Okada (University of Pittsburgh). TLR9−/− mice were a gift from Shizuo Akira (Osaka University). All mice were bred and maintained in accordance with the guidelines of the Local Administrative Panels on Laboratory Animal Care/Institutional Animal Care and Use Committee protocols. All mice used were between 6–8 weeks of age and were also strain and gender matched within experiments.
In Vivo Innate Immune Stimulation with Toll-like Receptor Ligands
Purified LPS (from E. coli K12), ODN1826 (CpG), R848 (resiquimod), poly (I:C), PAM3CSK4, and FSL1 were purchased from Invivogen (San Diego, California). Mice were injected via the tail vein with 20 μg of each ligand. Following stimulation in vivo, the spleens of recipient mice were harvested as previously described (Krutzik et al., 2005). In brief, mice were sacrificed at various time points between 15 minutes and 7 hours after stimulation. Spleens were excised and immediately dissociated into a 10-ml PBS solution containing 1.6% paraformaldehyde (Electron Microscopy Sciences). Following a 15-min fixation period, cell suspensions were transferred through a 70-μm pore size mesh and ice-cold methanol was directly added to a final concentration of 80% methanol. In some experiments mice were injected with 2 μg-15 μg of ligand.
Staining and Flow Cytometry
Fixed and permeabilized cells were prepared for intracellular analysis as previously described (Krutzik et al., 2005; Krutzik and Nolan 2003). The following Abs were used in 8-color panels (in parallel) to identify and analyze splenic cell populations (purchased from BD Biosciences except as noted): pSTAT1 (pY701, clone 4a), pSTAT3 (pY705, clone 4), pSTAT4 (pY693, clone 38), pSTAT5 (pY694, clone 47), pSTAT6 (pY641, clone J71-773.58.11) B220 (RA3-6B2), CD90.2 (BioLegend; 30-H12), CD4 (RM4-5), CD11b (M1/70), CD11c (HL3), anti-keyhole limpet hemocyanin control antibody (X40). All antibody concentrations were titrated for optimal staining in a 100 μl staining volume with no more than 4 × 106 methanol-permeabilized cells per sample. The percent pSTAT positivity represents the percentage of activated cells showing a pSTAT median fluorescence intensity greater than the highest 1% of that of PBS controls.
Viral Infection
Vaccinia strain Western Reserve was purchased from ATCC. The Western Reserve strain expressing luciferase was kindly provided by Dr Gary Luker (University of Michigan) and the Ectromelia Virus (Moscow strain) was kindly provided by Mark Buller (University of St Louis). Purified viral preparations were delivered via tail vein injection at 1 × 107 pfu/mouse unless otherwise stated. In some experiments the virus was filter sterilized (to remove viral particles) and used as a control to verify that no contaminants from the viral preparation were activating TLRs. Mice were sacrificed and tissues were collected for determination of activation of signaling pathways by flow cytometry or viral load by plaque assay. Alternatively, bioluminescence imaging (BLI) was performed to determine levels of viral luciferase gene expression. Mice were injected with luciferin intraperitoneally (300 mg/kg), anesthetized (2% isoflurane) and imaged in an IVIS200 (Xenogen/Caliper Life Sciences).
Serum cytokine measurements by ELISA
Production of IL-6 in vivo in response to viral infection was detected in sera by ELISA kits purchased from R&D (Minneapolis, MN) according to manufacturer’s protocols.
Neutralizing Antibody Quantification
A standard neutralizing antibody assay was performed. Briefly, serial dilutions of heat-inactivated plasma samples were mixed with 1 × 103 pfu of vaccinia strain Western Reserve expressing luciferase, and neutralization was allowed to occur for 2 hours. Virus and plasma were then layered over a monolayer of A2780 cells (from ATCC), and infection and viral gene expression were allowed to take place for 24 hours. At the end of this time, the level of non-neutralized virus was determined by bioluminescence imaging (IVIS200, Xenogen). The relative percentage neutralization was determined relative to virus with no plasma (0% neutralization) and plasma with no virus (100% neutralization). Levels of neutralizing antibody were defined by the dilution of plasma required to achieve 50% neutralization of the virus.
In vitro TLR2 activation assay
HEK-293 cells and HEK-293 cells stably transfected to express murine TLR2 were purchased from Invivogen. These cells were transiently transfected with the pNIFTY plasmid (Invivogen), containing a luciferase reporter driven by an NFkB responsive promoter. These cells were exposed to control ligands or UV-inactivated vaccinia virus (MOI of 5 viral particles per cell). Luciferase expression was determined at indicated times after stimulation by bioluminescence imaging (IVIS 200, Xenogen, part of Caliper) subsequent to addition of luciferin.
Statistical Analysis
A two-tailed, unpaired t-test analysis was used in some experiments to determine significance for averaged values with noted standard deviations. At least 3 mice/group and often as many as 7 mice/group were analyzed.
Supplementary Material
Supplemental Figure 1. IFN-γ and IL-10 play minor roles in early pSTAT1 and pSTAT3 activation.
(A) Wild-type and IL-10−/− mice of the BALB/c background do not differ in the early pSTAT1 and pSTAT3 response to LPS. Wild-type and IL-10−/− mice were injected with 20 μg LPS; after 1 hour spleens were extracted, and splenocytes were analyzed by flow cytometry.
(B) IFNγ is necessary for wild-type levels of pSTAT1 activation. Wild-type C57BL/6 and IFNγ −/− C57BL/6 mice were injected with 20ug LPS, after 1hr spleens were extracted and analyzed by flow cytometry.
Supplemental Figure 2. TLR2−/− mice experience late and reduced pSTAT3 activation after vaccinia infection.
(A) pSTAT3 pathway activation in TLR2−/− mice is delayed. Wild-type and TLR2−/− mice were intravenously injected with 1 × 107 pfu of vaccinia virus (Western Reserve strain) or with PBS (negative control). Spleens were harvested at 1 hour, 3 hours, and 7 hours following infection (data represented as mean ± SD).
Supplemental Figure 3. Vaccinia induced pSTAT1 responses are dependent on type I IFN and IFN- γ
(A) pSTAT1 signaling after vaccinia infection is impaired in IFNAR−/− mice and almost absent in IFN-γ −/− mice. Three hours following infection, mice were sacrificed and splenic single-cell suspensions were prepared for flow cytometry. pSTAT1 x pSTAT3 profiles for CD11c+ cells are shown.
Supplemental Figure 4. Vaccinia presents a far more potent TLR2 ligand than ectromelia; TLR2 does not confer resistance to lethal ectromelia infection.
(A) HEK-293 cells transfected with mouse TLR2 and an NFκ B-driven luciferase reporter and untransfected control cells with the NFκ B-driven luciferase reporter, alone, were exposed to UV inactivated vaccinia virus or ectromelia virus (5 viral particles per cell). NFkB-driven luciferase expression evaluated by bioluminescence signal (after addition of luciferase) was determined 24 hours later (data represented as mean ± SD).
(B) TLR2 is dispensable for resistance to ectromelia virus infection in C57BL/6 mice. Wild-type and TLR2−/− mice of the C57BL/6 background were intravenously infected with 1 × 105 pfu of ectromelia virus. Survival was monitored for 10 days following infection.
Supplemental Figure 5. PAM3CSK4 pretreatment does not reduce ectromelia burden in IL6−/− mice.
(A) PBS or 20 μg of PAM3CSK4 was intravenously injected into C57BL/6 IL6−/− mice one hour before systemic infection with 1 × 107 pfu of ectromelia. Spleens were excised 48 hours later and ectromelia burden was quantified using a plaque-forming assay.
Acknowledgments
We would like to thank Hideho Okada and Shizuo Akira for providing transgenic mouse strains and Gary Luker and Mark Buller for providing viral strains used in this work. We also thank Abul Abbas, Bruce Beutler, and Sean Bendall for critical review and discussion. Dr. Thorne is supported by an Alliance of Cancer Gene Therapy Young Investigator award. This work is part of the systems immunology initiative that can be accessed at www.systemsimmunology.org.
Supported by NIH grants 2U19 AI057229 (GPN), HHSN272200700038C (GPN), U01 AI-074512 (DBL), and the Hillman Foundation (SHT).
Footnotes
Competing Interests Statement
Technologies associated with phospho-flow are licensed in part to BD Biosciences, and Dr. Garry P. Nolan is a consultant for BD Biosciences, a supplier of the reagents used in this report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adamson AS, Collins K, Laurence A, O'Shea JJ. The Current STATus of lymphocyte signaling: new roles for old players. Curr Opin Immunol. 2009;21:161–166. doi: 10.1016/j.coi.2009.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Beutler B, Eidenschenk C, Crozat K, Imler JL, Takeuchi O, Hoffmann JA, Akira S. Genetic analysis of resistance to viral infection. Nat Rev Immunol. 2007;7:753–766. doi: 10.1038/nri2174. [DOI] [PubMed] [Google Scholar]
- Buller RM, Palumbo GJ. Poxvirus pathogenesis. Microbiol Rev. 1991;55:80–122. doi: 10.1128/mr.55.1.80-122.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdin N, Galibert L, Garrone P, Durand I, Banchereau J, Rousset F. Inability to produce IL-6 is a functional feature of human germinal center B lymphocytes. J Immunol. 1996;156:4107–4113. [PubMed] [Google Scholar]
- Chaudhri G, Panchanathan V, Bluethmann H, Karupiah G. Obligatory requirement for antibody in recovery from a primary poxvirus infection. J Virol. 2006;80:6339–6344. doi: 10.1128/JVI.00116-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri G, Panchanathan V, Buller RML, van den Eertwegh AJM, Claassen E, Zhou J, de Chazal R, Laman JD, Karupiah G. Polarized type 1 cytokine response and cell-mediated immunity determine genetic resistance to mousepox. Proc Natl Acad Sci USA. 2004;101:9057–9062. doi: 10.1073/pnas.0402949101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaloye J, Roger T, Steiner-Tardivel QG, Le Roy D, Knaup Reymond M, Akira S, Petrilli V, Gomez CE, Perdiguero B, Tschopp J, et al. Innate immune sensing of modified vaccinia virus Ankara (MVA) is mediated by TLR2-TLR6, MDA-5 and the NALP3 inflammasome. PLoS Pathog. 2009;5:e1000480. doi: 10.1371/journal.ppat.1000480. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Dienz O, Eaton SM, Bond JP, Neveu W, Moquin D, Noubade R, Briso EM, Charland C, Leonard WJ, Ciliberto G, et al. The induction of antibody production by IL-6 is indirectly mediated by IL-21 produced by CD4+ T cells. J Exp Med. 2009;206:69–78. doi: 10.1084/jem.20081571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenner F. Mouse-pox; infectious ectromelia of mice; a review. J Immunol. 1949;63:341–373. [PubMed] [Google Scholar]
- Fenner F. Mousepox (infectious ectromelia): past, present, and future. Lab Anim Sci. 1981;31:553–559. [PubMed] [Google Scholar]
- Finbloom DS, Winestock KD. IL-10 induces the tyrosine phosphorylation of tyk2 and Jak1 and the differential assembly of STAT1 alpha and STAT3 complexes in human T cells and monocytes. J Immunol. 1995;155:1079–1090. [PubMed] [Google Scholar]
- Galmiche MC, Goenaga J, Wittek R, Rindisbacher L. Neutralizing and protective antibodies directed against vaccinia virus envelope antigens. Virology. 1999;254:71–80. doi: 10.1006/viro.1998.9516. [DOI] [PubMed] [Google Scholar]
- Gitlin L, Barchet W, Gilfillan S, Cella M, Beutler B, Flavell RA, Diamond MS, Colonna M. Essential role of mda-5 in type I IFN responses to polyriboinosinic:polyribocytidylic acid and encephalomyocarditis picornavirus. Proc Natl Acad Sci U S A. 2006;103:8459–8464. doi: 10.1073/pnas.0603082103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gretz JE, Norbury CC, Anderson AO, Proudfoot AE, Shaw S. Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J Exp Med. 2000;192:1425–1440. doi: 10.1084/jem.192.10.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T, Taga T, Nakano N, Yasukawa K, Kashiwamura S, Shimizu K, Nakajima K, Pyun KH, Kishimoto T. Purification to homogeneity and characterization of human B-cell differentiation factor (BCDF or BSFp-2) Proc Natl Acad Sci USA. 1985;82:5490–5494. doi: 10.1073/pnas.82.16.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung V, Ellegast J, Kim S, Brzózka K, Jung A, Kato H, Poeck H, Akira S, Conzelmann KK, Schlee M, et al. 5'-Triphosphate RNA is the ligand for RIG–I. Science. 2006;314:994–997. doi: 10.1126/science.1132505. [DOI] [PubMed] [Google Scholar]
- Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- Kisseleva T, Bhattacharya S, Braunstein J, Schindler CW. Signaling through the JAK/STAT pathway, recent advances and future challenges. Gene. 2002;285:1–24. doi: 10.1016/s0378-1119(02)00398-0. [DOI] [PubMed] [Google Scholar]
- Kopf M, Baumann H, Freer G, Freudenberg M, Lamers M, Kishimoto T, Zinkernagel R, Bluethmann H, Köhler G. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature. 1994;368:339–342. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- Kopf M, Herren S, Wiles MV, Pepys MB, Kosco-Vilbois MH. Interleukin 6 influences germinal center development and antibody production via a contribution of C3 complement component. J Exp Med. 1998;188:1895–1906. doi: 10.1084/jem.188.10.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn T, Reddy J, Gao W, Bettelli E, Awasthi A, Petersen TR, Bäckström BT, Sobel RA, Wucherpfennig KW, Strom TB, et al. Myelin-specific regulatory T cells accumulate in the CNS but fail to control autoimmune inflammation. Nat Med. 2007;13:423–431. doi: 10.1038/nm1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama S, Ishii KJ, Coban C, Akira S. Innate immune response to viral infection. Cytokine. 2008;43:336–341. doi: 10.1016/j.cyto.2008.07.009. [DOI] [PubMed] [Google Scholar]
- Krutzik PO, Hale MB, Nolan GP. Characterization of the murine immunological signaling network with phosphospecific flow cytometry. J Immunol. 2005;175:2366–2373. doi: 10.4049/jimmunol.175.4.2366. [DOI] [PubMed] [Google Scholar]
- Krutzik PO, Nolan GP. Intracellular phosphoprotein staining techniques for flow cytometry: monitoring single cell signaling events. Cytometry A. 2003;55:61–70. doi: 10.1002/cyto.a.10072. [DOI] [PubMed] [Google Scholar]
- Lane JM, Ruben FL, Neff JM, Millar JD. Complications of smallpox vaccination. N Engl J Med. 1969;281:1201–1208. doi: 10.1056/NEJM196911272812201. [DOI] [PubMed] [Google Scholar]
- Luker KE, Hutchens M, Schultz T, Pekosz A, Luker GD. Bioluminescence imaging of vaccinia virus: effects of interferon on viral replication and spread. Virology. 2005;341:284–300. doi: 10.1016/j.virol.2005.06.049. [DOI] [PubMed] [Google Scholar]
- Manrique M, Kozlowski P, Wang S, Wilson R, Micewicz E, Montefiori D, Mansfield K, Carville A, Aldovini A. Nasal DNA-MVA SIV vaccination provides more significant protection from progression to AIDS than a similar intramuscular vaccination. Mucosal immunology. 2009 doi: 10.1038/mi.2009.103. [DOI] [PubMed] [Google Scholar]
- Martinez J, Huang X, Yang Y. Direct action of type I IFN on NK cells is required for their activation in response to vaccinia viral infection in vivo. J Immunol. 2008;180:1592–1597. doi: 10.4049/jimmunol.180.3.1592. [DOI] [PubMed] [Google Scholar]
- McFadden G. Poxvirus tropism. Nat Rev Microbiol. 2005;3:201–213. doi: 10.1038/nrmicro1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B. Poxviridae: The viruses and their replication. In: KDM, Fields BN, Howley PM, editors. Field's Virology. Ch 84 Philadelphia: Lippincott-Raven; 2001. [Google Scholar]
- Muller U, Steinhoff U, Reis LFL, Hemmi S, Pavlovic J, Zinkernagel RM, Auget M. Functional role of type I and type II interferons in antiviral defense. Science. 1994;264:1918–1921. doi: 10.1126/science.8009221. [DOI] [PubMed] [Google Scholar]
- Pandiyan P, Zheng L, Ishihara S, Reed J, Lenardo MJ. CD4+CD25+Foxp3+ regulatory T cells induce cytokine deprivation-mediated apoptosis of effector CD4+ T cells. Nat Immunol. 2007;8:1353–1362. doi: 10.1038/ni1536. [DOI] [PubMed] [Google Scholar]
- Ramsay AJ, Husband AJ, Ramshaw IA, Bao S, Matthaei KI, Koehler G, Kopf M. The role of interleukin-6 in mucosal IgA antibody responses in vivo. Science. 1994;264:561–563. doi: 10.1126/science.8160012. [DOI] [PubMed] [Google Scholar]
- Ramshaw IA, Ramsay AJ, Karupiah G, Rolph MS, Mahalingam S, Ruby JC. Cytokines and immunity to viral infections. Immunol Rev. 1997;159:119–135. doi: 10.1111/j.1600-065x.1997.tb01011.x. [DOI] [PubMed] [Google Scholar]
- Rodríguez A, Turk G, Pascutti M, Ferrer F, Nájera J, Mónaco D, Esteban M, Salomón H, Calamante G, Gherardi M. Characterization of DNA and MVA vectors expressing Nef from HIV-1 CRF12_BF revealed high immune specificity with low cross-reactivity against subtype B. Virus Res. 2009 doi: 10.1016/j.virusres.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Samuelsson C, Hausmann J, Lauterbach H, Schmidt M, Akira S, Wagner H, Chaplin P, Suter M, O'Keeffe M, Hochrein H. Survival of lethal poxvirus infection in mice depends on TLR9, and therapeutic vaccination provides protection. J Clin Invest. 2008;118:1776–1784. doi: 10.1172/JCI33940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selin LK, Santolucito PA, Pinto AK, Szomolanyi-Tsuda E, Welsh RM. Innate immunity to viruses: control of vaccinia virus infection by γ δ T cells. J Immunol. 2001;166:6784–6794. doi: 10.4049/jimmunol.166.11.6784. [DOI] [PubMed] [Google Scholar]
- Spriggs MK, Koller BH, Sato T, Morrissey PJ, Fanslow WC, Smithies O, Voice RF, Widmer MB, Maliszewski CR. β2-microglobulin-, CD8+ T-cell-deficient mice survive inoculation with high doses of vaccinia virus and exhibit altered IgG responses. Proc Natl Acad Sci USA. 1992;89:6070–6074. doi: 10.1073/pnas.89.13.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suematsu S, Matsuda T, Aozasa K, Akira S, Nakano N, Ohno S, Miyazaki J, Yamamura K, Hirano T, Kishimoto T. IgG1 plasmacytosis in interleukin 6 transgenic mice. Proc Natl Acad Sci USA. 1989;86:7547–7551. doi: 10.1073/pnas.86.19.7547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symons JA, Alcami A, Smith GL. Vaccinia virus encodes a soluble type I interferon receptor of novel structure and broad species specificity. Cell. 1995;81:551–560. doi: 10.1016/0092-8674(95)90076-4. [DOI] [PubMed] [Google Scholar]
- Takeda K, Kaisho T, Yoshida N, Takeda J, Kishimoto T, Akira S. Stat3 activation is responsible for IL-6-dependent T cell proliferation through preventing apoptosis: generation and characterization of T cell-specific Stat3-deficient mice. J Immunol. 1998;161:4652–4660. [PubMed] [Google Scholar]
- Wallace GD, Buller RM, Morse HC. Genetic determinants of resistance to ectromelia (mousepox) virus-induced mortality. J Virol. 1985;55:890–891. doi: 10.1128/jvi.55.3.890-891.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, Johnson AJ, Liggitt D, Bevan MJ. Cellular and humoral immunity against vaccinia virus infection of mice. J Immunol. 2004;172:6265–6271. doi: 10.4049/jimmunol.172.10.6265. [DOI] [PubMed] [Google Scholar]
- Zhu J, Martinez J, Huang X, Yang Y. Innate immunity against vaccinia virus is mediated by TLR2 and requires TLR-independent production of IFN-beta. Blood. 2007;109:619–625. doi: 10.1182/blood-2006-06-027136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. IFN-γ and IL-10 play minor roles in early pSTAT1 and pSTAT3 activation.
(A) Wild-type and IL-10−/− mice of the BALB/c background do not differ in the early pSTAT1 and pSTAT3 response to LPS. Wild-type and IL-10−/− mice were injected with 20 μg LPS; after 1 hour spleens were extracted, and splenocytes were analyzed by flow cytometry.
(B) IFNγ is necessary for wild-type levels of pSTAT1 activation. Wild-type C57BL/6 and IFNγ −/− C57BL/6 mice were injected with 20ug LPS, after 1hr spleens were extracted and analyzed by flow cytometry.
Supplemental Figure 2. TLR2−/− mice experience late and reduced pSTAT3 activation after vaccinia infection.
(A) pSTAT3 pathway activation in TLR2−/− mice is delayed. Wild-type and TLR2−/− mice were intravenously injected with 1 × 107 pfu of vaccinia virus (Western Reserve strain) or with PBS (negative control). Spleens were harvested at 1 hour, 3 hours, and 7 hours following infection (data represented as mean ± SD).
Supplemental Figure 3. Vaccinia induced pSTAT1 responses are dependent on type I IFN and IFN- γ
(A) pSTAT1 signaling after vaccinia infection is impaired in IFNAR−/− mice and almost absent in IFN-γ −/− mice. Three hours following infection, mice were sacrificed and splenic single-cell suspensions were prepared for flow cytometry. pSTAT1 x pSTAT3 profiles for CD11c+ cells are shown.
Supplemental Figure 4. Vaccinia presents a far more potent TLR2 ligand than ectromelia; TLR2 does not confer resistance to lethal ectromelia infection.
(A) HEK-293 cells transfected with mouse TLR2 and an NFκ B-driven luciferase reporter and untransfected control cells with the NFκ B-driven luciferase reporter, alone, were exposed to UV inactivated vaccinia virus or ectromelia virus (5 viral particles per cell). NFkB-driven luciferase expression evaluated by bioluminescence signal (after addition of luciferase) was determined 24 hours later (data represented as mean ± SD).
(B) TLR2 is dispensable for resistance to ectromelia virus infection in C57BL/6 mice. Wild-type and TLR2−/− mice of the C57BL/6 background were intravenously infected with 1 × 105 pfu of ectromelia virus. Survival was monitored for 10 days following infection.
Supplemental Figure 5. PAM3CSK4 pretreatment does not reduce ectromelia burden in IL6−/− mice.
(A) PBS or 20 μg of PAM3CSK4 was intravenously injected into C57BL/6 IL6−/− mice one hour before systemic infection with 1 × 107 pfu of ectromelia. Spleens were excised 48 hours later and ectromelia burden was quantified using a plaque-forming assay.