Abstract
Interstitial pneumonitis (IP) is an uncommon pulmonary complication associated with interferon (IFN) therapy for chronic hepatitis C virus (HCV) infection. Pneumonitis can occur at any stage of HCV treatment, ranging from 2 to 48 wk, usually in the first 12 wk. Its most common symptoms are dyspnoea, dry cough, fever, fatigue, arthralgia or myalgia, and anorexia, which are reversible in most cases after cessation of IFN therapy with a mean subsequent recovery time of 7.5 wk. Bronchoalveolar lavage in combination with chest high resolution computed tomography has a high diagnostic value. Prompt discontinuation of medication is the cornerstone, and corticosteroid therapy may not be essential for patients with mild-moderate pulmonary functional impairment. The severity of pulmonary injury is associated with the rapid development of IP. We suggest that methylprednisolone pulse therapy followed by low dose prednisolone for a short term is necessary to minimize the risk of fatal pulmonary damage if signs of significant pulmonary toxicity occur in earlier stage. Clinicians should be aware of the potential pulmonary complication related to the drug, so that an early and opportune diagnosis can be made.
Keywords: Chronic hepatitis C, Interferon α, Interstitial pneumonitis, Management, Corticosteroid therapy
INTRODUCTION
Combination of pegylated interferon (PEG-IFN) and ribavirin has been the standard program for hepatitis C virus (HCV) infection. Pulmonary complications, although uncommon, are associated with the use of IFN for HCV infection[1-21]. The overall incidence of pulmonary toxicities is < 1%[10], and the unusual wide spectrum includes pulmonary sarcoidosis, interstitial pneumonitis (IP), bronchiolitis obliterans organizing pneumonia, pleural effusion, and exacerbation of bronchial asthma and acute respiratory distress syndrome[8,9,18-21]. The precise incidence rate of IFN-induced IP is still unclear. In a Japanese study by Okanoue et al[6], 3 of 667 IFN-treated patients with HCV infection developed IP, with an incidence of 0.45%, which is significantly higher than the reported annual incidence rate in the general populations of Japan, United States and other countries. Thus, the incidence rate of this complication is estimated to be 0.01%-0.3%[22].
With the increasing use of IFN, more and more IP cases will present. Based on this background, the present review was to investigate the clinical data, chest radiography, chest high resolution computed tomography (HRCT), pulmonary function test (PFT), histological findings, treatment and prognosis, with an attempt to propose an algorithm for the evaluation of suspected IP patients.
PATHOGENESIS
The mechanism underlying IFN-induced IP has not yet been clarified. It has been reported that IFN can provoke lung tissue fibrosis by inhibiting suppressor T cells, increasing cytotoxic T cells, inducing proinflammatory cytokines, and exaggerating release of fibrinogenic cytokines[23,24]. PEG-IFN provides a higher IFN level because of its prolonged half-life and increased area under the curve. However, increased risk of IP due to the use of PEG-IFN has not been reported since its first application for HCV in 2000[25,26]. The major side effects of ribavirin are dose-dependent hemolytic anemia, cough, dyspnoea, rash, depression, and dyspepsia. No case of IP caused by ribavirin has yet been described, and whether ribavirin plays a potential role in the development of IP remains theoretical.
CLINICAL FEATURES
English papers were searched from PubMed, Blackwell, Elsevier, Springer and OVID in 1986-2009 using various combinations of “IFN”, “HCV”, “IP” and “pulmonary toxicity”. References in the papers were reviewed as additional sources.
Twenty-four cases (with detailed data available from 20) reported in 17 articles were included in this review. Their main characteristics are shown in Table 1. Of these cases, 11 (46%) were males and 13 (54%) were females. Their mean age was 54.2 years (range 39-72 years). IP occurred in a comparable proportion of females and males, and was more frequent in older patients. The mean IFN treatment time before the diagnosis of IP was 11.8 wk (range 2-48 wk) and less than 12 wk in 73% of them. Of the 24 cases, 3 were given Sho-saiko-to (a Chinese herbal drug that increases the risk of IP), 12 were treated with ribavirin and IFN, and 9 were given IFN without any other medications. The most common symptoms were dyspnoea (80%, 16/20), dry cough (75%, 15/20) and fever (55%, 11/20), followed by fatigue (25%, 5/20), arthralgia or myalgia (20%, 4/20), and anorexia (15%, 3/20). Physical examination revealed unilateral or bilateral lung field fine crackles in 10 out of 11 patients.
Table 1.
Case reports of interstitial pneumonitis associated with interferon therapy for hepatitis C virus
| Ref. | Yr | Age (yr)/sex | Administered | Duration of IFN therapy (wk) | Diagnostic method | Treatment | Outcome |
| [1] | 1993 | 60/F | IFNα-2b | 8 | C, R, B | D | Resolved |
| [2] | 1994 | 58/F | IFN-α | 12 | C, R, B | D | Resolved |
| 56/M | IFN-α | 6 | C, R, B | D + S | Resolved | ||
| 72/F | IFN-α | 3 | C, R | D + S | Improvement | ||
| [3] | 1994 | 48/F | IFN-α | 9 | C, R, B | D + S | Resolved |
| [4] | 1994 | 62/F | IFN-α | 3 | C, R, B | D + S | Resolved |
| [5] | 1996 | 49/F | IFNα-2b, Sho-saiko-to | 6 | C, R, B | D + S | Resolved |
| 59/M | IFNα-2b, Sho-saiko-to | 4 | C, R, B | D + S | Resolved | ||
| 42/M | IFNα-2b | 16 | C, R, B | D + S | Resolved | ||
| [6] | 1996 | 46/M | IFN-α, Sho-saiko-to | 4 | C, R | D | Resolved |
| 57/M | IFN-α | 5 | C, R | D | Resolved | ||
| 59/M | IFN-α | 23 | C, R | D | Resolved | ||
| [7] | 2001 | 57/M | IFN-α, ribavirin | 12 | C, R, B | D | Resolved |
| [8] | 2002 | 48/F | IFNα-2b, ribavirin | 24 | C, R | D | Resolved |
| PEGIFNα-2a, ribavirin | 6 | C, R, B | D + S | Improvement | |||
| 50/M | IFNα-2b, ribavirin | 4 | C, R, B | D + S | Resolved | ||
| [9] | 2003 | 49/M | PEGIFNα-2b, ribavirin | 2 | C, R, B | D + S | Death |
| [10] | 2004 | 71/F | PEGIFNα-2a, ribavirin | 20 | C, R, B | D | Resolved |
| [11] | 2004 | 51/M | PEGIFNα-2b, ribavirin | 5 | C, R, B | D + S | Death |
| [12] | 2005 | 58/F | PEGIFNα-2b, ribavirin | 12 | C, R, B | D + S | Resolved |
| PEGIFNα-2a, ribavirin | 12 | C, R | D | Resolved | |||
| [13] | 2006 | 68/F | IFNα-2b, ribavirin | 14 | C, R, B | D + S | Resolved |
| [14] | 2007 | 47/F | PEGIFNα-2b, ribavirin | 10 | C, R, B | D + S | Resolved |
| [15] | 2008 | 43/F | PEGIFNα-2b, ribavirin | 48 | C, R | D + S | Death |
| [16] | 2009 | 39/F | PEGIFNα-2a, ribavirin | 36 | C, R, B | D + S | Resolved |
| [17] | 2010 | 51/M | PEGIFNα-2b, ribavirin | 4 | C, R, B | D + S | Resolved |
B: Biopsy proven diagnosis; C: Clinical diagnosis; R: Radiological diagnosis; PEGIFN-α: Pegylated interferon α; D: Discontinuation IFN-α; S: Corticosteroids therapy.
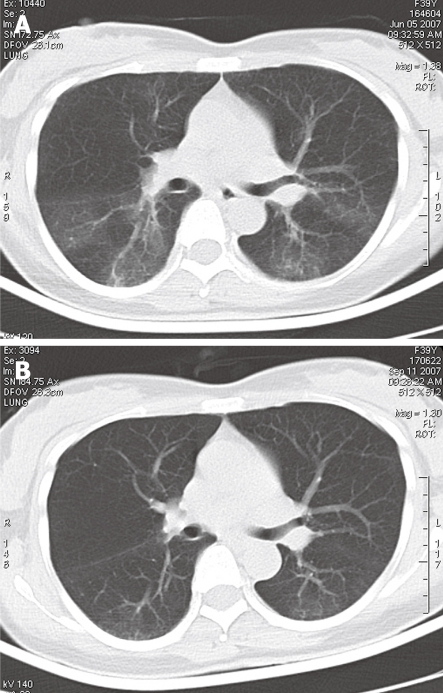
Detailed PFT information was available from 14 cases with a restrictive pulmonary functional impairment pattern. Of the 14 cases, 8 were accompanied with an obstructive pathological change. Arterial blood gas (ABG) analysis showed different extents of hypoxemia in 88% (15/17) of the patients. Chest radiography showed no evidence of lung disease and HRCT showed diffuse infiltration in 4 cases after pulmonary symptoms occurred[10-12,16]. Chest CT scanning showed symmetrical, bilateral interstitial abnormality with areas of ground glass opacity predominantly involving middle lung fields and lung bases (Figure 1A), and visible resolution of inflammatory infiltration following symptom improvement with less dyspnoea and cough (Figure 1B), in 14 out of 21 cases.
Figure 1.
Chest computed tomography scanning showing symmetrical, bilateral interstitial abnormality with areas of ground glass opacity predominantly involving middle lung fields and lung bases (A), and visible resolution of inflammatory infiltration following symptom improvement with less dyspnoea and cough (B) in 14 out of 21 cases.
Bronchoscopy with bronchoalveolar lavage (BAL) was performed for 19 cases. BAL fluid (BALF) analysis demonstrated that the number of lymphocytes, macrophages and other inflammatory cells was increased with an elevated proportion of CD8+/CD4+ cells[27]. Culture and staining of bronchial lavage were negative for bacteria, fungi, acid fast bacilli (AFB) and viruses in these cases. Of the 24 cases included in the review, 17 were diagnosed as IP, which was confirmed by lung biopsies including transbronchial, thoracoscopic lung and open lung biopsies. A biopsy was considered positive for IP when histology demonstrated interstitial infiltration, principally of lymphocytes with macrophages, interstitial fibrosis with thickening of alveolar walls and without evidence of granulomatous lesions in parenchyma[28], although bronchial biopsy showed normal epithelium in 3 cases[12,13,16].
DIAGNOSIS
The diagnosis of IFN-induced IP is not easy since no uniform international diagnostic criteria for IFN-induced IP are available at present. Based upon the classification of causal criteria for adverse effects of medications from the World Health Organization[29], we suggest that the diagnostic criteria for IFN-induced IP should include (1) dyspnoea, dry cough, fever, fatigue, arthralgia or myalgia, and anorexia during antiviral treatment; (2) patchy shadows on chest radiograph and ground glass shadows and/or reticular opacities on HRCT; (3) a restrictive pulmonary functional impairment pattern with/without hypoxemia; (4) no pulmonary infection with bacteria, fungi, AFB and viruses, and congestive heart failure; (5) symptom improvement of pneumonitis after cessation of IFN therapy; and (6) one of the following positive results, including bronchoscopy with BAL, lung biopsy and IP recurrence on re-challenge with IFN therapy. The diagnosis was certain, probable and possible, respectively, when the patient had the 6 major features, the first 5 features and the first 4 features.
EVALUATION OF DISEASE SEVERITY
The ATS/ERS guidelines[30] can accurately predict the clinical severity or prognosis of individual patients, and are generally used in patients with obstructive, restrictive and mixed pulmonary defects. Severe pulmonary functional impairment is defined as FEV1%pred < 50% in PFT or arterial oxygen pressure < 60 mmHg in ABG[30]. Mild-moderate pulmonary functional impairment is defined as FEV1%pred > 50% and arterial oxygen pressure > 60 mmHg. The 8 patients with severe pulmonary functional impairment[2,4,5,7,9,11] received IFN therapy for a shorter time (5.9 wk vs 14.4 wk, P < 0.05) and more of them were treated with corticosteroids than other cases (75% vs 56%). No significant difference was found in age and sex between the two subgroups (Table 2).
Table 2.
Clinical data about patients in two subgroups
| Severity of IP | Cases, n (%) | Sex (M/F) | Age (yr, mean) | Duration of IFN therapy (wk, mean) | Steroid therapy |
| Severe group | 8 (36) | 4/4 | 57.1 | 5.9 | 6/2 |
| M and M group | 14 (64) | 7/7 | 52.1 | 14.4 | 9/7 |
| P value | -- | 1.00 | 0.2011 | 0.0412 | 0.43 |
U = 31.5, W = 109.5;
U = 20.5, W = 53.5, P < 0.05 by Mann-Whitney test. M and M group: Mild or moderate pulmonary functional impairment group. IP: Interstitial pneumonitis; IFN: Interferon.
TREATMENT
The standard strategy for managing drug-induced IP is to immediately discontinue the drug and start corticosteroid pulse therapy, combined with supportive therapy for severe respiratory condition[6]. IFN therapy should be discontinued in all cases following the diagnosis of IP. Corticosteroids were prescribed for pneumonitis in 67% (16/24) cases included in our review. The dosage and length of corticosteroids are highly variable, usually started at a relatively high dosage. IP in a 39-year-old female patient was treated with methylprednisolone (160 mg/d for 3 d, 80 mg/d for 4 d), followed by prednisolone (30 mg/d), with the dosage gradually tapered over the next 12 wk[16]. A 56-year-old male patient was treated with methylprednisolone (2 g/d for 3 d) and prednisolone (40 mg/d for 2 d)[2]. Prednisolone (60 mg/d) and azathioprine (100 mg/d) were given after relapse of IP initially treated with prednisolone (30 mg/d)[4]. Some patients started on oral corticosteroid therapy (40 mg/d)[8,13,17], while other patients started on prednisolone (125 mg/d)[11,15] or on corticosteroid by inhalation[12]. Of the 8 out of the 24 patients (33%) included in our review, 6 suffering from mild-moderate pulmonary functional impairment had a resolution of IP after the drug was discontinued without administration of corticosteroids.
Corticosteroid treatment of IP associated with IFN therapy for HCV infection is controversial. Which patients need corticosteroid therapy, what dosage of corticosteroids should be chosen and how long the patients should be treated are unclear. Based on this review, corticosteroid therapy may not be essential for patients with mild-moderate pulmonary functional impairment. Methylprednisolone pulse therapy (160 mg/d for 3 d) followed by prednisolone (30 mg/d) for a short term may be a good choice for patients with signs of significant pulmonary toxicity in earlier stage.
OUTCOMES
In our review, the prognosis of IFN-induced IP was different. Of the 24 patients, 19 had a complete resolution in a follow-up time of 7.5 wk (range 2-12 wk), 2 required permanent corticosteroid treatment to control their respiratory symptoms[2,8], 3 were treated with PEG-IFNα-2b and died due to acute respiratory failure, chronic hypoxia-induced cerebral edema and acute cholestatic hepatitis, respectively[9,11,15]. Of the 11 patients who developed IP within 6 wk, two cases died[9,11] and 2 cases required permanent corticosteroid therapy[2,8]. The other 13 patients who developed IP over 6 wk, one case died[15] and none required permanent corticosteroid therapy.
PROPOSED ALGORITHM
In general, IP occurs within the first 12 wk after IFN therapy for HCV infection. Clinical presentations such as dyspnoea, dry cough, fever, fatigue, arthralgias or myalgias, and anorexia during the treatment are attributed to the common side effects of IFN and ribavirin therapy. It is worth noting that these symptoms are usually present or exacerbated after elimination or remission of the general side effects of antiviral treatment. Laboratory assessment should include PFT and ABG analysis, blood test, culture of blood, sputum specimens, chest radiography and HRCT, BALF analysis and lung biopsy.
Chest X-ray plays an important role in the diagnosis of IP. Of the 24 patients included in this review, 4 showed no evidence of lung disease on chest radiograph after pulmonary symptoms occurred[10-12,16], indicating that chest CT scan should be performed for such patients. The occurrence of fatal evolution in the patients confirms this recommendation[11]. HRCT scanning may provide a high diagnostic value as it can show the lung interstitial infiltrations in earlier stage[31]. BAL has become a standard diagnostic procedure for the majority of patients with drug-induced interstitial lung diseases[27]. BAL with a pattern of lymphocytes, neutrophils, and eosinophils, or a mixed cellular pattern can be used as an adjunct to diagnosis[27]. Besides, negative culture and staining of bronchial washing help to exclude pneumonia resulting from infection with bacteria, fungi, AFB and viruses. BAL in combination with HRCT can increase the diagnostic power of both methods, and may even obviate more invasive procedures in some cases[27,31].
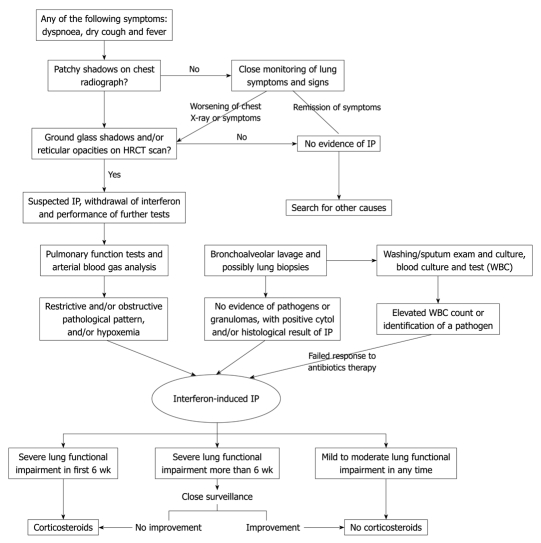
There is convincing evidence that corticosteroids increase HCV replication[32,33]. Whether corticosteroids should be included in the treatment modality for IP associated with IFN therapy for HCV infection remains controversial. We, thus, suggest an algorithm for the evaluation of suspected IP patients (Figure 2) and clinicians should be aware of the potential pulmonary complication related to the drug, so that an early and opportune diagnosis can be made.
Figure 2.
Algorithm for the diagnosis and management of interstitial pneumonitis associated with interferon therapy for hepatitis C virus infection. HRCT: High resolution computed tomography; IP: Interstitial pneumonitis.
CONCLUSION
There are some limitations in this review. All the information came from case reports, whereby cannot be controlled for confounding factors. Detailed data were not available from some cases, thus making it difficult to identify the risk factors for the occurrence of IP. Despite the limitations, several conclusions can be made based upon the available data.
IP can occur at any stage of IFN therapy for HCV infection. Close monitoring is necessary for the treatment of HCV with IFN in early stage, especially for older cases. Prompt discontinuation of medication is the cornerstone, and corticosteroid therapy may not be essential for patients with mild- moderate pulmonary functional impairment. The severity of pulmonary injury may be associated with the rapid development of IP, indicating that methylprednisolone pulse therapy, followed by low dose prednisolone therapy for a short term, is necessary to minimize the risk of fatal pulmonary damage if signs of significant pulmonary toxicity occur in earlier stage (within 6 wk) during antiviral therapy.
Footnotes
Supported by Natural Science Foundation of Shaanxi Province, China, No. 2008K09-05
Peer reviewers: Dr. Yoshiaki Iwasaki, MD, Department of Gastroenterology and Hepatology, Okayama University Graduate School of Medicine, Dentistry, and Pharmaceutical Sciences, 2-5-1, Shikata-cho, Okayama 700-8558, Japan; Dr. Paolo Del Poggio, MD, Hepatology Unit, Department of Internal Medicine, Treviglio Hospital, Piazza Ospedale 1, Treviglio Bg 24047, Italy
S- Editor Tian L L- Editor Wang XL E- Editor Zheng XM
References
- 1.Kamisako T, Adachi Y, Chihara J, Yamamoto T. Interstitial pneumonitis and interferon-alfa. BMJ. 1993;306:896. doi: 10.1136/bmj.306.6882.896-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chin K, Tabata C, Sataka N, Nagai S, Moriyasu F, Kuno K. Pneumonitis associated with natural and recombinant interferon alfa therapy for chronic hepatitis C. Chest. 1994;105:939–941. doi: 10.1378/chest.105.3.939. [DOI] [PubMed] [Google Scholar]
- 3.Moriya K, Yasuda K, Koike K, Ichinose Y, Yotsuyanagi H, Kurokawa K, Iino S. Induction of interstitial pneumonitis during interferon treatment for chronic hepatitis C. J Gastroenterol. 1994;29:514–517. doi: 10.1007/BF02361253. [DOI] [PubMed] [Google Scholar]
- 4.Hizawa N, Kojima J, Kojima T, Sukoh N, Yamaguchi E, Kawakami Y, Matsushima T. A patient with chronic hepatitis C who simultaneously developed interstitial pneumonia, hemolytic anemia and cholestatic liver dysfunction after alpha-interferon administration. Intern Med. 1994;33:337–341. doi: 10.2169/internalmedicine.33.337. [DOI] [PubMed] [Google Scholar]
- 5.Ishizaki T, Sasaki F, Ameshima S, Shiozaki K, Takahashi H, Abe Y, Ito S, Kuriyama M, Nakai T, Kitagawa M. Pneumonitis during interferon and/or herbal drug therapy in patients with chronic active hepatitis. Eur Respir J. 1996;9:2691–2696. doi: 10.1183/09031936.96.09122691. [DOI] [PubMed] [Google Scholar]
- 6.Okanoue T, Sakamoto S, Itoh Y, Minami M, Yasui K, Sakamoto M, Nishioji K, Katagishi T, Nakagawa Y, Tada H, et al. Side effects of high-dose interferon therapy for chronic hepatitis C. J Hepatol. 1996;25:283–291. doi: 10.1016/s0168-8278(96)80113-9. [DOI] [PubMed] [Google Scholar]
- 7.Karim A, Ahmed S, Khan A, Steinberg H, Mattana J. Interstitial pneumonitis in a patient treated with alpha-interferon and ribavirin for hepatitis C infection. Am J Med Sci. 2001;322:233–235. doi: 10.1097/00000441-200110000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Kumar KS, Russo MW, Borczuk AC, Brown M, Esposito SP, Lobritto SJ, Jacobson IM, Brown RS Jr. Significant pulmonary toxicity associated with interferon and ribavirin therapy for hepatitis C. Am J Gastroenterol. 2002;97:2432–2440. doi: 10.1111/j.1572-0241.2002.05999.x. [DOI] [PubMed] [Google Scholar]
- 9.Abi-Nassif S, Mark EJ, Fogel RB, Hallisey RK Jr. Pegylated interferon and ribavirin-induced interstitial pneumonitis with ARDS. Chest. 2003;124:406–410. doi: 10.1378/chest.124.1.406. [DOI] [PubMed] [Google Scholar]
- 10.Midturi J, Sierra-Hoffman M, Hurley D, Winn R, Beissner R, Carpenter J. Spectrum of pulmonary toxicity associated with the use of interferon therapy for hepatitis C: case report and review of the literature. Clin Infect Dis. 2004;39:1724–1729. doi: 10.1086/425746. [DOI] [PubMed] [Google Scholar]
- 11.Fuhrmann V, Kramer L, Bauer E, Laferl H, Tucek G, Dekan G, Schenk P. Severe interstitial pneumonitis secondary to pegylated interferon alpha-2b and ribavirin treatment of hepatitis C infection. Dig Dis Sci. 2004;49:1966–1970. doi: 10.1007/s10620-004-9602-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Renou C, Germain S, Harafa A, Martin S, Larroque O, Muller P, Bertrand JJ, Halfon P. Interstitial pneumonia recurrence during chronic hepatitis C treatment. Am J Gastroenterol. 2005;100:1625–1626. doi: 10.1111/j.1572-0241.2005.50006_9.x. [DOI] [PubMed] [Google Scholar]
- 13.Hillier AE, Mand J, Raza A, Markov M, Nadir A. Consensus interferon induced interstitial pneumonitis in a patient with HCV. Am J Gastroenterol. 2006;101:200–202. doi: 10.1111/j.1572-0241.2006.00393_3.x. [DOI] [PubMed] [Google Scholar]
- 14.Chen YC, Lu SN, Lin MC. Interstitial pneumonitis after combination therapy with pegylated interferon alpha-2b and ribavirin for chronic hepatitis C. Chang Gung Med J. 2007;30:92–97. [PubMed] [Google Scholar]
- 15.Carrillo-Esper R, González-Avila D, Uribe-Ríos M, Méndez-Sánchez N. Interstitial pneumonitis associated with pegylated interferon alpha-2b therapy for chronic hepatitis C: case report. Ann Hepatol. 2008;7:87–90. [PubMed] [Google Scholar]
- 16.Ji FP, Li ZX, Deng H, Xue HA, Liu Y, Li M. [Clinical features of interstitial pneumonitis due to interferon alpha therapy for chronic hepatitis C] Nanfang Yike Daxue Xuebao. 2009;29:667–670. [PubMed] [Google Scholar]
- 17.Slavenburg S, Heijdra YF, Drenth JP. Pneumonitis as a consequence of (peg)interferon-ribavirin combination therapy for hepatitis C: a review of the literature. Dig Dis Sci. 2010;55:579–585. doi: 10.1007/s10620-009-0797-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alazemi S, Campos MA. Interferon-induced sarcoidosis. Int J Clin Pract. 2006;60:201–211. doi: 10.1111/j.1742-1241.2005.00651.x. [DOI] [PubMed] [Google Scholar]
- 19.Monsuez JJ, Carcelain G, Charniot JC, Barrière J, Duclos-Vallée JC, Parent F, Autran B. T cells subtypes in a patient with interferon-alpha induced sarcoidosis. Am J Med Sci. 2009;337:60–62. doi: 10.1097/01.MAJ.0000308932.93221.68. [DOI] [PubMed] [Google Scholar]
- 20.Arora A, Vargas L, Kuzniar TJ. Pleural effusion associated with pegylated interferon alpha and ribavirin treatment for chronic hepatitis C. J Hosp Med. 2009;4:E45–E46. doi: 10.1002/jhm.452. [DOI] [PubMed] [Google Scholar]
- 21.Bini EJ, Weinshel EH. Severe exacerbation of asthma: a new side effect of interferon-alpha in patients with asthma and chronic hepatitis C. Mayo Clin Proc. 1999;74:367–370. doi: 10.4065/74.4.367. [DOI] [PubMed] [Google Scholar]
- 22.Solsky J, Liu J, Peng M, Schaerer M, Tietz A. Rate of interstitial pneumonitis among hepatitis virus C-infected patients treated with pegylated interferon. J Hepatol. 2009;50 Suppl 1:S238. [Google Scholar]
- 23.Tilg H. New insights into the mechanisms of interferon alfa: an immunoregulatory and anti-inflammatory cytokine. Gastroenterology. 1997;112:1017–1021. doi: 10.1053/gast.1997.v112.pm9041265. [DOI] [PubMed] [Google Scholar]
- 24.Borden EC, Parkinson D. A perspective on the clinical effectiveness and tolerance of interferon-alpha. Semin Oncol. 1998;25:3–8. [PubMed] [Google Scholar]
- 25.Zeuzem S, Feinman SV, Rasenack J, Heathcote EJ, Lai MY, Gane E, O'Grady J, Reichen J, Diago M, Lin A, et al. Peginterferon alfa-2a in patients with chronic hepatitis C. N Engl J Med. 2000;343:1666–1672. doi: 10.1056/NEJM200012073432301. [DOI] [PubMed] [Google Scholar]
- 26.Glue P, Rouzier-Panis R, Raffanel C, Sabo R, Gupta SK, Salfi M, Jacobs S, Clement RP. A dose-ranging study of pegylated interferon alfa-2b and ribavirin in chronic hepatitis C. The Hepatitis C Intervention Therapy Group. Hepatology. 2000;32:647–653. doi: 10.1053/jhep.2000.16661. [DOI] [PubMed] [Google Scholar]
- 27.Costabel U, Uzaslan E, Guzman J. Bronchoalveolar lavage in drug-induced lung disease. Clin Chest Med. 2004;25:25–35. doi: 10.1016/S0272-5231(03)00143-6. [DOI] [PubMed] [Google Scholar]
- 28.Katzenstein AL, Myers JL. Idiopathic pulmonary fibrosis: clinical relevance of pathologic classification. Am J Respir Crit Care Med. 1998;157:1301–1315. doi: 10.1164/ajrccm.157.4.9707039. [DOI] [PubMed] [Google Scholar]
- 29.Edwards IR, Aronson JK. Adverse drug reactions: definitions, diagnosis, and management. Lancet. 2000;356:1255–1259. doi: 10.1016/S0140-6736(00)02799-9. [DOI] [PubMed] [Google Scholar]
- 30.Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, Coates A, van der Grinten CP, Gustafsson P, Hankinson J, Jensen R, Johnson DC, MacIntyre N, McKay R, Miller MR, Navajas D, Pedersen OF, Wanger J. Interpretative strategies for lung function tests. Eur Respir J. 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 31.Sharma S, Maycher B. Is HRCT the best way to diagnose idiopathic interstitial fibrosis? Curr Opin Pulm Med. 2006;12:323–330. doi: 10.1097/01.mcp.0000239548.62949.66. [DOI] [PubMed] [Google Scholar]
- 32.Magy N, Cribier B, Schmitt C, Ellero B, Jaeck D, Boudjema K, Wolf P, Labouret N, Doffoel M, Kirn A, et al. Effects of corticosteroids on HCV infection. Int J Immunopharmacol. 1999;21:253–261. doi: 10.1016/s0192-0561(99)00005-3. [DOI] [PubMed] [Google Scholar]
- 33.Henry SD, Metselaar HJ, Van Dijck J, Tilanus HW, Van Der Laan LJ. Impact of steroids on hepatitis C virus replication in vivo and in vitro. Ann N Y Acad Sci. 2007;1110:439–447. doi: 10.1196/annals.1423.046. [DOI] [PubMed] [Google Scholar]