Abstract
Multisite phosphorylation is a common form of posttranslational protein regulation which has been used to increase the switchlike behavior of the protein response to increasing kinase concentrations. In this letter, we show that the switchlike response of multisite phosphoproteins is strongly enhanced by nonessential phosphorylation sites, a mechanism that is robust to parameter changes and easily implemented in nature. We obtained analytic estimates for the Hill exponent (or coefficient) of the switchlike response, and we observed that a tradeoff exists between the switch and the kinase threshold for activation. This also suggests a possible evolutionary mechanism for the relatively large numbers of phosphorylation sites found in various proteins.
It has been argued that a large number of phosphorylation sites could form the basis for a strong switchlike response (large Hill exponent) to an increase in kinase concentration (1,2). Such a property is desirable in situations where a highly ultrasensitive response is expected (such as cell division in response to a growth factor), or in the design of multistable systems (3). However, it was demonstrated in Gunawardena (4) through a mathematical model that the dose-response curve of the fully phosphorylated protein cannot be switchlike. Several plausible mechanisms have been suggested to enhance the switchlike response, including cooperativity among different sites (4), temporal cooperativity (5), or introducing other players such as membrane (6) and scaffold proteins (7).
Here, we propose a simple mechanism that substantially improves ultrasensitivity of a dose response. Instead of considering only the fully phosphorylated substrate as in Gunawardena (4), Qian and Cooper (5), and Serber and Ferrell (6), we include substrates with at least k phosphorylated sites, out of a total of n sites, as active (Fig. 1). We call k the minimal activation number. A possible way to achieve this threshold protein activation is through an entropic binding mechanism, as described in Lenz and Swain (8). The potential improvement of ultrasensitivity for such mechanism has been suggested in a recent study on bistability by multisite phosphorylation (9). The steady-state fraction of activated substrates is given by
| (1) |
where si denotes the steady-state concentration of the corresponding protein. There is increasing evidence supporting this assumption. A classical example is the yeast cell cycle regulator, Sic1, which has nine phosphorylation sites, among which any combination of six is sufficient to trigger the onset of S phase (1,10,11). At first glance, the inclusion of partially phosphorylated substrates as active seems futile, because a system with minimal activation number k out of a total of n sites seems similar to a system with a total of k sites where full phosphorylation is required for activation. Rather surprisingly, we find that the remaining n – k sites markedly improve the ultrasensitivity of the dose-response curve (Fig. 2 A).
Figure 1.
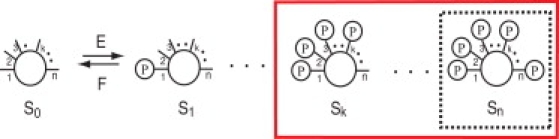
A schematic diagram of n-site phosphorylations and dephosphorylations under the sequential and distributive mechanism by kinase E and phosphatase F, respectively. Inside the solid box is the generalized definition of active substrates; inside the dashed box is the conventional definition.
Figure 2.

Ultrasensitivity and threshold under the sequential mechanism. (A) Dose-response curve. (B) The Hill exponents' estimation by Eq. 2 (curve) and computed by ln(81)/ ln(EC90/EC10) (dots). (C) Random λi (black) versus λi ≈ λ (red). (D) Threshold. (Green) n = 10, λi = 1. (Red) n = 20, λi = 1. (Black) n = 20; λ randomly chosen between 0.1 and 10.
In general, phosphorylation and dephosphorylation can follow either a sequential or a nonsequential mechanism. In the sequential mechanism, (de)phosphorylation takes place in a specific order (Fig. 1) (12). Denote by λi the relative efficiency of a substrate being phosphorylated versus being dephosphorylated at the ith residue (detailed definitions given in Text S1 A in the Supporting Material). When λi ≈ λ, the effective Hill exponent of the dose-response curve can be estimated as (see Text S1 A in the Supporting Material)
| (2) |
where α = k/(n + 1). In particular, when n = 2k – 1, i.e., approximately half of the phosphorylation sites are nonessential, and Eq. 1 can be rewritten as (see Text S1 A in the Supporting Material)
| (3) |
where u (dose) is the ratio of the steady-state free kinase to phosphatase. Clearly, Eq. 3 is a Hill function with Hill exponent k, consistent with the Hill exponent estimated by Eq. 2 when n = 2k – 1. In comparison, the effective Hill exponent of the traditional k-site phosphorylation model in Gunawardena (4) is 2k/(k + 1), a number always smaller than k for k > 1.
Moreover, based on Eq. 2, we can conclude (see Text S1 A in the Supporting Material):
-
1.
For fixed n, as k increases, the ultrasensitivity first increases then decreases (Fig. 2 B).
-
2.
When k is fixed, the ultrasensitivity is improved by including more nonessential phosphorylation sites (black versus red for the same k in Fig. 2 B).
-
3.
For fixed α, the ultrasensitivity increases linearly in n (points on the same ray in Fig. 2 B).
Similar trends are observed through numerical simulations when the λi values are chosen randomly for each site (Fig. 2 C and Fig. S1 in the Supporting Material).
As an illustration of Eq. 2, consider the Wee1 protein in the Xenopus egg cell cycle. It is known that the first three sites (Ser38, Thr53, and Ser62) tend to be phosphorylated before the other two (Thr104 and Thr150), although the other two are essential to the activity of Wee1 (13). When the first four sites are mutated, only Thr150 can be phosphorylated, and this is equivalent to k = n = 1. The estimated Hill exponent by Eq. 2 is 1, close to the observed experimental value of 1.1 (14). When the Thr104 site is mutated alone (Wee1-T104E), the Hill exponent increases in experiments to 1.4 (14). Assuming for simplicity that the first three sites are phosphorylated sequentially, which corresponds to k = n = 4 in our model, one obtains a comparable value of 1.6.
Next, we study the effect of nonessential phosphorylation sites to the threshold of switches under the sequential mechanism. One possible definition of the threshold is EC10, i.e., the input value such that the output reaches 10% of its maximum (6). Our analytical study (see Text S1 C in the Supporting Material) for λi ≈ λ reveals that the threshold increases in k (Fig. 2 D) and decreases in n (green versus red for the same k in Fig. 2 D). The threshold plot of randomly chosen λi shows the same trend (Fig. 2 D, black).
The above analysis focuses on the sequential mechanism. In the nonsequential case, we assume that any subset of at least k phosphorylated sites is sufficient to activate the protein, regardless of the exact position of the sites. The unordered mechanism seems to be especially applicable in the context of bulk electrostatics: when a protein is sufficiently phosphorylated, it may cease to bind to negatively charged or hydrophobic regions, such as the cell membrane, regardless of exactly which sites are involved. This can alter the activity of a protein, as illustrated by the Ste5 protein in yeast (15). It has been shown that phosphorylation sites on the substrate tend to be located in poorly conserved (16) and predominantly disordered (17) regions, which suggests that the exact location of the sites can often be unessential for activation and may support mechanisms such as bulk electrostatics.
In the λi ≈ λ case, the Hill exponent of the steady-state fraction of the active substrates is estimated by (Text S1 C in the Supporting Material)
| (4) |
which is approximately the square-root of H in the sequential case (Fig. S3 A). Therefore, the conclusions of the sequential case still hold (Fig. S3 B), but the ultrasensitivity increases in
instead of n (Text S1 D in the Supporting Material). Conclusions on the threshold under the sequential mechanism also carry over to the nonsequential case (Fig. S3 C), except that for fixed α, the threshold increases in n, regardless of the value of α (Text S1 E in the Supporting Material).
In summary, under both sequential and nonsequential mechanisms, the introduction of nonessential sites appears to have opposite effects on ultrasensitivity and threshold (Table S1). Because a good switch is expected to have both high ultrasensitivity and large threshold, there seems to be an optimal range for the number of nonessential sites when the total number of sites is fixed. Thus, one possible explanation of the requirement of six phosphorylated sites in Sic1 is through the optimization of ultrasensitivity and threshold. If the phosphorylation of Sic1 follows a sequential mechanism and λi ≈ λ, then the largest Hill exponent is achieved when k = 5 (Fig. S4 A). If the phosphorylation of Sic1 is random, k could be further increased to achieve a better threshold without much sacrifice in the ultrasensitivity (Fig. S4 B).
In this new model, by decoupling the total number of sites from the number of phosphorylations needed for activation, we may look at the evolution of multisite phosphorylation, because a given mutation can easily change one without altering the other. For instance, a series of mutations (or a single insertion event), that lead to doubled number of sites while leaving k unchanged, may increase the ultrasensitivity dramatically (a versus b in Fig. 3) while slightly decreasing the threshold (green versus red at k = 5 in Fig. 2 D). Moreover, the evolutionary pressure may drive k to increase (b versus c in Fig. 3). This process can continue to repeat itself over time, and one can speculate that this could sometimes lead to a runaway increase in the number of sites.
Figure 3.

Evolutionary race. (a) k = 5, n = 10; (b) k = 5, n = 20; and (c) k = 10, n = 20.
Intuitively, why will the addition of the nonessential sites increase the switchlike behavior of a system?
Imagine the phosphorylation of an individual protein as a biased random walk between 0 and n phosphorylations. The propensities for phosphorylation and dephosphorylation are given by the constants E and F, respectively. When E is larger than F, the biased random walk gravitates toward states with large phosphorylated residues, and the probability of it falling in Sk,…,Sn is higher than that in Sn. Conversely, even if E is slightly smaller than F, the biased random walk now favors states with few phosphorylated residues, and this accounts for the ultrasensitive behavior of the switch (Text S1 B in the Supporting Material).
In conclusion, we have proposed a mechanism through the use of nonessential sites that could account for the high ultrasensitivity observed in many multisite phosphorylation systems. For given values of the total number of sites and the minimal number of phosphorylations for activation, we have obtained estimates of the effective Hill exponent under both sequential and nonsequential mechanisms. The effect of nonessential sites on both the effective Hill exponent and the threshold of a dose-response curve are analyzed. Our results suggest that inclusion of nonessential phosphorylation sites improves ultrasensitivity, but decreases the threshold (Table S1 in the Supporting Material). Thus, to achieve a good activation switch, there is a balance between the number of nonessential sites and the total number of sites. This new mechanism could be extended to other contexts such as methylation, acetylation, or even the binding of multiple transcription factors on a promoter region.
Acknowledgments
We thank Karen Sachs for the many stimulating conversations, and Jim Ferrell and Lee Bardwell for useful advice.
Q.N. was supported by National Institutes of Health grant Nos. R01GM75309, R01GM67247, and P50GM76516, and National Science Foundation grant No. DMS-0917492.
Supporting Material
References and Footnotes
- 1.Nash P., Tang X., Tyers M. Multisite phosphorylation of a CDK inhibitor sets a threshold for the onset of DNA replication. Nature. 2001;414:514–521. doi: 10.1038/35107009. [DOI] [PubMed] [Google Scholar]
- 2.Welcker M., Singer J., Roberts J.M. Multisite phosphorylation by Cdk2 and GSK3 controls cyclin E degradation. Mol. Cell. 2003;12:381–392. doi: 10.1016/s1097-2765(03)00287-9. [DOI] [PubMed] [Google Scholar]
- 3.Angeli D., Ferrell J.E., Jr., Sontag E.D. Detection of multistability, bifurcations, and hysteresis in a large class of biological positive-feedback systems. Proc. Natl. Acad. Sci. USA. 2004;101:1822–1827. doi: 10.1073/pnas.0308265100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gunawardena J. Multisite protein phosphorylation makes a good threshold but can be a poor switch. Proc. Natl. Acad. Sci. USA. 2005;102:14617–14622. doi: 10.1073/pnas.0507322102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Qian H., Cooper J.A. Temporal cooperativity and sensitivity amplification in biological signal transduction. Biochemistry. 2008;47:2211–2220. doi: 10.1021/bi702125s. [DOI] [PubMed] [Google Scholar]
- 6.Serber Z., Ferrell J.E., Jr. Tuning bulk electrostatics to regulate protein function. Cell. 2007;128:441–444. doi: 10.1016/j.cell.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 7.Liu X.F., Bardwell L., Nie Q. A combination of multisite phosphorylation and substrate sequestration produces switchlike responses. Biophys. J. 2010;98:1396–1407. doi: 10.1016/j.bpj.2009.12.4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lenz P., Swain P.S. An entropic mechanism to generate highly cooperative and specific binding from protein phosphorylations. Curr. Biol. 2006;16:2150–2155. doi: 10.1016/j.cub.2006.09.013. [DOI] [PubMed] [Google Scholar]
- 9.Kapuy O., Barik D., Novák B. Bistability by multiple phosphorylation of regulatory proteins. Prog. Biophys. Mol. Biol. 2009;100:47–56. doi: 10.1016/j.pbiomolbio.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deshaies R.J., Ferrell J.E., Jr. Multisite phosphorylation and the countdown to S phase. Cell. 2001;107:819–822. doi: 10.1016/s0092-8674(01)00620-1. [DOI] [PubMed] [Google Scholar]
- 11.Orlicky S., Tang X., Sicheri F. Structural basis for phosphodependent substrate selection and orientation by the SCFCdc4 ubiquitin ligase. Cell. 2003;112:243–256. doi: 10.1016/s0092-8674(03)00034-5. [DOI] [PubMed] [Google Scholar]
- 12.Furdui C.M., Lew E.D., Anderson K.S. Autophosphorylation of FGFR1 kinase is mediated by a sequential and precisely ordered reaction. Mol. Cell. 2006;21:711–717. doi: 10.1016/j.molcel.2006.01.022. [DOI] [PubMed] [Google Scholar]
- 13.Kim S.Y., Song E.J., Ferrell J.E., Jr. Multisite M-phase phosphorylation of Xenopus Wee1A. Mol. Cell. Biol. 2005;25:10580–10590. doi: 10.1128/MCB.25.23.10580-10590.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Strickfaden S.C., Winters M.J., Pryciak P.M. A mechanism for cell-cycle regulation of MAP kinase signaling in a yeast differentiation pathway. Cell. 2007;128:519–531. doi: 10.1016/j.cell.2006.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown C.J., Takayama S., Dunker A.K. Evolutionary rate heterogeneity in proteins with long disordered regions. J. Mol. Evol. 2002;55:104–110. doi: 10.1007/s00239-001-2309-6. [DOI] [PubMed] [Google Scholar]
- 16.Iakoucheva L.M., Radivojac P., Dunker A.K. The importance of intrinsic disorder for protein phosphorylation. Nucleic Acids Res. 2004;32:1037–1049. doi: 10.1093/nar/gkh253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawaguchi K., Ishiwata S. Thermal activation of single kinesin molecules with temperature pulse microscopy. Cell Motil. Cytoskeleton. 2001;49:41–47. doi: 10.1002/cm.1019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


