Abstract
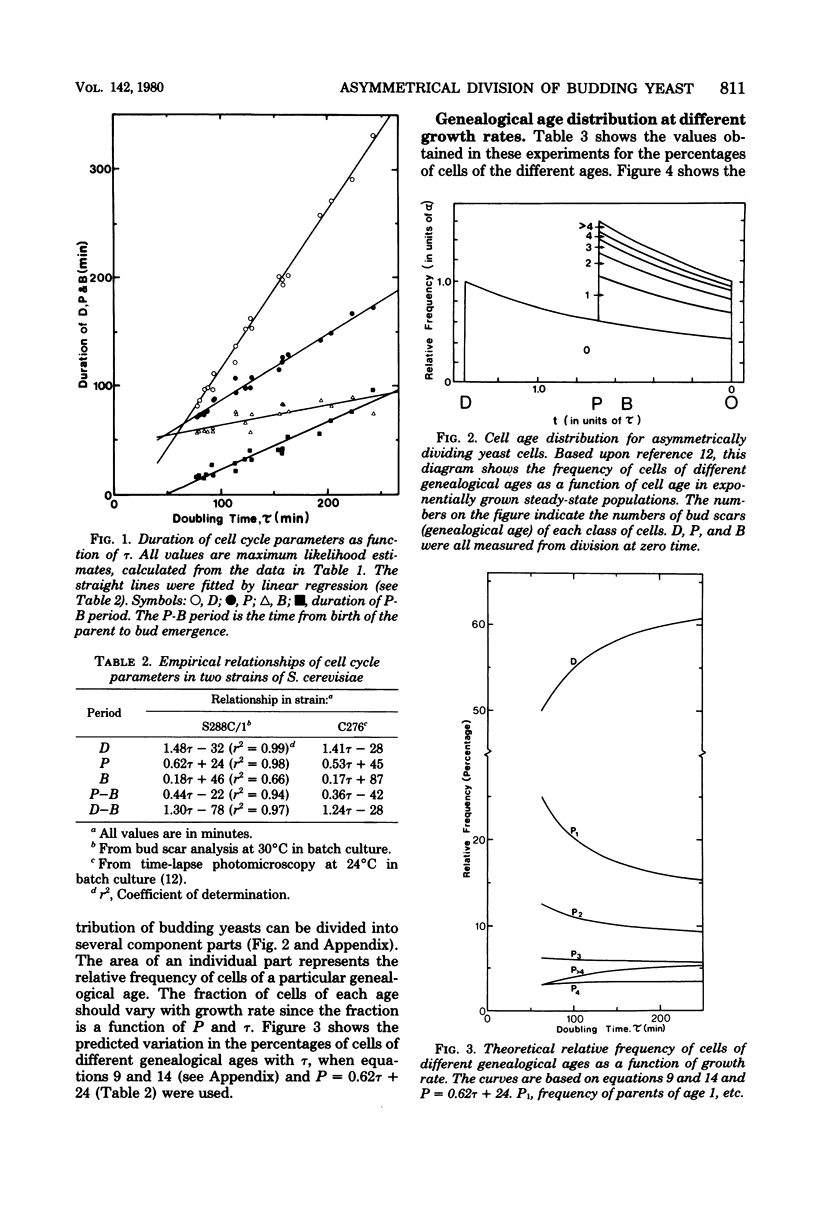
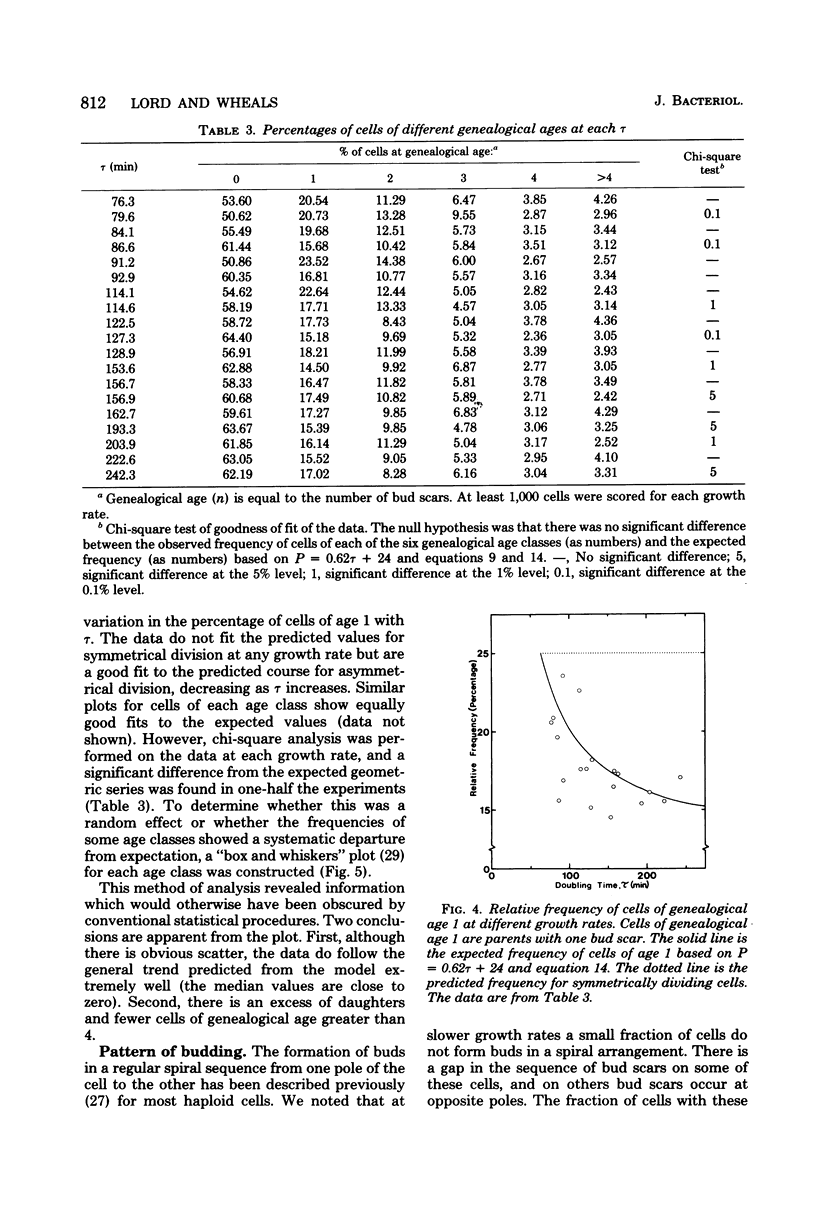
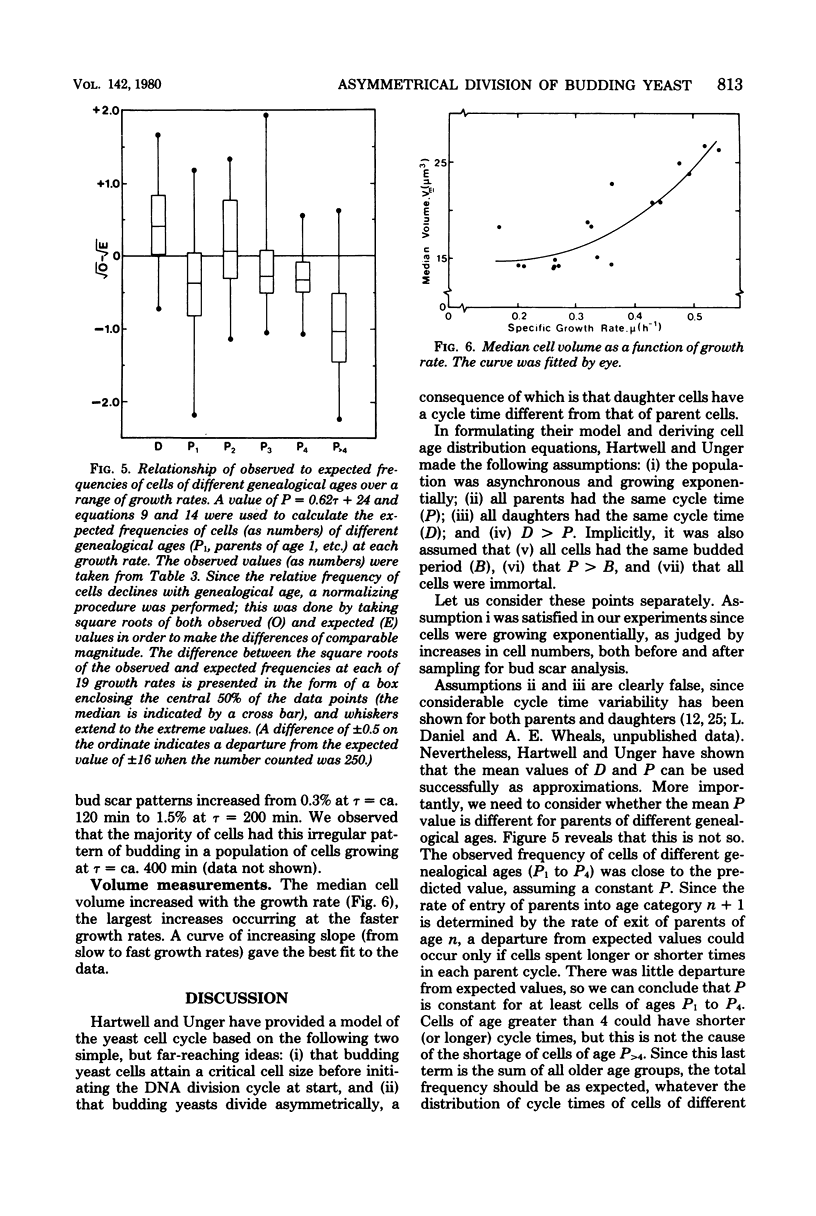
The unequal division model proposed for budding yeast (L. H. Hartwell and M. W. Unger, J. Cell Biol. 75:422-435, 1977) was tested by bud scar analyses of steady-state exponential batch cultures of Saccharomyces cerevisiae growing at 30 degrees C at 19 different rates, which were obtained by altering the carbon source. The analyses involved counting the number of bud scars, determining the presence or absence of buds on at least 1,000 cells, and independently measuring the doubling times (gamma) by cell number increase. A number of assumptions in the model were tested and found to be in good agreement with the model. Maximum likelihood estimates of daughter cycle time (D), parent cycle time (P), and the budded phase (B) were obtained, and we concluded that asymmetrical division occurred at all growth rates tested (gamma, 75 to 250 min). D, P, and B are all linearly related to gamma, and D, P, and gamma converge to equality (symmetrical division) at gamma = 65 min. Expressions for the genealogical age distribution for asymmetrically dividing yeast cells were derived. The fraction of daughter cells in steady-state populations is e-alpha P, and the fraction of parent cells of age n (where n is the number of buds that a cell has produced) is (e-alpha P)n-1(1-e-alpha P)2, where alpha = IN2/gamma; thus, the distribution changes with growth rate. The frequency of cells with different numbers of bud scars (i.e., different genealogical ages) was determined for all growth rates, and the observed distribution changed with the growth rate in the manner predicted. In this haploid strain new buds formed adjacent to the previous buds in a regular pattern, but at slower growth rates the pattern was more irregular. The median volume of the cells and the volume at start in the cell cycle both increased at faster growth rates. The implications of these findings for the control of the cell cycle are discussed.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURNS V. W. Temporal studies of cell division. I. The influence of ploidy and temperature on cell division in S. cerevisiae. J Cell Physiol. 1956 Jun;47(3):357–375. doi: 10.1002/jcp.1030470305. [DOI] [PubMed] [Google Scholar]
- Barford J. P., Hall R. J. Estimation of the length of cell cycle phases from asynchronous cultures of Saccharomyces cerevisiae. Exp Cell Res. 1976 Oct 15;102(2):276–284. doi: 10.1016/0014-4827(76)90043-4. [DOI] [PubMed] [Google Scholar]
- Byers B., Goetsch L. Behavior of spindles and spindle plaques in the cell cycle and conjugation of Saccharomyces cerevisiae. J Bacteriol. 1975 Oct;124(1):511–523. doi: 10.1128/jb.124.1.511-523.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter B. L., Jagadish M. N. The relationship between cell size and cell division in the yeast Saccharomyces cerevisiae. Exp Cell Res. 1978 Mar 1;112(1):15–24. doi: 10.1016/0014-4827(78)90520-7. [DOI] [PubMed] [Google Scholar]
- FREIFELDER D. Bud position in Saccharomyces cerevisiae. J Bacteriol. 1960 Oct;80:567–568. doi: 10.1128/jb.80.4.567-568.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegel T. W. Difference in generation times for mother and daughter cells in yeasts. Can J Microbiol. 1978 Jul;24(7):827–833. doi: 10.1139/m78-138. [DOI] [PubMed] [Google Scholar]
- Gani J., Saunders I. W. Fitting a model to the growth of yeast colonies. Biometrics. 1977 Mar;33(1):113–120. [PubMed] [Google Scholar]
- Hartwell L. H. Saccharomyces cerevisiae cell cycle. Bacteriol Rev. 1974 Jun;38(2):164–198. doi: 10.1128/br.38.2.164-198.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H., Unger M. W. Unequal division in Saccharomyces cerevisiae and its implications for the control of cell division. J Cell Biol. 1977 Nov;75(2 Pt 1):422–435. doi: 10.1083/jcb.75.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagadish M. N., Carter B. L. Genetic control of cell division in yeast cultured at different growth rates. Nature. 1977 Sep 8;269(5624):145–147. doi: 10.1038/269145a0. [DOI] [PubMed] [Google Scholar]
- Johnson B. F., Gibson E. J. Autoradiographic analysis of regional cell wall growth of yeasts. III. Saccharomyces cerevisiae. Exp Cell Res. 1966 Mar;41(3):580–591. doi: 10.1016/s0014-4827(66)80108-8. [DOI] [PubMed] [Google Scholar]
- Johnston G. C., Ehrhardt C. W., Lorincz A., Carter B. L. Regulation of cell size in the yeast Saccharomyces cerevisiae. J Bacteriol. 1979 Jan;137(1):1–5. doi: 10.1128/jb.137.1.1-5.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston G. C., Pringle J. R., Hartwell L. H. Coordination of growth with cell division in the yeast Saccharomyces cerevisiae. Exp Cell Res. 1977 Mar 1;105(1):79–98. doi: 10.1016/0014-4827(77)90154-9. [DOI] [PubMed] [Google Scholar]
- Müller I. Experiments on ageing in single cells of Saccharomyces cerevisiae. Arch Mikrobiol. 1971;77(1):20–25. doi: 10.1007/BF00407985. [DOI] [PubMed] [Google Scholar]
- NICKERSON W. J. SYMPOSIUM ON BIOCHEMICAL BASES OF MORPHOGENESIS IN FUNGI. IV. MOLECULAR BASES OF FORM IN YEASTS. Bacteriol Rev. 1963 Sep;27:305–324. doi: 10.1128/br.27.3.305-324.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard R. H. Review lecture on the growth and form of a bacterial cell. Philos Trans R Soc Lond B Biol Sci. 1974 Feb 21;267(886):303–336. doi: 10.1098/rstb.1974.0003. [DOI] [PubMed] [Google Scholar]
- Slater M. L., Sharrow S. O., Gart J. J. Cell cycle of Saccharomycescerevisiae in populations growing at different rates. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3850–3854. doi: 10.1073/pnas.74.9.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streiblová E. Study of scar formation in the life cycle of heterothallic Saccharomyces cerevisiae. Can J Microbiol. 1970 Sep;16(9):827–831. [PubMed] [Google Scholar]
- Tyson C. B., Lord P. G., Wheals A. E. Dependency of size of Saccharomyces cerevisiae cells on growth rate. J Bacteriol. 1979 Apr;138(1):92–98. doi: 10.1128/jb.138.1.92-98.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrana D., Beran K. Tsitomorfologicheskaia kharakteristika Saccharomyces cerevisiae i Candida utilis kak pokazatel' fiziologicheskogo sostoianiia kul'tury. Mikrobiologiia. 1977 Jan-Feb;46(1):161–164. [PubMed] [Google Scholar]
- Vraná D. Daughter cells as an important factor in determining the physiological state of yeast populations. Biotechnol Bioeng. 1976 Mar;18(3):297–309. doi: 10.1002/bit.260180303. [DOI] [PubMed] [Google Scholar]
- Vraná D. Some morphological and physiological properties of Candida utilis growing "hypertrophically" in excess of substrate in a two-stage continuous cultivation. Biotechnol Bioeng Symp. 1973;0(4-1):161–173. [PubMed] [Google Scholar]



