Abstract
Rapid and accurate assembly of the ribosomal subunits, which are responsible for protein synthesis, is required to sustain cell growth. Our best understanding of the interaction of 30S ribosomal subunit components (16S ribosomal RNA [rRNA] and 20 ribosomal proteins [r-proteins]) comes from in vitro work using Escherichia coli ribosomal components. However, detailed information regarding the essential elements involved in the assembly of 30S subunits still remains elusive. Here, we defined a set of rRNA nucleotides that are critical for the assembly of the small ribosomal subunit in E. coli. Using an RNA modification interference approach, we identified 54 nucleotides in 16S rRNA whose modification prevents the formation of a functional small ribosomal subunit. The majority of these nucleotides are located in the head and interdomain junction of the 30S subunit, suggesting that these regions are critical for small subunit assembly. In vivo analysis of specific identified sites, using engineered mutations in 16S rRNA, revealed defective protein synthesis capability, aberrant polysome profiles, and abnormal 16S rRNA processing, indicating the importance of these residues in vivo. These studies reveal that specific segments of 16S rRNA are more critical for small subunit assembly than others, and suggest a hierarchy of importance.
Keywords: ribosome assembly, modification interference, 16S rRNA, RNA–protein interactions
INTRODUCTION
The ribosome is an enormous ribonucleoprotein particle (RNP) composed of two-thirds RNA and one-third protein. The quick and accurate biogenesis of this large protein-synthesizing machine is necessary for the viability and growth of all living cells (Warner et al. 2001). Bacterial ribosomes contain three RNAs (16S, 23S, and 5S ribosomal RNA [rRNA]) and more than 50 ribosomal proteins (r-proteins). The Escherichia coli ribosome has been extensively studied and has been a model for assembly studies as functional particles that can be reconstituted in vitro (Traub and Nomura 1968). The E. coli ribosomes can rapidly assemble in vivo in a matter of minutes (Schlessinger 1974; Lindahl 1975); by comparison, in vitro assembly features slow kinetics and a marked dependence on nonphysiological temperature and ionic conditions (Nomura and Traub 1968; Traub and Nomura 1968). This temperature dependence has been exploited to study ribosome assembly, with major thrusts focused on small subunit assembly (Sykes and Williamson 2009). At a low temperature (0°C–15°C), the 30S subunit reconstitution stalls and a reconstitution intermediate (RI) is formed (Fig. 1; Traub and Nomura 1969; Held and Nomura 1973). This intermediate can be converted to a functional 30S particle upon incubation with additional r-proteins at an elevated temperature (42°C). Intermediates with similar r-protein compositions have been observed in vivo, indicating the relevance of these intermediates to the 30S subunit in vivo assembly (Guthrie et al. 1969; Lindahl 1975).
FIGURE 1.
Modified in vitro 30S ribosomal subunit assembly map (Mizushima and Nomura 1970; Culver 2003; Grondek and Culver 2004). 16S rRNA is shown in 5′ to 3′ direction. 16S rRNA, composed of 5′, central, and 3′ major domains forms the body, platform, and head of 30S subunit, respectively. R-proteins are categorized into early, mid- and late-binding proteins. The arrows represent hierarchical binding of r-proteins. Red, green and blue arrows indicate the assembly of the body, platform and head of the small subunit, respectively. At stage I of assembly, early and mid-binding r-proteins bind to 16S rRNA at low temperature to form RI. RI upon heating incorporates late-binding ribosomal proteins to form functional 30S particles at stage II of assembly.
Previous studies of 30S subunit assembly, including kinetic analyses (Talkington et al. 2005) and equilibrium titration experiments (Mizushima and Nomura 1970), have established an assembly sequence of the r-protein addition to 16S rRNA (Fig. 1). Many experiments suggest that small subunit assembly can be considered to have an early stage, stage I, where early binding proteins (S4, S7, S8, S15, S17, and S20) and mid-binding proteins (S5, S6, S9, S11, S12, S13, S16, S18, and S19) bind to 16S rRNA, and a late stage, stage II, where late-binding proteins (S2, S3, S10, S14, and S21) bind to 16S rRNA. In both stages, the r-protein association is accompanied by significant conformational changes in 16S rRNA both locally and globally during the formation of a functional 30S particle (Holmes and Culver 2004, 2005; Ramaswamy and Woodson 2009). Footprinting experiments have provided snapshots of 16S rRNA conformations during the small subunit assembly process, and from these studies, the conformational changes of individual 16S rRNA nucleotides have been revealed (Moazed et al. 1986; Stern et al. 1988). Nevertheless, the 16S rRNA nucleotides that are critical to these rearrangements, and thus to the assembly of functional 30S subunits, have yet to be defined. To address this problem, we have used a modification interference and selection scheme to identify the 16S rRNA nucleotides critical for the 30S subunits assembly in vitro (Fig. 2). We have identified 54 nucleotides (nt) that interfere with the 30S subunit assembly. The majority of these nucleotides mapped to the 3′ domain and interdomain junction regions, indicating that assembly of these regions are less plastic than the assembly of other domains and that r-protein addition prior to modification does not greatly shift the modification patterns. Moreover, we engineered mutations in 16S rRNA at five individual sites identified in our in vitro selection and examined the mutants for variation in growth, ribosome biogenesis, and protein synthesis in vivo. The majority of plasmid encoded 16S rRNA of mutants when expressed supported E. coli viability. However, when using a specialized ribosome system (Abdi and Fredrick 2005), most of these mutants had detrimental effects on protein synthesis. In addition, many of the mutants had aberrant polysome profiles and defects in 16S rRNA maturation, suggesting the importance of these residues in ribosome assembly.
FIGURE 2.
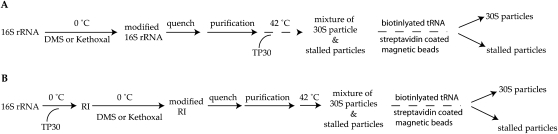
Experimental schemes for modification interference to identify critical nucleotides for stage I (A) and stage II (B) of 30S subunit assembly.
RESULTS
In vitro analysis
Experimental design and overview
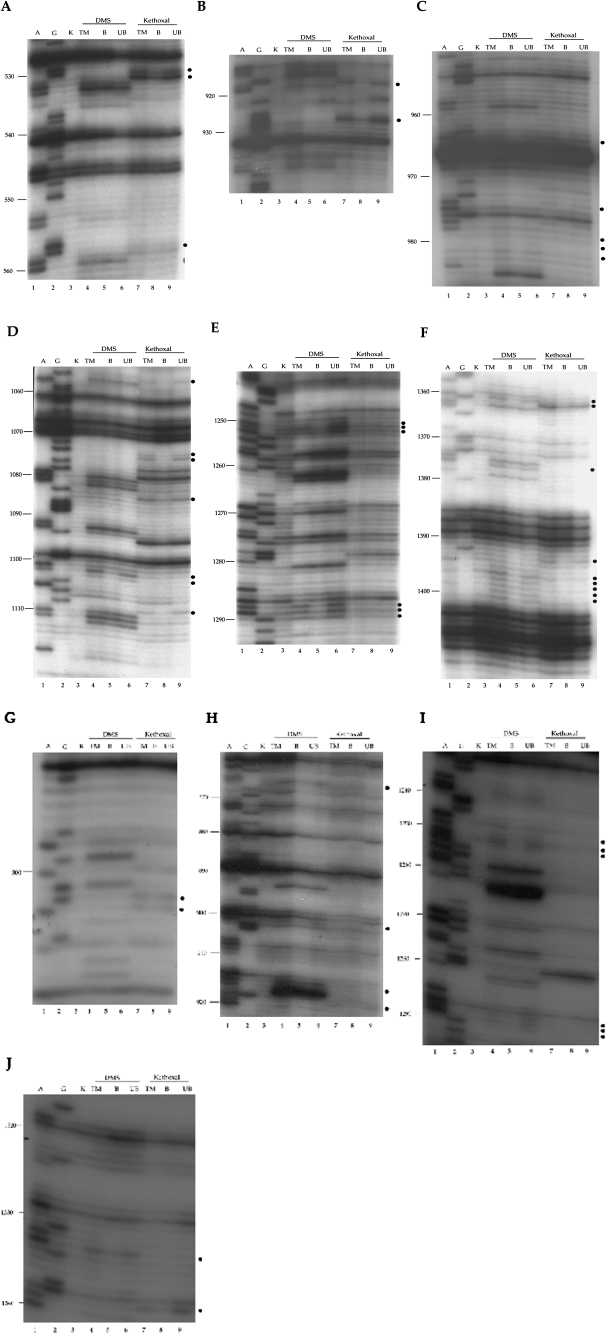
Modification interference has been used to identify nucleotides that are integral for RNP function and the interaction with appropriate ligands. For example, modification interference was used to identify nucleotides within the 30S subunits important for subunit association (Pulk et al. 2006) and for tRNA binding (von Ahsen and Noller 1995). Our experimental design is an extension of this later procedure. To determine which nucleotides are important for the 30S subunit assembly, nucleobase-specific chemical reagents, kethoxal (3-ethoxy-α-ketobutyraldehyde) and DMS (dimethyl sulfate), were used to modify bases within the 16S rRNA at two stages of the 30S subunit reconstitution in vitro (Xu and Culver 2009). First, for investigating early stages (stage I) of small subunit assembly, naked 16S rRNA was modified prior to addition of r-proteins, and assembly was then allowed to proceed, otherwise unimpeded (Materials and Methods; Fig. 2A). Second, for investigating later stages (stage II) of small subunit assembly, 16S rRNA and r-proteins were assembled at low temperature to form RI, and then this heterogeneous population of intermediates was modified (Materials and Methods; Fig. 2B) prior to a temperature shift to allow full assembly. Differences in tRNA binding capability were used to distinguish fully assembled, functional 30S subunits from the stalled nonfunctional particles. Sites of modification in both the functional and stalled populations were then identified by primer extension analysis (Fig. 3). Comparison of 16S rRNA modification patterns in the starting population with those of assembled/bound and the stalled/unbound particles revealed that 54 nt had reduced reactivity in the functional population (Materials and Methods; Fig. 3), indicating that the 30S subunit assembly is impaired by modification of these nucleotides.
FIGURE 3.
Primer extension analysis to identify DMS and kethoxal modified residues in 16S rRNA. The lanes labeled A and G (lanes 1,2) are dideoxy sequencing lanes. The lane labeled K (lane 3) is unmodified 16 S rRNA. The lanes marked with DMS and kethoxal denote samples were treated with those chemicals, respectively. TM (lanes 4,7) indicates total modified population of naked16S rRNA (stage I) or RI (stage II). B (lanes 5,8) indicates particles bound to tRNA, i.e., functional 30S particles. UB (lanes 6,9) indicates particles unable to bind to tRNA, i.e., misassembled particles. Residues that have different reactivity in the TM, B, and UB lanes are marked with dots. (A–F) Stage I assembly; (G–J) stage II assembly. The primers used are as follows: (A) 656, (B) 1046, (C)1046, (D) 1190, (E) 1390, (F) 1508, (G) 323, (H)1046, (I) 1390, and (J) 1508.
Given that our means of partitioning the fully assembled 30S subunits from the stalled particles is the ability to bind tRNA, the identification of nucleotides critical for this function was expected. Both G926 and G1338 were identified as critical sites for tRNA binding to the 30S subunits in a previous modification interference experiment (von Ahsen and Noller 1995). We also identified these residues, demonstrating that changes in our modification and selection protocol did not alter these findings. Nucleotides G529, G530, G926, G1338, and A1493 were all identified in our experiments and have been shown to change reactivity upon P-site tRNA binding (Moazed and Noller 1986, 1990), suggesting that these nucleotides may participate in the dynamic tRNA binding environment of the 30S subunit. Thus, it is possible that the dynamic nature of this region may be important for 30S subunit assembly and function. However, given our inability to distinguish effects due to loss of tRNA binding or assembly, these nucleotides were not examined in detail.
In general, modification of nucleotides in the body and platform of 30S subunits appears to have minimal effects on assembly, with only 11 out of 915 nt (1.2%) identified in these domains. In contrast, modification of 43 out of 627 nt (6.8%) in the 3′ domain alters assembly. These results are consistent with the polarity of assembly (Powers et al. 1993) and with the relatively high density of late-binding r-proteins that associate with the head of the small subunit (Powers et al. 1988; Wimberly et al. 2000; Schuwirth et al. 2005). Intriguingly, the majority of nucleotides whose modification impedes assembly are located not only in the head but also near the functional center of the 30S subunit (Fig. 4), suggesting that interaction with r-proteins and folding events in these regions may be particularly important for functional 30S subunit formation.
FIGURE 4.
Nucleotides identified as critical for the small subunit assembly shown on secondary (Cannone et al. 2002) and tertiary (Protein Data Bank [PDB] 2AW7) (Schuwirth et al. 2005) structure of 16S rRNA in 30S subunit. (A) The 5′, central, 3′ major, and 3′ minor domain boundaries are indicated in red and are defined in the text. Red circles denote to residues refractory to small subunit assembly when modified at stage I, blue circles denote to residues refractory to small subunit assembly when modified at stage II, purple circles denote those residues whose modification are refractory to both stages of assembly, and gray circles denote to residues critical to tRNA binding (von Ahsen and Noller 1995). (B) 16S rRNA from 30S subunit is shown in gray. Nucleotides that are critical for early stage of the small subunit assembly are shown in red spheres. Nucleotides that are critical for late stage of the small subunit assembly are shown in blue spheres. Nucleotides that are critical for both stages of the small subunit assembly are shown in purple spheres. (C) Nucleotides refractory to the small subunit assembly interacting with S12 are marked. S12 is shown in dark cartoon form. (D) Base interactions identified as important in helix 41, which is colored in dark. (E) Base triple interactions between G1053, G1057, and C1203, which appear to be important for assembly. All the figures are generated using PyMOL and PDB 2AW7 (Schuwirth et al. 2005).
Our data from the two modification schemes did not reveal clear delineations of regions critical at stage I or stage II. Generally, the data for the modification of naked 16S rRNA or RI are interspersed. However, the findings that the 16S rRNA domains have differences in sensitivity to modification interference are clear and indicate that changes in the 3′domain are least tolerated.
Nucleotides within the 5′ domain critical for 30S subunit assembly
The 5′ domain of 16S rRNA (nucleotides 1–566) is the RNA component of the body of 30S subunits that runs from the neck to the toe. Our data are consistent with earlier studies suggesting that the 5′ domain folds early during assembly and is mainly stabilized by RNA–RNA interactions (Stern et al. 1989; Adilakshmi et al. 2005). Five nucleotides (G301, G302, G529, G530, and G558) in the 5′ domain were identified as critical for formation of functional 30S subunits (Fig. 4; Table 1). G529, whose modification could affect tRNA binding (Moazed and Noller 1990), was identified in both modification schemes. Modification of nucleotides G530 and G558 was refractory to stage I of assembly of the 30S subunit. Modification of nucleotides G301 and G302 shows an inhibitory effect to stage II assembly. Interestingly, all of identified nucleotides (G301, G302, and G529) whose modifications interfere with stage II assembly are indicated as interacting with S12 in the crystal structure of the E. coli small subunit (Fig. 4C; Schuwirth et al. 2005). These are the only modification sites in the body where r-protein/16S rRNA interactions appear to be disrupted. Also, S12 is poised on the functional face of the small subunit, and thus, an importance in assembly is expected.
TABLE 1.
Nucleotides critical for 30S subunit assembly as identified by modification interference
Nucleotides within the central domain critical for 30S subunit assembly
The central domain of 16S rRNA (nucleotides 567–915) with five associated r-proteins folds into the platform of the small subunit. Overall, there are 6 nt, which when modified, block assembly in the central domain (Fig. 4). G575, which is located in the single-stranded 570 loop, when modified in stage I, disrupts the assembly of small subunits. The crystal structure indicates that G575 interacts with C880 (Table 1; Schuwirth et al. 2005); thus, modification of this nucleotide could cause direct interruption of the base-pairing interface and result in stalled assembly. Modification of C737 and C738 in stage I of assembly impedes assembly, and these residues interact directly with S6 in the 30S subunit (Table 1; Schuwirth et al. 2005), supporting the known role of S6 in assembly (Traub and Nomura 1968). In addition, nucleotide A913, which interacts with S12 (Fig. 4C), when modified in stage II is refractory to small subunit formation. Moreover, modification of G791, located in the functionally important 790 loop (Lee et al. 1997), and G818, located in the single-stranded 820 loop, inhibits the formation of functional 30S subunits. While these 2 nt do not have direct identifiable interactions in the E. coli 30S subunit crystal structure (Schuwirth et al. 2005), they could be involved in transient interactions during assembly of functional particles.
Nucleotides within the 3′ major domain critical for 30S subunit assembly
The 3′ major domain of 16S rRNA (nucleotides 916–1396) with associated r-proteins composes the head of the small subunit. The 3′ major domain features irregular packing interactions in contrast to the extensive helix stacking interaction in the 5′ domain and the central domain (Wimberly et al. 2000; Schuwirth et al. 2005). In our collection of modified nucleotides that limit 30S subunit assembly, 30 nt are part of the 3′ major domain (Table 1; Fig. 4). Overall, it appears that assembly of the head of the 30S subunit is most susceptible to nucleotide modification interference.
Twenty nucleotides in the 3′ major domain identified as important for assembly interact directly with r-proteins. Most of these residues map to mid-late-binding r-proteins in the 3′ major domain (Table 1). The only exceptions are nucleotides A1289 and A1375, which interact with the early binding r-protein S7. Many other residues (G963, C979, A983, G1074, A1101, A1102, G1108, G1316, and C1359) have direct interaction with S2, S3, S10, and S14 (Table 1; Schuwirth et al. 2005), and modifications of these residues can be linked to stalled assembly. Thus, accommodation of these proteins in the formation of the head of the 30S subunit is likely to be a critical stage of assembly.
In addition to the r-protein/16S rRNA interaction, the 14 nt identified in the 3′ major domain are implicated in specific base pairs (Table 1; Schuwirth et al. 2005). Modification of such nucleotides could cause direct disruption of the base-pairing interface and thus result in stalled assembly. For example, modification of residues A1288 and C1249 restricts closing an internal loop in helix 41 (Fig. 4D); modifications that could block a base triple interaction between G1053, G1057, and C1203 are also refractory to assembly (Fig. 4E).
Nucleotides within the 3′ minor domain critical for 30S subunit assembly
The 3′ minor domain (nucleotide 1397–1542) comprises the penultimate and ultimate stem of 16S rRNA, which spans from the body to the head of the small subunit. In the 3′ minor domain, the modification of 13 nt disrupts the assembly of 30S subunit (Table 1; Fig. 4). These residues are critical for stage II or at both stages of assembly. Although, we cannot pinpoint any RNA/r-protein interaction with these residues, 6 nt (G1392, C1395, A1396, C1399, G1416, and G1494) are implicated in a specific base-pairing interaction in the crystal structure of the E. coli small subunits (Schuwirth et al. 2005). The percentage of residues refractory to assembly in the 3′ minor domain (9%) suggests that limiting the extension or stabilization of helix 44 may be detrimental to assembly. In addition, residues G1392, C1395, and A1396 in helix 28, which are predicted to be in base pairs in the secondary structure (Cannone et al. 2002), are susceptible to modification, and such modifications limit assembly. These data suggest that formation of both helix 26 and helix 44 is a critical event in functional 30S subunit formation. Overall, the data presented herein indicate that the individual domains of 16S rRNA have different sensitivity to nucleotide modification in vitro.
In vivo studies
To further verify the importance of identified nucleotides in the small subunit ribosome assembly, we constructed mutations at several selected residues in 16S rRNA for in vivo analysis. Thus, examination of the effect caused by the mutation of these residues in vivo may produce insight into a broad range of assembly defects. Given that our in vitro data pointed to the importance of the 3′ domain in assembly, we focused on these residues for in vivo analysis. Three approaches to study the effect of mutations at five residues (917, 1053, 1084, 1101, and 1108) in vivo were undertaken. First, the overall impact of mutation was examined by assessing translation competency. Second, the ribosome profiles from strains bearing mutations were examined. Lastly, the 16S rRNA maturation was examined.
We used a specialized ribosome system to monitor translation capability. This system uses a plasmid encoded 16S rRNA gene with an altered anti-Shine-Dalgarno sequence (GGGGU), and an E. coli strain KLF10, with a chromosomal copy of the β-galactosidase gene (lacZ) with an altered Shine-Dalgarno sequence (the ribosome binding site, AUCCC) (Abdi and Fredrick 2005). Thus, the 30S subunits assembled from the plasmid-encoded 16S rRNA translate only the lacZ mRNA, while the wild-type cellular ribosomes translate the wild-type cellular mRNAs. The level of β-galactosidase activity mirrors the quality of the 30S subunits composed of plasmid-derived borne 16S rRNA. The β-galactosidase activity assay revealed that various mutations at position G1084 reduced the protein synthesis capabilities to less than 40% of that for ribosomes with control 16S rRNA (Fig. 5). By comparison, mutations at position G917, G1053, A1101, and G1108 resulted in nearly undetectable levels of translation (Fig. 5). These results suggest the importance of the specific identity of nucleotides for protein synthesis and possibly for assembly.
FIGURE 5.
Effect of mutations at indicated positions in 16S rRNA on translation activity as monitored by β-galactosidase activity using a specialized ribosome system (Abdi and Fredrick 2005). All values are normalized to 16S rRNA with anti-SD mutations but otherwise wild-type sequence.
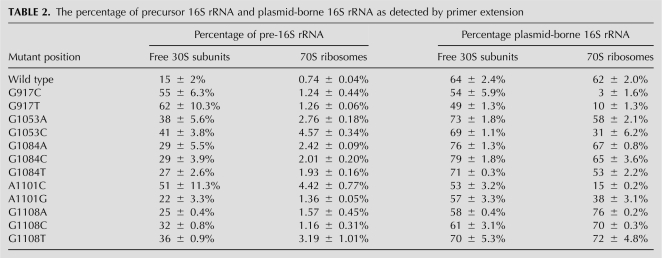
To examine if these mutations directly alter ribosome biogenesis, we analyzed polysome profiles using a system that allows the regulated expression of plasmid-derived 16S rRNA with mutations at positions G917, G1053, A1101, and G1108. Upon induction, plasmid encoded 16S rRNA accounts for around 60% of total cellular 16S rRNA in wild-type strains (Table 2; Powers and Noller 1991; Yassin et al. 2005). In contrast, the percentage of plasmid encoded 16S rRNA in mutant strains varies (see Discussion; Table 2). For each tested mutation, the polysome profiles were altered compared to plasmid-derived wild-type 16S rRNA. A reduction in the 70S peak area with concurrent increase in free subunits was observed, with variations ranging from a slight increase in free 30S subunits of 1.6-fold (1084 and 1108) to nearly 3.5-fold (1053 and 1101). Moreover, strains containing the 16S rRNA mutations G1053A, G1053C, or A1101C not only exhibited diminished 70S particles relative to free 30S subunits but also aberrant 30S subunit peaks (Fig. 6). Thus, these mutations clearly have an impact on the 30S subunit assembly in vivo. These results pinpoint the importance of these specific residues in this process. Additionally, we wished to address whether the accumulated ribosomal particles were fully mature. Analysis of the rRNA from 30S subunit and 70S ribosome fractions from strains carrying specific mutants (G1053A, G1053C, G1084T, A1101C, A1101G, and G1108T) revealed that 16S rRNA processing was abnormal compared with wild type. The percentage of pre-16S rRNA (17S rRNA) in the ribosomal 30S and 70S fractions from the mutant strains increased, indicating defective 16S rRNA maturation (Table 2). While these changes are modest, when the relative proportions of chromosomally encoded (wild type) versus plasmid-borne (mutant) 16S rRNA is considered, these differences are more significant. These results corroborate our in vitro data and indicate that these residues are critical for accurate and appropriate 30S subunit biogenesis in vivo.
TABLE 2.
The percentage of precursor 16S rRNA and plasmid-borne 16S rRNA as detected by primer extension
FIGURE 6.
Effects of mutations at indicated positions in 16S rRNA on polysome profiles. The scale of all profiles is the same. The percentage of free 30S subunits (dark gray) relative to 30S subunits in 70S ribosomes (light gray) is given.
DISCUSSION
We have identified nucleotides that are critical for 30S subunit assembly by using modification interference and in vivo analysis. These data are consistent with the polar nature of the assembly of the 30S subunit (Powers et al. 1993; Holmes and Culver 2005). It appears that the assembly of the 5′ domain and central domain is mostly resistant to modification interference, and this is not due to differences in the number of modified residues observed in the total pool for these domains (data not shown). Conversely, modifications within the 3′ major and minor domains as well as at the tertiary interaction sites appear to be refractory for assembly. Moreover, in vivo studies support the importance of these residues for small subunit assembly and functional 70S ribosome formation. Overall, these data might suggest that specific modification within the 5′ and central domains can be overcome during the assembly process, while this is not the case for the 3′ major and minor domains.
Our data indicate that the binding of many early and mid-associating r-proteins cannot be disrupted by modification interference. It is possible, as suggested for the 5′ domain, that much of the functional architecture can be formed within naked 16S rRNA and that protein association is secondary (Stern et al. 1988). Alternatively, it is possible that there are redundant, alternative pathways for assembly of the 5′ and central domains such that specific rearrangements can be circumvented. However, the assembly of the 3′ domain appears to be more restricted. In previous work, pulse-chase quantitative mass spectrometry of kinetics accessing r-protein association (Talkington et al. 2005) and time-resolved hydroxyl radical probing of 16S rRNA nucleotides during 30S subunit assembly (Adilakshmi et al. 2008) have suggested that assembly of the 3′ domain is slow relative to the other domains. Thus, assembly kinetics may reflect differences in parallel pathways that allow functional 30S subunits to be formed. Consistent with this hypothesis, three of the 6 nt in the 5′ domain identified in this work interact with S12, which is one of the slowest binding r-proteins (Talkington et al. 2005). Thus, the flexibility of assembly of the 5′ and central domains when nucleotides are modified may be linked to redundant folding pathways and could further explain the fast assembly/folding kinetics observed in these other experiments (Talkington et al. 2005; Adilakshmi et al. 2008). The susceptibility of the 3′ domain to modification, which restricts assembly, is significantly more pronounced, and in light of the slower kinetics for assembly of this domain (Talkington et al. 2005; Adilakshmi et al. 2008), it is plausible that there is a more restricted assembly landscape for the head of the 30S subunit.
Alternative explanations for the differences between the domains are also possible. The 3′ domain may be more susceptible to folding traps and require the action of extra ribosomal factors for proper formation. This might suggest that many alternative conformations of the 3′ domain are not available. Examination of RNA backbone protection rates (available for only a subset of the nucleotides identified herein) (Adilakshmi et al. 2008) suggests that critical residues belong to either the very fast or slow kinetic groupings (see Table 1). These correlations may suggest that the intermediate landscape is more plastic and that early (fast) or late (slow) events are more critical to the process.
Our in vivo results strongly support our in vitro selection data. The results from translation read-out, polysome profiles, and precursor 16S rRNA analysis reflect the importance of these nucleotides in the small subunit assembly in vivo. However, there are subtleties revealed by the mutational analysis data. Defects of most analyzed mutants (G917C, G917T, G1053A, G1053C, A1101C, and G1108T) appeared to be most likely caused by ribosome biogenesis defects (Figs. 5 and 6; Table 2). Conversely, mutations at residues 1084 and 1108 (except G1108T) probably impair function of the ribosome (Figs. 5 and 6; Table 2).
Previous studies have shown that mutation of the residue G917 significantly perturbs the translational activity of ribosomes (Brink et al. 1993). This is consistent with our results using the specialized ribosome system (Fig. 5). Our analysis of mutations at position 917 reveal that there is still a significant 70S population (Fig. 6); however, these 70S ribosomes contain very little mutant 16S rRNA (see Table 2). Thus, this lack of function could result from a problem in completing 30S subunit biogenesis, as suggested by the increase in precursor 16S rRNA in both free and associated subunits in these strains (Table 2) and possibly defects in translation initiation.
Mutations at residue 1053 resulted in no translational activity using the specialized ribosome system (Fig. 5) in addition to increased precursor 16S rRNA in 70S ribosome (approximately sixfold compared with wild type in G1053C strain) (Table 2). Changes in the percentage of mutant 16S rRNA presented in the 70S ribosome compared with wild type are relatively mild. Thus, the lack of function of these mutant strains could be caused by pre-rRNA processing and/or assembly defects. This mutation may disrupt the triple base interaction between G1053, G1057, and C1203 (Fig. 4E). This indicates architectural changes during assembly are complex and multifaceted.
Variant mutations at the same position (residues 1108 and 1084) appear to have different effects. Mutation from guanine to adenine at residue 1108 decreased the translation activity to around 50% of wild type (Fig. 5). In contrast, mutation from guanine 1108 to cytosine or uracil decreased the translation activity to less than 10% of wild type (Fig. 5). Accordingly, polysome profiles of G1108A are similar to the wild-type profiles, while G1108C is slightly different (Fig. 6). In addition, changes in 16S rRNA processing in the G1108A mutant strain are rather subtle (Table 2). Thus, it appears that a purine at this position is better tolerated and may be important for interaction with S3 (Schuwirth et al. 2005). Change of precursor 16S rRNA in these strains (except G1108T) is modest compared with wild type in both 30S subunits and 70S ribosomes. Therefore, mutations at these positions are most likely defective in ribosome function. However, we cannot eliminate the possibility of these mutations being disruptive to ribosome assembly.
Our data suggest that individual domains of 16S rRNA have distinct assembly properties. Assembly of the 5′ and central domains appears to be more plastic, while assembly of the 3′ major and minor domains is more complex. These differences could explain the high density of late-binding r-proteins (four of five) that interact with the 3′ major domain. These r-proteins and perhaps biogenesis factors as well, may act to direct assembly and appropriate folding of the 3′ major domain.
MATERIALS AND METHODS
Biotinylation of tRNA
Biotinylation of tRNA was performed as described (von Ahsen and Noller 1995) with the following modification: 100 nmol of S. cerevisiae tRNAphe was incubated with 10 mM potassium periodate in 4 mL of 100 mM sodium acetate solution (pH 5.5) for 30 min on ice in the dark. Extra sodium periodate was removed by washing the oxidized tRNA with 100 mM sodium acetate solution (pH 5.5) on a Centricon-10 at 6000 rpm for 2 h at 4°C to a volume of 1 mL. The above-treated oxidized tRNA was then incubated with 100 μL of 50 mM biotin hydrazide (in DMSO) for 2 h at room temperature in the dark. Additional biotin hydrazide was removed on a Microcon-10 column at 8000 rpm, and biotinlyated tRNA was stabilized by adding an equal volume of 0.2 M sodium borahydride and a twofold volume of 1 M Tris-Cl (pH 8.2) with incubation on ice for 30 min in the dark. After ethanol precipitation, the biotinylated tRNA was redissolved in 200 μL 100 mM sodium acetate (pH 5.5). Excess salt was removed by dialyzing the biotinylated tRNA in 100 mM sodium acetate. The concentration of the biotinylated tRNA was determined by absorbance at A260 and Pierce biotin quantitation kit.
Modification interference
Chemical probing conditions were determined empirically by titrating the modification reagent to a fixed concentration of RNA followed by tRNA binding. Conditions were chosen that approximately half of the total RNA retained its ability to assemble into a functional 30S subunit. Accordingly, for assessing early stage of 30S subunit assembly, 200 pmol of E. coli 16S rRNA in RecA buffer (80 mM K+-HEPES at pH 7.6, 20 mM MgCl2, 330 mM KCl and 0.01% Nikkol) purified as described (Culver and Noller 1999) was incubated with 2.9 μL of 3.5 M DMS (made by diluting 4 μL of 10.6 M stock into 8 μL 95% ethanol) per 200 μL reaction or 3.2 μL of 10% kethoxal per 200 μL reaction. Modification reactions were carried out on ice for 5 min, which were subsequently quenched by addition of 10 μL of 14.4 M 2-mercaptoethanol for DMS reactions or 10 μL of 500 mM potassium borate for kethoxal reactions. Modification reagents were further removed by gel filtration through a 2-mL Sephadex G-25 column equilibrated in RecA buffer. 30S particles were reconstituted as described (Culver and Noller 2000) with modified 16S rRNA and TP30 (prepared as in Traub and Nomura 1968 and Holmes and Culver 2004). To separate the functional 30S particles from the misassembled particles, a tRNA binding assay was carried as described (von Ahsen and Noller 1995). Before the tRNA binding assay, a buffer exchange by gel filtration with Sephadex G-25 column equilibrated in TBB buffer (50 mM HEPES at pH 7.6, 10 mM MgCl2, 100 mM KCl) was performed. An aliquot of modified reconstituted subunits before tRNA binding assay was kept on ice as a total modified control for primer extension.
To assess References 36 and 15 late stage of 30S subunit assembly, 200 pmol 16S rRNA was incubated with 400 pmol TP30 in a standard reconstitution assay (Culver and Noller 1999) at 0°C to form RI, which was subsequently subjected to 6.5 μL of 880 mM DMS (made by diluting 10 μL of 10.6 M stock into 110 μL 95% ethanol) per 400 μL reaction or 16 μL of 0.4% kethoxal per 400 μL reaction. Excess DMS or kethoxal was quenched and removed as described above. Modified particles were incubated for 15 min at 42°C followed by gel filtration with Sephadex G-25 column equilibrated in TBB buffer. Separation of functional fraction and nonfunctional particles was completed as described (von Ahsen and Noller 1995).
Modified residues in 16S rRNA were identified by primer extension analysis as described (Moazed et al. 1986; Xu and Culver 2009). The concentration of rRNA extracted from the unmodified, total modified, unselected, and selected pool was normalized to 0.8 pmol/μL.
In vivo analysis of selected residues by specialized ribosome system
Individual 16S rRNA mutations were generated by site-directed mutagenesis using the Stratagene Quick-change kit in the plasmid pKF207. This plasmid contains a single copy of 16S rRNA gene with an altered anti–Shine-Dalgarno sequence (5′-GGGGU-3′) under an arabinose inducible promoter (Abdi and Fredrick 2005). Plasmids carrying individual mutations were transformed into KLF10 competent cells that carry a chromosomally encoded lacZ reporter gene with an altered Shine–Dalgarno sequence (5′-AUCCC-3′). A volume of 50 μL of saturated overnight cells grown at 37°C with 100 μg/mL Amp was used to inoculate 5 mL of LB with 100 μg/mL Amp and 1 mM arabinose. Growth at 37°C was continued until OD600 of ∼0.5. The β-galactosidase activity was determined as described (Lancaster and Noller 2005).
Polysome profile analysis of individual 16S rRNA mutations
pLK35 plasmids containing individual 16S rRNA mutations introduced by site-directed mutagenesis were transformed into the POP2136 strain (Yassin et al. 2005), which encodes a temperature-sensitive λ repressor gene. The rrnB operon carried by the pLK35 plasmid is under control of a λ promoter that can be regulated by temperature (Douthwaite et al. 1989). POP2136 strains containing mutant rRNA were grown at 30°C with 100 μg/mL Amp overnight. Fifty milliliters of fresh LB (containing 100 μg/ml Amp) was inoculated with 100 μl of the above overnight culture at 42°C until OD600 of ∼0.5 to allow expression of mutant 16S rRNA. Polysomes were isolated and analyzed as described (Connolly et al. 2008). The ratio of free 30S subunits to total 30S subunits was calculated as described (Connolly et al. 2008). The 16S rRNA extracted from the 30S and 70S ribosomal fractions was analyzed by primer extension using a Cye5-GCGGTATTAGCTACCGT sequence primer. The band intensity of mature 16S rRNA and 17S rRNA was quantified using the BioRad VersaDoc and Image J software. The percentage of precursor 16S rRNA to 16S rRNA was determined as a ratio of band intensity of 17S rRNA to the sum of band intensity of 17S rRNA and mature 16S rRNA.
Quantification of plasmid 16S rRNA, distinguished by a C-to-U mutation at 1192, was performed as described (Powers and Noller 1991).
ACKNOWLEDGMENTS
Z.X. and G.M.C designed and interpreted the experiments and wrote the manuscript. Z.X. performed the experiments. We thank H.F. Noller (University of Santa Cruz) for providing pKF207 plasmid and KLF10 strain; K. Fredrick (Ohio State University) for providing POP2136 strain; and all the Culver laboratory members for comments on the manuscript. This work was supported by NIH funding (GM06432 to G.M.C.).
Footnotes
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.2246710.
REFERENCES
- Abdi NM, Fredrick K 2005. Contribution of 16S rRNA nucleotides forming the 30S subunit A and P sites to translation in Escherichia coli. RNA 11: 1624–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adilakshmi T, Ramaswamy P, Woodson SA 2005. Protein-independent folding pathway of the 16S rRNA 5′ domain. J Mol Biol 351: 508–519 [DOI] [PubMed] [Google Scholar]
- Adilakshmi T, Bellur DL, Woodson SA 2008. Concurrent nucleation of 16S folding and induced fit in 30S ribosome assembly. Nature 455: 1268–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allers J, Shamoo Y 2001. Structure-based analysis of protein–RNA interactions using the program ENTANGLE. J Mol Biol 311: 75–86 [DOI] [PubMed] [Google Scholar]
- Brink MF, Verbeet MP, de Boer HA 1993. Formation of the central pseudoknot in 16S rRNA is essential for initiation of translation. EMBO J 12: 3987–3996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannone JJ, Subramanian S, Schnare MN, Collett JR, D'Souza LM, Du Y, Feng B, Lin N, Madabusi LV, Muller KM, et al. 2002. The comparative RNA web (CRW) site: An online database of comparative sequence and structure information for ribosomal, intron, and other RNAs. BMC Bioinformatics 3: 2 doi: 10.1186/1471-2105-3-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly K, Rife JP, Culver G 2008. Mechanistic insight into the ribosome biogenesis functions of the ancient protein KsgA. Mol Microbiol 70: 1062–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culver GM 2003. Assembly of the 30S ribosomal subunit. Biopolymers 68: 234–249 [DOI] [PubMed] [Google Scholar]
- Culver GM, Noller HF 1999. Efficient reconstitution of functional Escherichia coli 30S ribosomal subunits from a complete set of recombinant small subunit ribosomal proteins. RNA 5: 832–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culver GM, Noller HF 2000. In vitro reconstitution of 30S ribosomal subunits using complete set of recombinant proteins. Methods Enzymol 318: 446–460 [DOI] [PubMed] [Google Scholar]
- Douthwaite S, Powers T, Lee JY, Noller HF 1989. Defining the structural requirements for a helix in 23 S ribosomal RNA that confers erythromycin resistance. J Mol Biol 209: 655–665 [DOI] [PubMed] [Google Scholar]
- Grondek JF, Culver GM 2004. Assembly of the 30S ribosomal subunit: Positioning ribosomal protein S13 in the S7 assembly branch. RNA 10: 1861–1866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guthrie C, Nashimoto H, Nomura M 1969. Studies on the assembly of ribosomes in vivo. Cold Spring Harb Symp Quant Biol 34: 69–75 [DOI] [PubMed] [Google Scholar]
- Held WA, Nomura M 1973. Rate determining step in the reconstitution of Escherichia coli 30S ribosomal subunits. Biochemistry 12: 3273–3281 [DOI] [PubMed] [Google Scholar]
- Holmes KL, Culver GM 2004. Mapping structural differences between 30S ribosomal subunit assembly intermediates. Nat Struct Mol Biol 11: 179–186 [DOI] [PubMed] [Google Scholar]
- Holmes KL, Culver GM 2005. Analysis of conformational changes in 16 S rRNA during the course of 30 S subunit assembly. J Mol Biol 354: 340–357 [DOI] [PubMed] [Google Scholar]
- Lancaster L, Noller HF 2005. Involvement of 16S rRNA nucleotides G1338 and A1339 in discrimination of initiator tRNA. Mol Cell 20: 623–632 [DOI] [PubMed] [Google Scholar]
- Lee K, Varma S, SantaLucia J Jr, Cunningham PR 1997. In vivo determination of RNA structure-function relationships: Analysis of the 790 loop in ribosomal RNA. J Mol Biol 269: 732–743 [DOI] [PubMed] [Google Scholar]
- Lindahl L 1975. Intermediates and time kinetics of the in vivo assembly of Escherichia coli ribosomes. J Mol Biol 92: 15–37 [DOI] [PubMed] [Google Scholar]
- Mizushima S, Nomura M 1970. Assembly mapping of 30S ribosomal proteins from E. coli. Nature 226: 1214–1218 [DOI] [PubMed] [Google Scholar]
- Moazed D, Noller HF 1986. Transfer RNA shields specific nucleotides in 16S ribosomal RNA from attack by chemical probes. Cell 47: 985–994 [DOI] [PubMed] [Google Scholar]
- Moazed D, Noller HF 1990. Binding of tRNA to the ribosomal A and P sites protects two distinct sets of nucleotides in 16 S rRNA. J Mol Biol 211: 135–145 [DOI] [PubMed] [Google Scholar]
- Moazed D, Stern S, Noller HF 1986. Rapid chemical probing of conformation in 16 S ribosomal RNA and 30 S ribosomal subunits using primer extension. J Mol Biol 187: 399–416 [DOI] [PubMed] [Google Scholar]
- Nomura M, Traub P 1968. Structure and function of Escherichia coli ribosomes. 3. Stoichiometry and rate of the reconstitution of ribosomes from subribosomal particles and split proteins. J Mol Biol 34: 609–619 [DOI] [PubMed] [Google Scholar]
- Powers T, Noller HF 1991. A functional pseudoknot in 16S ribosomal RNA. EMBO J 10: 2203–2214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers T, Stern S, Changchien LM, Noller HF 1988. Probing the assembly of the 3′ major domain of 16 S rRNA. Interactions involving ribosomal proteins S2, S3, S10, S13 and S14. J Mol Biol 201: 697–716 [DOI] [PubMed] [Google Scholar]
- Powers T, Daubresse G, Noller HF 1993. Dynamics of in vitro assembly of 16 S rRNA into 30 S ribosomal subunits. J Mol Biol 232: 362–374 [DOI] [PubMed] [Google Scholar]
- Pulk A, Maivali U, Remme J 2006. Identification of nucleotides in E. coli 16S rRNA essential for ribosome subunit association. RNA 12: 790–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswamy P, Woodson SA 2009. S16 throws a conformational switch during assembly of 30S 5′ domain. Nat Struct Mol Biol 16: 438–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger D 1974. Ribosome formation in Escherichia coli. Cold Spring Harbor, NY [Google Scholar]
- Schuwirth BS, Borovinskaya MA, Hau CW, Zhang W, Vila-Sanjurjo A, Holton JM, Cate JH 2005. Structures of the bacterial ribosome at 3.5 Å resolution. Science 310: 827–834 [DOI] [PubMed] [Google Scholar]
- Stern S, Weiser B, Noller HF 1988. Model for the three-dimensional folding of 16 S ribosomal RNA. J Mol Biol 204: 447–481 [DOI] [PubMed] [Google Scholar]
- Stern S, Powers T, Changchien LM, Noller HF 1989. RNA–protein interactions in 30S ribosomal subunits: Folding and function of 16S rRNA. Science 244: 783–790 [DOI] [PubMed] [Google Scholar]
- Sykes MT, Williamson JR 2009. A complex assembly landscape for the 30S ribosomal subunit. Annu Rev Biophys 38: 197–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talkington MW, Siuzdak G, Williamson JR 2005. An assembly landscape for the 30S ribosomal subunit. Nature 438: 628–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub P, Nomura M 1968. Structure and function of E. coli ribosomes. V. Reconstitution of functionally active 30S ribosomal particles from RNA and proteins. Proc Natl Acad Sci 59: 777–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub P, Nomura M 1969. Structure and function of Escherichia coli ribosomes. VI. Mechanism of assembly of 30S ribosomes studied in vitro. J Mol Biol 40: 391–413 [DOI] [PubMed] [Google Scholar]
- von Ahsen U, Noller HF 1995. Identification of bases in 16S rRNA essential for tRNA binding at the 30S ribosomal P site. Science 267: 234–237 [DOI] [PubMed] [Google Scholar]
- Warner JR, Vilardell J, Sohn JH 2001. Economics of ribosome biosynthesis. Cold Spring Harb Symp Quant Biol 66: 567–574 [DOI] [PubMed] [Google Scholar]
- Wimberly BT, Brodersen DE, Clemons WM Jr, Morgan-Warren RJ, Carter AP, Vonrhein C, Hartsch T, Ramakrishnan V 2000. Structure of the 30S ribosomal subunit. Nature 407: 327–339 [DOI] [PubMed] [Google Scholar]
- Xu ZL, Culver GM 2009. Chemical probing of RNA and RNA/protein complexes. Meth Enzymol 468: 147–165 [DOI] [PubMed] [Google Scholar]
- Yassin A, Fredrick K, Mankin AS 2005. Deleterious mutations in small subunit ribosomal RNA identify functional sites and potential targets for antibiotics. Proc Natl Acad Sci 102: 16620–16625 [DOI] [PMC free article] [PubMed] [Google Scholar]