Abstract
In search of novel control parameters for the polymerization of sickle cell hemoglobin (HbS), the primary pathogenic event of sickle cell anemia, we explore the role of free heme, which may be excessively released in sickle erythrocytes. We show that the concentration of free heme in HbS solutions typically used in the laboratory is 0.02–0.04 mole heme/mole HbS. We show that dialysis of small molecules out of HbS solutions arrests HbS polymerization. The addition of 100–260 μM of free heme to dialyzed HbS solutions leads to rates of nucleation and polymer fiber growth faster by two orders of magnitude than before dialysis. Toward an understanding of the mechanism of nucleation enhancement by heme, we show that free heme at a concentration of 66 μM increases by two orders of magnitude the volume of the metastable clusters of dense HbS liquid, the locations where HbS polymer nuclei form. These results suggest that spikes of the free heme concentration in the erythrocytes of sickle cell anemia patients may be a significant factor in the complexity of the clinical manifestations of sickle cell anemia. The prevention of free heme accumulation in the erythrocyte cytosol may be a novel avenue to sickle cell therapy.
Introduction
The polymerization of sickle cell hemoglobin (HbS) (1–3) is the primary event (4,5) in the pathophysiology of sickle cell anemia (6,7). The polymerization has been described via a double-nucleation mechanism, an elaboration of the nucleation-and-growth scenario typical of first-order phase transitions, according to which homogeneous nucleation of single fibers is followed by their growth and branching by heterogeneous nucleation of new fibers on top of existing ones (3,8,9). This mechanism explains many of the clinical features of the disease (2,10), yet discrepancies between the clinical facts and the predictions of the polymerization-based scenario of the pathophysiology abound (11,12). At the root of these discrepancies are features of the disease that cannot be explained by the solubility and activity of HbS, the only parameters in the double-nucleation mechanism (4). It has been found that elevated levels of erythrocytes containing higher HbS concentrations, the so-called dense cells, in which polymerization should be faster, do not correlate with painful events, the main clinical manifestation of the disease (13). Although the rate of polymerization in individual cells was found to scale with the concentration of HbS in them (14), several studies have shown that the clinical manifestations of sickle cell anemia are dramatically different in patients with identical concentrations of HbS in their red blood cells (15–18). It was suggested that these discrepancies point to the action of factors for the disease severity, which are unrelated to HbS polymerization: erythrocyte adhesivity, endothelial activation, platelet count, and others (12,19).
In this study, we test whether the presence of free heme in supersaturated solutions of HbS may affect the rate of HbS polymerization and in this way be another factor in the disease pathophysiology. Heme may be released in sickle erythrocytes after autoxidation of hemoglobin to methemoglobin (20), but the presence of free heme in sickle cell erythrocytes has seen controversy on several levels:
-
1.
It has been argued that the reductive enzymes present in the red cell cytosol would protect HbS from autoxidation and in this way prevent the release of heme. The current consensus appears to be that although normal red cells are indeed protected, sickle red cells may succumb to the stronger oxidative stress (21) due to chelated and membrane-bound Fe3+ ions, oxygen radicals, and others, found in them (22).
-
2.
The propensity of HbS to autoxidize more avidly than the normal adult hemoglobin, HbA, has been questioned. A definitive study (21) showed that HbS has an exaggerated intrinsic oxidation rate compared with HbA. Of greater importance, the same study found that the exogenous stimuli discussed in the previous paragraph, which act at abnormal levels in sickle red cells, further enhance HbS autoxidation.
-
3.
Since the heme molecule is amphiphilic, with well-defined charged and hydrophobic nonpolar parts, it has been argued that it attaches to the phospholipid bilayer of the red cell membrane or to the cytoskeleton, similar to many other detergents (23,24). In fact, most detergents are soluble in water as single molecules and their solubility is several millimoles/liter (25). It is not surprising that the heme solubility, determined below, is ∼2 mM. The heme solubility is the equilibrium concentration for the exchange between heme in solution and in a solid phase, which is likely to be crystalline and is called hemozoin or β-hematin. The crystals consist of layers of parallel porphyrin rings in hydrophobic contact with one another, cross-linked by iron-oxygen and hydrogen bonds (26,27). When embedded in the phospholipid bilayer, the heme only establishes hydrophobic contacts with the lipid molecules, lacking the stronger iron-oxygen and hydrogen bonds (28), and the free energy of this interaction should be lower in magnitude than that between heme molecules in the hemozoin crystal. Hence, the equilibrium concentration of heme in contact with a hydrophobic surface would be higher than the solubility value above. Since the phospholipid bilayer is a dynamic structure that constantly exchanges molecules with the surrounding solution, arguments that the heme may be permanently trapped by this layer so that the heme concentration is kept lower than the equilibrium value are unfounded.
It appears that the controversial issues have been resolved in favor of the presence of free heme in the red cell cytosol. Correspondingly, free heme has been directly detected in ghostless sickle hemolysate and its average concentration in the tested samples was four or five times higher than in normal red cells (29).
This free heme in the erythrocytes has been implicated in damage of the red cell membrane, leading to higher red cell adhesion to the endothelium (30). Existing evidence suggests that higher frequency and severity of sickle cell crises in patients with equal expression of HbS, such as monozygotic twins, may be related to enhanced release of heme, exhibited as membrane-bound iron (17).
The experiments discussed here illustrate another potential role of free heme in the pathophysiology of sickle cell anemia: as a crucial cofactor of sickle cell hemoglobin polymerization. We show that removal of free heme completely prevents polymerization and that its addition at micromolar concentrations enhances the polymerization of sickle cell hemoglobin by orders of magnitude. We propose a biophysical mechanism of action of heme that explains the high efficacy of heme at low concentrations.
Below, we test solutions with free heme completely removed and with free heme concentration in the range 100–260 μM; for discussion of the methods employed, see the Supporting Material. The chosen free heme concentrations are 10–20× lower than the molar concentrations of hemoglobin in the tested solutions. We show that these heme concentrations are representative of the free heme present during typical determinations of the rates of HbS polymerization. The physiological relevance of the chosen heme concentrations is more nuanced: although the results obtained in the absence of heme may be relevant to in vivo processes in some sickle cell patients, the concentrations of the added heme are significantly higher than the average free heme concentration in sickle red cells of <1 μM, according to the single determination in Liu et al. (29). However, although no determinations of the consistency and steadiness of heme concentration in individual red cells have been made, it is unlikely that the heme concentration is uniform, equal to this average, and constant in time throughout all red cells. Thus, our experiments may be viewed as models of the HbS polymerization response to spikes in intraerythrocytic heme concentration.
Results
Removal of the free heme from HbS solutions
Free heme may be present in the donated red blood cells used as a source of HbS. However, since the HbS purification procedures include ion exchange chromatography and dialysis (31,32), no free heme is expected in freshly purified HbS solutions. Indeed, the low amounts of free heme and met-HbS in solutions prepared from freshly lysed red cells were verified by the absorbance in the 600–650 nm range, where absorbance of these two species was significantly stronger than that of CO- or oxy-HbS (for details, see Galkin et al. (31)). Hence, any free heme present in the HbS solutions used in the studies of the polymerization kinetics must have been released during storage, freezing, thawing, and refreezing of the oxy-HbS stock, and during the preparation of the CO-HbS solutions used in the kinetics experiments.
To prepare a solution without this released heme, a solution sample was thawed, diluted with 0.15 M phosphate buffer, and dialyzed overnight through a membrane with a molecular mass (MM) cutoff of 2000 g mol−1. Thus, during dialysis, the HbS was in oxy-state. The free heme, which at near-neutral pH exists mostly as a dimer (33) for which MM = 1232 g mol−1, is expected to dialyze out. To slow any processes of HbS decay, such as autoxidation, release of heme, denaturation, etc., during dialysis, it was carried out at 3°C. Since our method of characterization of the polymerization kinetics uses CO-HbS, spectroscopic characterization of the dialyzed HbS solutions was carried out after conversion of HbS to this species and the addition of sodium dithionite (see Methods). This characterization showed that spectra of the dialyzed solutions were identical to those of the undialyzed solution, and to the expected spectra of CO-HbS (Fig. 1), except at λ > 600 nm, as discussed below. The similarity of the spectra indicates that in the undialyzed solutions, the amounts of both free heme and apoglobin produced upon heme release are small (for quantification, see below and Supporting Material).
Figure 1.
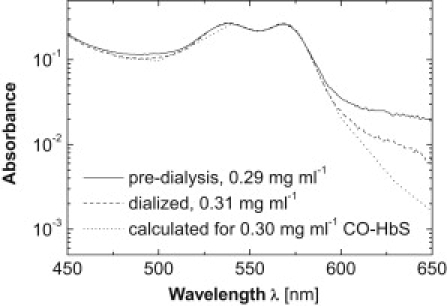
Spectra of HbS before and after dialysis through a membrane with a 2000 g mol−1 molecular mass cutoff are compared to a spectrum calculated for a similar HbS concentration using data from Zjilstra et al. (70). HbS was converted to a CO-state before spectrophotometry characterization, and 50 mM of sodium dithionite were added. Because of the low HbS concentration, absorbance at wavelengths of λ > 600 nm is low and affected by noise.
It is possible that dialysis could affect solution pH, chloride, and 2,3-diphosphoglycerate (DPG) binding to HbS, methemoglobin production, changes in the iron ligands, and other factors. Changes of pH as a result of dialysis are unlikely: the buffer against which dialysis is performed is identical to the buffer in which HbS is dissolved. Since during its purification by anion exchange chromatography and dialysis HbS is in oxy-state, to which DPG binds weakly (34,35), this allosteric effector of oxygen release is likely not present in the solution at all. Since the chromatography and dialysis media used during purification contain no chloride ions, it is likely that the purified HbS would be stripped of any Cl– that might be bound to it in the red cells. The lack of met-HbS production is evident from the similarity of the solution spectra after different dialysis times, from 12 to 24 h: production of met-hemoglobin would have yielded increased absorbance in the wavelength range 600–650 nm (31). The suppression of met-HbS production is likely due to the low temperature maintained during dialysis. Since the solution contains only phosphate and hydroxyl anions, which are in huge excess of the heme iron cations both before and after dialysis, the liganded state of the iron cation is likely preserved. Hence, we conclude that none of the potential modifications of HbS molecules listed above are likely during dialysis.
To test whether heme is indeed removed from the dialyzed solution, we added albumin to the dialysis buffer: heme binds to albumin, and the complex has a characteristic peak at 404 nm (36–38). Evidence presented in the Supporting Material demonstrates the removal of significant amounts of heme from the HbS solutions.
As another test of the dialysis procedure, we varied the length of dialysis from 12 to 24 h. Quantification of the free heme removed from the solution by dialysis (see Supporting Material for details) revealed that the concentration of free heme found was independent of the dialysis length within these limits. An important conclusion form this observation is that the heme removed from the solution by dialysis was not released during dialysis but was present in the HbS solution before dialysis.
Quantification of free heme concentration in polymerizing HbS solutions
The absorption of light by heme and its derivatives in aqueous solutions has not been extensively studied: the measurements that exist were carried out mostly in organic solvents or, if in aqueous solutions, used heme encapsulated in detergent micelles (39). The spectra of heme in solutions with compositions identical to that used in the quantification of HbS polymerization kinetics (see Supporting Material) are similar to those of met-Hb and indicate that the heme iron is in Fe3+ form and heme is in the form of hemin. Since the release of heme from the available met-HbS is partial, and certain amounts of met-HbS remain in the solution, direct spectroscopic determination of the heme concentration would be inaccurate. To detect free heme and quantify its concentration in the presence of met-HbS, we employed three methods: spectroscopy after dialysis, binding to albumin, and conversion of met-HbS to cyanmet-HbS. The details and a discussion of the merits and errors of these methods are provided in the Supporting Material.
Fig. 2 shows a summary of the determinations made using the dialysis and cyanide methods, in which the concentration of free heme is plotted as a function of the HbS concentration. The scatter in the free heme concentrations in Fig. 2 reflects an inherent error of the cyanide method of ∼50%; the two points determined after dialysis have lower errors. With this caveat, the results of the two methods are consistent. Fig. 2 indicates that the concentration of free heme increases with higher HbS concentration. The ratio of the free heme to HbS concentration is in the range 0.02–0.04 mol heme/mole HbS. If we extrapolate this ratio to the HbS concentrations used in the quantification of the polymerization kinetics discussed below, 200–290 mg ml−1 or 3–4.5 mM, we learn that the respective free heme concentrations would be in the range 120–180 μM.
Figure 2.
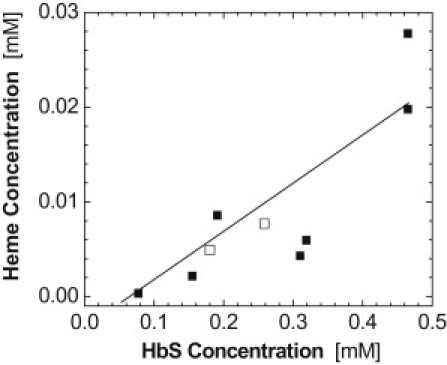
The concentration of free heme in solutions of HbS as a function of HbS concentration. Solid symbols represent determinations using the cyanide method (see text and Supporting Material for details); open symbols represent determinations using the dialysis method. The line is a guide for the eye.
The effects of heme on HbS polymerization: phenomenology
Fig. 3 shows a representative series of images that trace the evolution of HbS polymerization in slides of controlled thickness containing HbS solution (see Methods in the Supporting Material). The images were taken with differential interference contrast (DIC) microscopy, which allows detection of all HbS polymers nucleated in the illuminated area (31,32). At each solution composition and temperature, 80–100 series of images were collected: each series started with flash-photolysis of the CO-HbS to produce a supersaturated solution of deoxy-HbS and ended after 70–120 images were collected, which took up to 150 s. Although the exact number of polymers existing at a given time in a series is random, as expected for a stochastic process such as nucleation (32,40), we chose series that represent the evolution of the number of polymers averaged over ∼100 repetitions, as illustrated in Galkin and Vekilov (32).
Figure 3.
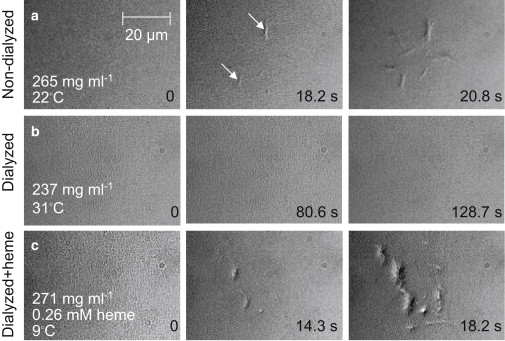
Effects of heme on the polymerization of sickle cell hemoglobin. Solution of CO-HbS is held in slides of uniform 5-μm thickness (32). Photolysis by illumination with a wavelength of 532 nm in the center of the viewfield produces deoxy-HbS, which polymerizes if its concentration is above the solubility point (31,32). Each row represents the evolution of polymerization under a set of conditions. Time after start of photolysis is indicated at each evolution step. HbS concentration and temperature are indicated for each row. White arrows point to some of the HbS polymer domains. (a) HbS polymerization in a solution freshly prepared from stock stored under liquid nitrogen for 1 week. (b) Removal of small molecules by dialysis with a molecular mass cutoff of 2000 g mol−1 prevents polymerization; increasing temperature to 31°C does not lead to polymerization even though, as discussed in the text, higher temperature enhances polymerization; HbS concentration is lower because of dilution during dialysis. (c) After heme at the concentration shown is added to another dialyzed solution sample, polymerization is extremely fast; the temperature was lowered to slow down polymer nucleation and growth so that nucleated polymers would be distinguishable.
In Fig. 3 a, the HbS solution is similar to that normally used in HbS polymerization studies, i.e., without extra dialysis after storage. We see that after starting with no polymers, two polymers have formed at 18.2 s after initiation of polymerization by photolysis, and approximately eight at 20.8 s. Although this polymerization evolution is somewhat slower (see Supporting Material), it is close to the typical behavior of HbS solutions at this temperature with this concentration (31).
A surprising finding was that in the solutions dialyzed after thawing of the stock, no polymers formed at 22°C. According to numerous experimental observations, higher temperatures lead to faster polymerization (8,9,41). To compensate for the solution dilution during dialysis, and to test whether polymers might nucleate at higher temperature in the absence of heme, the temperature was raised in three steps (Table 1) to 36.5°C. At each temperature, 20–50 series of images were collected. No polymerization occurred at any of the temperature steps during any of the repetitions; the lack of polymers at 31°C is illustrated in Fig. 3 b. Table 1 provides a summary of all combinations of HbS concentration and temperature at which polymers appeared within a few seconds, the so-called nucleation delay time, in normally prepared HbS solutions, but did not appear for extended periods in experiments repeated 20–50 times in freshly dialyzed solutions. The lack of polymerization under the conditions listed in the first row of Table 1—CHbS = 280 mg ml−1, T = 24.7°C, and no heme—compared to the moderately fast polymerization at similar CHbS and temperature in the presence of heme in Fig. 3 a demonstrates unambiguously that it is the absence of heme, and not lower concentration or different temperature, that leads to the arrest of polymerization.
Table 1.
Summary of tested combinations of HbS concentration and temperature of solutions for which there was no polymerization after dialysis
| HbS concentration (mg ml−1) | Temperature (°C) | Duration of exposure to supersaturation (s) | θ in nondialyzed solution (s) |
|---|---|---|---|
| 280 | 24.7 | 77.4 | 1.5 |
| 27.7 | 77.4 | 0.7 | |
| 237 | 22 | 77.4 | 8–38 |
| 30.9 | 77.4 | 6–33 | |
| 31.0 | 128.7 | 6–33 | |
| 36.5 | 129.9 | 5–28 | |
| 235 | 33 | 129.9 | 4–27 |
| 35 | 129.9 | 3–22 | |
| 36.5 | 129.9 | 2–18 |
Dialysis was performed through a membrane with 2000 g mol−1 molecular mass cutoff, and polymers did not appear after exposure to supersaturation for extended times. For comparison, the nucleation delay times, θ, from Galkin and Vekilov (31) are shown in the last column. Since aging of HbS solutions shortens θ, ranges of this variable are shown where data are available.
The addition of 260 μM of heme (this concentration is comparable to, though somewhat higher than, the concentration predicted based on the heme/HbS ratio from Fig. 2) to the dialyzed solution led to explosive polymerization at 22°C. Since lower temperature leads to slower nucleation and growth, the temperature was lowered to 9°C, where individual polymers could be distinguished. Because of the higher solubility at this low temperature (3) and the Arrhenius slowing of polymerization kinetics, one expects significantly lower rates of nucleation and growth than in Fig. 3 a. Yet the number of nucleated fibers and their dimensions after ∼20 s (Fig. 3 c) are about the same as in Fig. 3 a.
The observations in Fig. 3, a–c, show that free heme accelerates HbS polymerization; in the absence of heme, polymerization is prevented under the conditions tested here and is likely significantly delayed in a broader range of conditions.
Kinetics of nucleation and growth of HbS polymer fibers in the presence of heme
Quantifications of the kinetics of nucleation and growth are presented in Fig. 4. Since no HbS polymers nucleate after dialysis through a membrane with a cutoff of 2000 g mol−1, J = 0, θ = ∞, and R is undefined. The addition of micromolar concentrations of heme to dialyzed solutions yields nucleation and growth rates that are significantly faster and delay times that are shorter: For instance, in the presence of 260–280 μM heme, at CHbS = 271 and 267 mg ml−1, values of J ≈ 108 cm−3 s−1, θ ≈ 10–30 s, and R ≈ 0.2–0.6 μm s−1 are achieved at temperatures lower by 10–15°C than those required for similar rates at CHbS = 265 mg ml−1 without added heme. Since higher temperature leads to faster polymerization (8,9,41), this observation shows that heme significantly accelerates nucleation and growth of HbS polymers. The slight concentration difference in the experiments with and without specially added free heme is insufficient to explain this significant difference. To quantify the acceleration, we extrapolate the data in Fig. 4, a–c, to temperature ranges where the rates at different solution compositions can be compared (because of the high sensitivity to temperature and concentration of the kinetics of nucleation and growth, the ranges of J, θ, and R accessible by the methods used (31,32) limit direct comparison). The good correspondence of the J(T), θ(T), and R(T) dependencies to the exponential function (Fig. 4) (32,42,43) justifies such extrapolations. The comparison reveals that the addition of heme enhances J by more than two orders of magnitude and R by a factor of ∼10 and shortens θ by approximately two orders of magnitude. Note that the action of heme is different for J and θ; which is expected, since J and θ are independent characteristics of nucleation (42,44).
Figure 4.
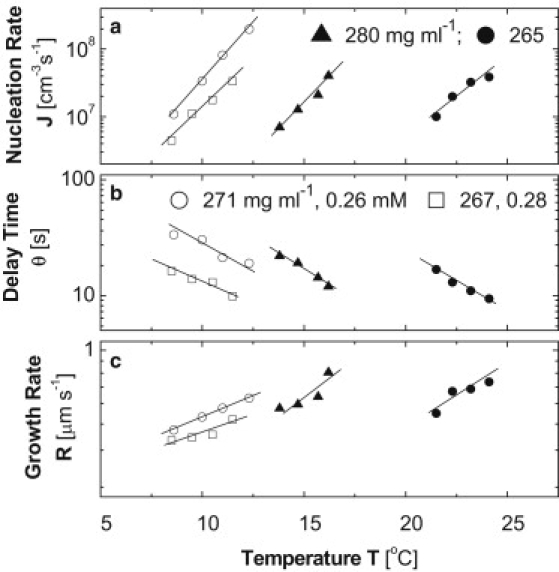
Effect of free heme on the nucleation rate, J (a), delay time, θ (b), and growth rate, R (c) in HbS polymer fibers. All determinations were carried out as in previous studies (31,32). HbS concentrations (mg ml−1) are shown in a and b. Solid symbols represent HbS solution in 0.15 M phosphate buffer with no heme added. Open symbols represent dialyzed solutions with heme added at the concentrations (mM) shown in b. Lines are guides for the eye.
Free heme and the precursors for HbS polymer nuclei
In solutions of deoxy-HbS of concentration 67 mg ml−1 and higher, clusters of dense liquid exist; their size is ∼300 nm and it weakly depends on HbS concentration (45). Fig. 5 a shows the fraction of the solution volume occupied by these clusters, ϕ2, determined by dynamic light scattering, as in Pan et al. (45). Since the clusters are metastable (45), ϕ2 is steady (the volume occupied by a stable new phase is expected to increase in time). As HbS concentration, C, is increased from 67 to 96 mg ml−1, ϕ2 increases from ∼10−6 to ∼10−5. Further increase of HbS concentration to 121, 131, and 178 mg ml−1 (deoxy-HbS solubility at 22°C is 185 mg ml−1 (3)) does not lead to increasing ϕ2 (Fig. 5 b). This behavior is in agreement with a recent theory of the metastable dense liquid clusters in protein solutions (46). According to this theory, clusters as large as several hundred nanometers are kinetically stabilized by specific short-range attraction between the molecules; for the significant role played by short-range interactions in HbS polymerization, see Vekilov et al. (43). This theory predicts cluster size weakly dependent on C, increasing ϕ2 for relatively low C, and ϕ2 insensitive to C at intermediate C (46). From the ϕ2(C) dependence in Fig. 5 b, we conclude that the cluster volume fraction complies with the predictions of this theory. Hence, ϕ2, which is ∼10−5 at C = 96–178 mg ml−1, likely has a similar value at 201 mg ml−1 < C < 280 mg ml−1, the HbS concentration range in Fig. 4.
Figure 5.
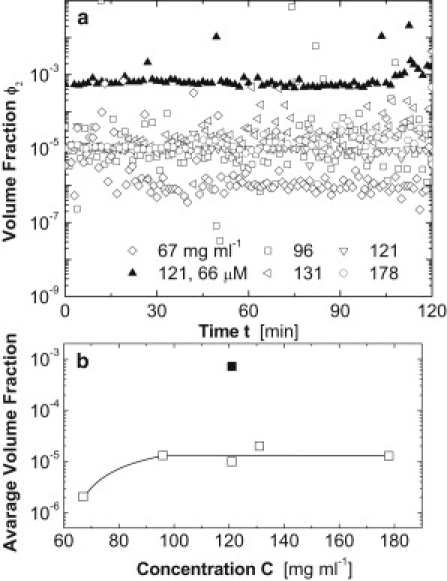
The effect of free heme on the metastable dense liquid clusters in deoxy-HbS solutions. Volume fraction, ϕ2, occupied by clusters was determined as in Pan et al. (45) at the five HbS concentrations shown in the legend, and in the presence of heme at one of these concentrations. The temperature was 22°C. (a) Time dependence of the volume fraction at five solution compositions, as indicated in the plot. (b) Dependence of the average volume fraction from a on HbS concentration. The solid square at 121 mg ml−1 indicates the volume fraction in the presence of 66 μM of free heme; open symbols indicate volume fraction in the absence of heme. The line is a guide for the eye.
In the experiments depicted in Figs. 3 and 4, the volume of the supersaturated HbS solution is ∼10−8 cm3 (31). Since the volume of one cluster is ∼10−13 cm3, with the above ϕ2, the solution contains about one cluster at steady state. Since the fibers nucleate inside the clusters, this observation indicates that cluster formation is the rate-determining step in the nucleation of HbS polymers (see also Galkin et al. (42)). The volume of an erythrocyte is ∼100× smaller than the test volume, so the probability of finding a cluster in a red cell is low, and cluster formation may be the rate-limiting step of nucleation of HbS polymers in vivo.
Fig. 5 shows that the addition of heme leads to an increase in the cluster volume fraction, ϕ2, by two orders of magnitude. The cluster radius is the same as in solutions without heme, i.e., the cluster number density also increases by two orders of magnitude. Since the nuclei of HbS fibers form inside the metastable dense liquid clusters (42,47), the faster J and shorter θ in Fig. 4 directly correlate to the increase in ϕ2.
Discussion
The mechanism of action of free heme
The above results show that micromolar amounts of free heme lead to orders-of-magnitude acceleration of the nucleation and growth of sickle cell polymers. To understand the biophysical mechanisms by which such low heme concentrations cause significant acceleration of HbS polymerization, in a previous article (48), we characterized the effects of heme on the intermolecular interactions in hemoglobin solutions. We applied static light scattering and differential refractometry to solutions of deoxy-HbS, deoxy-HbA, and CO-HbA in the absence and presence of free heme. The data reveal that in the presence of heme, the attraction between the molecules of all three hemoglobin variants is significantly stronger. Analyses based on the Kirkwood-Goldberg theory of light scattering in three-component systems (49) indicate that this attraction results from a combination of electrostatic and hydrophobic behaviors and molecular dynamics: the negative heme molecules electrostatically repel the negative hemoglobin molecules. The repulsion by the heme excludes parts of the volume for the hemoglobin molecules and induces effective attraction between them. The long-range nature of the electrostatic repulsion and the high mobility of the relatively small heme, which allow a single heme molecule to interact with many hemoglobin molecules, explain the high efficacy of the heme.
An alternative explanation of heme effects on the kinetics of polymerization is that their presence or absence affects the conversion of CO-HbS to deoxy-HbS (similar to the effects of DPG): CO-HbS does not polymerize and does not partake in polymerization of deoxy-HbS in mixed solutions. Hence, if the absence of heme leads to slower or incomplete photolysis of CO-HbS, this could result in slower, or potentially no, polymerization. Tests of the heme effects on oxygen binding to hemoglobin were carried out with HbA in the laboratory of R. Briehl at Albert Einstein College of Medicine (R. Briehl, Albert Einstein College of Medicine, Bronx, NY;personal communication, 2010). These preliminary control results showed that free heme has no significant DPG-like effect on the oxyhemoglobin dissociation curve. Although binding of oxygen to HbA is different from binding of carbon monoxide to HbS, it is still unlikely that heme may affect the binding of CO to HbS to an extent sufficient to explain the dramatic effects of free heme on HbS polymerization.
Thus, heme effects on nucleation and growth rate are likely due to the effects of heme on interactions between HbS molecules. To understand the acceleration of the nucleation rate, we show in Fig. 5 that in HbS solutions, free heme enhances the volume fraction of the metastable dense liquid clusters. Since the dense liquid clusters are where HbS polymers nucleate, this increase is likely the cause of faster nucleation of HbS polymers. In turn, the higher cluster volume fraction is directly related to the enhanced attraction, which leads to lower excess free energy of the clusters compared to the HbS solution.
To understand the acceleration of the growth rate in the presence of heme, we note that Vekilov et al. (43) showed that the hydration shell around the HbS molecules largely determines their rate of attachment to the growing polymer fiber. The action of heme on the growth rate likely occurs through modification of this shell, discussed above and in Pan et al. (48).
It is likely that in addition to its effects on the kinetics of HbS polymerization, heme strongly affects the equilibrium between the HbS polymers and the HbS solution. The enhanced attraction between the HbS molecules in the presence of heme could lead to lower solubility; such correlations between intermolecular attraction and solubility have been recorded for many proteins (50,51) and theoretically justified (52,53). With the lower solubility, heme-containing HbS solutions would have higher supersaturation with respect to the polymers than would solutions of equal HbS concentration in the absence of heme. This scenario can readily be tested by determining the solubility in the presence of heme and comparing it to data for solutions without heme, e.g., those of Eaton and Hofrichter (3). The potential heme effects on HbS solubility do not exclude, and likely work in concert with, its kinetic effects on the rates of nucleation and growth discussed above.
Free heme and HbS polymerization in red cells
The results presented here show that the complete removal of free heme leads to the arrest of HbS polymerization. From this point of view, it appears that the observed presence of free heme in the red cell cytosol, even at its low average concentration (29), is crucial for the known mild sickling of large fractions of the red cells that occurs on each passage through the venous circulation and does not lead to vasoocclusive crises (54). The rates of nucleation and growth of sickle cell polymers in the presence of such low concentrations of heme are still to be tested at complete deoxygenation and at physiological ranges of oxygen partial pressure; however, it is likely that they are low. The low rates may be the reason for the vast discrepancy between the frequencies of deoxygenation of an erythrocyte (∼1/min) during which substantial numbers sickle and of vasoocclusive crises of from once weekly to one to four per year in severely sick patients. The likely nonuniformity of the free heme concentration may be the reason why only some of the red cells in the venous circulation of sickle-cell patients undergo the mild sickling seen upon each deoxygenation event (54).
Several explanations of the discrepancy between the sickling and painful crisis frequencies have been proposed (11,12,55). The strong effects of free heme on the nucleation and growth of HbS polymers allow us to speculate that a vasoocclusive crisis might also be triggered by a spike in the intraerythrocyte free heme concentration due to a failure of the antioxidative defense. Since a vasoocclusion requires the coordinated catastrophic sickling (rigidification and deformation) of many red blood cells (56), the spike in free heme concentration should occur simultaneously in numerous erythrocytes. Physiological factors that might lead to concerted release of heme in numerous red blood cells are discussed below.
In addition to the antioxidative defense system, the heme concentration in the red cells is regulated by three removal processes: diffusion through the cell membrane (28), association to the membrane or the cytoskeleton (57), and intracellular degradation by glutathione (22). The former two mechanisms are concentration-dependent, and since the heme concentration, even during the suspected spike, would be below its solubility of 2 mM, excessive binding of heme to the membranes is unlikely. The third mechanism requires the availability of glutathione in the red blood cells at concentrations up to 2 mM (58) and a fast rate of degradation reaction (22). Thus, if a spike in the concentration of free heme occurs as a result of a failure of the antioxidative defense, the removal processes may fail to wipe out the heme excess.
As positive evidence for spikes in the free heme concentration, one could consider the observation of free heme and nonheme iron (produced by the degradation of heme by glutathione (22)) in the red cell membranes (59,60), which is unlikely if the concentration is fixed at 1 μM. Thus, the higher severity of sickle cell anemia in one of the monozygotic twins discussed in the Introduction and in Amin et al. (17) might have been related to enhancement of catastrophic sickling by the spikes of free heme concentration, evidenced as membrane-bound iron.
Possible external triggers of free heme concentration spikes
Vasoocclusive crises in sickle cell anemia are precipitated by many factors: hypoxia, acidosis, infection, and others; often no specific cause is identified (61,62). The mechanism of action of those factors can be related to polymerization, endothelial activation, and vasoconstriction (61). The extremely strong effects of relatively low concentrations of free heme reported here suggest that these factors might act also through formation of met-HbS with subsequent heme release causing a spike in the free heme concentration.
A general mechanism for met-HbS formation is the depletion of the reducing equivalents in the red cells and increase of oxidant stress. Hypoxia appears to apply this mechanism directly: at low-oxygen partial pressures, the autooxidation of hemoglobin is faster (33) and the production of met-Hb is accelerated. This counterintuitive finding has been attributed to the trapping of oxidative radicals and ions by byproducts of the O2 molecule (33). The effects of acidosis have been attributed to shifts of the oxygen dissociation curve that favor deoxy-HbS formation. In addition to this, metabolic acidosis has been associated with, among other factors, significant oxidative stress and acquired methemoglobinemia (63), and this might be an additional mechanism linking acidosis to sickle crises.
Vasoocclusion is often precipitated by infection. Many mechanisms by which infection enhances sickling are discussed, including fever leading to dehydration, lack of food intake leading to acidosis, and others (61). One should keep in mind that the immune system response to most viral and bacterial infections relies, to a large degree, on the release of reactive oxygen species as a weapon against foreign genetic material (64). Some infectious organisms might produce reactive oxygen species on their own. Irrespective of its origin, the significant oxidative stress during infection may lead to an increase in hemoglobin autoxidation. Emotional stress results in adrenalin discharges, which leads to vasoconstriction. However, adrenalin has a multitude of effects, and a correlation between adrenaline and reactive oxygen species production on the cellular level has been established: adrenaline has been found to promote hydroxyl radical generation in isolated rat hepatocytes (65).
The variability of sickle cell anemia and the effects of free heme
The vast variability of sickle cell anemia has been linked to cellular, vascular, and phenotypic factors (11,12,19,66,67). The strong effects of free heme discussed above suggest that it might be another factor for this variability. It is likely that the differences in the antioxidative defense system in patients would lead to different responses to peak oxidative stress and, hence, to stronger or weaker spikes of the free heme concentration. Because of the high sensitivity of the rates of nucleation and growth of sickle cell polymers to the concentration of free heme, demonstrated in this article, this variability could lead to significant variability in the prognosis of patients with identical HbS expression. Thus, the differences in antioxidative defense of different patients may emerge as a new factor (in addition to differences in red-cell adhesion, endothelial activation, platelet count, and others) in the vast variability of clinical outcome of sickle cell anemia (67).
A more significant suggestion emerging from our results is that if the resistance of the red cell cytosol to spikes in free heme concentration in the erythrocytes is enhanced, this might result in milder sickling and less severe sickle cell symptoms. Thus, the regulation of free heme concentration spikes might be an alternative route in the search for sickle cell anemia treatments. Such regulation might be achieved by enhancement of heme degradation, e.g., by glutathione, or of the reductive pathways that prevent formation of met-HbS.
The larger field of fundamental research of the pathophysiology of sickle cell is largely focused on the search for genetic modifiers of the behavior of the cells in the microcirculation (18,66,67). The results presented here on the effects of the heme on sickle cell hemoglobin polymerization suggest that some of the genes identified as modifiers of sickle cell severity might act through regulation of the amount of free heme in the erythrocytes: by suppressing HbS autoxidation or by enhancing heme degradation.
The link between sickle cell disease and malaria
Last, it is possible that the heme released in red blood cells infected by malaria plasmodia as a byproduct of the parasites' metabolism of hemoglobin (68) causes the known sickling of sickle-trait erythrocytes (which would not sickle in the absence of heme because of lower HbS activity) and enables their selective removal from the circulation (69). This may be a novel explanation of the link between malaria and sickle cell disease.
Acknowledgments
We are deeply indebted to R. W. Briehl, W. A. Eaton, and R. Krishnamoorti for critical reading of the manuscript, to R. Briehl, again, for sharing unpublished results, and to V. Lubchenko and A. Kolomeisky for helpful suggestions. We thank B. Dinu, G. Airewele, and B. Mueller for discussions on the medical relevance of the result and for the supply of sickle cell blood.
This work was supported by the National Science Foundation (grants CBET 0609387 and MCB 0843726) and The Welch Foundation (grant E-1641).
Supporting Material
References
- 1.Pauling L., Itano H.A., Wells I.C. Sickle cell anemia a molecular disease. Science. 1949;110:543–548. doi: 10.1126/science.110.2865.543. [DOI] [PubMed] [Google Scholar]
- 2.Eaton W.A. Linus Pauling and sickle cell disease. Biophys. Chem. 2003;100:109–116. doi: 10.1016/s0301-4622(02)00269-7. [DOI] [PubMed] [Google Scholar]
- 3.Eaton W.A., Hofrichter J. Sickle cell hemoglobin polymerization. Adv. Protein Chem. 1990;40:63–279. doi: 10.1016/s0065-3233(08)60287-9. [DOI] [PubMed] [Google Scholar]
- 4.Vekilov P.G. Sickle-cell haemoglobin polymerization: is it the primary pathogenic event of sickle-cell anaemia? Br. J. Haematol. 2007;139:173–184. doi: 10.1111/j.1365-2141.2007.06794.x. [DOI] [PubMed] [Google Scholar]
- 5.Pawliuk R., Westerman K.A., Leboulch P. Correction of sickle cell disease in transgenic mouse models by gene therapy. Science. 2001;294:2368–2371. doi: 10.1126/science.1065806. [DOI] [PubMed] [Google Scholar]
- 6.Herrick J.B. Peculiar elongated and sickle-shaped red blood corpuscles in a case of severe anemia. 1910. Yale J. Biol. Med. 2001;74:179–184. [PMC free article] [PubMed] [Google Scholar]
- 7.Stuart M.J., Nagel R.L. Sickle-cell disease. Lancet. 2004;364:1343–1360. doi: 10.1016/S0140-6736(04)17192-4. [DOI] [PubMed] [Google Scholar]
- 8.Ferrone F.A., Hofrichter J., Eaton W.A. Kinetics of sickle hemoglobin polymerization. I. Studies using temperature-jump and laser photolysis techniques. J. Mol. Biol. 1985;183:591–610. doi: 10.1016/0022-2836(85)90174-3. [DOI] [PubMed] [Google Scholar]
- 9.Ferrone F.A., Hofrichter J., Eaton W.A. Kinetics of sickle hemoglobin polymerization. II. A double nucleation mechanism. J. Mol. Biol. 1985;183:611–631. doi: 10.1016/0022-2836(85)90175-5. [DOI] [PubMed] [Google Scholar]
- 10.Ferrone F.A. Polymerization and sickle cell disease: a molecular view. Microcirculation. 2004;11:115–128. doi: 10.1080/10739680490278312. [DOI] [PubMed] [Google Scholar]
- 11.Embury S.H. The not-so-simple process of sickle cell vasoocclusion. Microcirculation. 2004;11:101–113. doi: 10.1080/10739680490278277. [DOI] [PubMed] [Google Scholar]
- 12.Hebbel R.P. Beyond hemoglobin polymerization: the red blood cell membrane and sickle disease pathophysiology. Blood. 1991;77:214–237. [PubMed] [Google Scholar]
- 13.Nagel R.L. Sickle cell anemia is a multigene disease: sickle painful crises, a case in point. Am. J. Hematol. 1993;42:96–101. doi: 10.1002/ajh.2830420119. [DOI] [PubMed] [Google Scholar]
- 14.Coletta M., Hofrichter J., Eaton W.A. Kinetics of sickle haemoglobin polymerization in single red cells. Nature. 1982;300:194–197. doi: 10.1038/300194a0. [DOI] [PubMed] [Google Scholar]
- 15.el-Hazmi M.A. Heterogeneity and variation of clinical and haematological expression of haemoglobin S in Saudi Arabs. Acta Haematol. 1992;88:67–71. doi: 10.1159/000204654. [DOI] [PubMed] [Google Scholar]
- 16.Bridges K.R., Barabino G.D., Eaton W.A. A multiparameter analysis of sickle erythrocytes in patients undergoing hydroxyurea therapy. Blood. 1996;88:4701–4710. [PubMed] [Google Scholar]
- 17.Amin B.R., Bauersachs R.M., Westerman M.P. Monozygotic twins with sickle cell anemia and discordant clinical courses: clinical and laboratory studies. Hemoglobin. 1991;15:247–256. doi: 10.3109/03630269109027877. [DOI] [PubMed] [Google Scholar]
- 18.Weatherall M.W., Higgs D.R., Serjeant G.R. Phenotype/genotype relationships in sickle cell disease: a pilot twin study. Clin. Lab. Haematol. 2005;27:384–390. doi: 10.1111/j.1365-2257.2005.00731.x. [DOI] [PubMed] [Google Scholar]
- 19.Hebbel R.P. Special issue of Microcirculation: examination of the vascular pathobiology of sickle cell anemia. Foreword. Microcirculation. 2004;11:99–100. [PubMed] [Google Scholar]
- 20.Hebbel R.P., Morgan W.T., Hedlund B.E. Accelerated autoxidation and heme loss due to instability of sickle hemoglobin. Proc. Natl. Acad. Sci. USA. 1988;85:237–241. doi: 10.1073/pnas.85.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sheng K., Shariff M., Hebbel R.P. Comparative oxidation of hemoglobins A and S. Blood. 1998;91:3467–3470. [PubMed] [Google Scholar]
- 22.Atamna H., Ginsburg H. Heme degradation in the presence of glutathione. A proposed mechanism to account for the high levels of non-heme iron found in the membranes of hemoglobinopathic red blood cells. J. Biol. Chem. 1995;270:24876–24883. doi: 10.1074/jbc.270.42.24876. [DOI] [PubMed] [Google Scholar]
- 23.Solar I., Shaklai N. Association of hemin with protein 4.1 as compared to spectrin and actin. Biochim. Biophys. Acta. 1989;983:199–204. doi: 10.1016/0005-2736(89)90234-4. [DOI] [PubMed] [Google Scholar]
- 24.Jarolim P., Lahav M., Palek J. Effect of hemoglobin oxidation products on the stability of red cell membrane skeletons and the associations of skeletal proteins: correlation with a release of hemin. Blood. 1990;76:2125–2131. [PubMed] [Google Scholar]
- 25.Dill K., Bromberg S. Garland Science; New York: 2003. Molecular Driving Forces: Statistical Thermodynamics in Chemistry and Biology. [Google Scholar]
- 26.Weissbuch I., Leiserowitz L. Interplay between malaria, crystalline hemozoin formation, and antimalarial drug action and design. Chem. Rev. 2008;108:4899–4914. doi: 10.1021/cr078274t. [DOI] [PubMed] [Google Scholar]
- 27.Pagola S., Stephens P.W., Madsen S.K. The structure of malaria pigment β-haematin. Nature. 2000;404:307–310. doi: 10.1038/35005132. [DOI] [PubMed] [Google Scholar]
- 28.Cannon J.B., Kuo F.S., Muller-Eberhard U. Kinetics of the interaction of hemin liposomes with heme binding proteins. Biochemistry. 1984;23:3715–3721. doi: 10.1021/bi00311a022. [DOI] [PubMed] [Google Scholar]
- 29.Liu S.C., Zhai S., Palek J. Detection of hemin release during hemoglobin S denaturation. Blood. 1988;71:1755–1758. [PubMed] [Google Scholar]
- 30.Hebbel R.P. Perspectives series: cell adhesion in vascular biology. Adhesive interactions of sickle erythrocytes with endothelium. J. Clin. Invest. 1997;99:2561–2564. doi: 10.1172/JCI119442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galkin O., Nagel R.L., Vekilov P.G. The kinetics of nucleation and growth of sickle cell hemoglobin fibers. J. Mol. Biol. 2007;365:425–439. doi: 10.1016/j.jmb.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 32.Galkin O., Vekilov P.G. Mechanisms of homogeneous nucleation of polymers of sickle cell anemia hemoglobin in deoxy state. J. Mol. Biol. 2004;336:43–59. doi: 10.1016/j.jmb.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 33.Antonioni E., Brunori M. North Holland; Amsterdam: 1971. Hemoglobin and Mioglobin in Their Reactions with Ligands. [Google Scholar]
- 34.Benesch R., Benesch R.E. The effect of organic phosphates from the human erythrocyte on the allosteric properties of hemoglobin. Biochem. Biophys. Res. Commun. 1967;26:162–167. doi: 10.1016/0006-291x(67)90228-8. [DOI] [PubMed] [Google Scholar]
- 35.Nelson D.L., Cox M.M. 3rd ed. W. H. Freeman; New York: 2000. Lehninger's Principles of Biochemistry. [Google Scholar]
- 36.Zunszain P.A., Ghuman J., Curry S. Crystal structural analysis of human serum albumin complexed with hemin and fatty acid. BMC Struct. Biol. 2003;3:6. doi: 10.1186/1472-6807-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuzelová K., Mrhalová M., Hrkal Z. Kinetics of heme interaction with heme-binding proteins: the effect of heme aggregation state. Biochim. Biophys. Acta. 1997;1336:497–501. doi: 10.1016/s0304-4165(97)00062-7. [DOI] [PubMed] [Google Scholar]
- 38.Bunn H.F., Jandl J.H. Exchange of heme among hemoglobins and between hemoglobin and albumin. J. Biol. Chem. 1968;243:465–475. [PubMed] [Google Scholar]
- 39.Falk J.E. Elsevier; Amsterdam: 1964. Porphyrins and Metalloporphyrins. [Google Scholar]
- 40.Hofrichter J. Kinetics of sickle hemoglobin polymerization. III. Nucleation rates determined from stochastic fluctuations in polymerization progress curves. J. Mol. Biol. 1986;189:553–571. doi: 10.1016/0022-2836(86)90324-4. [DOI] [PubMed] [Google Scholar]
- 41.Briehl R.W., Ewert S. Effects of pH, 2,3-diphosphoglycerate and salts on gelation of sickle cell deoxyhemoglobin. J. Mol. Biol. 1973;80:445–458. doi: 10.1016/0022-2836(73)90415-4. [DOI] [PubMed] [Google Scholar]
- 42.Galkin O., Pan W., Vekilov P.G. Two-step mechanism of homogeneous nucleation of sickle cell hemoglobin polymers. Biophys. J. 2007;93:902–913. doi: 10.1529/biophysj.106.103705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vekilov P.G., Galkin O., Nagel R.L. Determination of the transition-state entropy for aggregation suggests how the growth of sickle cell hemoglobin polymers can be slowed. J. Mol. Biol. 2008;377:882–888. doi: 10.1016/j.jmb.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kashchiev D., Vekilov P.G., Kolomeisky A.B. Kinetics of two-step nucleation of crystals. J. Chem. Phys. 2005;122:244706. doi: 10.1063/1.1943389. [DOI] [PubMed] [Google Scholar]
- 45.Pan W., Galkin O., Vekilov P.G. Metastable mesoscopic clusters in solutions of sickle-cell hemoglobin. Biophys. J. 2007;92:267–277. doi: 10.1529/biophysj.106.094854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pan W., Vekilov P.G., Lubchenko V. The origin of anomalous mesoscopic phases in protein solutions. J. Phys. Chem. B. 2010;114:7620–7630. doi: 10.1021/jp100617w. [DOI] [PubMed] [Google Scholar]
- 47.Galkin O., Chen K., Vekilov P.G. Liquid-liquid separation in solutions of normal and sickle cell hemoglobin. Proc. Natl. Acad. Sci. USA. 2002;99:8479–8483. doi: 10.1073/pnas.122055299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pan W., Uzunova V.V., Vekilov P.G. Free heme in micromolar amounts enhances the attraction between sickle cell hemoglobin molecules. Biopolymers. 2009;91:1108–1116. doi: 10.1002/bip.21191. [DOI] [PubMed] [Google Scholar]
- 49.Kirkwood J.G., Goldberg R.J. Light scattering arising from composition fluctuations in multi-component systems. J. Chem. Phys. 1950;18:54–57. [Google Scholar]
- 50.George A., Wilson W.W. Predicting protein crystallization from a dilute solution property. Acta Crystallogr. D Biol. Crystallogr. 1994;50:361–365. doi: 10.1107/S0907444994001216. [DOI] [PubMed] [Google Scholar]
- 51.Guo B., Kao S., Combs L.L. Correlation of second virial coefficients and solubilities useful in protein crystal growth. J. Cryst. Growth. 1999;196:424–433. [Google Scholar]
- 52.Haas C., Drenth J., Wilson W.W. Relation between the solubility of proteins in aqueous solutions and the second virial coefficient of the solution. J. Phys. Chem. B. 1999;103:2808–2811. [Google Scholar]
- 53.Petsev D.N., Thomas B.R., Vekilov P.G. Temperature-independent solubility and interactions between apoferritin monomers and dimers in solution. J. Cryst. Growth. 2001;232:21–29. [Google Scholar]
- 54.Serjeant G.R., Petch M.C., Serjeant B.E. The in vivo sickle phenomenon: a reappraisal. J. Lab. Clin. Med. 1973;81:850–856. [PubMed] [Google Scholar]
- 55.Hebbel R.P., Osarogiagbon R., Kaul D. The endothelial biology of sickle cell disease: inflammation and a chronic vasculopathy. Microcirculation. 2004;11:129–151. [PubMed] [Google Scholar]
- 56.Higgins J.M., Eddington D.T., Mahadevan L. Sickle cell vasoocclusion and rescue in a microfluidic device. Proc. Natl. Acad. Sci. USA. 2007;104:20496–20500. doi: 10.1073/pnas.0707122105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rank B.H., Carlsson J., Hebbel R.P. Abnormal redox status of membrane-protein thiols in sickle erythrocytes. J. Clin. Invest. 1985;75:1531–1537. doi: 10.1172/JCI111857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kondo T., Dale G.L., Beutler E. Glutathione transport by inside-out vesicles from human erythrocytes. Proc. Natl. Acad. Sci. USA. 1980;77:6359–6362. doi: 10.1073/pnas.77.11.6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kuross S.A., Hebbel R.P. Nonheme iron in sickle erythrocyte membranes: association with phospholipids and potential role in lipid peroxidation. Blood. 1988;72:1278–1285. [PubMed] [Google Scholar]
- 60.Kuross S.A., Rank B.H., Hebbel R.P. Excess heme in sickle erythrocyte inside-out membranes: possible role in thiol oxidation. Blood. 1988;71:876–882. [PubMed] [Google Scholar]
- 61.Beutler E. The sickle cell diseases and related disorders. In: Beutler E., Lichtman M.A., Coller B.S., Kipps T.J., Seligsohn U., editors. Williams Hematology. 6th ed. MaGraw Hill; New York: 2001. pp. 581–605. [Google Scholar]
- 62.Schnog J.B., Duits A.J., Brandjes D.P. Sickle cell disease; a general overview. Neth. J. Med. 2004;62:364–374. [PubMed] [Google Scholar]
- 63.Golden P.J., Weinstein R. Treatment of high-risk, refractory acquired methemoglobinemia with automated red blood cell exchange. J. Clin. Apher. 1998;13:28–31. doi: 10.1002/(sici)1098-1101(1998)13:1<28::aid-jca6>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 64.Wu W., Hsu Y.M., Lin X. CARD9 facilitates microbe-elicited production of reactive oxygen species by regulating the LyGDI-Rac1 complex. Nat. Immunol. 2009;10:1208–1214. doi: 10.1038/ni.1788. [DOI] [PubMed] [Google Scholar]
- 65.Díaz-Cruz A., Guinzberg R., Piña E. Adrenaline stimulates H2O2 generation in liver via NADPH oxidase. Free Radic. Res. 2007;41:663–672. doi: 10.1080/10715760701268751. [DOI] [PubMed] [Google Scholar]
- 66.Steinberg M.H. Predicting clinical severity in sickle cell anaemia. Br. J. Haematol. 2005;129:465–481. doi: 10.1111/j.1365-2141.2005.05411.x. [DOI] [PubMed] [Google Scholar]
- 67.Nabel E.G., Shurin S.B. A recommitment to sickle cell disease research. Blood. 2008;111:4852–4853. doi: 10.1182/blood-2008-03-143685. [DOI] [PubMed] [Google Scholar]
- 68.Jani D., Nagarkatti R., Rathore D. HDP-a novel heme detoxification protein from the malaria parasite. PLoS Pathog. 2008;4:e1000053. doi: 10.1371/journal.ppat.1000053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nagel R.L. Malaria and hemoglobinopathies. In: Steinberg M.H., Forget B.G., Higgs D.R., Nagel R.L., editors. Disorders of Hemoglobin: Genetics, Pathophysiology and Clinical Management. Cambridge University; Cambridge, United, Kingdom: 2001. pp. 832–860. [Google Scholar]
- 70.Zijlstra W.G., Buursma A., Meeuwsen-van der Roest W.P. Absorption spectra of human fetal and adult oxyhemoglobin, de-oxyhemoglobin, carboxyhemoglobin, and methemoglobin. Clin. Chem. 1991;37:1633–1638. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


