Abstract
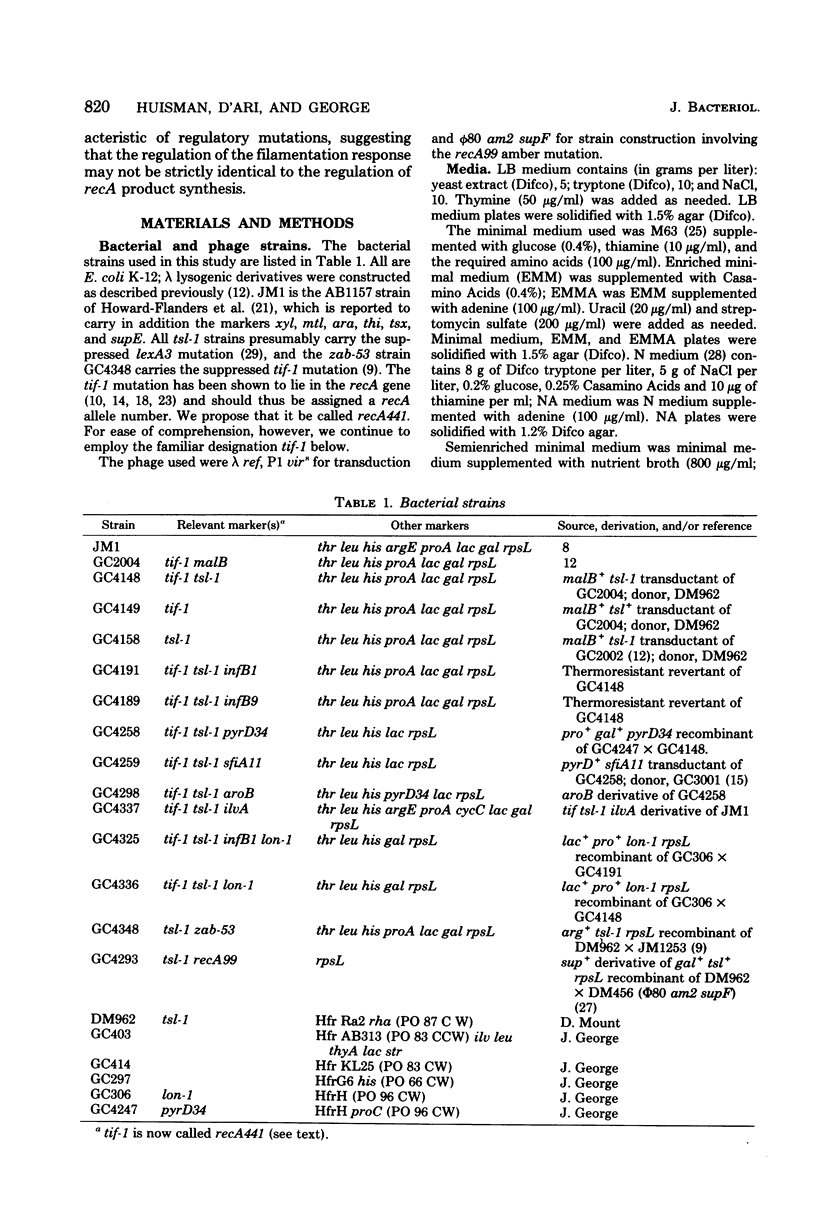
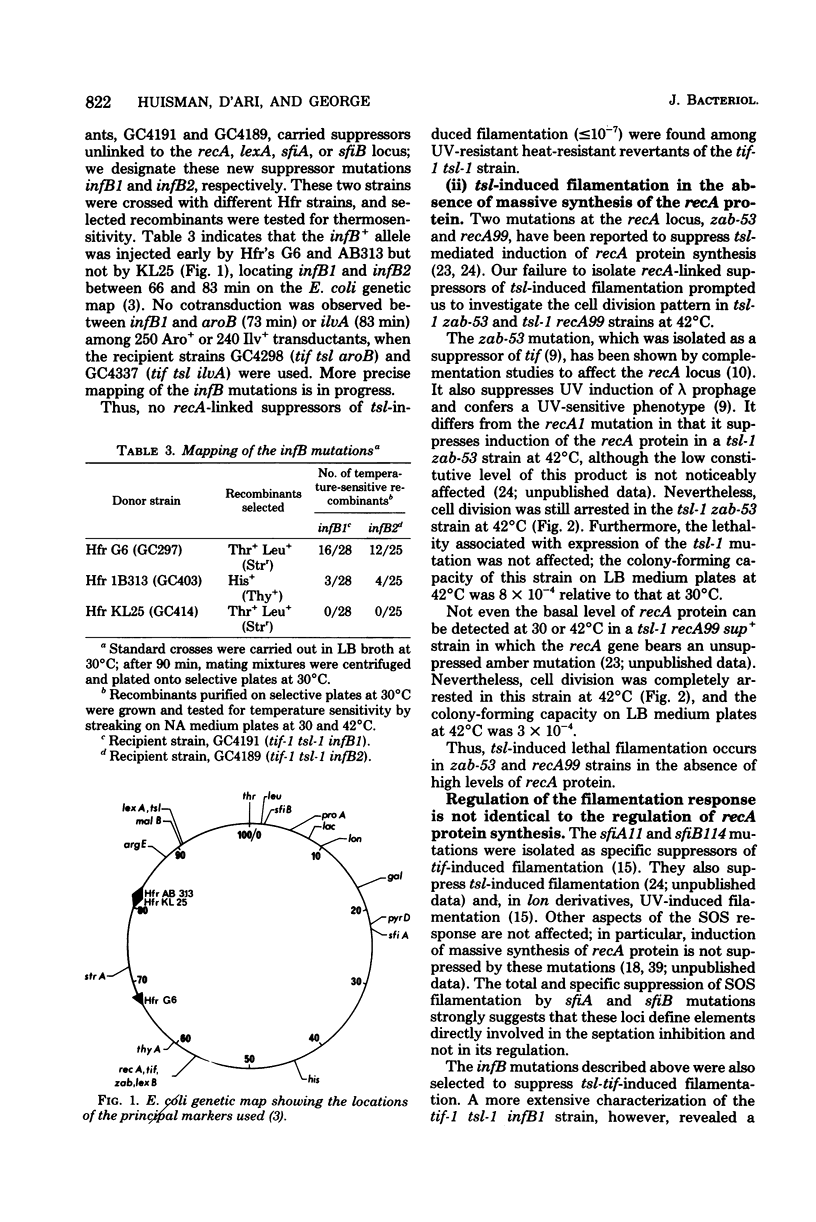
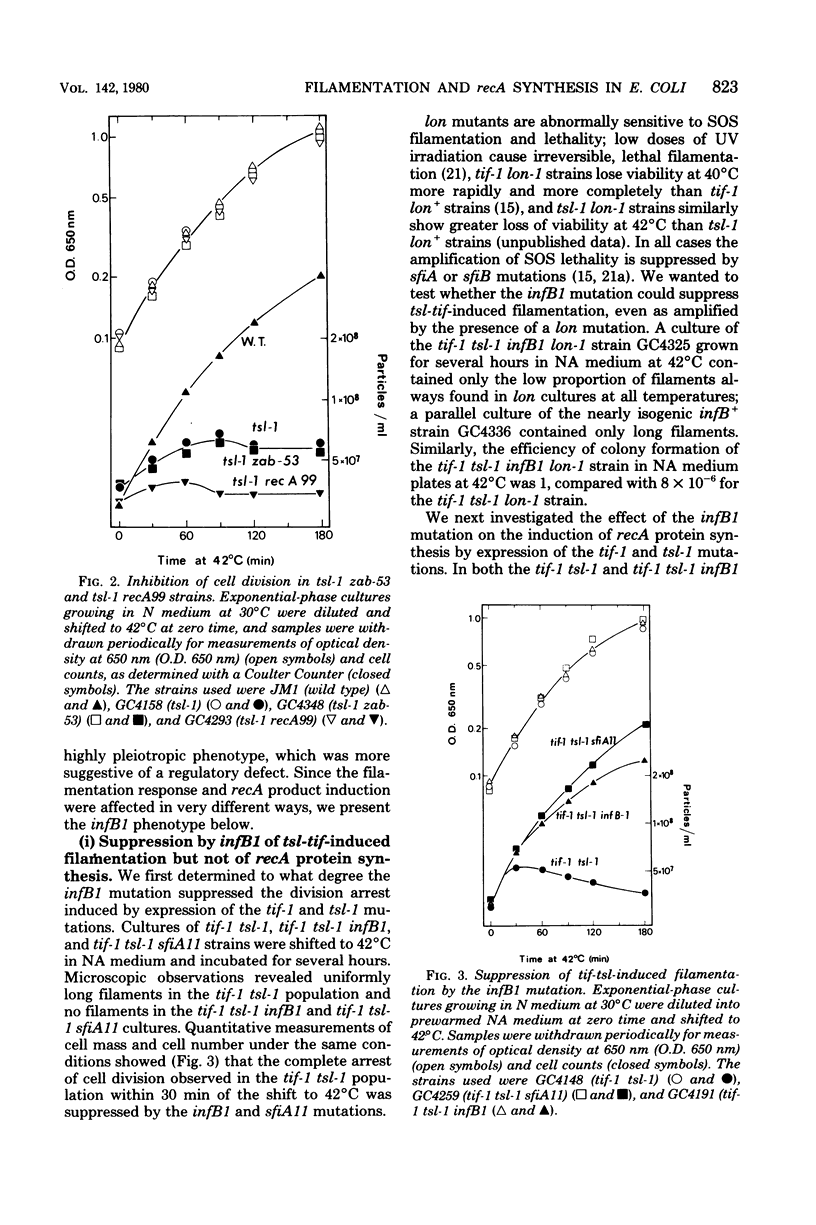
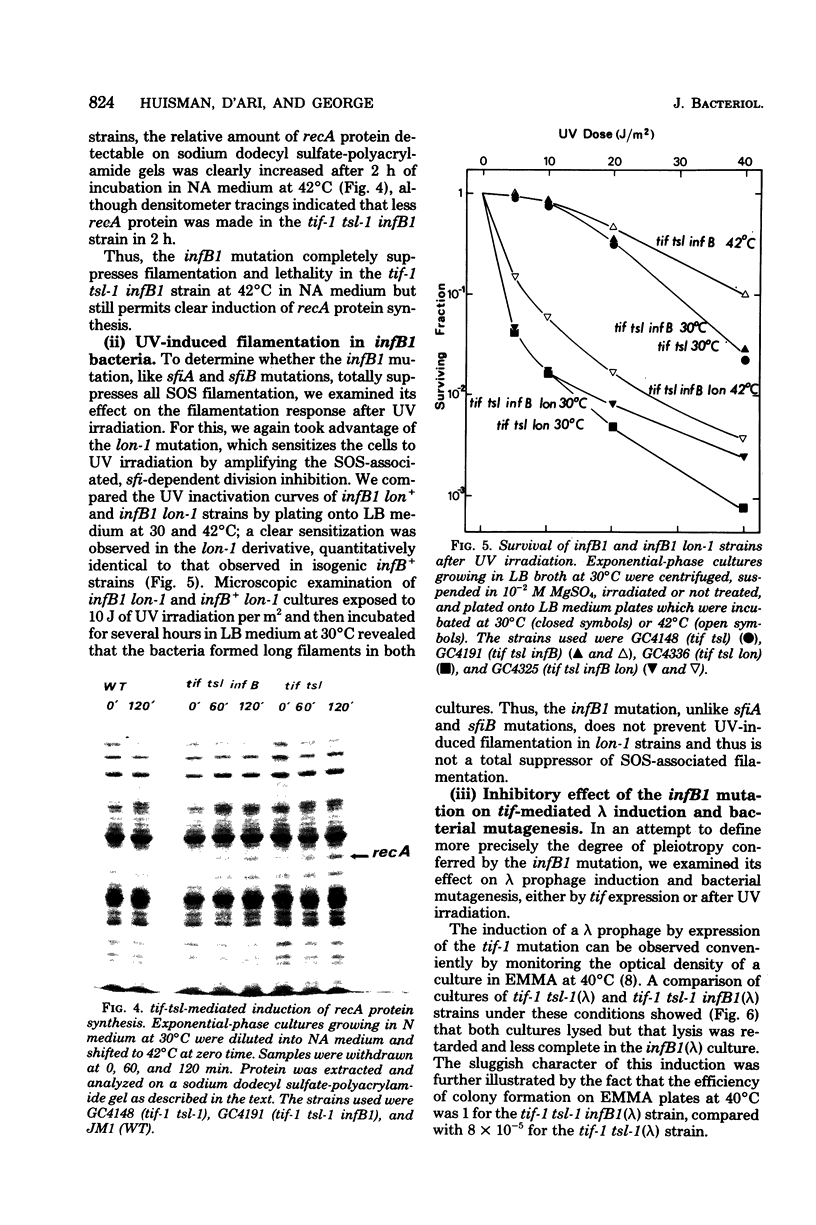
In Escherichia coli, expression of the tif-1 mutation (in the recA gene) induces the “SOS response” at 40°C, including massive synthesis of the recA(tif) protein, cell filamentation, appearance of new repair and mutagenic activities, and prophage induction. Expression of the tsl-1 mutation (in the lexA gene) induces massive synthesis of the recA protein and cell filamentation at 42°C, although other SOS functions are not induced. In this paper we show that the septation inhibition induced in tif and tsl strains at 42°C is not due to the presence of a high concentration of recA protein since (i) no recA mutants (≤10−8) were isolated among thermoresistant nonfilamenting revertants of a tif-1 tsl-1 strain, (ii) in a tsl-1 zab-53 strain, only the low basal level of recA protein was synthesized at 42°C, yet cell division was inhibited, and (iii) in a tsl-1 recA99 (amber) strain, no recA protein could be detected at 42°C, yet cell division was inhibited. Among suppressors of tsl-tif-induced lethality are mutations at a locus which we call infB, located in the 66- to 83-min region. The infB1 mutation confers a highly pleiotropic phenotype, which is suggestive of a regulatory defect; it suppressed tsl-tif-induced filamentation but not recA protein synthesis, it did not suppress ultraviolet-induced filamentation (in a lon derivative), and it reduced but did not abolish tif-mediated induction of λ prophage and bacterial mutagenesis. The dissociation of tsl-tif-induced septation inhibition and recA protein synthesis in the tif-1 tsl-1 infB1 strain suggests that the control of SOS filamentation may not be strictly identical to the control of recA protein synthesis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andoh T., Ozeki H. Suppressor gene Su3+ of E. coli, a structural gene for tyrosine TRNA. Proc Natl Acad Sci U S A. 1968 Mar;59(3):792–799. doi: 10.1073/pnas.59.3.792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armengod M. E., Blanco M. Influence of the recF143 mutation of Escherichia coli K12 on prophage lambda induction. Mutat Res. 1978 Oct;52(1):37–47. doi: 10.1016/0027-5107(78)90093-3. [DOI] [PubMed] [Google Scholar]
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailone A., Blanco M., Devoret R. E. coli K12 inf: a mutant deficient in prophage lambda induction and cell filamentation. Mol Gen Genet. 1975;136(4):291–307. doi: 10.1007/BF00341714. [DOI] [PubMed] [Google Scholar]
- Bailone A., Levine A., Devoret R. Inactivation of prophage lambda repressor in vivo. J Mol Biol. 1979 Jul 5;131(3):553–572. doi: 10.1016/0022-2836(79)90007-x. [DOI] [PubMed] [Google Scholar]
- Bridges B. A., Mottershead R. P., Green M. H. Cell division in Escherichia coli BS-12 is hypersensitive to deoxyribonucleic acid damage by ultraviolet light. J Bacteriol. 1977 May;130(2):724–728. doi: 10.1128/jb.130.2.724-728.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellazzi M., George J., Buttin G. Prophage induction and cell division in E. coli. I. Further characterization of the thermosensitive mutation tif-1 whose expression mimics the effect of UV irradiation. Mol Gen Genet. 1972;119(2):139–152. doi: 10.1007/BF00269133. [DOI] [PubMed] [Google Scholar]
- Castellazzi M., George J., Buttin G. [Prophage induction and cell division in E. coli. II. Linked (recA, zab) and unlinked (lex) suppressors of tif-1-mediated induction and filamentation]. Mol Gen Genet. 1972;119(2):153–174. doi: 10.1007/BF00269134. [DOI] [PubMed] [Google Scholar]
- Castellazzi M., Morand P., George J., Buttin G. Prophage induction and cell division in E. coli. V. Dominance and complementation analysis in partial diploids with pleiotropic mutations (tif, recA, zab and lexB) at the recA locus. Mol Gen Genet. 1977 Jun 24;153(3):297–310. doi: 10.1007/BF00431595. [DOI] [PubMed] [Google Scholar]
- D'Ari R., George J., Huisman O. Suppression of tif-mediated induction of SOS functions in Escherichia coli by an altered dnaB protein. J Bacteriol. 1979 Nov;140(2):381–387. doi: 10.1128/jb.140.2.381-387.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby V., Holland I. B. A kinetic analysis of cell division, and induction and stability of recA protein in U.V. Irradiated ion+ and ion-strains of Escherichia coli K12. Mol Gen Genet. 1979 Oct 2;176(1):121–128. doi: 10.1007/BF00334303. [DOI] [PubMed] [Google Scholar]
- Emmerson P. T., West S. C. Identification of protein X of Escherichia coli as the recA+/tif+ gene product. Mol Gen Genet. 1977 Sep 21;155(1):77–85. doi: 10.1007/BF00268563. [DOI] [PubMed] [Google Scholar]
- GOLDTHWAIT D., JACOB F. SUR LE M'ECANISME DE L'INDUCTION DU D'EVELOPPEMENT DU PROPHAGE CHEZ LES BACT'ERIES LYSOG'ENES. C R Hebd Seances Acad Sci. 1964 Jul 20;259:661–664. [PubMed] [Google Scholar]
- George J., Castellazzi M., Buttin G. Prophage induction and cell division in E. coli. III. Mutations sfiA and sfiB restore division in tif and lon strains and permit the expression of mutator properties of tif. Mol Gen Genet. 1975 Oct 22;140(4):309–332. [PubMed] [Google Scholar]
- Gudas L. J., Mount D. W. Identification of the recA (tif) gene product of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5280–5284. doi: 10.1073/pnas.74.12.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudas L. J., Pardee A. B. DNA synthesis inhibition and the induction of protein X in Escherichia coli. J Mol Biol. 1976 Mar 15;101(4):459–477. doi: 10.1016/0022-2836(76)90240-0. [DOI] [PubMed] [Google Scholar]
- Gudas L. J. The induction of protein X in DNA repair and cell division mutants of Escherichia coli. J Mol Biol. 1976 Jul 5;104(3):567–587. doi: 10.1016/0022-2836(76)90121-2. [DOI] [PubMed] [Google Scholar]
- HOWARD-FLANDERS P., SIMSON E., THERIOT L. THE EXCISION OF THYMINE DIMERS FROM DNA, FILAMENT FORMATION AND SENSITIVITY TO ULTRAVIOLET LIGHT IN ESCHERICHIA COLI K-12. Mutat Res. 1964 Oct;106:219–226. doi: 10.1016/0027-5107(64)90002-8. [DOI] [PubMed] [Google Scholar]
- Horii Z., Clark A. J. Genetic analysis of the recF pathway to genetic recombination in Escherichia coli K12: isolation and characterization of mutants. J Mol Biol. 1973 Oct 25;80(2):327–344. doi: 10.1016/0022-2836(73)90176-9. [DOI] [PubMed] [Google Scholar]
- Huisman O., D'Ari R., George J. Inducible sfi dependent division inhibition in Escherichia coli. Mol Gen Genet. 1980;177(4):629–636. doi: 10.1007/BF00272673. [DOI] [PubMed] [Google Scholar]
- Kneser H. Repair of ultraviolet lesions and induction of lambda prophage. Virology. 1966 Apr;28(4):701–706. doi: 10.1016/0042-6822(66)90254-6. [DOI] [PubMed] [Google Scholar]
- McEntee K. Protein X is the product of the recA gene of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5275–5279. doi: 10.1073/pnas.74.12.5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morand P., Blanco M., Devoret R. Characterization of lexB mutations in Escherichia coli K-12. J Bacteriol. 1977 Aug;131(2):572–582. doi: 10.1128/jb.131.2.572-582.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mound D. W., Walker A. C., Kosel C. Suppression of lex mutations affecting deoxyribonucleic acid repair in Escherichia coli K-12 by closely linked thermosensitive mutations. J Bacteriol. 1973 Nov;116(2):950–956. doi: 10.1128/jb.116.2.950-956.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount D. W. Isolation and genetic analysis of a strain of Escherichia coli K-12 with an amber recA mutation. J Bacteriol. 1971 Jul;107(1):388–389. doi: 10.1128/jb.107.1.388-389.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount D. W., Low K. B., Edmiston S. J. Dominant mutations (lex) in Escherichia coli K-12 which affect radiation sensitivity and frequency of ultraviolet lght-induced mutations. J Bacteriol. 1972 Nov;112(2):886–893. doi: 10.1128/jb.112.2.886-893.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount D. W., Walker A. C., Kosel C. Effect of tsl mutations in decreasing radiation sensitivity of a recA- strain of Escherichia coli K-12. J Bacteriol. 1975 Mar;121(3):1203–1207. doi: 10.1128/jb.121.3.1203-1207.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radman M. SOS repair hypothesis: phenomenology of an inducible DNA repair which is accompanied by mutagenesis. Basic Life Sci. 1975;5A:355–367. doi: 10.1007/978-1-4684-2895-7_48. [DOI] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W., Craig N. L. Escherichia coli recA gene product inactivates phage lambda repressor. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4714–4718. doi: 10.1073/pnas.75.10.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. W., Roberts C. W. Proteolytic cleavage of bacteriophage lambda repressor in induction. Proc Natl Acad Sci U S A. 1975 Jan;72(1):147–151. doi: 10.1073/pnas.72.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgwick S. G., Levine A., Bailone A. Induction of recA+-protein synthesis in Escherichia coli. Mol Gen Genet. 1978 Apr 17;160(3):267–276. doi: 10.1007/BF00332970. [DOI] [PubMed] [Google Scholar]
- Wackernagel W., Winkler U. A mutation in Escherichia coli enhancing the UV-mutability of phage lambda but not of its infectious DNA in a spheroplast assay. Mol Gen Genet. 1972;114(1):68–79. doi: 10.1007/BF00268748. [DOI] [PubMed] [Google Scholar]
- Walker J. R., Smith J. A. Cell division of the Escherichia coli lon- mutant. Mol Gen Genet. 1970;108(3):249–257. doi: 10.1007/BF00283355. [DOI] [PubMed] [Google Scholar]
- Wechsler J. A., Gross J. D. Escherichia coli mutants temperature-sensitive for DNA synthesis. Mol Gen Genet. 1971;113(3):273–284. doi: 10.1007/BF00339547. [DOI] [PubMed] [Google Scholar]
- West S. C., Emmerson P. T. Induction of protein synthesis in Escherichia coli following UV- or gamma-irradiation, mitomycin C treatment or tif Expression. Mol Gen Genet. 1977 Feb 28;151(1):57–67. doi: 10.1007/BF00446913. [DOI] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]



