Abstract
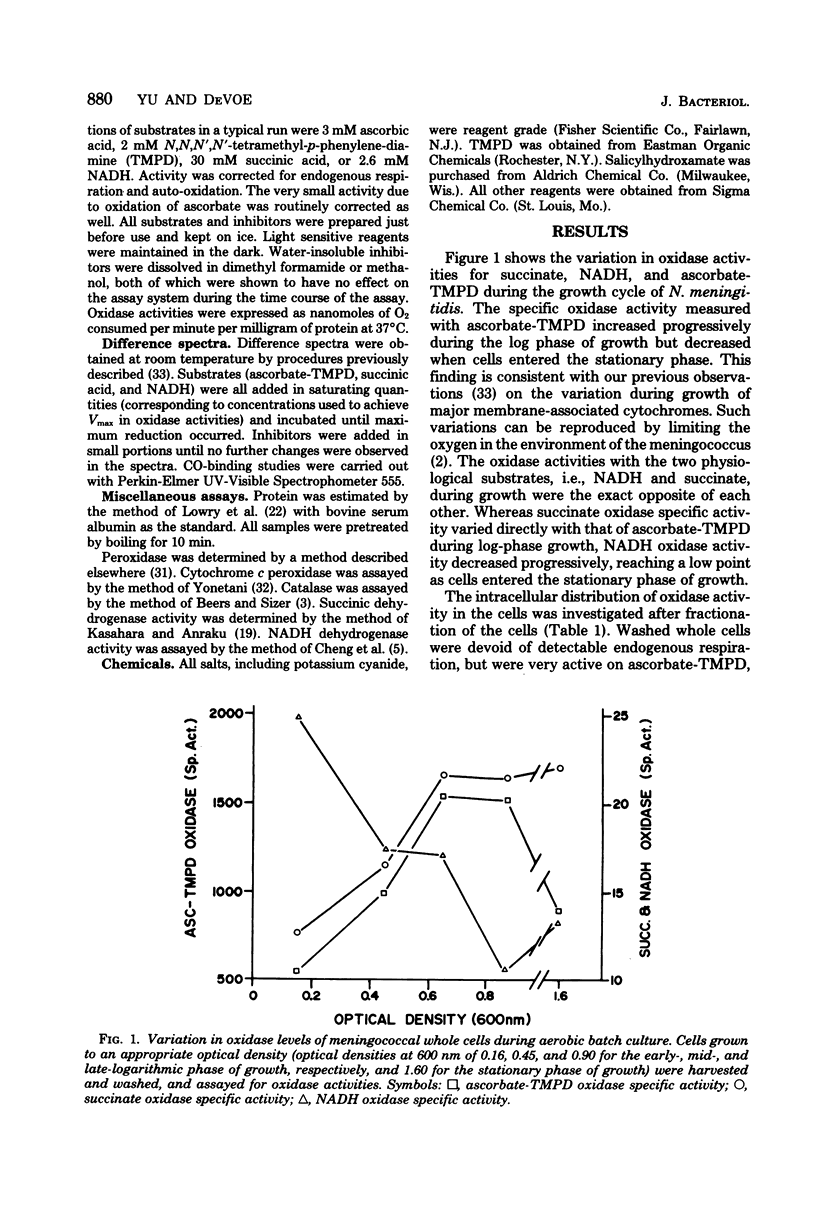
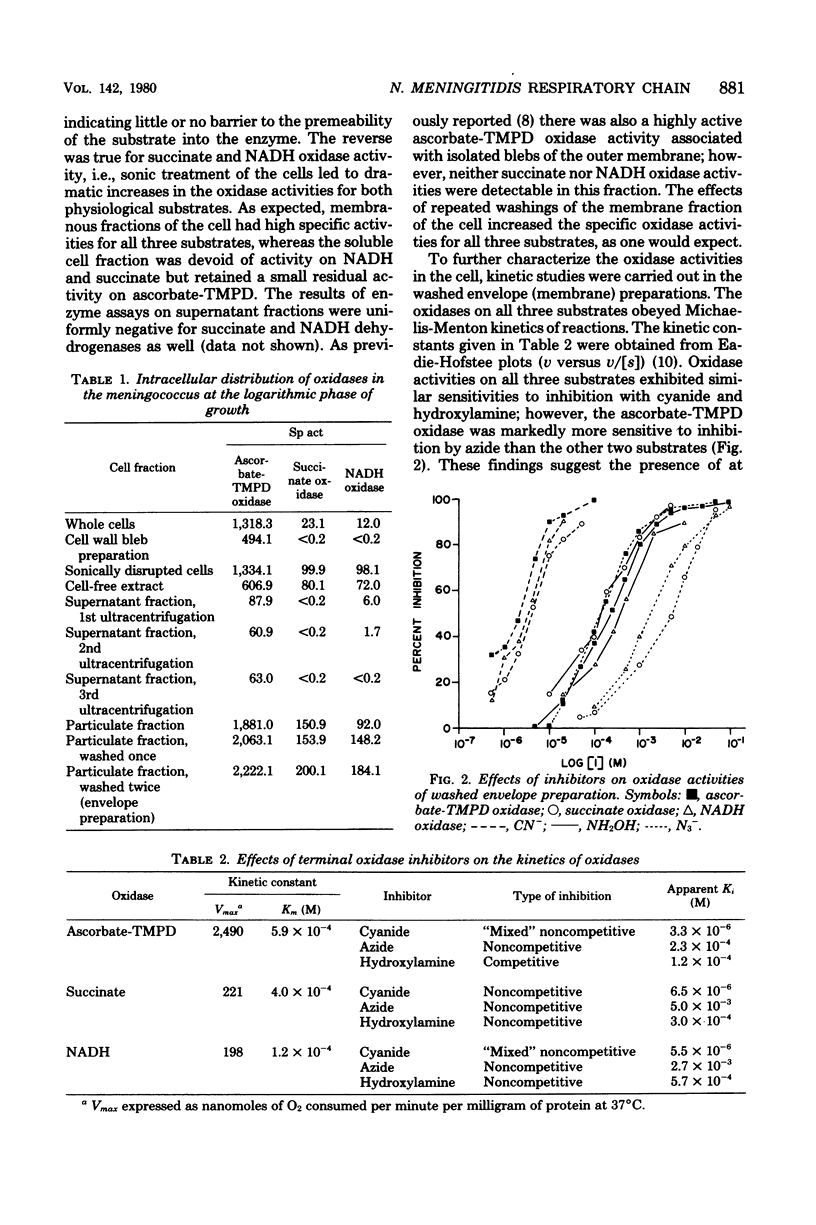
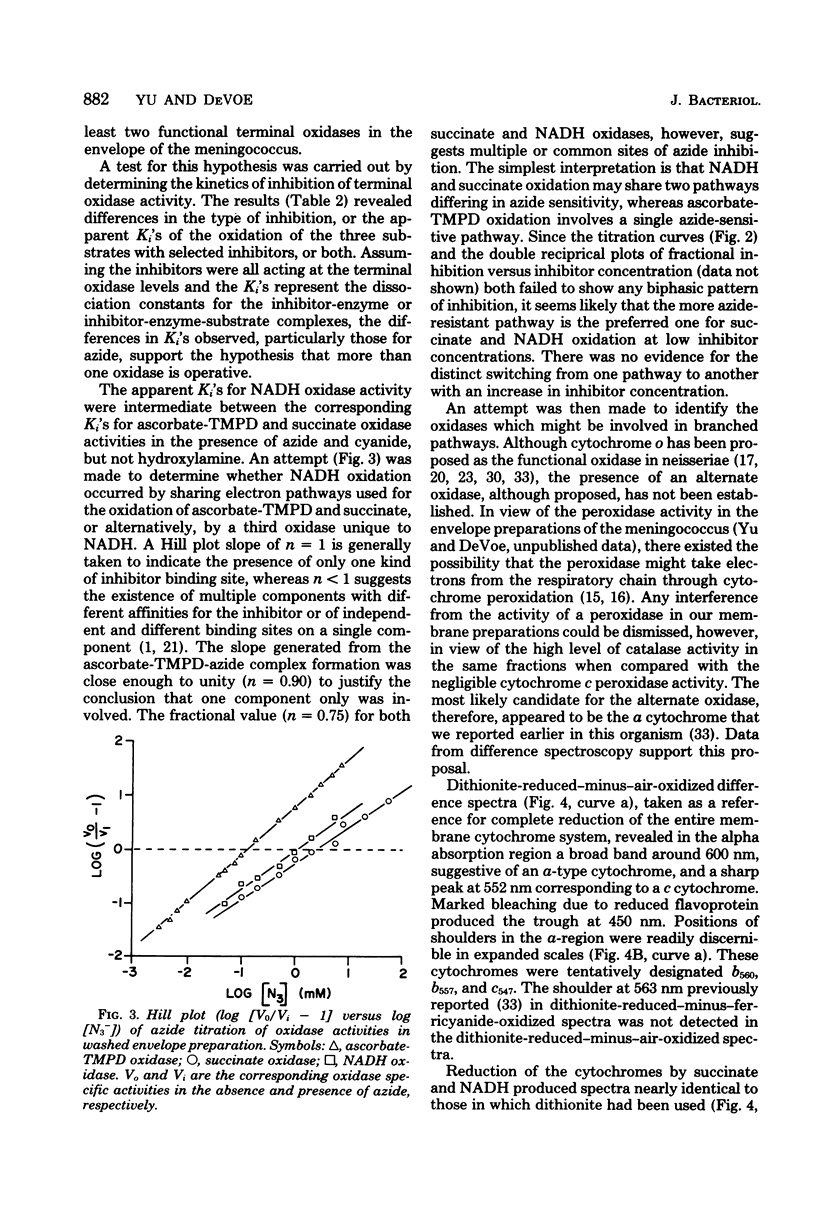
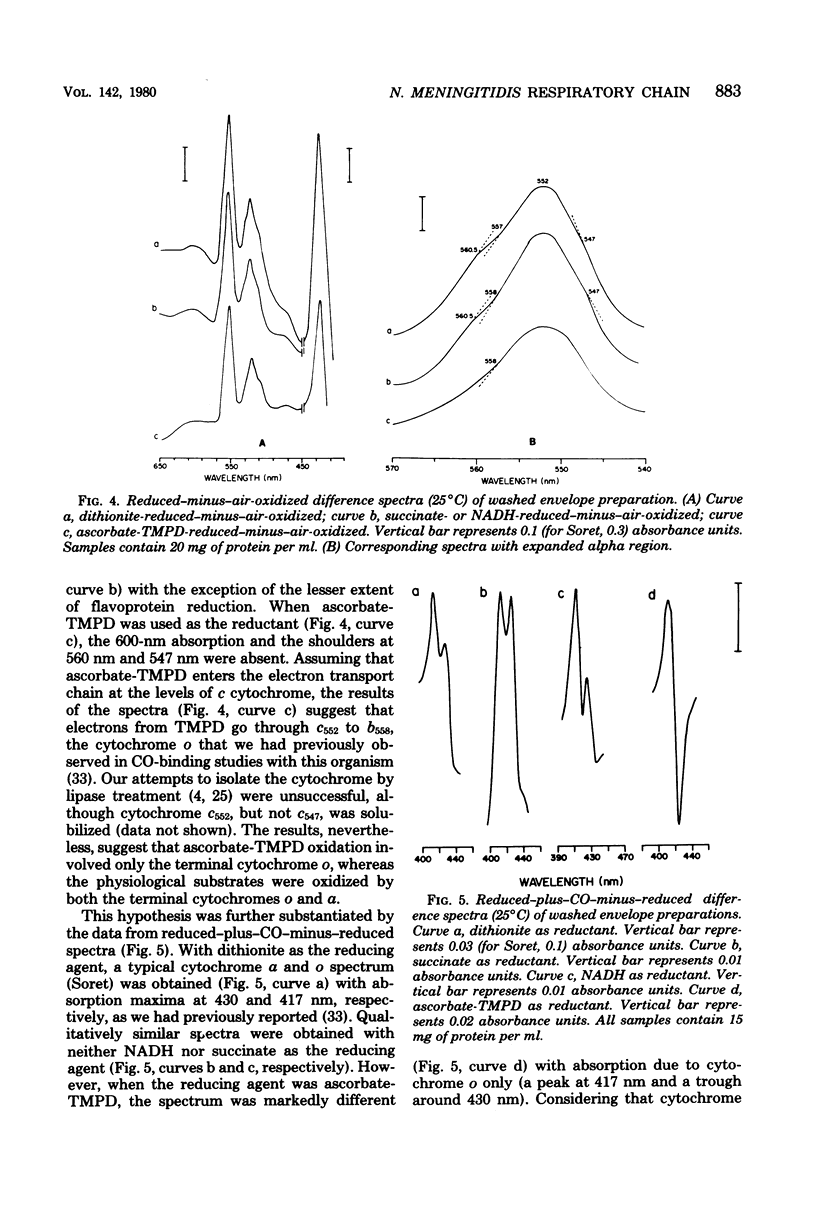
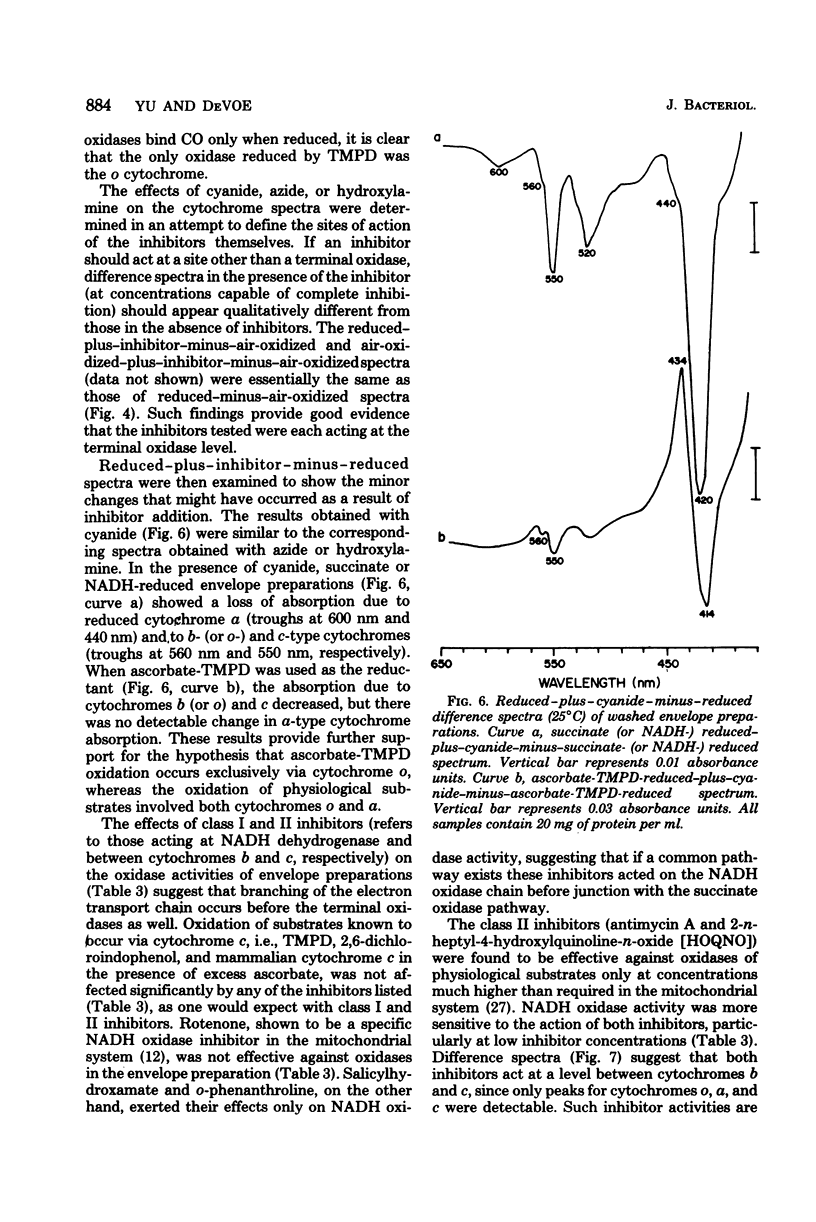
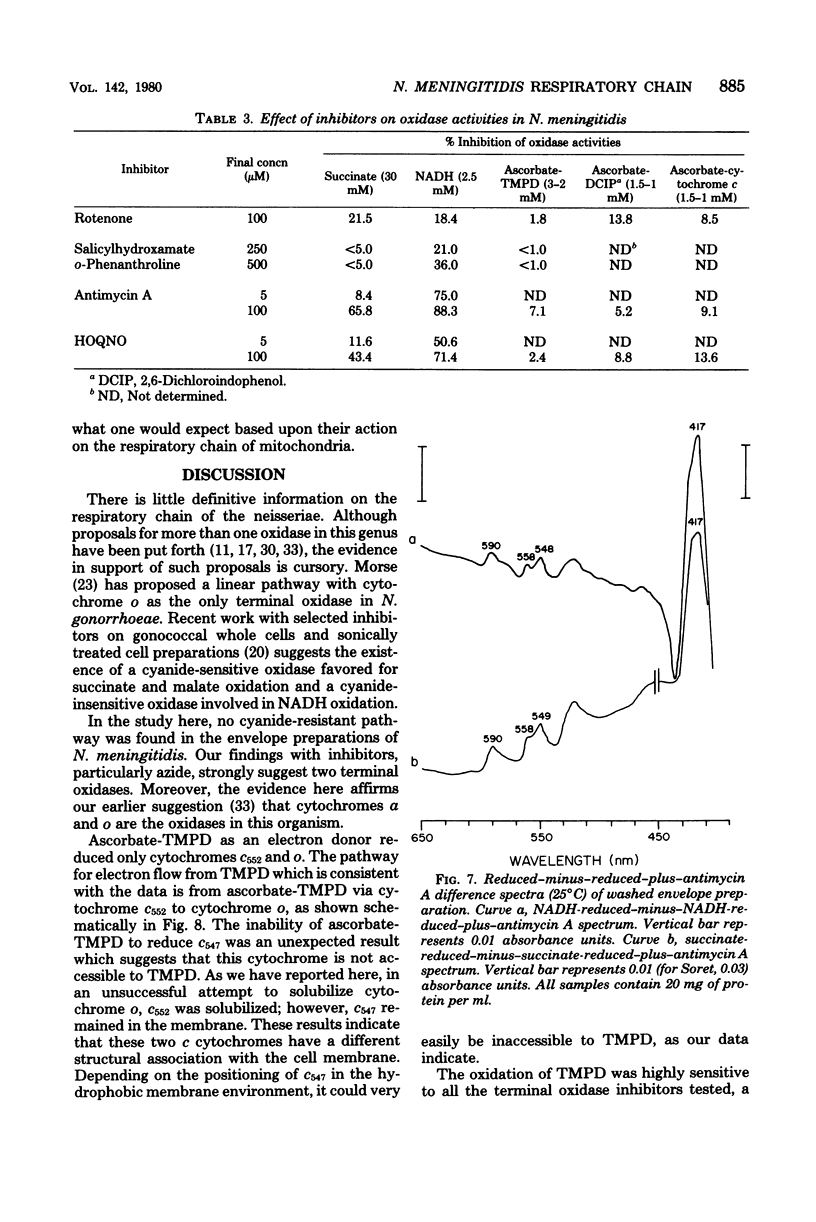
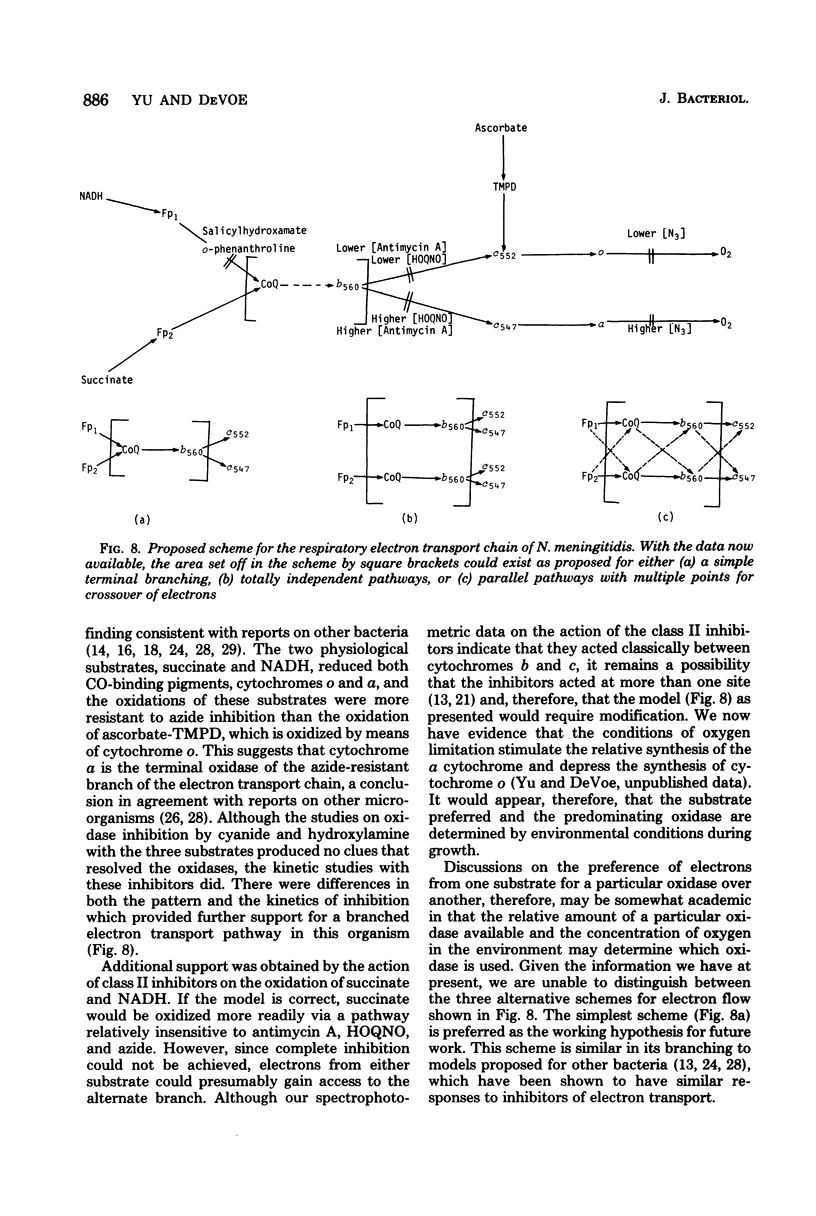
The respiratory components of the envelope membrane preparation of Neisseria meningitidis were investigated. Oxidase activities were demonstrated in this fraction in the presence of succinic acid, reduced nicotinamide adenine dinucleotide, and ascorbate-N,N,N',N'-tetramethyl-p-phenylene-diamine (TMPD). Differences in the kinetics of inhibition by terminal oxidase inhibitors on the three oxidase activities indicated that ascorbate-TMPD oxidation involved only an azide-sensitive oxidase, whereas oxidation of the physiological substrates involved two oxidases, one of which was relatively azide resistant. Spectrophotometric studies revealed that ascorbate-TMPD donated its electrons exclusively to cytochrome o, whereas the physiological substrates were oxidized via both cytochromes o and a. The effects of class II inhibitors on the oxidases suggest terminal branching of the electron transport chain at the cytochrome b level. A model of the respiratory system in N. meningitidis is proposed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleby C. A. Electron transport systems of Rhizobium japonicum. I. Haemoprotein P-450, other CO-reactive pigments, cytochromes and oxidases in bacteroids from N2-fixing root nodules. Biochim Biophys Acta. 1969 Jan 14;172(1):71–87. doi: 10.1016/0005-2728(69)90093-0. [DOI] [PubMed] [Google Scholar]
- Archibald F. S., DeVoe I. W. Iron in Neisseria meningitidis: minimum requirements, effects of limitation, and characteristics of uptake. J Bacteriol. 1978 Oct;136(1):35–48. doi: 10.1128/jb.136.1.35-48.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEERS R. F., Jr, SIZER I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952 Mar;195(1):133–140. [PubMed] [Google Scholar]
- Broberg P. L., Smith L. The cytochrome system of Bacillus megaterium KM. The presence and some properties of two CO-binding cytochromes. Biochim Biophys Acta. 1967 May 9;131(3):479–489. doi: 10.1016/0005-2728(67)90007-2. [DOI] [PubMed] [Google Scholar]
- Cheng K. J., Ingram J. M., Costerton J. W. Release of alkaline phosphatase from cells of Pseudomonas aeruginosa by manipulation of cation concentration and of pH. J Bacteriol. 1970 Nov;104(2):748–753. doi: 10.1128/jb.104.2.748-753.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOWD J. E., RIGGS D. S. A COMPARISON OF ESTIMATES OF MICHAELIS-MENTEN KINETIC CONSTANTS FROM VARIOUS LINEAR TRANSFORMATIONS. J Biol Chem. 1965 Feb;240:863–869. [PubMed] [Google Scholar]
- DeVoe I. W. Egestion of degraded meningococci by polymorphonuclear leukocytes. J Bacteriol. 1976 Jan;125(1):258–266. doi: 10.1128/jb.125.1.258-266.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVoe I. W., Gilchrist J. E. Piliation and colonial morphology among laboratory strains of meningococci. J Clin Microbiol. 1978 Apr;7(4):379–384. doi: 10.1128/jcm.7.4.379-384.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoe I. W., Gilchrist J. E. Release of endotoxin in the form of cell wall blebs during in vitro growth of Neisseria meningitidis. J Exp Med. 1973 Nov 1;138(5):1156–1167. doi: 10.1084/jem.138.5.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoe I. W., Golchrist J. E. Localization of tetramethylphenylenediamine-oxidase in the outer cell wall layer of Neisseria meningitidis. J Bacteriol. 1976 Oct;128(1):144–148. doi: 10.1128/jb.128.1.144-148.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones C. W., Redfearn E. R. Electron transport in Azotobacter vinelandii. Biochim Biophys Acta. 1966 Mar 7;113(3):467–481. doi: 10.1016/s0926-6593(66)80005-x. [DOI] [PubMed] [Google Scholar]
- Jones M. V., Hughes D. E. The oxidation of nicotinic acid by Pseudomonas ovalis Chester. The terminal oxidase. Biochem J. 1972 Sep;129(3):755–761. doi: 10.1042/bj1290755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. V. Physiological role for the membrane bound ascorbate-TMPD oxidase in pseudomonas putida. Arch Microbiol. 1975 Mar 10;102(3):275–279. doi: 10.1007/BF00428378. [DOI] [PubMed] [Google Scholar]
- Jurtshuk P., Aston P. R., Old L. Enzymatic oxidation of tetramethyl-p-phenylenediamine and p-phenylenediamine by the electron transport particulate fraction of Azotobacter vinelandii. J Bacteriol. 1967 Mar;93(3):1069–1078. doi: 10.1128/jb.93.3.1069-1078.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurtshuk P., Jr, Mueller T. J., Acord W. C. Bacterial terminal oxidases. CRC Crit Rev Microbiol. 1975 May;3(4):399–468. doi: 10.3109/10408417509108757. [DOI] [PubMed] [Google Scholar]
- Jurtshuk P., Milligan T. W. Preliminary characterization studies on the Neisseria catarrhalis respiratory electron transport chain. J Bacteriol. 1974 Oct;120(1):552–555. doi: 10.1128/jb.120.1.552-555.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasahara M., Anraku Y. Succinate dehydrogenase of Escherichia coli membrane vesicles. Activation and properties of the enzyme. J Biochem. 1974 Nov;76(5):959–966. [PubMed] [Google Scholar]
- Kenimer E. A., Lapp D. F. Effects of selected inhibitors on electron transport in Neisseria gonorrhoeae. J Bacteriol. 1978 May;134(2):537–545. doi: 10.1128/jb.134.2.537-545.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knook D. L., Kauffman H. F., Van 'T Riet J. Different effects of 2-n-heptyl-4-hydroxyquinoline-N-oxide on oxygen and nitrate respiration in Klebsiella aerogenes. Arch Biochem Biophys. 1974 Dec;165(2):449–455. doi: 10.1016/0003-9861(74)90270-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Morse S. A. The biology of the gonococcus. CRC Crit Rev Microbiol. 1978;7(2):93–189. doi: 10.3109/10408417909083071. [DOI] [PubMed] [Google Scholar]
- Niven D. F., Collins P. A., Knowles C. J. The respiratory system of Chromobacterium violaceum grown under conditions of high and low cyanide evolution. J Gen Microbiol. 1975 Oct;90(2):271–285. doi: 10.1099/00221287-90-2-271. [DOI] [PubMed] [Google Scholar]
- Sasaki T., Motokawa Y., Kikuchi G. Occurrence of both a-type and o-type cytochromes as the functional terminal oxidases in Rhodopseudomonas spheroides. Biochim Biophys Acta. 1970 Mar 3;197(2):284–291. doi: 10.1016/0005-2728(70)90039-3. [DOI] [PubMed] [Google Scholar]
- Weston J. A., Collins P. A., Knowles C. J. The respiratory system of the marine bacterium Beneckea natriegens. II. Terminal branching of respiration to oxygen and resistance to inhibition by cyanide. Biochim Biophys Acta. 1974 Nov 19;368(2):148–157. doi: 10.1016/0005-2728(74)90145-5. [DOI] [PubMed] [Google Scholar]
- White D. C., Sinclair P. R. Branched electron-transport systems in bacteria. Adv Microb Physiol. 1971;5:173–211. doi: 10.1016/s0065-2911(08)60407-5. [DOI] [PubMed] [Google Scholar]
- Winter D. B., Morse S. A. Physiology and metabolism of pathogenic Neisseria: partial characterization of the respiratory chain of Neisseria gonorrhoeae. J Bacteriol. 1975 Aug;123(2):631–636. doi: 10.1128/jb.123.2.631-636.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]



