Abstract
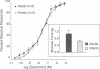
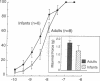
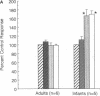
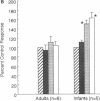
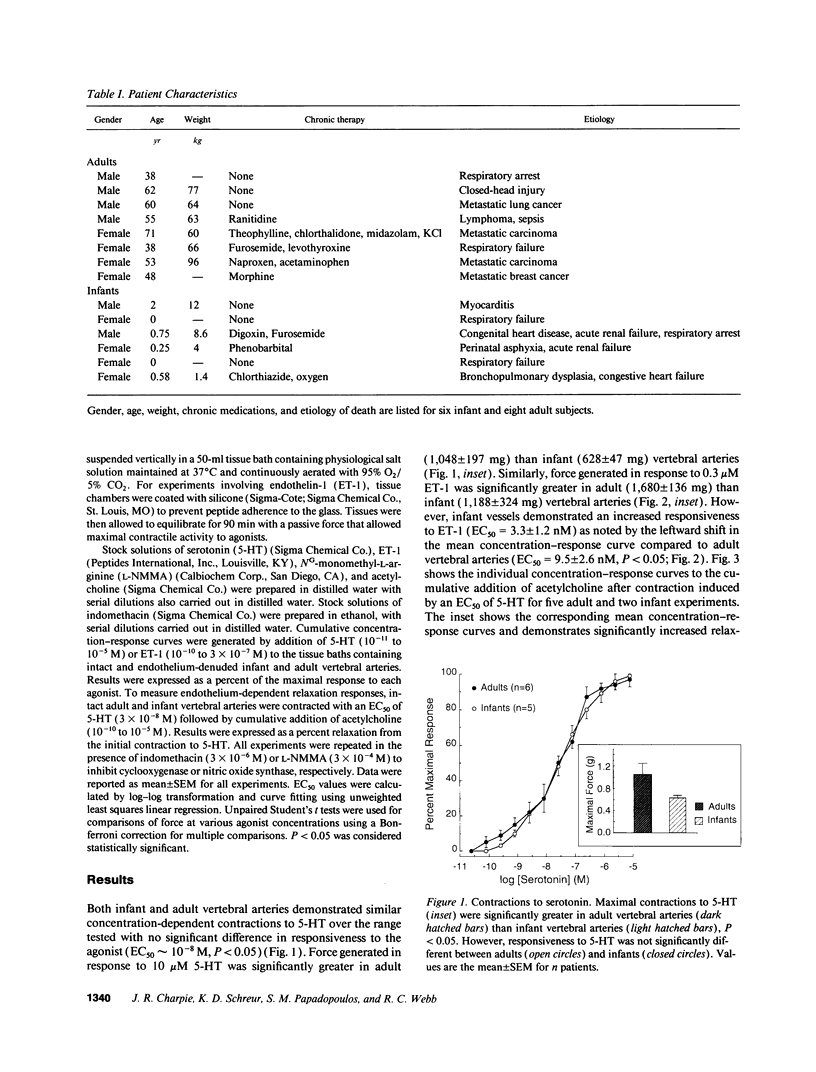
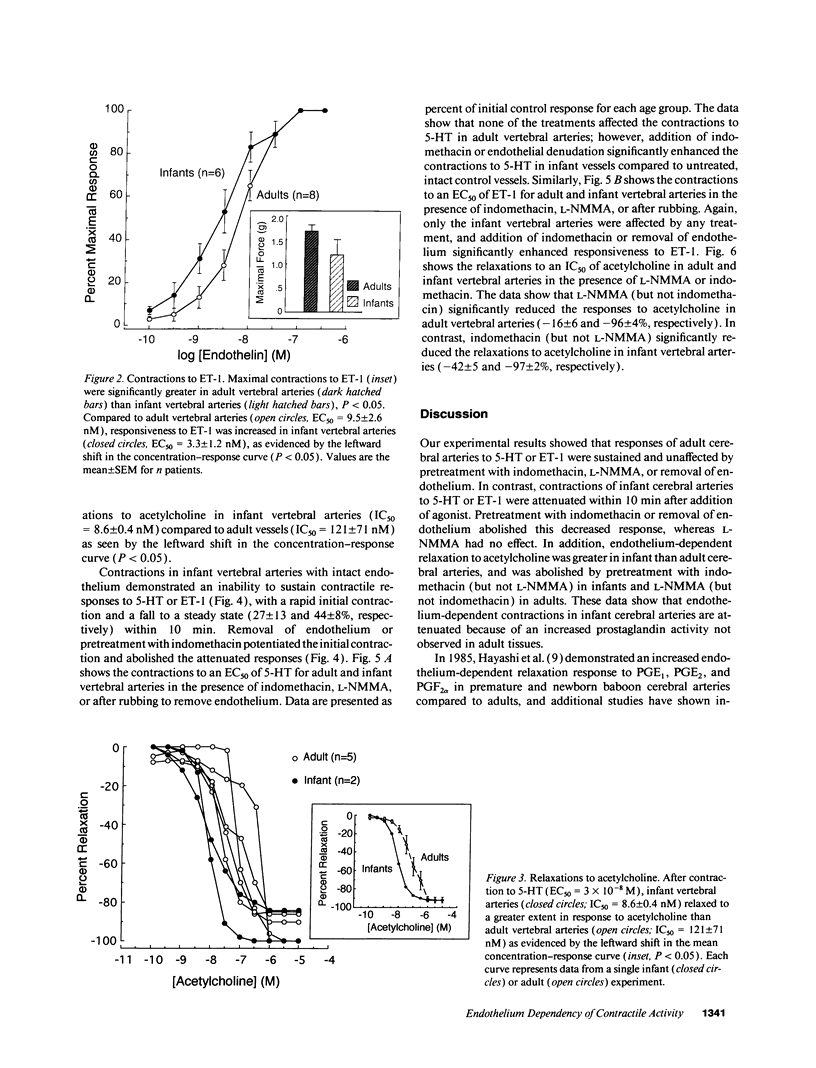
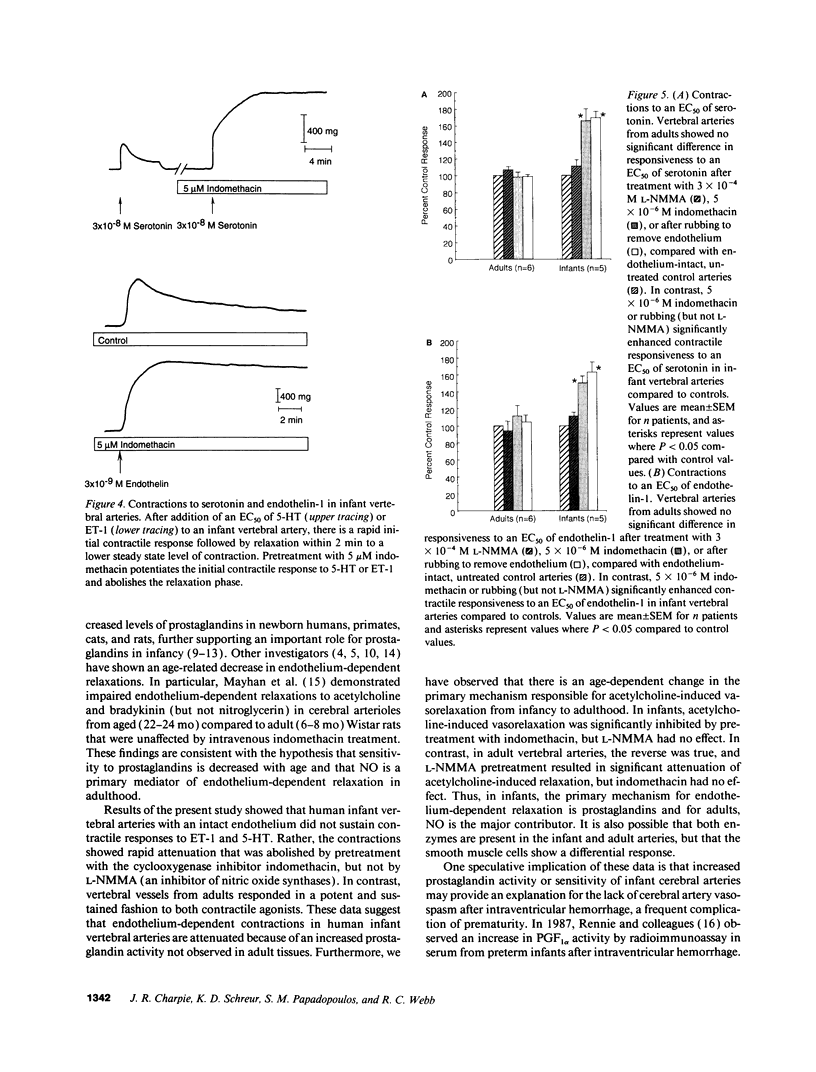
Contractions to serotonin (5-HT) and endothelin-1 (ET-1) in infant (0-2 yr) and adult (38-71 yr) vertebral arteries were examined in the presence of either the cyclooxygenase inhibitor indomethacin or NG-monomethyl-L-arginine (L-NMMA), an inhibitor of nitric oxide production. In addition, endothelium-dependent relaxations to acetylcholine were characterized in arteries contracted with agonist. The results showed that: (a) Contractions of infant arteries to 5-HT or ET-1 decreased to 44 +/- 8% and 27 +/- 13%, respectively, within 10 min. Indomethacin or removal of endothelium abolished this decreased response, whereas L-NMMA had no effect. (b) Adult arteries produced sustained contractions to 5-HT or ET-1 that were unaffected by indomethacin, endothelium denudation, or L-NMMA. (c) Endothelium-dependent relaxations to acetylcholine were greater in infant than adult arteries and were abolished by indomethacin (but not L-NMMA) in infants and L-NMMA (but not indomethacin) in adults. Thus, endothelium-dependent responses in infant arteries are attenuated because of increased prostaglandin activity not observed in adult tissues. Additionally, there is an age-dependent change in the primary mechanism responsible for acetylcholine-induced vasodilation. Apparently, endothelium dependency of acetylcholine-induced relaxation is highly dependent on cyclooxygenase activity in the infant vertebral artery, but in the adult artery, nitric oxide is linked to the vasodilator response.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brandt L., Ljunggren B., Andersson K. E., Hindfelt B., Uski T. Effects of indomethacin and prostacyclin on isolated human pial arteries contracted by CSF from patients with aneurysmal SAH. J Neurosurg. 1981 Dec;55(6):877–883. doi: 10.3171/jns.1981.55.6.0877. [DOI] [PubMed] [Google Scholar]
- Bär T. Morphometric evaluation of capillaries in different laminae of rat cerebral cortex by automatic image analysis: changes during development and aging. Adv Neurol. 1978;20:1–9. [PubMed] [Google Scholar]
- Clyman R. I. Developmental responses to oxygen, arachidonic acid, and indomethacin in the fetal lamb ductus arteriosus in vitro. Prostaglandins Med. 1978 Aug;1(2):167–174. doi: 10.1016/0161-4630(78)90044-7. [DOI] [PubMed] [Google Scholar]
- Clyman R. I., Mauray F., Rudolph A. M., Heymann M. A. Age-dependent sensitivity of the lamb ductus arteriosus to indomethacin and prostaglandins. J Pediatr. 1980 Jan;96(1):94–98. doi: 10.1016/s0022-3476(80)80338-6. [DOI] [PubMed] [Google Scholar]
- Hayashi S., Park M. K., Kuehl T. J. Effects of prostaglandins and arachidonic acid on baboon cerebral and mesenteric arteries. Prostaglandins. 1986 Oct;32(4):587–596. doi: 10.1016/0090-6980(86)90040-7. [DOI] [PubMed] [Google Scholar]
- Hayashi S., Park M. K., Kuehl T. J. Relaxant and contractile responses to prostaglandins in premature, newborn and adult baboon cerebral arteries. J Pharmacol Exp Ther. 1985 Jun;233(3):628–635. [PubMed] [Google Scholar]
- Heistad D. D., Mayhan W. G., Coyle P., Baumbach G. L. Impaired dilatation of cerebral arterioles in chronic hypertension. Blood Vessels. 1990;27(2-5):258–262. doi: 10.1159/000158817. [DOI] [PubMed] [Google Scholar]
- Hongo K., Nakagomi T., Kassell N. F., Sasaki T., Lehman M., Vollmer D. G., Tsukahara T., Ogawa H., Torner J. Effects of aging and hypertension on endothelium-dependent vascular relaxation in rat carotid artery. Stroke. 1988 Jul;19(7):892–897. doi: 10.1161/01.str.19.7.892. [DOI] [PubMed] [Google Scholar]
- Hynes M. R., Duckles S. P. Effect of increasing age on the endothelium-mediated relaxation of rat blood vessels in vitro. J Pharmacol Exp Ther. 1987 May;241(2):387–392. [PubMed] [Google Scholar]
- Kassell N. F., Sasaki T., Colohan A. R., Nazar G. Cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Stroke. 1985 Jul-Aug;16(4):562–572. doi: 10.1161/01.str.16.4.562. [DOI] [PubMed] [Google Scholar]
- Moise K. J., Jr, Mari G., Kirshon B., Huhta J. C., Walsh S. W., Cano L. The effect of indomethacin on the pulsatility index of the umbilical artery in human fetuses. Am J Obstet Gynecol. 1990 Jan;162(1):199–202. doi: 10.1016/0002-9378(90)90849-3. [DOI] [PubMed] [Google Scholar]
- Moritoki H., Hosoki E., Ishida Y. Age-related decrease in endothelium-dependent dilator response to histamine in rat mesenteric artery. Eur J Pharmacol. 1986 Jul 15;126(1-2):61–67. doi: 10.1016/0014-2999(86)90738-7. [DOI] [PubMed] [Google Scholar]
- Rennie J. M., Doyle J., Cooke R. W. Elevated levels of immunoreactive prostacyclin metabolite in babies who develop intraventricular haemorrhage. Acta Paediatr Scand. 1987 Jan;76(1):19–23. doi: 10.1111/j.1651-2227.1987.tb10408.x. [DOI] [PubMed] [Google Scholar]
- Shirasaki Y., Su C., Lee T. J., Kolm P., Cline W. H., Jr, Nickols G. A. Endothelial modulation of vascular relaxation to nitrovasodilators in aging and hypertension. J Pharmacol Exp Ther. 1986 Dec;239(3):861–866. [PubMed] [Google Scholar]
- Stewart P. A., Magliocco M., Hayakawa K., Farrell C. L., Del Maestro R. F., Girvin J., Kaufmann J. C., Vinters H. V., Gilbert J. A quantitative analysis of blood-brain barrier ultrastructure in the aging human. Microvasc Res. 1987 Mar;33(2):270–282. doi: 10.1016/0026-2862(87)90022-7. [DOI] [PubMed] [Google Scholar]
- Uski T., Andersson K. E., Brandt L., Edvinsson L., Ljunggren B. Responses of isolated feline and human cerebral arteries to prostacyclin and some of its metabolites. J Cereb Blood Flow Metab. 1983 Jun;3(2):238–245. doi: 10.1038/jcbfm.1983.32. [DOI] [PubMed] [Google Scholar]