Abstract
Purpose
Recent studies suggest that donor B cells as well as T cells contribute to immune pathology in patients with chronic graft-versus-host disease (GVHD). B-cell activating factor (BAFF) promotes survival and differentiation of activated B cells. Thus, we tested whether BAFF correlated with chronic GVHD disease activity and time of onset after allogeneic hematopoietic stem cell transplantation (HSCT).
Experimental Design
Patients who had undergone allogeneic HSCT between 1994 and 2005 for hematologic malignancies were studied. ELISA was used to measure plasma BAFF levels and flow cytometry was used to assess BAFF receptor expression on B cells in patients with or without chronic GVHD.
Results
In 104 patients, BAFF levels were significantly higher in patients with active chronic GVHD compared with those without disease (P = 0.02 and 0.0004, respectively).Treatment with high-dose prednisone (≥30 mg/d) was associated with reduced BAFF levels in patients with active chronic GVHD (P = 0.0005). Serial studies in 24 patients showed that BAFF levels were high in the first 3 months after HSCT but subsequently decreased in13 patients who never developed chronic GVHD. In contrast, BAFF levels remained elevated in 11 patients who developed chronic GVHD. Six-month BAFF levels ≥10 ng/mL were strongly associated with subsequent development of chronic GVHD (P < 0.0001). Following transplant, plasma BAFF levels correlated inversely with BAFF receptor expression on B cells (P = 0.01), suggesting that soluble BAFF affected B cells through this receptor.
Conclusion
These results suggest that elevated BAFF levels contribute to B-cell activation in patients with active chronic GVHD.
Chronic graft-versus-host disease (GVHD) continues to be a significant cause of morbidity and mortality following allogeneic hematopoietic stem cell transplantation (HSCT; refs. 1, 2). Approximately 30% to 50% of patients with HLA-matched sibling donors and 50% to 70% of patients with unrelated stem cell donors develop chronic GVHD at a median time to onset of 4 to 6 months after HSCT (3–5). Chronic GVHD has a substantial adverse effect on survival that has not improved substantially over the last 30 years (6, 7). Murine models and clinical studies of chronic GVHD have described various immunologic abnormalities, including alloreactivity, autoreactivity, lymphopenia associated with cellular and humoral immune deficiency, and deficiency of regulatory T cells (1, 8–10). Nevertheless, the immunopathology of human chronic GVHD remains poorly understood, making targeted immune therapies difficult to develop (11, 12).
The role of effector T cells in both acute and chronic GVHD is widely known (13, 14). However, recent studies suggest that B cells also play a role in the development of chronic GVHD (15). In male recipients of hematopoietic stem cells from female donors, HY minor histocompatibility antigens are targets of donor T cells (14). Our laboratory previously showed that HY proteins also elicit specific antibody responses. HY antibodies develop 4 to 8 months following transplant in male recipients with female donors but not in patients with sex-matched donors (16, 17). HY antibodies are capable of distinguishing between HY and highly homologous HX proteins and patients who develop B-cell responses also develop coordinated T-cell responses to different epitopes within the same HY protein (16–19). Consistent with these findings, the presence of antibodies specific for HY proteins were found to correlate with the subsequent development of chronic GVHD (18). Autoantibodies have also been described in patients with chronic GVHD (19, 20). An immunopathologic role for B cells in this disease is further emphasized by the observation that treatment with anti-CD20 monoclonal antibody, rituximab, has resulted in clinical improvement in some patients with steroid-resistant chronic GVHD (21–23).
Although antibodies specific for recipient antigens seem to play a role in chronic GVHD, the mechanisms whereby donor B cells and the antibodies produced by these cells contribute to the immune pathology of this disease are not known. B-cell activating factor (BAFF; also known as BLys) was recently identified as a key regulator of normal B-cell homeostasis in mice and humans (21, 22), and high BAFF levels have been found in patients with a variety of autoimmune diseases (23–25). In the setting of normal BAFF levels, transitional B cells with low-affinity, self-reactive B-cell receptors fail to develop in the periphery (26). In contrast, high BAFF levels rescue low-affinity, self-reactive B cells from deletion by attenuating apoptosis during peripheral B-cell recirculation after germinal center reactions (27, 28). High BAFF levels have also been found to prevent apoptosis of normal human B cells and induce differentiation of memory B cells into antibody-secreting cells (29). Thus, we hypothesized that BAFF pathways might be involved in human chronic GVHD. In this report, we identified a strong correlation between high BAFF levels and active chronic GVHD. Importantly, high BAFF levels preceded the onset of clinically evident disease activity and were markedly reduced in patients receiving high-dose corticosteroids. These results suggest the BAFF/BAFF receptor (BAFF-R) pathway plays a role in the development of chronic GVHD and may represent a novel therapeutic target for either treatment or prevention of this disease.
Materials and Methods
Patient characteristics
All patient samples were collected after written informed consent on protocols approved by the Institutional Review Board of the Dana-Farber/Harvard Cancer Center. One hundred and four patients who had undergone allogeneic HSCT between 1994 and 2005 for hematologic malignancies were studied. Clinical characteristics of these patients are summarized in Table 1. The study included patients who received nonmyeloablative or myeloablative conditioning regimens and bone marrow or mobilized peripheral blood stem cell grafts. Patients whose primary disease relapsed within 1 year of transplant were excluded from this study. Only 14 patients (13%) received T-cell–depleted stem cells. Forty-eight patients had active chronic GVHD at the time of primary analysis. This included patients with either limited or extensive chronic GVHD based on clinical severity and target organs affected per modified Seattle criteria (12). Fifty-six patients had either no GVHD or inactive GVHD at the time of analysis. Patients who never developed chronic GVHD after HSCT were designated as “ no chronic GVHD.” Inactive chronic GVHD was determined by clinical assessment and included patients who had achieved a complete response to immunosuppressive therapy. Patients with active chronic GVHD were more likely to be receiving high-dose oral prednisone (P < 0.0001), defined as ≥30 mg/d, and/or sirolimus (P = 0.03) or tacrolimus (P = 0.01). Median time of analysis was 16 months after transplant for patients with active chronic GVHD compared with 23 months after transplant for patients without active disease (P = 0.06). All other clinical characteristics of patients with active and inactive chronic GVHD were similar in terms of age, gender, type of transplant, and underlying hematologic malignancy.
Table 1.
Clinical characteristics of patients who underwent allogeneic HSCT
| Chronic GVHD | P | ||
|---|---|---|---|
| Extensive/limited, n (%) | Inactive/none, n (%) | ||
| n | 48 | 56 | |
| Age, median (range) | 50 (27–68) | 50 (21–68) | 0.95 |
| Female gender | 20 (42) | 26 (46) | 0.69 |
| Type of transplant | |||
| Myeloablative | 29 (60) | 40 (71) | 0.30 |
| Nonmyeloablative | 19 (40) | 16 (29) | |
| Cell source | |||
| Peripheral blood stem cells | 40 (83) | 39 (70) | 0.11 |
| Bone marrow | 8 (17) | 17 (30) | |
| HLA matching | |||
| Matched (unrelated) | 21 (44) | 20 (36) | 0.68 |
| Matched (related) | 24 (50) | 33 (59) | |
| Mismatched | 3 (6) | 3 (5) | |
| Prednisone dose (mg/d) | |||
| 0 | 15 (31) | 39 (70) | <0.0001 |
| 0–30 | 19 (40) | 15 (27) | |
| ≥30 | 14 (29) | 2 (3) | |
| Sirolimus | 33 (69) | 26 (46) | 0.03 |
| Tacrolimus | 16 (33) | 6 (11) | 0.01 |
| Mycophenolate mofetil | 9 (19) | 4 (7) | 0.13 |
| Acute GVHD | |||
| Grade 1 or none | 31 (65) | 43 (77) | 0.20 |
| Grade 2–4 | 17 (35) | 13 (23) | |
| Months posttransplant, median (range) | 16 (5–79) | 23 (4–135) | 0.06 |
| Acute GVHD prophylaxis* | |||
| Sirolimus containing (group 1) | 33 (69) | 26 (46) | 0.07 |
| Nonsirolimus (group 2) | 10 (21) | 21 (38) | |
| T-cell depletion (group 3) | 5 (10) | 9 (16) | |
| BAFF level (ng/mL), median (range) | 9.9 (1.6–46.4) | 4.8 (1.5–28.0) | 0.0002 |
| Disease | |||
| AML/AML from MDS | 13 (17) | 12 (21) | 0.16 |
| ALL | 3 (6) | 5 (9) | |
| CML | 7 (15) | 16 (29) | |
| CLL | 8 (17) | 1 (2) | |
| MDS | 7 (15) | 7 (12) | |
| NHL | 6 (12) | 8 (14) | |
| HL | 1 (2) | 1 (2) | |
| MM | 0 (0) | 2 (4) | |
| Aplastic anemia | 0 (0) | 1 (2) | |
| Other | 3 (6) | 3 (5) | |
| Good prognosis† | 11 (23) | 13 (23) | >0.99 |
Abbreviations: AML, acute myelogenous leukemia; MDS, myelodysplastic syndrome; CML, chronic myelogenous leukemia; CLL, chronic lymphocytic leukemia, NHL, non–Hodgkin’s lymphoma; HL, Hodgkin’s lymphoma; MM, multiple myeloma.
Group 1: sirolimus and tacrolimus or sirolimus, tacrolimus, and methotrexate; group 2: combinations of ATG, methotrexate, cyclosporine, corticosteroids, tacrolimus, and mycophenolate mofetil; group 3: in vitro depletion of CD6-or CD8-positive T cells.
Patients with acute myelogenous leukemia or acute lymphoblastic leukemia in first complete remission or chronic myelogenous leukemia in chronic phase or patients with aplastic anemia or myelodysplastic syndrome with refractory anemia or refractory anemia with ringed sideroblast.
Processing of patient plasma and peripheral blood cells
Whole blood was drawn into standard EDTA-containing collection tubes. Plasma was separated from whole blood cells by centrifugation at 600 × g. Plasma was stored in aliquots at −80°C and used after first thaw for BAFF measurements. Whole blood for flow cytometry studies was also collected on day of use in EDTA-containing tubes.
BAFF ELISA
Soluble BAFF/BLys levels in patient plasma samples were measured using a commercially available ELISA and the manufacturer’s recommended procedures (R&D Systems).
Flow cytometric analysis of peripheral B cells
Whole blood was processed for flow cytometry using the Prep Plus 2 system (Beckman Coulter, Inc.). Isotype controls were used to set positive and negative gates for both BAFF-R and CD19. Anti-CD19 PC7 (Beckman Coulter) was used per manufacturer’s instructions. BR3 FITC (clone 8A7; R&D Systems) was used to detect surface expression of BAFF-R. Twenty microliter per 106 cells of a 1:10 dilution of BR3 FITC were used because we had determined that this titer allowed detection of BAFF-R on B cells in both patient and healthy donor samples (data not shown). RBCs were lysed and leukocytes were fixed using the Beckman Coulter TQPrep system before fluorescence-activated cell sorting. Cells were analyzed using a Cytomics FC 500 instrument and CXP Analysis 2.0 software (Beckman Coulter).
Statistical analyses
For 2 × 2 table analysis, a two-sided Fisher’s exact test was done. For two-sample comparison of continuous variables, a two-sided Wilcoxon-rank sum test was done. Multiple comparisons were not adjusted for comparisons of various groups. Multivariable logistic regression analysis was done to further assess potential predictors of chronic GVHD as well as potential factors that might affect elevated BAFF levels. Spearman’s rank test was used for correlation analysis. Locally weighted least squares (Lowess) and smoothing spline techniques for smoothing curve estimation were used to characterize change of BAFF levels over time, the relationship between BAFF level and BAFF-R expression, and the relationship between BAFF level and the total number of CD19+ B cells.
Results
Quantitative measurement of BAFF after allogeneic HSCT
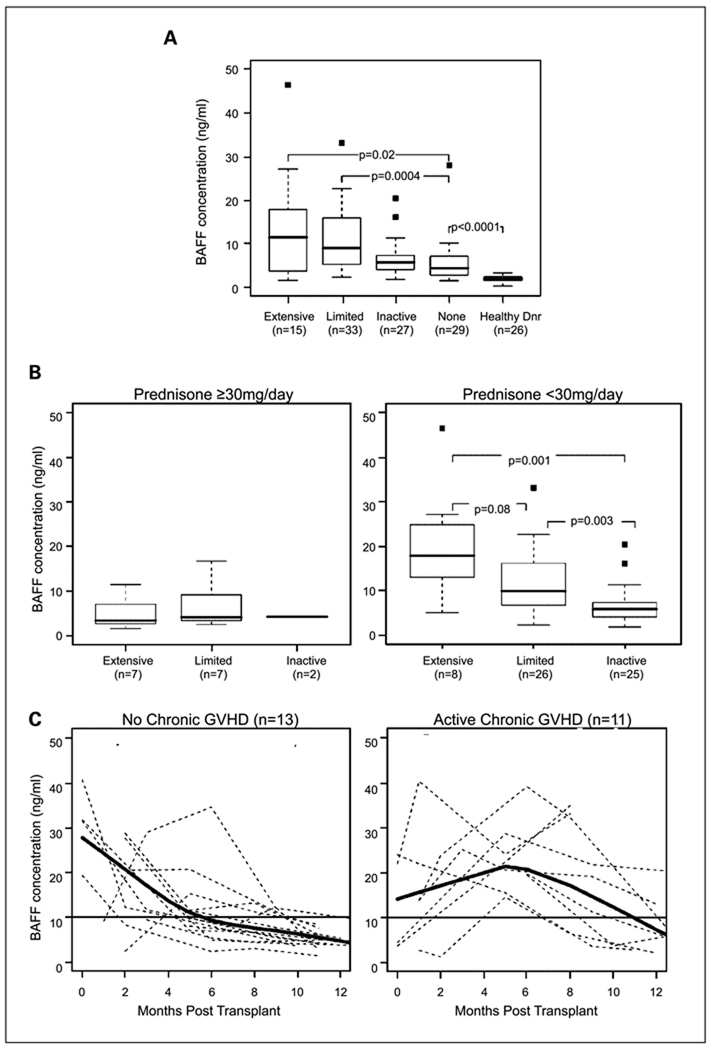
To examine whether BAFF plays a role in chronic GVHD, we measured plasma BAFF levels in 104 patients after HSCT (Table 1). As shown in Fig. 1A, 15 patients with extensive GVHD and 33 patients with limited chronic GVHD were compared with 27 patients with inactive chronic GVHD, 29 patients who never developed chronic GVHD, and 26 healthy donors. All post-HSCT patients had significantly higher BAFF levels than healthy individuals (P < 0.0001 for each group versus healthy individuals). BAFF levels were significantly higher in patients with either extensive (median, 11.5 ng/mL) or limited (median, 9.0 ng/mL) chronic GVHD compared with patients without active chronic GVHD (median, 5.7 ng/mL; P = 0.02 and 0.0004, respectively). When combined, patients with limited and extensive active chronic GVHD had significantly higher BAFF levels (median, 9.9 ng/mL) than patients with inactive or no chronic GVHD (median, 4.8 ng/mL; P = 0.0002).
Fig. 1.
Plasma BAFF levels in patients with chronic GVHD. A, BAFF levels measured by ELISA in 104 patients with and without chronic GVHD after allogeneic HSCT and in healthy donors. The patients with chronic GVHD were further categorized into limited or extensive disease groups according to the Seattle criteria (12). Lines within box plots, median value for each group; bottom and top whiskers, 25th and 75th percentiles, respectively; black squares, outliers.
B, effect of high-dose prednisone on plasma BAFF levels. BAFF levels in the same cohort of 104 patients with extensive, limited, or inactive chronic GVHD after allogeneic HSCT, separated into groups of patients receiving ≥30 mg/d prednisone versus patients receiving <30 mg/d prednisone. C, serial plasma BAFF measurements of samples from 13 patients who never developed chronic GVHD and from 11 patients who developed chronic GVHD between 7 and 12 mo after HSCT.
Because corticosteroids are an effective treatment for chronic GVHD, and several patients with active chronic GVHD were taking high doses of prednisone at the time of analysis (Table 1), we examined whether prednisone treatment affected BAFF levels. Overall, 16 patients were receiving oral prednisone at doses ≥30 mg/d (7 had extensive, 7 had limited, and 2 had inactive chronic GVHD) at the time of analysis. Figure 1B shows that the 16 patients receiving ≥30 mg/d prednisone had uniformly lower median BAFF levels. In contrast, among individuals not receiving corticosteroids or <30 mg/d prednisone, BAFF levels were significantly elevated in patients with extensive or limited chronic GVHD compared with inactive GVHD. Because some patients with active chronic GVHD were receiving sirolimus or tacrolimus (Table 1), we also examined BAFF levels in patients receiving each of these immunosuppressive agents. There was no significant difference in BAFF levels between patients receiving or not receiving these agents (data not shown). There was also no correlation between BAFF levels (≥10 or <10 ng/mL) and administration of these other medications (data not shown). Thus, relatively high-dose steroid therapy was the only immunosuppressive treatment that resulted in lower BAFF levels. Because of this finding, subsequent correlations of BAFF with other variables excluded patients receiving ≥30 mg prednisone beyond 100 days after transplantation.
To examine changes in BAFF levels at different times following allogeneic HSCT, we measured BAFF in a group of 24 patients who were not receiving prednisone 3 months following HSCT. Samples were tested 1, 3, 6, 9, and 12 months after HSCT. Serial BAFF levels in 13 patients who never developed chronic GVHD are compared with BAFF levels in 11 patients who developed limited chronic GVHD between 7 and 12 months after transplantation (Fig. 1C). In both groups, BAFF levels were elevated in most patients in the first 3 months after transplant. Lowess analysis with spline smoothing curves (in bold) show that BAFF concentrations generally decreased to <10 ng/mL by 6 months after transplant in patients who did not develop chronic GVHD. In contrast, BAFF levels remained >10 ng/mL for at least 10 to 12 months in patients who developed chronic GVHD.
BAFF levels 6 months after transplantation correlate with subsequent chronic GVHD
To ascertain whether BAFF levels ≥10 ng/mL might be predictive of subsequent chronic GVHD, we measured BAFF concentrations in 43 samples obtained 6 months after HSCT. We based the 10 ng/mL BAFF cutoff on the median BAFF levels associated with active systemic lupus erythematosus in previous studies (30). None of these patients had evidence of chronic GVHD at the time of analysis. With further follow-up of at least 1.5 years after HSCT (range, 1.5–5 years), 21 patients never developed chronic GVHD and 22 patients developed chronic GVHD between 7 and 12 months after HSCT. Seventy-seven percent of patients with BAFF levels ≥10 ng/mL at 6 months subsequently developed chronic GVHD compared with 12% of patients with BAFF levels <10 ng/mL at 6 months (P < 0.0001; Table 2).
Table 2.
Association of BAFF levels 6 mo after transplantation with subsequent development of chronic GVHD
| Disease status | ≥10 ng/mL BAFF, n (%) |
≤10 ng/mL BAFF, n (%) |
|---|---|---|
| No chronic GVHD (n = 21) | 6 (23) | 15 (88) |
| Chronic GVHD (n = 22) | 20 (77) | 2 (12) |
NOTE: P < 0.0001.
Examination of prognostic factors of chronic GVHD
Potential prognostic factors for active chronic GVHD were examined in univariate analysis as well as in logistic regression analysis. In univariate analysis, BAFF level (as a continuous variable) was the only factor found to be significantly associated with the development of chronic GVHD (P = 0.0002; Table 1). To further examine this observation, logistic regression analysis was done to adjust for potential prognostic factors of chronic GVHD, such as patient age, gender, conditioning regimen, acute GVHD prophylaxis or previous acute GVHD, stem cell source, HLA compatibility, month after transplant, and good prognosis (based on disease characteristics). In the model, elevated BAFF level was the only factor that was significantly associated with chronic GVHD (odds ratio, 1.16 for 1 unit increase of BAFF level; P = 0.0007; odds ratio, 11.55 for BAFF ≥10 ng/mL compared with <10 ng/mL; Table 3). Patients with BAFF ≥10 ng/mL had a shorter median time from HSCT (16 versus 23 months; P = 0.06), but this was not found to be significant in the adjusted model.
Table 3.
Logistic regression model to examine BAFF levels and chronic GVHD
| Effect | Unadjusted OR (95% CI) | Adjusted OR* (95% CI) | P |
|---|---|---|---|
| BAFF | NA | 1.16 (1.07–1.26) | 0.0007 |
| BAFF ≥10 vs BAFF <10 | 10.20 (3.21–37.56) | 11.55 (3.57–37.41) | <0.0001† |
Abbreviations: NA, not available; OR, odds ratio; 95 CI, 95% confidence interval.
Variables included in the model are patient age, gender, related versus unrelated donors, bone marrow versus peripheral blood stem cell source, myeloablative versus nonablative conditioning regiments, months posttransplant, acute GVHD prophylaxis, and good prognosis disease at conditioning.
For both adjusted and unadjusted odds ratio.
Examination of variables potentially affecting BAFF levels
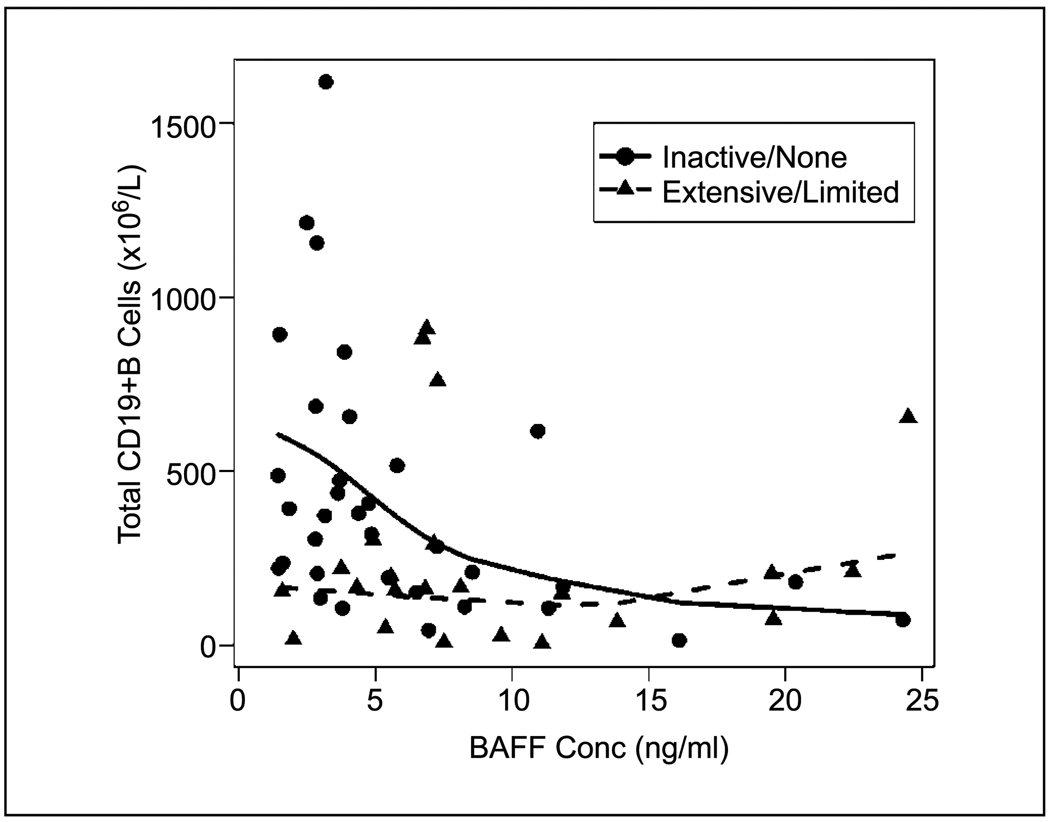
To examine potential factors that might affect the BAFF level, we compared patient age, gender, conditioning regimen, stem cell source, HLA compatibility, acute GVHD prophylaxis regimen, and previous acute GVHD. None of these factors was significantly associated with BAFF (data not shown). Finally, because BAFF plays an important role in B-cell homeostasis, we examined the correlation between the total number of CD19+ B cells in whole blood and BAFF concentrations in plasma in 31 patients with active chronic GVHD and in 35 patients who had no/inactive chronic GVHD at the time of flow cytometric analysis (Fig. 2). We found a significant inverse correlation between total CD19+ B-cell number and BAFF levels in patients with inactive/no chronic GVHD (n = 35; r = −0.51; P = 0.002), but there was no association in patients with active chronic GVHD (n = 31; r = −0.15; P = 0.41). In patients without chronic GVHD, this suggests that BAFF may play a role in reconstitution of B cells, with higher levels of BAFF reflecting a physiologic response to low numbers of circulating B cells. In contrast, high BAFF was not only a reflection of low B-cell numbers in patients with active chronic GVHD because total B-cell number remained low in these patients despite high BAFF levels.
Fig. 2.
Comparison of soluble BAFF to total CD19+ B-cell number in peripheral blood after allogeneic HSCT. Solid line, no/inactive chronic GVHD; dashed line, active chronic GVHD; circles, patients with no/inactive chronic GVHD; triangles, patients with active chronic GVHD at the time of sample collection.
BAFF-R expression on B cells following transplant
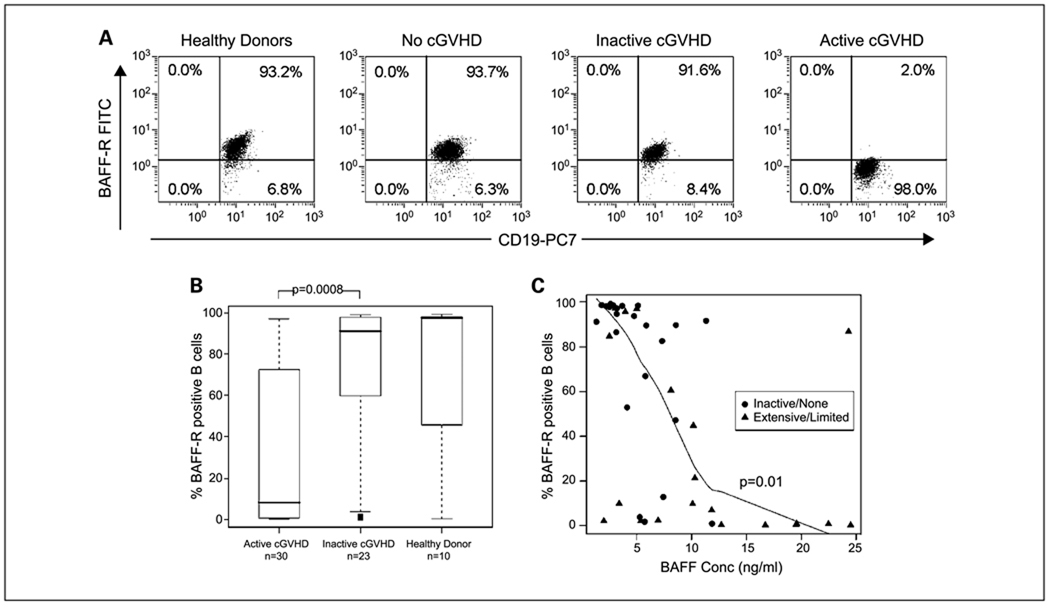
Because signaling via BAFF-R is the major survival and differentiation pathway for peripheral blood B cells in humans, we examined BAFF-R expression on B cells after transplant. As shown in representative flow cytometric analyses (Fig. 3A), >90% of CD19+ B cells express BAFF-R in normal donors, patients without chronic GVHD, and patients with inactive chronic GVHD. In contrast, BAFF-R expression on CD19+ B cells was markedly reduced in patients with active chronic GVHD. Expression of BAFF-R in 43 patients and 10 normal donors is summarized in Fig. 3B. In patients with active chronic GVHD (limited or extensive disease; n = 20), the median percentage of CD19+ cells expressing BAFF-R was lower than in patients with inactive or no chronic GVHD (n = 23; P = 0.0008) or healthy donors (n = 10). Finally, to ascertain whether soluble BAFF affected circulating B cells in vivo, we examined the correlation between BAFF concentration and BAFF-R expression on B cells for each patient. The percentage of CD19+ B cells expressing cell surface BAFF-R was inversely correlated with plasma BAFF levels for all patients after transplantation (r = −0.68; P < 0.0001; Fig. 3C). When the groups were examined separately, both the inactive/no and the active chronic GVHD groups had a significant association between BAFF-R expression on B cells and plasma BAFF concentration (r = −0.66; and P < 0.0001 and r = −0.56; P = 0.01, respectively). Thus, reduced expression of BAFF-R on B cells was associated with both high BAFF levels and disease activity in patients with chronic GVHD.
Fig. 3.
Expression of BAFF-R on the surface of CD19+ B cells after allogeneic HSCT. Monoclonal anti-human BAFF-R (BR3 FITC) and anti-human CD19+PC7 were used to detect cell surface BAFF-R expression on B cells in whole blood samples (see Materials and Methods). A, representative flow cytometry dot blot analyses of CD19+ and BAFF-R expression are shown for representative samples from a healthy donor, a patient without chronic GVHD (cGVHD), a patient with an inactive chronic GVHD, and a patient with active chronic GVHD. B, whisker plot showing median percentage of CD19+ B cells that coexpress BAFF-R in a healthy donors, patients with inactive/no chronic GVHD, and patients with active chronic GVHD. Lines within box plots, median value for each group; bottom and top whiskers, 25th and 75th percentiles, respectively; black squares, outliers. C, soluble BAFF levels in plasma are compared with the percentage of CD19+ B cells expressing cell surface BAFF-R in 43 patient samples after HSCT. Solid heavy line, smoothing spline curve fit for extensive/limited values; circles, patients with no/inactive chronic GVHD; triangles, patients with extensive/limited chronic GVHD at the time of sample collection.
Discussion
In this report, we undertook a detailed analysis of BAFF concentrations in patients after allogeneic HSCT. These studies showed that the highest BAFF levels were found in patients with active chronic GVHD, and these were significantly higher than in patients with inactive disease or in patients who never developed chronic GVHD after HSCT. Further studies in a cohort of patients who were tested at regular intervals showed that BAFF levels were elevated in almost all patients in the first 3 months after HSCT but persistence of high BAFF levels occurred primarily in those patients who went on to develop chronic GVHD. High BAFF levels 6 months after transplantation were then shown to be highly predictive of the subsequent clinical onset of chronic GVHD. Finally, high BAFF levels in plasma correlated with reduced levels of BAFF-R expression on B cells, suggesting that the BAFF/BAFF-R pathway is actively modulated in vivo in patients with active chronic GVHD. For the first time, these studies implicate BAFF and its associated effects on B-cell function in immune pathology of human chronic GVHD.
BAFF is required for B reconstitution after myeloablation in animal models (31). Similarly, increased BAFF levels in all patients early after stem cell transplantation likely contribute to normal recovery of the B-cell compartment. In patients without chronic GVHD, higher BAFF levels were significantly correlated with low numbers of B cells, suggesting that high BAFF levels in these individuals reflected a normal compensatory response to B-cell lymphopenia. As donor B cells reconstitute after HSCT, BAFF levels gradually decline, providing evidence that this cytokine contributes to B-cell reconstitution and homeostasis after HSCT. In contrast, high BAFF levels in patients with active chronic GVHD did not correlate with B-cell numbers in peripheral blood, suggesting that high BAFF in these patients is not only a reflection of B lymphopenia. Studies in animal models suggest that persistently high levels of BAFF in the setting of low B-cell number contribute to the generation and maintenance of autoreactive B cells and loss of B-cell tolerance (21, 26). Similarly, persistence of high BAFF levels in patients with low numbers of B cells may contribute to the development and persistence of active chronic GVHD. Importantly, high BAFF levels were present at 6 months after HSCT in patients who subsequently developed chronic GVHD but not in patients who never developed chronic GVHD. The finding that high BAFF levels precede the clinical manifestations of alloimmunity suggests that this cytokine plays a pathogenic role in chronic GVHD that may be similar to its role in autoimmunity. Thus, persistent high BAFF levels may contribute to a break in B-cell tolerance resulting in perpetuation of alloantibody-producing B cells following HSCT.
Although the precise mechanisms whereby donor B cells contribute to the immune pathology of chronic GVHD have not been clarified, there are several functional effects of BAFF that could play an important role. We have previously shown that donor B cells are capable of generating antibody responses specific for recipient minor histocompatibility antigens and that the presence of high-titer minor histocompatibility antigen antibodies is significantly associated with chronic GVHD (18). High levels of BAFF can support the survival and differentiation of B cells into alloantibody-producing cells (29), and this may be one mechanism whereby BAFF supports the development of chronic GVHD. Alloantibodies can also facilitate the cross-presentation of antigens to effector T cells, thereby amplifying T-cell responses to minor histocompatibility antigen (32, 33). In addition, B cells and the BAFF-R, TACI, are required for efficient CTL generation in mice (34). Finally, B cells could act directly as antigen-presenting cells in their ability to process and present limiting amounts of antigen for presentation to T cells (35). Thus, BAFF is able to support B-cell functions that may contribute to the development of chronic GVHD at many levels.
BAFF is produced by a variety of hematopoietic and nonhematopoietic cell types, including granulocytes, macrophages, natural killer cells, dendritic cells, and marrow stromal cells. Importantly, inflammation as well as lymphopenia are known to promote BAFF secretion (36) and local BAFF production at sites of inflammation can also lead to autoimmunity (37). It is therefore likely that production of BAFF following HSCT is at least partially driven by both systemic and local inflammation. In this regard, patients receiving relatively high-dose corticosteroids had uniformly lower BAFF levels even if they had active chronic GVHD. Although corticosteroids are thought to affect chronic GVHD through a direct effect on T lymphocytes (38), our results suggest that steroids may also act through attenuating an inflammatory component of this disease, with subsequent reduction in secretion of BAFF. In contrast, BAFF levels were not reduced in patients who were receiving other immunosuppressive agents, such as sirolimus or tacrolimus, known to preferentially affect T lymphocytes through other pathways. Although the sources of soluble BAFF in patients with chronic GVHD are not known, identifying BAFF-producing cells following HSCT may have important implications for understanding both B-cell reconstitution as well as chronic GVHD pathogenesis.
The diverse functional effects of BAFF in vivo are mediated through three distinct receptors, BAFF-R, TACI, and BCMA. Our results showing a significant correlation between soluble BAFF levels and BAFF-R expression on B cells suggest that at least some of the effects of BAFF in active chronic GVHD are mediated through BAFF-R. Others have shown that chronic elevation of soluble BAFF in scleroderma and systemic lupus erythematosus results in decreased ability to detect cell surface BAFF-R and that this is associated with autoimmune disease activity. However, some of these findings have been controversial because anti-BAFF-R antibodies used to detect receptor expression in these studies can be blocked by endogenously bound BAFF (39, 40). Because we used whole blood flow cytometry to examine patient samples, decreased BAFF-R on B cells might, in part, also be due to occupation of the receptor with high-affinity soluble BAFF ligand. However, the antibody used in our study was not one of the antibodies found to be altered by BAFF-BAFF-R binding (41). Moreover, addition of high concentrations of soluble BAFF to whole blood samples from healthy donors did not effect BAFF-R staining using our methods (data not shown). In addition, analysis of patient cells after extensive washing to remove excess BAFF did not result in increased staining for BAFF-R (data not shown). Our results in patients with chronic GVHD therefore suggest that reduced levels of BAFF-R detected by flow cytometry reflect modulation of cell surface BAFF-R expression or occupancy of the receptor with high-affinity ligand (42). In two human studies describing BAFF-R expression in peripheral B-cell subsets, mature B-cell plasma blasts were found to have decreased expression of BAFF-R (32). Thus, it is also possible that lower BAFF-R expression in the total B-cell population might reflect a higher proportion of mature B cells (driven by excess BAFF) in patients with chronic GVHD (42). Further characterization of the peripheral B-cell compartment in patients with and without chronic GVHD will be required to assess the extent to which BAFF promotes survival and differentiation of mature B cells following HSCT. Although our data suggest that BAFF-R is a major downstream target of BAFF in chronic GVHD, it will also be important to investigate the potential roles of the other two BAFF-Rs, BCMA and TACI, in our patients (43).
Our analysis of BAFF in chronic GVHD also has potential clinical implications. First, the association of high plasma BAFF levels with active chronic GVHD suggests that an easily measured soluble factor may be used to corroborate clinical findings in a disease in which signs and symptoms are often protean. If persistent high BAFF levels are confirmed to predict the development of chronic GVHD, there may also be an opportunity to initiate treatment before the onset of clinical symptoms. Such a preemptive approach might reduce morbidity from both chronic GVHD and prolonged immunosuppressive therapy that is required once chronic GVHD is established. Moreover, monitoring of BAFF levels might be useful in determining when a reduction in immunosuppressive therapy may be undertaken safely (44). Finally, specific reagents that selectively target BAFF or BAFF-R may also represent new therapeutic options. Rituximab has recently been shown to have clinical efficacy in several autoimmune diseases (45–47), and preliminary results suggest that CD20 targeting may also be useful in chronic GVHD (48–50). As BAFF promotes B-cell maturation, these cells acquire a plasma cell phenotype and lose expression of CD20. Agents that target the BAFF/BAFF-R pathway may therefore have different functional activities than rituximab, resulting in distinct clinical effects in patients with chronic GVHD.
Acknowledgments
Grant support: Leukemia and Lymphoma Society, Jock and Bunny Adams Research and Education Endowment, Ted and Eileen Pasquarello Research Fund, and NIH grants AI29530 and HL70149.
References
- 1.Lee SJ, Vogelsang G, Flowers ME. Chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2003;9:215–233. doi: 10.1053/bbmt.2003.50026. [DOI] [PubMed] [Google Scholar]
- 2.Higman MA, Vogelsang GB. Chronic graft versus host disease. Br J Haematol. 2004;125:435–454. doi: 10.1111/j.1365-2141.2004.04945.x. [DOI] [PubMed] [Google Scholar]
- 3.Majolino I, Saglio G, Scime R, et al. High incidence of chronic GVHD after primary allogeneic peripheral blood stem cell transplantation in patients with hematologic malignancies. Bone Marrow Transplant. 1996;17:555–560. [PubMed] [Google Scholar]
- 4.Socie G, Salooja N, Cohen A, et al. Nonmalignant late effects after allogeneic stem cell transplantation. Blood. 2003;101:3373–3385. doi: 10.1182/blood-2002-07-2231. [DOI] [PubMed] [Google Scholar]
- 5.Cutler C, Antin JH. Chronic graft-versus-host disease. Curr Opin Oncol. 2006;18:126–131. doi: 10.1097/01.cco.0000208784.07195.84. [DOI] [PubMed] [Google Scholar]
- 6.Deeg HJ, Leisenring W, Storb R, et al. Long-term outcome after marrow transplantation for severe aplastic anemia. Blood. 1998;91:3637–3645. [PubMed] [Google Scholar]
- 7.Goerner M, Gooley T, Flowers ME, et al. Morbidity and mortality of chronic GVHD after hematopoietic stem cell transplantation from HLA-identical siblings for patients with aplastic or refractory anemias. Biol Blood Marrow Transplant. 2002;8:47–56. doi: 10.1053/bbmt.2002.v8.pm11858190. [DOI] [PubMed] [Google Scholar]
- 8.Zorn E, Kim HT, Lee SJ, et al. Reduced frequency of FOXP3+ CD4+CD25+ regulatory T cells in patients with chronic graft-versus-host disease. Blood. 2005;106:2903–2911. doi: 10.1182/blood-2005-03-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhushan V, Collins RH., Jr Chronic graft-vs-host disease. JAMA. 2003;290:2599–2603. doi: 10.1001/jama.290.19.2599. [DOI] [PubMed] [Google Scholar]
- 10.Ratanatharathorn V, Ayash L, Lazarus HM, Fu J, Uberti JP. Chronic graft-versus-host disease: clinical manifestation and therapy. Bone Marrow Transplant. 2001;28:121–129. doi: 10.1038/sj.bmt.1703111. [DOI] [PubMed] [Google Scholar]
- 11.Shlomchik WD, Lee SJ, Couriel D, Pavletic SZ. Transplantation’s greatest challenges: advances in chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2007;13 Suppl 1:2–10. doi: 10.1016/j.bbmt.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 12.Lee S. Chronic graft-versus-host disease. New York: Cambridge University Press; 2004. [Google Scholar]
- 13.Biedermann BC, Sahner S, Gregor M, et al. Endothelial injury mediated by cytotoxic T lymphocytes and loss of microvessels in chronic graft versus host disease. Lancet. 2002;359:2078–2083. doi: 10.1016/S0140-6736(02)08907-9. [DOI] [PubMed] [Google Scholar]
- 14.Mutis T, Gillespie G, Schrama E, Falkenburg JH, Moss P, Goulmy E. Tetrameric HLA class I-minor histocompatibility antigen peptide complexes demonstrate minor histocompatibility antigen-specific cytotoxic T lymphocytes in patients with graft-versus-host disease. Nat Med. 1999;5:839–842. doi: 10.1038/10563. [DOI] [PubMed] [Google Scholar]
- 15.Zhang C, Todorov I, Zhang Z, et al. Donor CD4+ T and B cells in transplants induce chronic graft-versus-host disease with autoimmune manifestations. Blood. 2006;107:2993–3001. doi: 10.1182/blood-2005-09-3623. [DOI] [PubMed] [Google Scholar]
- 16.Zorn E, Miklos DB, Floyd BH, et al. Minor histocompatibility antigen DBY elicits a coordinated B and T cell response after allogeneic stem cell transplantation. J Exp Med. 2004;199:1133–1142. doi: 10.1084/jem.20031560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miklos DB, Kim HT, Zorn E, et al. Antibody response to DBY minor histocompatibility antigen is induced after allogeneic stem cell transplantation and in healthy female donors. Blood. 2004;103:353–359. doi: 10.1182/blood-2003-03-0984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miklos DB, Kim HT, Miller KH, et al. Antibody responses to H-Y minor histocompatibility antigens correlate with chronic graft-versus-host disease and disease remission. Blood. 2005;105:2973–2978. doi: 10.1182/blood-2004-09-3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patriarca F, Skert C, Sperotto A, et al. The development of autoantibodies after allogeneic stem cell transplantation is related with chronic graft-vs-host disease and immune recovery. Exp Hematol. 2006;34:389–396. doi: 10.1016/j.exphem.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 20.Wechalekar A, Cranfield T, Sinclair D, Ganzckowski M. Occurrence of autoantibodies in chronic graft vs. host disease after allogeneic stem cell transplantation. Clin Lab Haematol. 2005;27:247–249. doi: 10.1111/j.1365-2257.2005.00699.x. [DOI] [PubMed] [Google Scholar]
- 21.Brink R. Regulation of B cell self-tolerance by BAFF. Semin Immunol. 2006;18:276–283. doi: 10.1016/j.smim.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 22.Tangye SG, Bryant VL, Cuss AK, Good KL. BAFF, APRIL and human B cell disorders. Semin Immunol. 2006;18:305–317. doi: 10.1016/j.smim.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Mariette X, Roux S, Zhang J, et al. The level of BLyS (BAFF) correlates with the titre of autoantibodies in human Sjogren’s syndrome. Ann Rheum Dis. 2003;62:168–171. doi: 10.1136/ard.62.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsushita T, Hasegawa M, Yanaba K, Kodera M, Takehara K, Sato S. Elevated serum BAFF levels in patients with systemic sclerosis: enhanced BAFF signaling in systemic sclerosis B lymphocytes. Arthritis Rheum. 2006;54:192–201. doi: 10.1002/art.21526. [DOI] [PubMed] [Google Scholar]
- 25.Cheema GS, Roschke V, Hilbert DM, Stohl W. Elevated serum B lymphocyte stimulator levels in patients with systemic immune-based rheumatic diseases. Arthritis Rheum. 2001;44:1313–1319. doi: 10.1002/1529-0131(200106)44:6<1313::AID-ART223>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 26.Thien M, Phan TG, Gardam S, et al. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity. 2004;20:785–798. doi: 10.1016/j.immuni.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 27.Goodnow CC, Cyster JG, Hartley SB, et al. Self-tolerance checkpoints in B lymphocyte development. Adv Immunol. 1995;59:279–368. doi: 10.1016/s0065-2776(08)60633-1. [DOI] [PubMed] [Google Scholar]
- 28.Cyster JG, Goodnow CC. Antigen-induced exclusion from follicles and anergy are separate and complementary processes that influence peripheral B cell fate. Immunity. 1995;3:691–701. doi: 10.1016/1074-7613(95)90059-4. [DOI] [PubMed] [Google Scholar]
- 29.Avery DT, Kalled SL, Ellyard JI, et al. BAFF selectively enhances the survival of plasmablasts generated from human memory B cells. J Clin Invest. 2003;112:286–297. doi: 10.1172/JCI18025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang J, Roschke V, Baker KP, et al. Cutting edge: a role for B lymphocyte stimulator in systemic lupus erythematosus. J Immunol. 2001;166:6–10. doi: 10.4049/jimmunol.166.1.6. [DOI] [PubMed] [Google Scholar]
- 31.Gorelik L, Gilbride K, Dobles M, Kalled SL, Zandman D, Scott ML. Normal B cell homeostasis requires B cell activation factor production by radiation-resistant cells. J Exp Med. 2003;198:937–945. doi: 10.1084/jem.20030789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Groh V, Li YQ, Cioca D, et al. Efficient cross-priming of tumor antigen-specific T cells by dendritic cells sensitized with diverse anti-MICA opsonized tumor cells. Proc Natl Acad Sci U S A. 2005;102:6461–6466. doi: 10.1073/pnas.0501953102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hon H, Oran A, Brocker T, Jacob J. B lymphocytes participate in cross-presentation of antigen following gene gun vaccination. J Immunol. 2005;174:5233–5242. doi: 10.4049/jimmunol.174.9.5233. [DOI] [PubMed] [Google Scholar]
- 34.Diaz-de-Durana Y, Mantchev GT, Bram RJ, Franco A. TACI-BLyS signaling via B-cell-dendritic cell cooperation is required for naive CD8+ T-cell priming in vivo. Blood. 2006;107:594–601. doi: 10.1182/blood-2004-12-4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.von Bergwelt-Baildon MS, Vonderheide RH, Maecker B, et al. Human primary and memory cytotoxic T lymphocyte responses are efficiently induced by means of CD40-activated B cells as antigen-presenting cells: potential for clinical application. Blood. 2002;99:3319–3325. doi: 10.1182/blood.v99.9.3319. [DOI] [PubMed] [Google Scholar]
- 36.Ueda Y, Yang K, Foster SJ, Kondo M, Kelsoe G. Inflammation controls B lymphopoiesis by regulating chemokine CXCL12 expression. J Exp Med. 2004;199:47–58. doi: 10.1084/jem.20031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schneider P. The role of APRIL and BAFF in lymphocyte activation. Curr Opin Immunol. 2005;17:282–289. doi: 10.1016/j.coi.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 38.Arce S, Luger E, Muehlinghaus G, et al. CD38 low IgG-secreting cells are precursors of various CD38 high-expressing plasma cell populations. J Leukoc Biol. 2004;75:1022–1028. doi: 10.1189/jlb.0603279. [DOI] [PubMed] [Google Scholar]
- 39.Carter RH, Zhao H, Liu X, et al. Expression and occupancy of BAFF-R on B cells in systemic lupus erythematosus. Arthritis Rheum. 2005;52:3943–3954. doi: 10.1002/art.21489. [DOI] [PubMed] [Google Scholar]
- 40.Gottenberg JE, Pallier C, Ittah M, et al. Failure to confirm coxsackievirus infection in primary Sjogren’s syndrome. Arthritis Rheum. 2006;54:2026–2028. doi: 10.1002/art.21906. [DOI] [PubMed] [Google Scholar]
- 41.Nakamura N, Hase H, Sakurai D, et al. Expression of BAFF-R (BR 3) in normal and neoplastic lymphoid tissues characterized with a newly developed monoclonal antibody. Virchows Arch. 2005;447:53–60. doi: 10.1007/s00428-005-1275-6. [DOI] [PubMed] [Google Scholar]
- 42.Sellam J, Miceli-Richard C, Gottenberg JE, et al. Decreased BAFF-R on peripheral lymphocytes associated with increased disease activity in primary Sjogren’s syndrome and systemic lupus erythematosus. Ann Rheum Dis. 2006;66:790–797. doi: 10.1136/ard.2006.065656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee SJ. New approaches for preventing and treating chronic graft-versus-host disease. Blood. 2005;105:4200–4206. doi: 10.1182/blood-2004-10-4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Atkinson K. Chronic graft-versus-host disease. Bone Marrow Transplant. 1990;5:69–82. [PubMed] [Google Scholar]
- 45.Edwards JC, Cambridge G. B-cell targeting in rheumatoid arthritis and other autoimmune diseases. Nat Rev Immunol. 2006;6:394–403. doi: 10.1038/nri1838. [DOI] [PubMed] [Google Scholar]
- 46.Edwards JC, Szczepanski L, Szechinski J, et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Enl J Med. 2004;350:2572–2581. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- 47.Okamoto H, Kamatani N. Rituximab for rheumatoid arthritis. N Engl J Med. 2004;351:1909. doi: 10.1056/NEJM200410283511820. author reply. [DOI] [PubMed] [Google Scholar]
- 48.Ratanatharathorn V, Ayash L, Reynolds C, et al. Treatment of chronic graft-versus-host disease with anti-CD20 chimeric monoclonal antibody. Biol Blood Marrow Transplant. 2003;9:505–511. doi: 10.1016/s1083-8791(03)00216-7. [DOI] [PubMed] [Google Scholar]
- 49.Cutler C, Miklos D, Kim HT, et al. Rituximab for steroid-refractory chronic graft-vs.-host disease. Blood. 2006;108:756–762. doi: 10.1182/blood-2006-01-0233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Okamoto M, Okano A, Akamatsu S, et al. Rituximab is effective for steroid-refractory sclerodermatous chronic graft-versus-host disease. Leukemia. 2006;20:172–173. doi: 10.1038/sj.leu.2403996. [DOI] [PubMed] [Google Scholar]