Abstract
Information on the immune response against H5N1 within the lung is lacking. Here we describe the sustained antiviral immune responses, as indicated by the expression of MxA protein and IFN-α mRNA, in autopsy lung tissue from an H5N1-infected patient. H5N1 infection of primary bronchial/tracheal epithelial cells and lung microvascular endothelial cells induced IP-10, and also up-regulated the retinoic acid-inducible gene-I (RIG-I). Down-regulation of RIG-I gene expression decreased IP-10 response. Co-culturing of H5N1-infected pulmonary cells with TNF-α led to synergistically enhanced production of IP-10. In the absence of viral infection, TNF-α and IFN-α also synergistically enhanced IP-10 response. Methylprednisolone showed only a partial inhibitory effect on this chemokine response. Our findings strongly suggest that both the H5N1 virus and the locally produced antiviral cytokines; IFN-α and TNF-α may have an important role in inducing IP-10 hyperresponse, leading to inflammatory damage in infected lung.
Keywords: H5N1 autopsy, H5N1-infected human pulmonary cells, MxA, TNF-α, IFN-α, IP-10
Introduction
Infection with highly pathogenic avian influenza H5N1 virus, unlike most human influenza infection, causes severe disease with a case-fatality rate of about 60%. In vitro infection of human alveolar and bronchial epithelial cells with H5N1 viruses led to higher levels production of IFN-β, IL-6, RANTES, and especially IP-10 than in cells infected with human influenza H1N1 virus [1]. We recently demonstrated that human plasmacytoid dendritic cells (PDCs) produced high levels of IFN-α and TNF-α after exposure to H5N1 viruses [2]. Several studies have consistently described elevated blood levels of IP-10 and other cytokine/chemokine in H5N1 patients [3–5]. The increase in IP-10, MCP-1, MIG, and IL-8 plasma levels was significantly associated with fatality [3].
These findings provide an important link between serum cytokine/chemokine levels and clinical severity of H5N1 infection. However, they do not provide detailed information concerning immunopathology in the lung, the primary target organ of H5N1 infection. Due to a lack of histological specimens from infected patients, it has been difficult to systemically investigate the immune response against H5N1 in the lung, and to evaluate the contribution of this response to the pathogenesis of H5N1 infection. In an attempt to determine the pathological mechanism within infected lung tissue, we examined the antiviral immune response in autopsy lung tissue of a patient who died with H5N1 infection. We also investigated the possible mechanisms underlying the hyperproduction of IP-10 in H5N1-infected human lung.
Materials and methods
Virus
H5N1 virus (A/open-billed stork/Nahkonsawan/BBD0104F/04) was isolated from cloacal swabs of live Asian open-billed storks and propagated in Madin-Darby canine kidney cells [2].
Cell culture and viral infection
Human primary bronchial/tracheal epithelial cells and human microvascular endothelial cells (Cambrex) were cultured in BEBM and EBM-2 growth media, respectively. Cells of passage 3 – 4 (5 × 104 cells /well) were co-cultured with H5N1 virus at MOI 1 in the absence or presence of IFN-α and/or TNF-α. After 24 h of incubation, culture supernatants were collected and assessed for production of IP-10, IL-8 and IL-6. Influenza infection was confirmed by staining with FITC-conjugated anti- NP and M antibodies [2]. Peripheral blood mononuclear cells (PBMCs) from healthy donors were obtained by centrifugation using Histopaque (Sigma-Aldrich) and cultured (4 × 105 cells/well) in RPMI 1640 supplemented with nonessential amino acids, 2 mM L-glutamine, 1 mM sodium pyruvate, 100 μg/ml penicillin, and 100 μg /ml streptomycin (all from Invitrogen Life Technologie) containing 10% FCS.
In some experiments, primary human pulmonary cells were infected with H5N1 (MOI 1) in the presence TNF-α (6 ng/ml) and methylprednisolone (100 μg/ml) or atorvastatin (0.25 – 2 μM). IP-10 response was measured at 24 h after infection. Preliminary experiments were conducted to determine non-toxic concentrations of methylprednisolone and atorvastatin.
Recombinant human IFN-α β2 and recombinant human TNF-α were from PBL Biomedical Laboratories and R&D Systems, respectively. Methylprednisolone and atorvastatin were obtained from Pfizer. E. coli LPS was purchased from InvivoGen.
Human tissue samples
Autopsy lung specimens from a H5N1 confirmed case and from a noninfectious patient were obtained from the archives of the Siriraj Hospital, Mahidol University. This investigation was approved by the Siriraj Ethics Committee, Mahidol University. The H5N1-infected patient was a 6-year-old boy who had progressive viral pneumonia leading to acute respiratory distress syndrome. He died on day 17 after onset of illness [6]. Autopsy lung tissue from one patient with no known respiratory infection was used as a negative control.
Real-time PCR
RNA was extracted from lung tissues as previously described [6]. cDNA was synthesized with AMV-RT (Promega, USA) using oligo-dT primer and then amplified by real-time PCR (Rotor Gene 3000, Corbett Research) with SYBR green I detection system. The sequences of IFN-α and IP-10 primers were as follows. IFN-α forward,5'-AGA ATC ACT CTC TAT CTG AAA GAG AAG AAA TA-3': IFN-α reverse, 5'-TCA TGA TTT CTG CTC TGA CAA CCT-3'; IP-10 forward, 5'-TCG AAG GCC ATC AAG AAT TT-3'; IP-10 reverse, 5'-GCT CCC CTC TGG TTT TAA GG-3'. Primers specific for the housekeeping genes; β-actin and GAPDH were as follows, β-actin forward, 5'-CCA CAC TGT GCC CAT CG-3'; β-actin reverse, 5'-AGG ATC TTC ATG AGG TAG TCA GTC AG-3'; GAPDH forward, 5'-GAT CAT CAG CAA TGC CTC CT-3'; GAPDH reverse, 5'-TGT GGT CAT GAG TCC TTC CA-3'. To assess RIG-I expression in human primary pulmonary cells, RNA of cells after 4 h of H5N1 infection was extracted with QIAGEN RNA easy kit (QIAGEN). cDNA was synthesized and amplified by real-time PCR with RIG-I forward primer, 5'-CTC TGC AGA AAG TGA AAG C-3' and reverse primer, 5'-GGC TTG GGA TGT GGT CTA CT-3'. Copy number of each gene of interest and housekeeping gene in each sample were calculated by Rotor gene software using standard curve of known copy plasmid containing its own specific gene sequence. The relative expression of each gene of interest/housekeeping gene of the same sample was presented. The fold difference in gene expression was compared to the control sample.
siRNA, transfection, and infection
Stealth siControl (UAA GUG GUU GAC UUG AAC CUA AUG G) and Stealth siRIG-I (UAA GGU UGU UCA CAA GAA UCU GUG G) were purchased from Invitrogen. Transfection with siRNA was performed using lipofectamine 2000. Briefly, semi-confluent cells were seeded with growth media without antibiotics one day before transfection. Cells were transfected with siRNA in the Opti-MEM media for 4 h, then replaced with growth media, and cultured overnight. Depletion of RIG-I expression by siRNA RIG-I was assessed by real-time PCR. Transfected cells which had their RIG-I expression been silence were co-cultured with H5N1 virus (MOI 1), culture supernatants were collected at 24 h post infection and then measured for IP-10 production.
Immunohistochemistry
Tissue sections were prepared from archived formalin-fixed, paraffin-embedded lung tissues and were stained by immunohistochemistry. Primary antibody specific to MxA (Dr. Haller, University of Freiburg) was used. Detection of primary antibody was conducted by using Polymer/HRP and DAB+ chromagen (EnVisionTM G/2 Doublestain System, Rabbit/Mouse, Dako).
Measurement of cytokines
Production of cytokines was measured by ELISA (R&D Systems).
Statistical analysis
Statistical comparisons among different treatment conditions with respect to production of IP-10 and TNF-α were conducted using SPSS 12.0 for Windows (SPSS Inc., Chicago, IL). The parametric Student's t test was used for normally distributed data, and the non-parametric Mann-Whitney rank-sum test was used for non-normally distributed data. A P value of < 0.05 was considered statistically significant.
Results
Expression of MxA, IFN-α, and IP-10 in H5N1-infected human lung
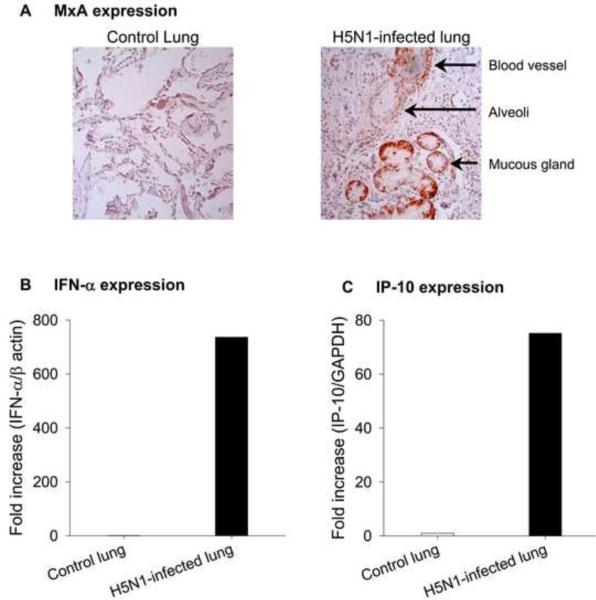
The type I interferon-induced Mx proteins play a critical role in protection against influenza A viral infection [7]. However, their expression in H5N1-infected human lung has not been investigated. We examined the expression of human MxA in lung tissue from a six-year-old boy who died on day 17 after onset of H5N1 illness. The histopathological findings, sites of viral replication, and clinical data from this patient have been previously reported [6]. Immunohistochemistry data in Fig. 1A revealed the expression of cytoplasmic MxA protein in several different cell types in the patient's lung. Expression was especially strong in mucous gland epithelium, and also occurred in vascular endothelial and smooth muscle cells as well as alveolar epithelial cells. MxA expression was negligible in control autopsy lung tissue. The observed MxA expression in the H5N1-infected patient led us to hypothesize that this expression may reflect local induction of type I IFN. Consistent with this, we found increased expression of IFN-α mRNA (> 700-fold increase) in H5N1-infected lung as compared to control lung (Fig. 1B). In addition to IFN-α, we detected increased expression of interferon-inducible protein IP-10 mRNA in H5N1-infected lung (>70-fold increase) as compared to control lung (Fig. 1C).
Fig. 1.
Expression of MxA (Panel A), IFN-α (Panel B), and IP-10 (Panel C) in autopsy lung tissue from human subjects with and without influenza H5N1 infection.
H5N1 infection stimulated IP-10 response which was mediated by RIG-I
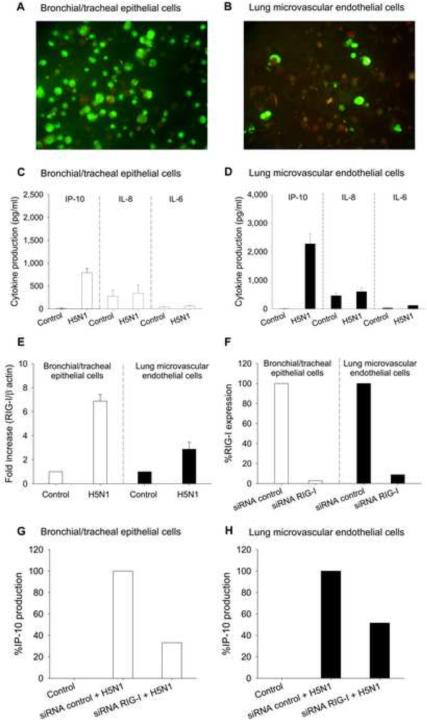
The finding of enhanced expression of IP-10 in H5N1-infected lung prompted us to evaluate in vitro IP-10 response in H5N1-infected primary human bronchial/tracheal epithelial and lung microvascular endothelial cells. Co-culture of these cells with H5N1 (MOI 1) resulted in infection, demonstrated by immunofluorescence staining of intracellular viral proteins (Figs. 2A and B). Infectious viruses were also detected at 24 h postinfection in culture supernatants of both cell types (data not shown). Analysis of supernatants at 24 h after infection revealed production of IP-10, but only negligible IL-8 and IL-6 production (Figs. 2C and D).
Fig. 2.
In vitro infectivity by influenza H5N1 virus and induction of IP-10, IL-8, and IL-6 in bronchial/tracheal epithelial cells (Panels A and C) and lung microvascular endothelial cells (Panels B and D). H5N1 up-regulated RIG-I expression in bronchial/tracheal epithelial cells and lung microvascular endothelial cells (Panel E). RIG-I expression (%) in epithelial and endothelial cells transfected with control siRNA or siRNA RIG-I after overnight culture (Panel F). IP-10 expression (%) in H5N1 infected epithelial cells (Panel G) and endothelial cells (Panel H), transfected with control siRNA or siRNA RIG-I. Data in Panels F, G and H are representative results from one of two experiments. Data in Panels C, D and E are shown as mean values ± SEM of 4 independent experiments using cells derived from two individual donors.
RIG-I is critical for influenza A viral sensing and is involved in IP-10 response [8]. To better understand the role of RIG-I in H5N1- infected pulmonary cells, we analyzed the effect of H5N1 infection on this viral recognition receptor. Both cell types constitutively expressed low levels of RIG-I. However, infection by H5N1 for 4 h increased RIG-I mRNA expression in bronchial/tracheal epithelial cells 7-fold, and in lung microvascular endothelial cells 3-fold, as compared to non-infected cells (Fig. 2E).
To explore the role of RIG-I in H5N1-mediated IP-10 production, we transfected cells with RIG-I-targeted siRNA or control siRNA, and then infected them with H5N1 virus. Compared to transfection with control siRNA, RIG-I-targeted siRNA with 90% gene knockdown (Fig. 2F) induced reduction in IP-10 expression of 67% and 49% in H5N1-infected bronchial/tracheal epithelium and lung microvascular endothelium, respectively (Figs. 2G and H). Control lung cells and control siRNA-treated lung cells produced similar levels of IP-10 upon H5N1 infection (data not shown).
The influence of TNF-α and /or IFN-α on IP-10 response of H5N1-infected human pulmonary cells
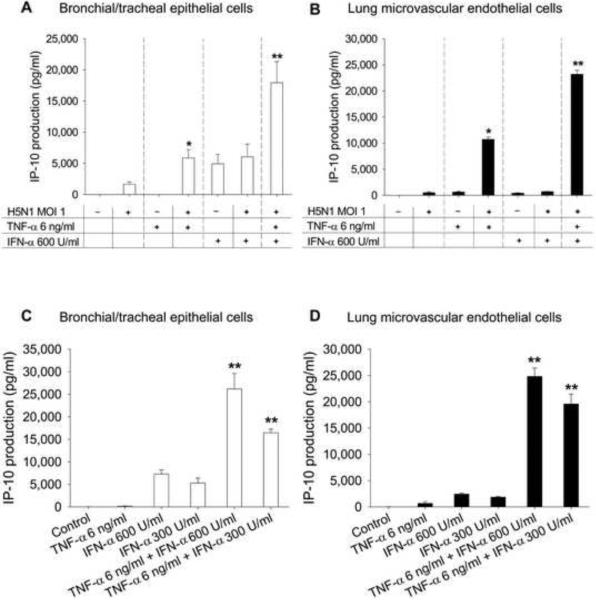
Expression of TNF-α in the lung of this patient has been reported previously [6]. To mimic the microenvironment found in the lungs of infected patients, primary human pulmonary cells were co-cultured with H5N1 and cytokines expressed in H5N1-infected lung, i.e. IFN-α and TNF-α. Both cytokines are known to have anti-H5N1 activity [2]. After 24 h, culture supernatants were measured for IP-10 production. TNF-α (6 ng/ml) alone poorly induced IP-10 response (Figs. 3A and B). Combining TNF-α and H5N1 infection synergistically enhanced IP-10 production in both bronchial/tracheal epithelial (P < 0.05) and lung microvascular endothelial cells (P < 0.05) (Figs. 3A and B). That is, the effect of combined TNF-α treatment and H5N1 infection was statistically significantly greater than the sum of the individual effects. Live H5N1 virus was required to produce enhanced IP-10 production, as heat-killed virus did not increase production (data not shown). IFN-α (600 U/ml) alone induced a modest IP-10 response (Figs. 3A and B) in bronchial/tracheal epithelial cells, but not in lung microvascular endothelium. Treatment with IFN-α and H5N1 failed to enhance IP-10 response in both cell types (Figs. 3A and B).
Fig. 3.
IP-10 induction with treatment by H5N1, TNF-α, IFN-α, or different combinations in bronchial/tracheal epithelial cells (Panel A) and lung microvascular endothelial cells (Panel B). Panels C and D show IP-10 production with treatment of TNF-α and IFN-α in the absence of viral infection. Data are shown as mean values ± SEM of 4 independent experiments using cells derived from two individual donors. P < 0.05 compared with the sum of the individual effects. **P < 0.01 compared with the sum of the individual effects.
A further increase in IP-10 response was observed in both types of pulmonary cells when adding IFN-α to the TNF-α and H5N1 treated cells (P < 0.01) (Figs. 3A and B). The increased response could result from the cooperative effect between IFN-α and TNF-α. To test this hypothesis, we co-cultured pulmonary cells in the presence of IFN-α and/or TNF-α in the absence of H5N1 infection. The combination of TNF-α (6 ng/ml) and IFN-α (600, 300 U/ml) synergistically induced high levels of IP-10 from both cell types (P < 0.01) (Figs. 3C and D).
Methylprednisolone was only partially effective in suppression of IP-10 response
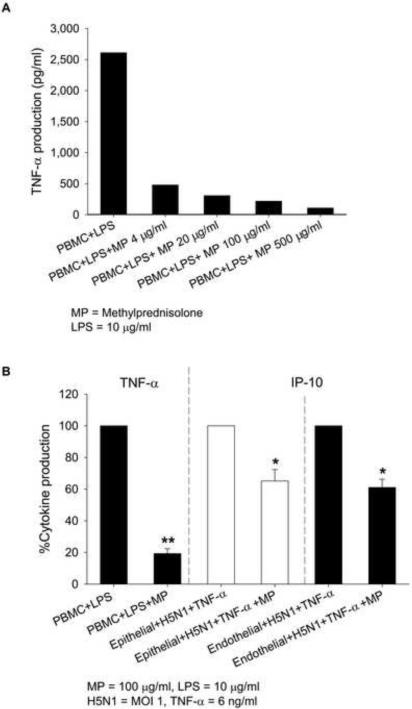
In addition to neuraminidase inhibitors, treatment of H5N1 infection often includes corticosteroids to suppress hypercytokinemia. Non-toxic concentrations of glucocorticoid methylprednisolone (4 – 500 μg/ml) markedly inhibited LPS-induced TNF-α production by PBMC (Fig. 4A). However, in pulmonary cell system, even a high dose of methylprednisolone (100 μg/ml) only partially reduced the synergistic production of IP-10 by TNF-α and H5N1 infection (35% in epithelial and 38% in endothelial cells) (Fig. 4B). Similarly, methylprednisolone only minimally inhibited the IP-10 response induced by the combination of IFN-α and TNF-α (Supplementary Fig.1).
Fig. 4.
TNF-α expression in LPS-treated PBMC in the presence of different concentrations of methylprednisolone (MP) (Panel A). Cytokine expression in stimulated PBMC (TNF-α), stimulated bronchial/tracheal epithelial cells (IP-10) and stimulated lung microvascular endothelial cells (IP-10) with and without MP treatment (Panel B). Data in panel A are representative results from one of two experiments. Cytokine expression in treated cells (panel B) is shown as mean percentage of TNF-α and IP-10 expression ± SEM of 4 independent experiments using cells derived from two individual donors. *P < 0.05 compared with the same cell type without MP treatment. **P < 0.01 compared with the same cell type without MP treatment.
Discussion
We observed increased expression of innate antiviral immune responses in the lung of a fatal H5N1 case, as measured by the expression of IFN-α and MxA protein. Recent observations in mouse model indicate that Mx protein played a critical role in protection against the pandemic 1918 H1N1 and H5N1 viruses [9]. The observed innate antiviral immunity in the H5N1-infected lung in this study seems to be sustained, since IFN-α and MxA could still be detected at day 17 after the onset of illness. The source of this IFN-α is not clear. Preliminary data from our group suggest that lung macrophages are not major IFN-α producer cells, since H5N1-induced IFN-α production was detected in macrophage-depleted lung immune cell cultures (data not shown). Viral infection of the lung often results in early recruitment of plasmacytoid dendritic cells (PDCs) [10]. We hypothesize that PDCs recruited to the H5N1- infected lung are responsible for the observed IFN-α response. This hypothesis is based on our previous findings which showed that PDCs produced very high levels of IFN-α in response to H5N1 viruses [2]. Production of H5N1-induced TNF-α was consistently detected in macrophages [11, 12], suggesting that they are the major source of TNF-α. Virus- sensing receptors, including RIG-I, TLR3, TLR7 and NLRP3 inflammasome are important in immune responses against influenza infection [8, 13]. Recognition of influenza virus by TLR7 is essential for IFN-α response by PDCs [14]. However, influenza virus-sensing receptor that is responsible for TNF-α production from macrophages remains unclear.
It is difficult to obtain autopsy samples from H5N1 patients. We measured expression of IFN-α and MxA in lung tissue from only one case of fatal H5N1 disease. Further research is required to confirm our findings. Nonetheless, our results agree with recent studies in ferret and non-human primate models, which demonstrated that H5N1 infection caused early increase, and sustained type I IFN responses in the infected lung [15, 16].
Our observation of IP-10 expression in an H5N1-infected human lung, but not in a control lung, is consistent with a recent study which demonstrated the presence of IP-10 in H5N1-infected human lung [12]. In our study, H5N1 virus was able to infect primary human lung microvascular endothelial cells. The data support recent observations which demonstrated that H5N1 virus could infect human lung endothelial both in vivo and in vitro [17, 18].
In the present study, we showed that H5N1 infection up-regulated RIG-I expression, and modestly up-regulated IP-10 (but not IL-8 and IL-6) in bronchial/tracheal epithelial cells and lung microvascular endothelial cells. Depletion of RIG-I expression led to inhibition of IP-10 response. Because this inhibition was not complete, other pathways are likely to be involved in addition to RIG-I.
The mechanisms underlying the enhanced IP-10 response in H5N1- infected lung are not clear. Treatment with TNF-α greatly enhanced IP-10 production in H5N1-infected bronchial/tracheal epithelial cells and lung microvascular endothelial cells. In contrast, treatment with IFN-α failed to increase IP-10 production in both infected cell types. It has been previously reported that TNF-α or IFN-α primed lung epithelial cell line A549 enhanced H3N2 virus-induced IP10 expression [19]. H5N1 infection and replication are required to produce enhanced IP-10 response since heat-killed virus showed no effect (data not shown). The quantity of IFN-α (600 U/ml) used in our study was higher than the previous report in epithelial cell line A549 [19] and could inhibit H5N1 replication in human pulmonary cells. This may be the reason why we did not detect an increased IP-10 response when IFN-α was added to H5N1-infected cells.
Combined treatment of cells with IFN-α and TNF-α without viral infection exerted a synergistic effect on IP-10 production, suggesting that uninfected bystander cells could also contribute to hyperchemokinemia in the lung. Our results suggest a complex interplay of H5N1 infection in the lung and locally released TNF-α and IFN-α in the excessive production of IP-10. At least three previous observations have indicated a possible link between IP-10 hyperresponse and severity of H5N1 disease; 1) strong and sustained IP-10 expression in the lung was detected during lethal H5N1 infection in ferrets, compared to H3N2 infection [16]; 2) H5N1-infected nonhuman primates showed sustained increase in IP-10 response in the infected lung [15]; 3) human data consistently show high blood levels of IP-10 and high plasma levels of IP-10 are strongly associated with fatal outcome in human H5N1 infection [3–5].
IP-10 is well known to chemoattract activated T cells and NK cells by signaling via the chemonkine receptor CXCR3 [20]. Other immune cells, including PDCs, mast cells, infiltrated lung macrophages, and infiltrated lung neutrophils also express CXCR3 [21–24], suggesting that IP-10 may involve in trafficking of these cells to the inflamed lung. The drug AMG487, an antagonist of CXCR3 was recently reported to reduce lung inflammation and increase survival time in H5N1-infected ferrets [16]. Better understanding of the role of IP-10/CXCR3 signaling in pathogenesis of H5N1 disease could well point the way to new and effective therapies.
Glucocorticoids are used in many inflammatory diseases. Our results indicate that methylprednisolone inefficiently blocked TNF-α-mediated IP-10 production by H5N1-infected lung cells. Our findings that methylprednisolone weakly inhibited IP-10 response could partially explain its ineffectiveness in the treatment of H5N1 infection [25]. However, this data should not be over interpreted and more investigations are required.
We hypothesize that initial H5N1 infection in the lung rapidly activates antiviral innate immune responses (IFN-α, MxA, and TNF-α), and IP-10 production. However, the broad tissue tropism of H5N1, combined with its high replication rate, may overwhelm this response. Subsequently, uncontrolled viral infection coupled with locally secreted TNF-α, and IFN-α trigger a massive IP-10 response in infected lungs, promoting inflammatory cells infiltration, resulting in extensive tissue damage. Also, the limited effectiveness of methylpredinisone to inhibit in vitro IP-10 response highlights the need for a new and effective anti-inflammatory agent that could be used in conjunction with anti-viral drugs to treat H5N1 infection.
Supplementary Material
Acknowledgments
This work was supported by Grant Y1-AI-5026-01 from the National Institutes of Health, National Institute of Allergy and Infectious Diseases. The views of the authors do not purport to reflect official policy of the U. S. Department of the Army or the Department of Defense.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Chan MC, Cheung CY, Chui WH, Tsao SW, Nicholls JM, Chan YO, Chan RW, Long HT, Poon LL, Guan Y, Peiris JS. Proinflammatory cytokine responses induced by influenza A (H5N1) viruses in primary human alveolar and bronchial epithelial cells. Respir Res. 2005;6:135. doi: 10.1186/1465-9921-6-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Thitithanyanont A, Engering A, Ekchariyawat P, Wiboon-ut S, Limsalakpetch A, Yongvanitchit K, Kum-Arb U, Kanchongkittiphon W, Utaisincharoen P, Sirisinha S, Puthavathana P, Fukuda MM, Pichyangkul S. High susceptibility of human dendritic cells to avian influenza H5N1 virus infection and protection by IFN-alpha and TLR ligands. J Immunol. 2007;179:5220–5227. doi: 10.4049/jimmunol.179.8.5220. [DOI] [PubMed] [Google Scholar]
- [3].de Jong MD, Simmons CP, Thanh TT, Hien VM, Smith GJ, Chau TN, Hoang DM, Chau NV, Khanh TH, Dong VC, Qui PT, Cam BV, Ha do Q, Guan Y, Peiris JS, Chinh NT, Hien TT, Farrar J. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med. 2006;12:1203–1207. doi: 10.1038/nm1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Peiris JS, Yu WC, Leung CW, Cheung CY, Ng WF, Nicholls JM, Ng TK, Chan KH, Lai ST, Lim WL, Yuen KY, Guan Y. Re-emergence of fatal human influenza A subtype H5N1 disease. Lancet. 2004;363:617–619. doi: 10.1016/S0140-6736(04)15595-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].To KF, Chan PK, Chan KF, Lee WK, Lam WY, Wong KF, Tang NL, Tsang DN, Sung RY, Buckley TA, Tam JS, Cheng AF. Pathology of fatal human infection associated with avian influenza A H5N1 virus. J Med Virol. 2001;63:242–246. doi: 10.1002/1096-9071(200103)63:3<242::aid-jmv1007>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- [6].Uiprasertkul M, Puthavathana P, Sangsiriwut K, Pooruk P, Srisook K, Peiris M, Nicholls JM, Chokephaibulkit K, Vanprapar N, Auewarakul P. Influenza A H5N1 replication sites in humans. Emerg Infect Dis. 2005;11:1036–1041. doi: 10.3201/eid1107.041313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Nagata K, Mibayashi M. The Mx protein that confers the resistance to influenza virus. Nippon Rinsho. 1997;55:2654–2659. [PubMed] [Google Scholar]
- [8].Le Goffic R, Pothlichet J, Vitour D, Fujita T, Meurs E, Chignard M, Si-Tahar M. Cutting Edge: Influenza A virus activates TLR3-dependent inflammatory and RIG-I-dependent antiviral responses in human lung epithelial cells. J Immunol. 2007;178:3368–3372. doi: 10.4049/jimmunol.178.6.3368. [DOI] [PubMed] [Google Scholar]
- [9].Tumpey TM, Szretter KJ, Van Hoeven N, Katz JM, Kochs G, Haller O, Garcia-Sastre A, Staeheli P. The Mx1 gene protects mice against the pandemic 1918 and highly lethal human H5N1 influenza viruses. J Virol. 2007;81:10818–10821. doi: 10.1128/JVI.01116-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Grayson MH, Ramos MS, Rohlfing MM, Kitchens R, Wang HD, Gould A, Agapov E, Holtzman MJ. Controls for lung dendritic cell maturation and migration during respiratory viral infection. J Immunol. 2007;179:1438–1448. doi: 10.4049/jimmunol.179.3.1438. [DOI] [PubMed] [Google Scholar]
- [11].Cheung CY, Poon LL, Lau AS, Luk W, Lau YL, Shortridge KF, Gordon S, Guan Y, Peiris JS. Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease? Lancet. 2002;360:1831–1837. doi: 10.1016/s0140-6736(02)11772-7. [DOI] [PubMed] [Google Scholar]
- [12].Deng R, Lu M, Korteweg C, Gao Z, McNutt MA, Ye J, Zhang T, Gu J. Distinctly different expression of cytokines and chemokines in the lungs of two H5N1 avian influenza patients. J Pathol. 2008;216:328–336. doi: 10.1002/path.2417. [DOI] [PubMed] [Google Scholar]
- [13].Ichinohe T, Pang IK, Iwasaki A. Influenza virus activates inflammasomes via its intracellular M2 ion channel. Nat Immunol. 11:404–410. doi: 10.1038/ni.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lund JM, Alexopoulou L, Sato A, Karow M, Adams NC, Gale NW, Iwasaki A, Flavell RA. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc Natl Acad Sci U S A. 2004;101:5598–5603. doi: 10.1073/pnas.0400937101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Baskin CR, Bielefeldt-Ohmann H, Tumpey TM, Sabourin PJ, Long JP, Garcia-Sastre A, Tolnay AE, Albrecht R, Pyles JA, Olson PH, Aicher LD, Rosenzweig ER, Murali-Krishna K, Clark EA, Kotur MS, Fornek JL, Proll S, Palermo RE, Sabourin CL, Katze MG. Early and sustained innate immune response defines pathology and death in nonhuman primates infected by highly pathogenic influenza virus. Proc Natl Acad Sci U S A. 2009;106:3455–3460. doi: 10.1073/pnas.0813234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cameron CM, Cameron MJ, Bermejo-Martin JF, Ran L, Xu L, Turner PV, Ran R, Danesh A, Fang Y, Chan PK, Mytle N, Sullivan TJ, Collins TL, Johnson MG, Medina JC, Rowe T, Kelvin DJ. Gene expression analysis of host innate immune responses during Lethal H5N1 infection in ferrets. J Virol. 2008;82:11308–11317. doi: 10.1128/JVI.00691-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chan MC, Chan RW, Yu WC, Ho CC, Chui WH, Lo CK, Yuen KM, Guan YI, Nicholls JM, Peiris JS. Influenza H5N1 virus infection of polarized human alveolar epithelial cells and lung microvascular endothelial cells. Respir Res. 2009;10:102. doi: 10.1186/1465-9921-10-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Liem NT, Nakajima N, Phat le P, Sato Y, Thach HN, Hung PV, San LT, Katano H, Kumasaka T, Oka T, Kawachi S, Matsushita T, Sata T, Kudo K, Suzuki K. H5N1-infected cells in lung with diffuse alveolar damage in exudative phase from a fatal case in Vietnam. Jpn J Infect Dis. 2008;61:157–160. [PubMed] [Google Scholar]
- [19].Veckman V, Osterlund P, Fagerlund R, Melen K, Matikainen S, Julkunen I. TNF-alpha and IFN-alpha enhance influenza-A-virus-induced chemokine gene expression in human A549 lung epithelial cells. Virology. 2006;345:96–104. doi: 10.1016/j.virol.2005.09.043. [DOI] [PubMed] [Google Scholar]
- [20].Booth V, Keizer DW, Kamphuis MB, Clark-Lewis I, Sykes BD. The CXCR3 binding chemokine IP-10/CXCL10: structure and receptor interactions. Biochemistry. 2002;41:10418–10425. doi: 10.1021/bi026020q. [DOI] [PubMed] [Google Scholar]
- [21].Brightling CE, Kaur D, Berger P, Morgan AJ, Wardlaw AJ, Bradding P. Differential expression of CCR3 and CXCR3 by human lung and bone marrow-derived mast cells: implications for tissue mast cell migration. J Leukoc Biol. 2005;77:759–766. doi: 10.1189/jlb.0904511. [DOI] [PubMed] [Google Scholar]
- [22].Grumelli S, Corry DB, Song LZ, Song L, Green L, Huh J, Hacken J, Espada R, Bag R, Lewis DE, Kheradmand F. An immune basis for lung parenchymal destruction in chronic obstructive pulmonary disease and emphysema. PLoS Med. 2004;1:e8. doi: 10.1371/journal.pmed.0010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hartl D, Krauss-Etschmann S, Koller B, Hordijk PL, Kuijpers TW, Hoffmann F, Hector A, Eber E, Marcos V, Bittmann I, Eickelberg O, Griese M, Roos D. Infiltrated neutrophils acquire novel chemokine receptor expression and chemokine responsiveness in chronic inflammatory lung diseases. J Immunol. 2008;181:8053–8067. doi: 10.4049/jimmunol.181.11.8053. [DOI] [PubMed] [Google Scholar]
- [24].Penna G, Sozzani S, Adorini L. Cutting edge: selective usage of chemokine receptors by plasmacytoid dendritic cells. J Immunol. 2001;167:1862–1866. doi: 10.4049/jimmunol.167.4.1862. [DOI] [PubMed] [Google Scholar]
- [25].Kandun IN, Wibisono H, Sedyaningsih ER, Yusharmen, Hadisoedarsuno W, Purba W, Santoso H, Septiawati C, Tresnaningsih E, Heriyanto B, Yuwono D, Harun S, Soeroso S, Giriputra S, Blair PJ, Jeremijenko A, Kosasih H, Putnam SD, Samaan G, Silitonga M, Chan KH, Poon LL, Lim W, Klimov A, Lindstrom S, Guan Y, Donis R, Katz J, Cox N, Peiris M, Uyeki TM. Three Indonesian clusters of H5N1 virus infection in 2005. N Engl J Med. 2006;355:2186–2194. doi: 10.1056/NEJMoa060930. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.