Abstract
Primary lung cancer is very heterogeneous in its clinical presentation, histopathology, and treatment response; and like other diseases, the prognosis consists of two essential facets: survival and quality of life (QOL). Lung cancer survival is mostly determined by disease stage and treatment modality, and the five-year survival rate has been in a plateau of 15% for three decades. QOL is focused on life aspects that are affected by health conditions and medical interventions; the balance of physical functioning and suffering from treatment side effects has long been a concern of care providers as well as patients. Obviously needed are easily-measurable biologic markers to stratify patients prior to treatment for optimal results in survival and QOL and to monitor treatment responses and toxicities. Targeted therapies towards the mechanisms of tumor development, growth, and metastasis are promising and actively translated into clinical practice. Long-term lung cancer (LTLC) survivors are people who are alive five years after the diagnosis. Knowledge about the health and QOL in LTLC survivors is limited because outcome research in lung cancer has been focused mainly on short-term survival. The independent or combined effects of lung cancer treatment, aging, smoking and drinking, comorbid conditions, and psychosocial factors likely cause late effects including organ malfunction, chronic fatigue, pain, or premature death among lung cancer survivors. New knowledge to be gained should help lung cancer survivors, their healthcare providers, and their caregivers by providing evidence for establishing clinical recommendations to enhance their long-term survival and health-related QOL.
Keywords: non small cell lung cancer, small cell lung cancer, survival, quality of life, pharmacogenomics, gene expression profiling, chemotherapy, radiation therapy, toxicity
2. Introduction
Lung cancer survivorship has two critical attributes: survival time or quantity and quality of life (QOL). The unparallel perception regarding the importance of QOL vs. survival after a lung cancer diagnosis was not appreciated by the medical research community until recently, mainly due to the leading battle to prolong patients’ lives and the devastating demand to find a cure. After decades of efforts focusing on reducing lung cancer incidence and mortality, we are now challenged by the lack of understanding of the health conditions and QOL among people who survived lung cancer. In this chapter, current yet limited knowledge is summarized in three major areas with varying levels of details that are available in the published literature: clinical epidemiology, genomic application towards patient-specific care, and psychosocial-behavioral characteristics of lung cancer survivors.
3. Clinical Epidemiology of Lung Cancer Survival
3.1. An Overview
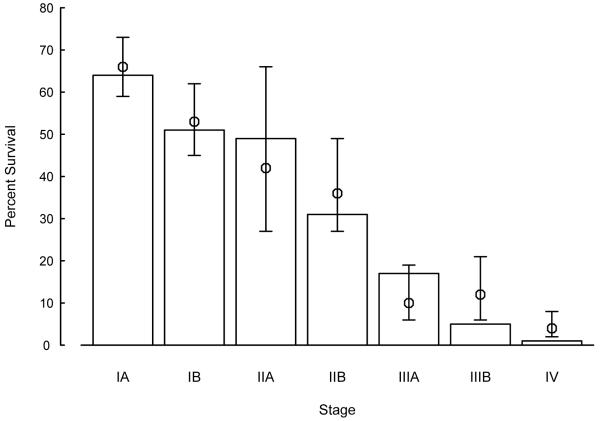
Primary lung cancer is very heterogeneous in its clinical presentation, histopathology, and treatment response. Conventionally, lung cancer has been divided into NSCLC and SCLC. For both groups, cancer stage is the most significant predictor of survival,1, 2 as illustrated in Figure 1. Five-year survival rates were comparable between two large clinical patient data resources that were from different institutions at different time frames: one from MD Anderson Cancer Center (diagnosed 1975-1988) and one from Mayo Clinic (diagnosed 1997-2002), indicating little improvement in NSCLC survival in the last 30 years. In SCLC, the five-year survival rate ranges from approximately 25% for limited disease to 1% - 5% for extensive disease.2-4
Figure 1. Five-year Survival Rates and 99% Confidence Intervals of Mayo Clinic NSCLC Patients Compared to MD Anderson Cancer Center’s Patients a.
a Open bars are average survival rates for each disease stage based on Mountain’s series of MD Anderson Cancer Center;1 circles with lines are estimated survival rates and 99% confidence intervals of Mayo Clinic’s series.2
Treatment modality, mostly dictated by lung cancer stage and patient’s performance status, directly determines disease survival. For NSCLC, surgical resection is the standard treatment for stage I-II disease; patients with IB or II disease are now being offered adjuvant chemotherapy.5 Some patients with a stage IIIA tumor are operable but often receive pre- or post-operative radiation and/or chemotherapy. For locally advanced, inoperable stage IIIA tumors, radiation with chemotherapy remains the standard of care; for selected patients with IIIB (with pleural effusion) or IV disease, chemotherapy remains the standard treatment in conjunction with supportive care. In SCLC, the majority of the patients with limited disease are treated with single-agent or combination chemotherapy with radiation therapy, and prophylactic cranial irradiation (for complete responders) is commonly used. In both NSCLC and SCLC, systemic dissemination can occur as solitary metastasis, oligometastasis, and multiple metastases. Solitary metastasis constitutes the majority of long-term survivors in advanced-stage lung cancer.6
At present, for any given chemo- or radiotherapy regimen, which is prescribed by the standard protocol with a fixed dosage, the response rate and toxicity range varies widely among patients. The disease-free survival time, even after adjusting for the well-established predictors, i.e., disease stage, histology, performance status, and treatment, varies significantly. Obviously needed are easily-measurable biologic markers to stratify patients prior to treatment for optimal results in survival and QOL and to monitor treatment responses and toxicities.
3.2. Lung Cancer Progression and Subsequent Cancers
Despite the generally-accepted diagnostic criteria, differentiation between lung cancer recurrence in the lung and occurrence of a new primary lung cancer is often difficult, especially when the subsequent tumor arises after more than two years from the initial diagnosis with the same tumor histology. 7, 8 Molecular biologic methods have not yet been proven to be a reliable tool to meet this challenge. Recognizing this uncertainty in differential diagnosis, the published literature suggests that a majority of NSCLC recurrences occur within two years after surgery and late recurrence may occur up to 10 years.9 Recurrent disease has been reported in 4% - 5% of five-year NSCLC survivors;9-12 up to 10% of recurrences may be discovered beyond five years following initial curative therapy.13 Two cohort studies of disease-free five-year survivors of NSCLC have reported a subsequent recurrence rate of 2% - 3% per patient-year.10, 14 Clinical management and outcome of recurrent lung cancer have been reported, demonstrating benefit of various treatments over no treatment.15, 16 Molecular characteristics or risk behaviors associated with late recurrent tumors have not been well described.
3.3. Additional Lung Cancer Prognostic Predictors
More and more factors emerged as independent lung cancer survival predictors in the recent decade. The importance of pathologic markers such as tumor size, cell type, lymphatic and blood vessel invasion, rate of proliferation and ploidy, and extent of tumor necrosis is apparent but inconsistent. Following is a highlight on the current knowledge of selected survival predictors beyond disease stage, treatment, and patient gender.17
Tumor Cell Differentiation
In a study of 5,018 hospital-based patients and 712 population-based patients, tumor grade was found to be significantly associated with survival after adjusting for the effects of age, gender, smoking history, tumor stage, histological cell type, and treatment modality. Patients with poorly/undifferentiated carcinoma had a 70% elevated risk of death compared to those with well differentiated carcinoma. A 40% elevated risk was observed for patients with moderately differentiated carcinoma.18
Smoking Cessation
In a study of 5,229 patients with NSCLC and SCLC, the median survival time among never, former, and current smokers with NSCLC was 1.4 years, 1.3 years, and 1.1 years, respectively (P < 0.01). Female NSCLC patients had a significantly lower risk of mortality with a longer duration of smoking abstinence. Specifically, the relative risk per 10 years of smoking abstinence is 0.85, supporting a direct biologic effect of smoking on survival.19
Dietary Supplements
In the general population, approximately 40% of people take vitamin/mineral supplements regularly; whereas, nearly 80% of cancer patients do so. Both clinical and laboratory data have shown that certain micronutrients effect the growth of malignant cells, i.e., vitamins and minerals may be modulators of tumor growth. In a study of more than 1,300 lung cancer patients, the use of vitamin/mineral supplements was found to be associated with improved survival among both NSCLC and SCLC patients.20, 21 Reduction in the death rate was 26% for NSCLC patients and 37% for SCLC patients, respectively.
4. Genomic and Biologic Markers: Advances and Applications in Lung Cancer Prognosis
Since the completion of the sequence of the whole human genome, the genomic approach in cancer research is playing a significant role in identifying diagnostic, prognostic, and therapeutic molecular markers. During the past two decades, there have been over 1,000 studies on biological markers including biochemical, histologic, and molecular, in hopes of identifying clinically useful prognostic markers and to assist in patient management.22 However, little scientific conclusion can be drawn because most of these studies are limited to small numbers or selected subgroups of patients, diverse methodology and type of specimens used, variable histogolic subtype, short and/or incomplete follow-up, univariate analysis, and various endpoints. The majority of the investigators have only looked at overall survival, mostly up to five years, with rare emphasis on disease-free survival, relapse rate, relationship to treatment responses, or long-term outcomes. In addition, there are only a few tumor target markers and virtually no host-specific predictive measures to base an optimal choice of the type, dosage, intensity, and combinations of the available drug(s).
4.1. Tumor Molecular Markers
Gene Expression Profiling: DNA Microarray Technology
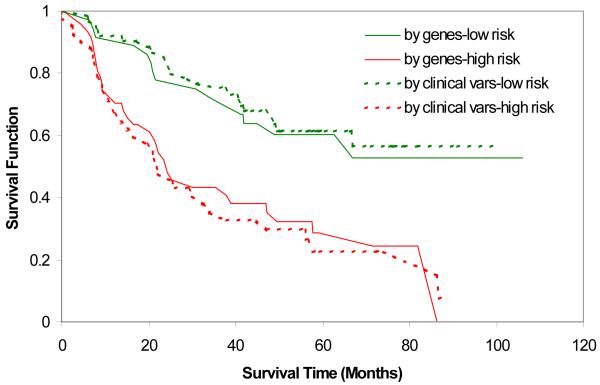
DNA microarray performs simultaneous interrogation of thousands of genes, which offers a unique opportunity to measure a tumor from multiple angles, which generally provides a more accurate measurement about biological behaviors than any single cellular or molecular parameter. As a high-throughput tool at the molecular level, DNA microarray has clear advantages over traditional histologic examinations and has been widely used in cancer research to better predict clinical outcomes and potentially improve patient management. The molecular measurement is more objective and often detects the difference that routine pathology fails to detect. More importantly, the DNA microarray provides a closer look at gene activities in tumors and creates an opportunity to find therapeutic targets. Studies show that this new approach provides accurate tumor sub-classification and outcome predictions such as tumor stage, metastatic status, patient survival, and offers some hope for individualized medicine.23 However, growing evidence suggests that gene-based prediction is not stable and little is known about the prediction power of the gene expression profile compared to well-known clinical and pathologic predictors. Overall, most studies lack an independent validation. When conventional predictors of age, gender, stage, cell type, and histologic grade are considered collectively, the predictive advantage of the gene expression profile diminishes. As shown in Figure 2, the gene panel derived from the DNA microarray achieved very good prediction for patient survival but did not outperform the prediction by the combination of five conventional variables (age, gender, stage, cell type, and tumor grade). This suggests that most prediction from the gene panel is reflected by tumor pathology information; thus, the enhanced predictive accuracy from the gene panel is limited at this point.
Figure 2. a23 Survival Curve Predicted by Gene Risk Index (50 genes) and Conventional Variables (stage, age, gender, cell type and grade).
a. Adapted from Sun and Yang, 2006, CEPB
Protein Expression Profiling: Mass Spectrometry
Only in the recent five years, technology in matrix-assisted laser desorption-ionization time-of-flight mass spectrometry (MALDI-TOF MS) made it possible to profile proteins in tissues. An obvious advantage over RNA expression profiling is that deciphering protein expression patterns would be closer to revealing the biological function of cells, and then to determining the pathologic mechanisms of disease development and progression. Meanwhile, greater challenges have been encountered in analyzing and interpreting protein profiling due to the immense throughput of data and ultra-sensitivity of signal detection. Pioneer researchers in applying protein profiling to predict lung cancer prognosis have reported a 15-protein peak-pattern 24 and a 25-signal proteomic signature25 that can distinguish lung cancer patients with poor vs. good outcome.
Genomic or proteomic signatures selected for predicting lung cancer recurrence and survival hold high hope to be used in clinical practice, particularly for those that have been vigorously validated in independent data sets. Like a clinical trial for a novel agent or a new treatment regimen before moving into the clinic, these markers need to be evaluated for their efficacy demonstrating better than currently available predictors and ease of use. Clearly, a gene signature or panel that does not provide improved prediction independent from well-known predictors will less likely be adopted clinically.
4.2. Development of Targeted Therapy
Tumor Marker Targeted Therapy
An ultimate goal of searching for DNA marker panels, molecular pathway signatures, or genomic patterns is to discover biological targets for therapeutics. Many drugs that target molecular markers have been evaluated in randomized controlled trials, but most of them failed to produce positive results.26 One of the important findings in cancer chemotherapy is the identification of somatic mutations in the tyrosine kinase domain of the epidermal growth factor receptor (EGFR) in NSCLC and a correlation with response to EGFR inhibitors.27-29 EGFR gene amplification is more prevalent in Western populations; whereas, the amplification of the closely related HER2 gene, which could also have implications for the treatment of NSCLC, is more frequent in East Asian patients. Ethnicity may indicate different genetic backgrounds in common tumors that may influence clinical outcome and response to therapy.30 Another important molecular marker for targeted therapy is the vascular endothelial growth factor (VEGF), which participates in the regulation of new vessel growth and promotion of immature vasculature survival.31 Drugs that inhibit the VEGF have been developed and tested successfully in randomized controlled trials, with the caveat of severe side effects including thrombocytopenia, hypertension, and neutropenia.32 The combined use of targeted therapy and chemotherapy is very promising in both improved treatment response and reduced toxicity.33
Immunotherapy
The fundamental basis of immunotherapy is that the immune system can distinguish cancerous cells from normal cells, and the immune machinery can be utilized to destroy cancer cells. Three strategies have been taken:34 The first is non-specific cellular immunotherapy, such as lymphokine-activated killer cells and interlukin-2 in treating renal cell carcinoma. The second is specific cellular immunotherapy that relies on but not limited to tumor antigen specific cytolytic lymphocytes and tumor infiltrating lymphocytes. The third strategy is therapeutic vaccination that incorporates tumor antigens with an adjuvant therapy for the recognition by the immune system. NSCLC has been not been considered as an immunogenic cancer and presently there is no standard immunotherapy in clinical practice, although optimism prevails on the progress of evaluating novel immuno-therapeutics.35
Gene Therapy
Improved molecular technology has made gene therapy an emerging and promising strategy for cancer treatment. Three experimental approaches have been used: immunotherapy that applies genetically modified cells and viral particles to stimulate the immune system to kill cancer cells; oncolytic virotherapy that causes cell death by replicating viral particles within cancer cells; and gene transfer that causes cell death or low growth by introducing new gene(s) to cancer cells or surrounding tissue.36 The promise of gene therapy is the identification of critical regulatory sequences that can selectively activate or inactivate the therapeutic genes in cancer cells; however, a major challenge is the development of the delivery of vector constructs that can reach the target effectively.
4.3. Host Genetic Predisposition in Treatment Response and Toxicity
Inherent and acquired drug resistance is a cause of chemotherapy failure, and pharmacogenomic studies have begun defining multiple gene variations responsible for varied drug metabolisms. Platinum-based drugs are the most commonly used in lung cancer treatment, and a major obstacle for the clinical use of platinum drugs is the development of tumor resistance. Maintaining an effective antineoplastic level of the platinum drug in the sera and tumor for a prolonged period could potentially eliminate acquired resistance; however, such an approach increases the risk for toxicity and drug side effects. Cisplatin-induced ototoxicity, for example, is a result of drug-induced neuron and hair cell destruction and a major dose-limiting adverse drug response (ADR).37 Other platinum drug related ADRs range from alopecia, neurotoxicity, myelotoxicity, to nephrotoxicity.38, 39 On the other hand, emerging evidence suggests that mild to moderate levels of specific ADRs may be a direct gauge for accurate dosing.40 An analysis of three randomized clinical trials39 showed a significant survival benefit for NSCLC patients who had mild or severe chemotherapy-induced neutropenia compared with those who did not. Other studies have shown a similar relation between ADRs and treatment outcomes for various cancers, such as colorectal, breast, testicular, and ovarian.41-44 The identification of patients most likely to benefit from chemotherapy through the incorporation of genomic information into treatment decisions may be a critical step in overcoming the barriers caused by ADRs.45, 46
The glutathione metabolic pathway is directly involved in the detoxification or inactivation of platinum compounds; and available evidence supports the role of the glutathione pathway in acquired and inherited drug resistance through rapid drug detoxification or through drug activation bypassing, which adversely impacts the treatment outcome of lung cancer.47 DNA repair is another critical mechanism of resistance to platinum-based chemotherapy. It is hypothesized that reduced DNA repair in tumor cells has a higher sensitivity to treatment and therefore has a better response and outcome after radio- and/or chemotherapy; whereas, increased repair capability causes tumor resistance and worse response.48 Clinical studies show that over-expression of excision repair cross-complementation group 1 (ERCC1) correlates with poor survival for gemcitabine/cisplatin-treated NSCLC patients, and the polymorphisms of ERCC1 or excision repair cross-complementation group 2 (ERCC2) are significantly associated with survival times for patients treated with platinum-based chemotherapy.49-52
Ionizing radiation is also a commonly used treatment modality for late stage lung cancer and acts on DNA, causing double strand and single strand breaks and base lesions, particularly double stand DNA breaks.53 The damages are repaired by at least two distinct pathways: Homologous recombination (HR) and nonhomologous end-joining (NHEJ). HR requires an undamaged template molecule that contains a homologous DNA sequence, generally from its sister chromatid; RAD51 and RAD52 proteins are involved in this pathway. For NHEJ, no undamaged partner DNA homologs are needed for rejoining of DNA breaks; RAD50 and DNA-dependent protein kinase may participate in the NHEJ repair process.54, 55 Genetic defects in HR or NHEJ can cause impaired DNA replication and enhanced radiation sensitivity.54
A key question is whether biological functions and mechanisms can be a clinically measurable determinant of a cancer patient’s response to chemotherapy and survival. Specifically, although evidence from laboratory studies supports a significant role for the glutathione system and DNA repair pathway in antitumor drug metabolism and resistance, the exploration of their clinical relevance and implications in cancer patients continues to evolve. Regulation of the glutathione metabolic and transport systems has been one of the targets for optimizing efficacy as well as minimizing toxicities of many chemotherapeutic agents.56-60 The premise for the clinical application of genomic polymorphic markers is most profound in identifying likely responsive patients and differentiating patients, for example, who could be sensitized with a glutathione and/or DNA repair modulator from those who should not be given the drug due to the predicted occurrence of severe side effects. Successful translation and application of these genetic polymorphic markers to treatment modulators or response predictors is an essential step toward individualized drug therapy.
5. Quality of Life: Psychosocial-Behavioral Characteristics among Lung Cancer Survivors
QOL is a subjective, dynamic, multi-dimensional measure encompassing all aspects that impact an individual’s life,61-63 and is often exchangeable with the term health-related QOL (HRQOL). Although there is no consensus on the definition, QOL or HRQOL is more focused on life aspects that are affected by health conditions and medical interventions. Commonly used QOL instruments are well organized and summarized by Li et al:63 from general tools such as the World Health Organization Quality of Life Assessment (WHOQOL)62 and Medical Outcome Study 36-Item Short-Form Health Survey (SF 36),64, 65 to cancer-specific measures such as the European Organization of Research and Treatment of Cancer (EORTC) QLQ C-30,66 and to lung cancer-specific scales such as the Functional Assessment of Cancer Therapy – Lung (FACT-L) version 3,67 and Lung Cancer Symptom Scale (LCSS).68 A comprehensive review of QOL in lung cancer patients evaluated 50 instruments and identified the best tool to be the EORTC Quality of Life Lung Cancer Questionnaire (EORTC-LC13) in conjunction with the core cancer questionnaire (QLQ-C30).69 LCSS and FACT-L are two additional instruments with good reliability and validity.
5.1. QOL Surrounding Lung Cancer Treatment
The balance of physical functioning and suffering from treatment side effects has long been a concern of patient care providers. Until two decades ago in the mid-1980s, supportive care plus combination chemotherapy (cisplatin and vinblastine) was not considered superior over supportive care only (palliative radiation, psychosocial support, analgesics, and nutritional support) for metastatic non-small cell lung cancer because of the non-significant survival benefit with serious toxicity.70 Ten years later, in a multi-center randomized phase III trial, for both QOL and survival, supportive care plus a different combination chemotherapy (carboplatin and etoposide) were shown superior to supportive care only.71 On the other hand, patients also have clear preference in value potential survival benefit and chemotherapy-induced toxicity.72 Among 81 patients previously treated with platinum drugs for stage III/IV NSCLC, over 50% of them would not choose chemotherapy for an estimated additional three-months of survival. Some patients would not choose chemotherapy to avoid any interference with their QOL even for an estimated 24-month additional survival period; whereas, others would take chemotherapy regardless of how long they would have to live as not to miss any opportunity to be cured. Lung cancer patients care about more than just survival and the majority of them would choose treatment that improves cancer symptoms.
Because surgical resection remains the treatment of choice for early-stage NSCLC, prospective evaluation and preservation of long-term QOL after the surgery is imperative. Before the resection, patients already have lower QOL, mainly in physical and emotional functioning; their QOL was further impaired after the resection, particularly in three to six months post operation.63 More specifically, pulmonary resection is known to cause postthoracotomy pain syndrome, commonly seen in approximately 50% of patients after thoracotomy. This chronic condition has been reported to last for more than four to five years in approximately 30% of patients,73 with no data beyond five years after surgery. Another study of 224 patients pathologically diagnosed with NSCLC reports a strong effect of psychosocial factors on mortality one year post diagnosis. These factors include high need for sympathy and devotion, reserved personality, and either very low or very high ego strength.74 Other known predictors in the study were disease stage, tumor cell type, and co-morbid condition on sex; disease stage was the only significant one.
Compared to NSCLC, SCLC is a fast growing tumor and in general is considered as a systemic disease. For numerous clinical trials of chemo-radiation therapy combinations, QOL has been measured, at the best, as secondary endpoints. In one of such phase III randomized trial of over 500 patients, the trial arm (TEC) and the control arm (VEC) showed comparable overall QOL, function, and symptom scores.75
A key domain of QOL is symptoms. Lung cancer patients experience high symptom burden and distress, even among high functioning patients.76 Fatigue, pain, dysnea, anorexia, and cachexia are the most common symptoms and can be caused by lung cancer as side effects of treatment. In addition, many patients experience emotional and psychosocial distress associated with their cancer diagnosis or non-response to therapy. Therefore, symptom control should be an important component of comprehensive and effective therapy.
5.2. Long-term Lung Cancer Survivors
People who are alive five years after a diagnosis of primary lung cancer are referred to as long-term lung cancer (LTLC) survivors.77 Although the chance is only 15%, over 25,000 individuals become LTLC survivors every year in the United States.78 Aging of the general population and advancements in early detection and treatment79, 80 will further increase the LTLC survivors in the population. Sometimes in the past, over a 30-month survival after a SCLC diagnosis was regarded as LTLC survivors.81 The majority of these LTLC survivors have undergone invasive treatment such as lung resection, radiation therapy, and/or chemotherapy; comorbidity burden in these survivors is especially high when compared to survivors of other cancer sites.82 Recurrent disease may occur in a sub-group of LTLC survivors up to over 10 years after diagnosis,10 and the survivors are extremely vulnerable (10-fold higher risk than other adult smokers) to developing new aerodigestive tract tumors,83 especially subsequent primary lung cancer (SPLC) and other smoking-related cancers. The Lung Cancer Study Group reported that the incidence of SPLC increased 2-fold after five years compared to the preceding five years after surgery. The cumulative risk of developing SPLC or other smoking-related cancers reaches 13%-20% at 6-8 years.84 Chest radiotherapy and continued smoking were found to significantly increase the risk of SPLC in these patients.85 Late effects of radiation and/or chemotherapy among LTLC survivors have not been defined.
Pulmonary Function Status
Two studies reported the impact of pulmonary function of LTLC survivors. The first study was based on 140 survivors with the observed average FEV1% predicted being 68% (SD, 23), one-fifth being under 50% predicted FEV1, and 36% with moderate to severe obstructive and/or restrictive ventilatory disorders.86 The second was a 15-year follow-up on the pulmonary status of 152 SCLC patients who had been treated with chest irradiation and chemotherapy that evaluated the time trend of symptoms, signs, and functions.87 Minimal changes have been found from 5-15 years after treatment.
Tobacco and Alcohol Use
Many smokers continue to smoke even after a diagnosis of lung cancer and even after receiving chemotherapy, radiation therapy, or surgery. Thirty percent to 60% of smokers will continue to smoke after their cancer diagnosis.88 In a study of 317 smokers diagnosed with stage I NSCLC, a two-month tobacco abstinence rate of 53% and a 24-month tobacco abstinence rate of 47% were observed.89 In a pilot study of 148 LTLC survivors, 19% were smoking at the time of diagnosis but only 5% were still smoking five years after diagnosis.90
Alcohol use was evaluated among lung cancer survivors in a cohort of 142 LTLC survivors.91 At lung cancer diagnosis, 69% consumed alcohol and then 16% reported changes in their alcohol use after diagnosis (either stopped or decreased their amount of alcohol intake). When compared to non-drinkers, an odds ratio of 9.0 showed that drinkers perceived themselves as having poorer health.
Self-assessed Quality of Life
In a cross-sectional survey of LTLC survivors, fatigue and anxiety were reported as major problems and their physical functioning scores were worse than other cancer survivors.92 The authors pointed out the importance of the QOL assessment and the pitfalls of assuming QOL findings in the absence of clinical data. Changes in QOL over time have been evaluated among 164 LTLC survivors in a pilot study; 34% of these survivors experienced a significant decline in their overall QOL at the five-year follow-up compared to their under three-year follow-up.93
6. Summary and Perspectives: Knowledge Gap and Research in Need
Outcome research in lung cancer has been focused mainly on short-term survival; there is a shortage of knowledge about the health and quality of life in LTLC survivors at present. Only occasionally in the past, systematic evaluation of survival predictors and QOL attributes were simultaneously conducted in the same study. One such study was carried out in 102 patients with inoperable NSCLC, in which disease symptoms and psychosocial well-being were the best predictors for survival.94 According to the limited information in the literature, the QOL of long-term survivors of lung cancer showed substantial deficits relative to other patient populations, indicating a need for targeted interventions.93 QOL has been suggested and should be considered to constitute a prognostic factor for lung cancer survival.
The independent or combined effects of lung cancer treatment, aging, smoking and drinking, comorbid conditions, and psychosocial factors likely cause late effects including organ malfunction, chronic fatigue, pain, or premature death among LTLC survivors.95 In the mid-1990’s, multi-dimensional models were proposed based on a conceptual framework of Wilson and Cleary96 to capture the most important QOL predictors.77 This framework encompasses the following five dimensions and domains, as illustrated in Figure 3: host-related factors (e.g., demographic and genomic), tumor-related factors (e.g., histology and markers of cell proliferation and apoptosis), disease- and treatment-related factors (e.g., adverse effects, symptoms, and disease recurrence), health-related behaviors (e.g., smoking status and physical activity level), co-morbid conditions, and psychosocial facets (e.g., emotional balance and spiritual well-being). With the advanced technology in genome era, more and more research initiatives are multi-disciplinary overarching basic, clinical, population, and behavioral sciences in achieving the goal of patient-specific medical care. New knowledge gained from these studies could help lung cancer survivors, their healthcare providers, and their caregivers by providing evidence for establishing clinical recommendations to enhance their long-term survival and health-related QOL.
Figure 3. An integrated View: Lung Cancer Outcome Research a.
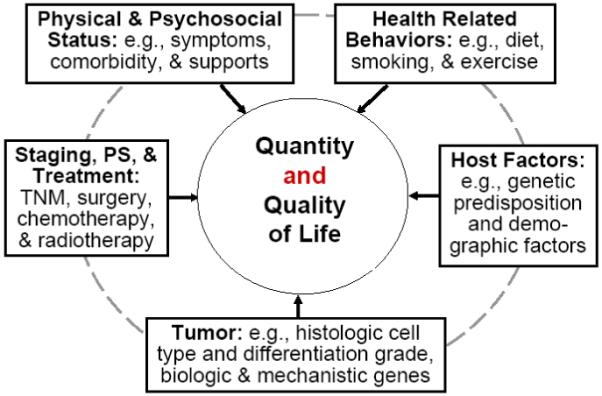
a Adapted from Sugimura & Yang, 2005, CHEST
References
- 1.Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest. 1997;111(6):1710–17. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- 2.Yang P, Allen MS, Aubry MC, Wampfler JA, Marks RS, Edell ES, Thibodeau SN, Adjei AA, Jett J, Deschamps C. Clinical Features of 5,628 Primary Lung Cancer Patients: Experience at Mayo Clinic from 1997-2003. Chest. 2005;128:452–62. doi: 10.1378/chest.128.1.452. [DOI] [PubMed] [Google Scholar]
- 3.Nesbitt JC, Lee JS, Komaki R, Roth JA. Cancer of the lung. In: Holland JF, Frei E III, Bast RJ, Kufe D, Morton D, Weichselbaum R, editors. Cancer Med. Williams & Wilkins; Baltimore: 1997. pp. 1723–803. [Google Scholar]
- 4.Feld R, Sagman U, LeBlanc M. Staging and prognostic factors: Small cell lung cancer. In: Pass HI, Mitchell JB, Johnson DH, Turrisi AT, editors. Lung Cancer: Principles and Practice. Lipincott-Raven Publishers; Philadelphia: 1996. pp. 495–509. [Google Scholar]
- 5.Wozniak AJ, Gadgeel SM. Adjuvant Treatment of Non-Small-Cell Lung Cancer: How Do We Improve the Cure Rates Further? Oncology. 2007;21:164–71. [PubMed] [Google Scholar]
- 6.Rubin P, Brasacchio R, Katz A. Solitary metastases: illusion versus reality. Semin. Radiat. Oncol. 2006;16:120–30. doi: 10.1016/j.semradonc.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Martini N, Melamed MR. Multiple primary lung cancers. J. Thorac. Cardiovasc. Surg. 1975;70:606–12. [PubMed] [Google Scholar]
- 8.Scott WJ. Metachronous lung cancer: The role of improved postoperative surveillance. J. Thorac. Cardiovasc. Surg. 2004;127:633–35. doi: 10.1016/j.jtcvs.2003.11.052. [DOI] [PubMed] [Google Scholar]
- 9.Martini N, Bains MS, Burt ME, Zakowski MF, McCormack P, Rusch VW, Ginsberg RJ. Incidence of local recurrence and second primary tumors in resected stage I lung cancer. J. Thorac. Cardiovasc. Surg. 1995;109(1):120–29. doi: 10.1016/S0022-5223(95)70427-2. [DOI] [PubMed] [Google Scholar]
- 10.The Lung Cancer Study Group Malignant disease appearing late after operation for T1 N0 non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 1993;106:1053–58. [PubMed] [Google Scholar]
- 11.Immerman SC, Vanecko RM, Fry WA, Head LR, Shields TW. Site of Recurrence in Patients with Stages I and II Carcinoma of the Lung Resected for Cure. Ann. Thorac. Surg. 1981;32:23–27. doi: 10.1016/s0003-4975(10)61368-9. [DOI] [PubMed] [Google Scholar]
- 12.Martini N, Rusch VW, Bains MS, Kris MG, Downey RJ, Flehinger BJ, Ginsberg RJ. Factors Influencing Ten-Year Survival in Resected Stages I to IIIA Non-Small Lung Cancer. J. Thorac. Cardiovasc. Surg. 1999;117:32–38. doi: 10.1016/s0022-5223(99)70467-8. [DOI] [PubMed] [Google Scholar]
- 13.Colice GL, Rubins J, Unger M. Follow-up and Surveillance of the Lung Cancer Patient Following Curative-Intent Therapy. Chest. 2003;123 doi: 10.1378/chest.123.1_suppl.272s. [DOI] [PubMed] [Google Scholar]
- 14.Thomas P, Rubinstein L, The Lung Cancer Study Group Cancer Recurrence After Resection: T1 N0 Non-Small Cell Lung Cancer. Ann. Thorac. Surg. 1990;49:242–47. doi: 10.1016/0003-4975(90)90145-v. [DOI] [PubMed] [Google Scholar]
- 15.Sugimura H, Nichols FC, Yang P, Allen MS, Deschamps C, Cassivi SD, Williams BA, Pairolero PC. Survival Following Recurrent Non-Small Cell Lung Cancer after Complete Pulmonary Resection. Annals of Thoracic Surgery. 2007;83:409–18. doi: 10.1016/j.athoracsur.2006.08.046. [DOI] [PubMed] [Google Scholar]
- 16.Williams BA, Sugimura H, Endo C, Nichols FC, Cassivi SD, Allen MS, Pairolero PC, Deschamps C, Yang P. Predicting Postrecurrence Survival Among Completely Resected Nonsmall-Cell Lung Cancer Patients. Annals of Thoracic Surgery. 2006;81:1021–27. doi: 10.1016/j.athoracsur.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 17.Visbal AL, Williams BA, Nichols FC, Marks RS, Jett JR, Aubry MC, Edell ES, Wampfler JA, Molina JR, Yang P. Gender Differences in Non-Small Cell Lung Cancer Survival: An Analysis of 4,618 Patients Diagnosed Between 1997-2002. Ann. Thorac. Surg. 2004;78(1):209–15. doi: 10.1016/j.athoracsur.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 18.Sun Z, Aubry MC, Deschamps C, Marks RS, Okuno SH, Williams BA, Sugimura H, Pankratz VS, Yang P. Histologic Grade is an Independent Prognostic Factor for Survival in Non-Small Cell Lung Cancer: An Analysis of 5018 Hospital- and 712 Population-Based Cases. Journal of Thoracic & Cardiovascular Surgery. 2006;131:1014–20. doi: 10.1016/j.jtcvs.2005.12.057. [DOI] [PubMed] [Google Scholar]
- 19.Ebbert JO, Yang P, Vachon CM, Vierkant RA, Cerhan JR, Folsom AR, Sellers TA. Lung Cancer Risk Reduction After Smoking Cessation: Observations From a Prospective Cohort of Women. J. Clin. Oncol. 2003;21(5):921–26. doi: 10.1200/JCO.2003.05.085. [DOI] [PubMed] [Google Scholar]
- 20.Jatoi A, Williams B, Nichols FC, Marks RS, Aubry MC, Wampfler J, Finke EE, Yang P. Is Voluntary Vitamin and Mineral Supplementation Associated with Better Outcome in Non-Small Cell Lung Cancer? Results from the Mayo Clinic Lung Cancer Cohort. Lung Cancer. 2005;49:77–84. doi: 10.1016/j.lungcan.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Jatoi A, Williams BA, Marks R, Nichols FC, Aubry MC, Wampfler J, Yang P. Exploring vitamin and mineral supplementation and purported clinical effects in patients with small cell lung cancer: results from the Mayo Clinic lung cancer cohort. Nutr. Cancer. 2005;51:7–12. doi: 10.1207/s15327914nc5101_2. [DOI] [PubMed] [Google Scholar]
- 22.Brundage MD, Davies D, Mackillop WJ. Prognostic Factors in Non-small Cell Lung Cancer. A Decade of Progress. Chest. 2002;122(3):1037–57. doi: 10.1378/chest.122.3.1037. [DOI] [PubMed] [Google Scholar]
- 23.Sun Z, Yang P. Gene expression profiling on lung cancer outcome prediction: Present clinical value and future premise. Cancer Epidemiology, Biomarkers & Prevention. 2006;15:2063–68. doi: 10.1158/1055-9965.EPI-06-0505. [DOI] [PubMed] [Google Scholar]
- 24.Yanagisawa K, Shyr Y, Xu BJ, Massion PP, Larsen PH, White BC, Roberts JR, Edgerton M, Gonzalez A, Nadaf S, Moore JH, Caprioli RM, Carbone DP. Proteomic patterns of tumour subsets in non-small-cell lung cancer. The Lancet. 2003;362:433–39. doi: 10.1016/S0140-6736(03)14068-8. [DOI] [PubMed] [Google Scholar]
- 25.Yanagisawa K, Tomida S, Shimada Y, Yatabe Y, Mitsudomi T, Takahashi T. A 25-Signal Proteomic Signature and Outcome for Patients with Resected Non-Small-Cell Lung Cancer. J. Natl. Cancer Inst. 2007;99:858–67. doi: 10.1093/jnci/djk197. [DOI] [PubMed] [Google Scholar]
- 26.Saijo N. Recent trends in the treatment of advanced lung cancer. Cancer. 2006;97:448–52. doi: 10.1111/j.1349-7006.2006.00198.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Byrne BJ, Garst J. Epidermal growth factor receptor inhibitors and their role in non-small-cell lung cancer. Curr. Oncol. Reports. 2005;7:241–47. doi: 10.1007/s11912-005-0045-6. [DOI] [PubMed] [Google Scholar]
- 28.Shigematsu H, Gazdar AF. Somatic mutations of epidermal growth factor receptor signaling pathway in lung cancers. Int. J. Cancer. 2006;118:257–62. doi: 10.1002/ijc.21496. [DOI] [PubMed] [Google Scholar]
- 29.Janne PA, Engelman JA, Johnson BE. Epidermal growth factor receptor mutations in non-small-cell lung cancer: implications for treatment and tumor biology. J. Clin. Oncol. 2005;23:3227–34. doi: 10.1200/JCO.2005.09.985. [DOI] [PubMed] [Google Scholar]
- 30.Calvo E, Baselga J. Ethnic differences in response to epidermal growth factor receptor tyrosine kinase inhibitors. J. Clin. Oncol. 2006;24:2158–63. doi: 10.1200/JCO.2006.06.5961. [DOI] [PubMed] [Google Scholar]
- 31.Jain RK. Molecular regulation of vessel maturation. Nat. Med. 2003;9:685–93. doi: 10.1038/nm0603-685. [DOI] [PubMed] [Google Scholar]
- 32.Molina JR, Adjei AA, Jett JR. Advance in Chemotherapy of Non-small Cell Lung Cancer. Chest. 2006;130:1211–19. doi: 10.1378/chest.130.4.1211. [DOI] [PubMed] [Google Scholar]
- 33.Stinchcombe TE, Socinski MA. Bevacizumab in the treatment of non-small-cell lung cancer. Oncogene. 2007;26:3691–98. doi: 10.1038/sj.onc.1210366. [DOI] [PubMed] [Google Scholar]
- 34.Ruttinger D, WInter H, van den Engel NK, Hatz RA, Schlemmer M, Phola H, Grutzner S, Schendel DJ, Fox BA, Jauch KW. Immunotherapy of Lung Cancer: An Update. Onkologie. 2006;29:33–38. doi: 10.1159/000090341. [DOI] [PubMed] [Google Scholar]
- 35.Blackhall FH, Shepherd FA. Small cell lung cancer and targeted therapies. Curr. Opin. Oncol. 2007;19:103–08. doi: 10.1097/CCO.0b013e328011bec3. [DOI] [PubMed] [Google Scholar]
- 36.Cross D, Burmester JK. Gene therapy for cancer treatment: past, present and future. Clin. Med. Res. 2006;4:218–27. doi: 10.3121/cmr.4.3.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boulikas T, Vougiouka M. Cisplatin and platinum drugs at the molecular level (Review) Oncol. Rep. 2003;10:1163–682. [PubMed] [Google Scholar]
- 38.Rogatko A, Babb JS, Wang H, Slifker MJ, Hudes GR. Patient Characteristics Compete with Dose as Predictors of Acute Treatment Toxicity in Early Phase Clinical Trials. Clin. Cancer Res. 2004;10:4645–51. doi: 10.1158/1078-0432.CCR-03-0535. [DOI] [PubMed] [Google Scholar]
- 39.Di Maio M, Gridelli C, Gallo C, Shepherd F, Piantedosi F, Cigolari S, Manzione L, Illiano A, Barbera S, Robbiati SF, Frontini L, Piazza E, Ianniello GP, Veltri E, Castiglione F, Rosetti F, Gebbia V, Seymour L, Chiodini P, Perrone F. Chemotherapy-induced neutropenia and treatment efficacy in advanced non-small-cell lung cancer: a pooled analysis of three randomised trials. Lancet Oncology. 2005;6:669–77. doi: 10.1016/S1470-2045(05)70255-2. [DOI] [PubMed] [Google Scholar]
- 40.Gurney H. I don’t underdose my patients … do I? Lancet Oncology. 2005;6:637–38. doi: 10.1016/S1470-2045(05)70296-5. [DOI] [PubMed] [Google Scholar]
- 41.Susman E. Rash correlates with tumour respsone after cetuximab. Lancet Oncol. 2004;5:647. doi: 10.1016/s1470-2045(04)01627-4. [DOI] [PubMed] [Google Scholar]
- 42.Gurney H. How to calculate the dose of chemotherapy. Br. J. Cancer. 2002;86:297–302. doi: 10.1038/sj.bjc.6600139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rankin EM, Mill L, Kaye SB, Atkinson R, Cassidy L, Cordiner J, Cruickshank D, Davis JR, Duncan ID, Fullerton W, Habeshaw T, Kennedy J, Kennedy R, Kitchener H, MacLean A, Paul J, Reed N, Sarker T, Soukop M, et al. A randomised study comapring standard dose carboplatin with chlorambucil and carboplatin in advanced ovarian cancer. Br. J. Cancer. 1992;65:275–81. doi: 10.1038/bjc.1992.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Poikonen P, Saarto T, Lundin J, Joensuu H, Blomqvist C. Leucocyte nadir as a marker for chemotherapy efficacy in node-positive breast cancer treated with adjuvant CMF. Br. J. Cancer. 1999;80:1763–66. doi: 10.1038/sj.bjc.6690594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ratain MJ, Relling MV. Gazing into a crystal ball-cancer therapy in the post-genomic era. Nat. Med. 2001;7:283–85. doi: 10.1038/85414. [DOI] [PubMed] [Google Scholar]
- 46.Lord PG, Papoian T. Genomics and Drug Toxicity. Science. 2004;306:57. doi: 10.1126/science.1105854. [DOI] [PubMed] [Google Scholar]
- 47.Yang P, Ebbert JO, Sun Z, Weinshilboum RM. A Role of the Glutathione Metabolic Pathway in Lung Cancer Treatment and Prognosis: A Review. J. Clin. Oncol. 2005;24:1761–69. doi: 10.1200/JCO.2005.02.7110. [DOI] [PubMed] [Google Scholar]
- 48.Fuertes MA, Castilla J, Alonso C, Perez JM. Cisplatin Biochemical Mechanism of Action: From Cytotoxicity to Induction of Cell Death Through Interconnections Between Apoptotic and Necrotic Pathways. Current Medicinal Chemistry. 2003;10:257–66. doi: 10.2174/0929867033368484. [DOI] [PubMed] [Google Scholar]
- 49.Rosell R, Taron M, Camps C, Lopez-Vivanco G. Influence of genetic markers on survival in non-small cell lung cancer. Drugs Today (Barc) 2003;39:775–86. doi: 10.1358/dot.2003.39.10.799471. [DOI] [PubMed] [Google Scholar]
- 50.Zhou W, Gurubhagavatula S, Liu G, Park S, Neuberg DS, Wain JC, Lynch TJ, Su L, Christiani DC. Excision repair cross-complementation group 1 polymorphism predicts overall survival in advanced non-small cell lung cancer patients treated with platinum-based chemotherapy. Clin Cancer Res. 2004;10:4939–43. doi: 10.1158/1078-0432.CCR-04-0247. [DOI] [PubMed] [Google Scholar]
- 51.Gurubhagavatula S, Liu G, Park S, Zhou W, Su L, Wain JC, Lynch TJ, Neuberg DS, Christiani DC. XPD and XRCC1 genetic polymorphisms are prognostic factors in advanced non-small-cell lung cancer patients treated with platinum chemotherapy. J. Clin. Oncol. 2004;22:2594–601. doi: 10.1200/JCO.2004.08.067. [DOI] [PubMed] [Google Scholar]
- 52.Ryu JS, Hong YC, Han HS, Lee JE, Kim S, Park YM, Kim YC, Hwang TS. Association between polymorphisms of ERCC1 and XPD and survival in non-small-cell lung cancer patients treated with cisplatin combination chemotherapy. Lung Cancer. 2004;44:311–6. doi: 10.1016/j.lungcan.2003.11.019. [DOI] [PubMed] [Google Scholar]
- 53.Connell PP, Kron SJ, Weichselbaum RR. Relevance and irrelevance of DNA damage response to radiotherapy. DNA Repair (Amst) 2004;3:1245–51. doi: 10.1016/j.dnarep.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 54.Willers H, Dahm-Daphi J, Powell SN. Repair of radiation damage to DNA. British of Journal of Cancer. 2004;90:1297–301. doi: 10.1038/sj.bjc.6601729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ross GM. Inducation of cell death by radiotherapy. Endocr. Relat. Cancer. 2002;6:41–44. doi: 10.1677/erc.0.0060041. [DOI] [PubMed] [Google Scholar]
- 56.Leslie EM, Deeley RG, Cole SPC. Toxicological relevance of the multidrug resistance protein 1, MRP1 (ABCC1) and related transporters. Toxicology. 2001;167:3–23. doi: 10.1016/s0300-483x(01)00454-1. [DOI] [PubMed] [Google Scholar]
- 57.Danesi R, De Braud F, Fogli S, Di Paolo A, Del Tacca M. Pharmacogenetic determinants of anti-cancer drug activity and toxicity. Trends in Pharmacological Sciences. 2001;22:420–26. doi: 10.1016/s0165-6147(00)01742-9. [DOI] [PubMed] [Google Scholar]
- 58.Strange RC, Jones PW, Fryer AA. Glutathione S-trnasferase: genetics and role in toxicology. Toxicol. Lett. 2000;112-113:357–63. doi: 10.1016/s0378-4274(99)00230-1. [DOI] [PubMed] [Google Scholar]
- 59.Eaton DL, Bammler TK. Concise Review of the Glutathione S-Transferases and their Significance to Toxicology. Toxicol. Sci. 1999;49:156–64. doi: 10.1093/toxsci/49.2.156. [DOI] [PubMed] [Google Scholar]
- 60.O’Brien ML, Tew KD. Glutathione and Related Enzymes in Multidrug Resistance. Eur. J. Cancer. 1996;6:967–78. doi: 10.1016/0959-8049(96)00051-2. [DOI] [PubMed] [Google Scholar]
- 61.Anonymous, The World Health Organization Quality of Life Assessment (WHOQOL) Position Paper from the World Health Organization. Social Science and Medicine. 1995;41:1403–09. doi: 10.1016/0277-9536(95)00112-k. [DOI] [PubMed] [Google Scholar]
- 62.Bonomi AE, Patrick DL, Bushnell DM, Martin M. Validation of the United States’ Version of the World Health Organization Quality of Life (WHOQOL) Instrument. J. Clin. Epidemiol. 2000;53:1–12. doi: 10.1016/s0895-4356(99)00123-7. [DOI] [PubMed] [Google Scholar]
- 63.Li WWL, Lee TW, Yim APC. Quality of life after lung cancer resection. Thorac. Surg. Clin. 2004;14:353–65. doi: 10.1016/S1547-4127(04)00023-4. [DOI] [PubMed] [Google Scholar]
- 64.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care. 1992;30:473–83. [PubMed] [Google Scholar]
- 65.McHorney CA, Ware JE, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med. Care. 1993;31:247–63. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 66.Bergman B, Aaronson NK. Quality-of-life and cost-effectiveness assessment in lung cancer. Curr. Opin. Oncol. 1995;7:138–43. doi: 10.1097/00001622-199503000-00008. [DOI] [PubMed] [Google Scholar]
- 67.Cella DF, Bonomi AE, Lloyd SR, Tulsky DS, Kaplan E, Bonomi P. Reliability and validity of the Functional Assessment of Cancer Therapy-Lung (FACT-L) quality of life instrument. Lung Cancer. 1995;12:199–200. doi: 10.1016/0169-5002(95)00450-f. [DOI] [PubMed] [Google Scholar]
- 68.Hollen PJ, Gralla RJ, Kris MG, Cox C. Quality of life during clinical trials: conceptual model for the Lung Cancer Symptom Scale (LCSS) Support. Care Cancer. 1994;2:213–22. doi: 10.1007/BF00365725. [DOI] [PubMed] [Google Scholar]
- 69.Montazeri A, Gillis CH, McEwen J. Quality of life in patients with lung cancer. A review of literature from 1970-1995. Chest. 1998;113(2):467–81. doi: 10.1378/chest.113.2.467. [DOI] [PubMed] [Google Scholar]
- 70.Ganz PA, Figlin RA, Haskell CM, La Soto N, Siau J, The UCLA Solid Tumor Study Group Supportive Care Versus Supportive Care and Combination Chemotherapy in Metastatic Non-Small Cell Lung Cancer. Cancer. 1989;63:1271–78. doi: 10.1002/1097-0142(19890401)63:7<1271::aid-cncr2820630707>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 71.Helsing M, Bergman B, Thaning L, Hero U. Quality of Life and survival in patients with advanced non-small cell lung cancer receiving supportive care plus chemotherapy with carboplatin and etoposide or supportive care only. A multicentre randomized phase III trial. Eur. J. Cancer. 1998;34(7):1036–44. doi: 10.1016/s0959-8049(97)10122-8. [DOI] [PubMed] [Google Scholar]
- 72.Silvestri G, Pritchard R, Weich HG. Preferences for chemotherapy in patients with advanced non-small cell lung cancer: descriptive study based on scripted interviews. Br. Med. J. 1998;317:771–75. doi: 10.1136/bmj.317.7161.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Karmakar MK, Ho AM. Postthoracotomy pain syndrome. Thorac. Surg. Clin. 2004;14:345–52. doi: 10.1016/S1547-4127(04)00022-2. [DOI] [PubMed] [Google Scholar]
- 74.Stavraky KM, Donner AP, Kincade JE, Stewart MA. The Effect of Psychosocial Factors on Lung cancer Mortality at One Year. J. Clin. Epidemiol. 1988;41:75–82. doi: 10.1016/0895-4356(88)90011-x. [DOI] [PubMed] [Google Scholar]
- 75.Reck M, von Pawel J, Macha HN, Kaukel E, Deppermann KM, Bonnet R, Ulm K, Hessler S, Gatzemeier U. Efficient palliation in patients with small-cell lung cancer by a combination of paclitaxel, etoposide and carboplatin: Quality of life and 6-years’-follow-up results from a randomised phase III trial. Lung Cancer. 2006;53:67–75. doi: 10.1016/j.lungcan.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 76.Temel JS, Pirl WF, Lynch TJ. Comprehensive symptom management in patients with advanced-stage non-small-cell lung cancer. Clin. Lung Cancer. 2006;7:241–49. doi: 10.3816/CLC.2006.n.001. [DOI] [PubMed] [Google Scholar]
- 77.Sugimura H, Yang P. Long-term Survivorship in Lung Cancer. A review. Chest. 2006;29:1088–97. doi: 10.1378/chest.129.4.1088. [DOI] [PubMed] [Google Scholar]
- 78.Institute NC, editor. SEER Cancer Statistics Review, 1973-1999. Bethesda, MD: 2002. http://seer.cancer.gov/csr/1973-1999/ [Google Scholar]
- 79.Winton TL, Livingston RB, Johnson D, et al. A prospective randomized trial of adjuvant vionrelbine (VIN) and cisplation (CIS) in completely resected stage 1B and II non small cell lung cancer (NSCLC) Intergroup JBR.10. 7018. 2004 [Google Scholar]
- 80.Strauss GM, Herndon J, Maddaus MA. Randomized clinical trial of adjuvant chemotherapy with paclitzxel and craboplatin following resection in Stage IB non-small cell lung cancer (NSCLC): Report of Cancer and Leukemia Group B (CALGB) Protocol 9633. 7019. 2004 al., e. [Google Scholar]
- 81.Jacoulet P, Depierre A, Moro D, Riviere A, Milleron B, Quoix E, Ranfaing E, Anthoine D, Lafitte JJ, Lebeau B, Kleisbauer JP,F,M, Fournel P, Zaegel M, Leclerc JP, Garnier G, Brambilla E, Capron F. Long-term survivors of small-cell lung cancer (SCLC): a French multicenter study. Groupe d’Oncologie de Langue Francaise. Ann. Oncol. 1997;8(10):1009–14. doi: 10.1023/a:1008287922285. [DOI] [PubMed] [Google Scholar]
- 82.Ko CY, Maggard M, Livingston EH. Evaluating health utility in patients with melanoma, breast cancer, colon cancer, and lung cancer: a nationwide, population-based assessment. J. Surg. Res. 2003;114:1–5. doi: 10.1016/s0022-4804(03)00167-7. [DOI] [PubMed] [Google Scholar]
- 83.Johnson BE, Cortazar P, Chute JP. Second Lung Cancers in Patients Successfully Treated for Lung Cancer. Semin. Oncol. 1997;24:492–99. [PubMed] [Google Scholar]
- 84.Johnson BE. Second Lung Cancers in Patients After Treatment for an Initial Lung Cancer. J. Natl. Cancer Inst. 1998;90(18):1335–45. doi: 10.1093/jnci/90.18.1335. [DOI] [PubMed] [Google Scholar]
- 85.Tucker MA, Murray N, Shaw EG, Ettinger DS, Mabry M, Huber MH, Feld R, Shepherd FA, Johnson DH, Grant SC, Aisner J, Johnson BE. Second Primary Cancers Related to Smoking and Treatment of Small-Cell Lung Cancer. J. Natl. Cancer Inst. 1997;89(23):1782–88. doi: 10.1093/jnci/89.23.1782. [DOI] [PubMed] [Google Scholar]
- 86.Sarna L, Evangelista L, Tashkin D, Padilla G, Holmes C, Brecht ML, Grannis F. Impact of Respiratory Symptoms and Pulmonary Function on Quality of Life of Long-term Survivors of Non-Small Cell Lung Cancer. Chest. 2004;125(2):439–45. doi: 10.1378/chest.125.2.439. [DOI] [PubMed] [Google Scholar]
- 87.Myers JN, O’Neil KM, Walsh TE, Hoffmeister KJ, Venzon DJ, Johnson BE. The Pulmonary Status of Patients with Limited-Stage Small Cell Lung Cancer 15 Years after Treatment with Chemotherapy and Chest Irradiation. Chest. 2005;128:3261–68. doi: 10.1378/chest.128.5.3261. [DOI] [PubMed] [Google Scholar]
- 88.Pinto BM, Eakin E, Maruyama N. Health behavior changes after a cancer diagnosis: what do we know and where do we go from here? Annals of Behavioral Medicine. 2000;22:38–52. doi: 10.1007/BF02895166. [DOI] [PubMed] [Google Scholar]
- 89.Gritz ER, Nisenbaum R, Elashoff RE, Holmes EC. Smoking behavior following diagnosis in patients with stage I non-small cell lung cancer. Cancer Causes & Control. 1991;2:105–12. doi: 10.1007/BF00053129. [DOI] [PubMed] [Google Scholar]
- 90.Yang P, Sugimura H, Ebbert JO, Nichols FC, Marks RS, Kelemen LE, Worra JB, Stoddard SM, Johnson ME, Sloan JA. Characteristics of Long-Term Lung Cancer Survivors. Cancer Survivorship: Pathways to Health After Treatment. 2004:51. [Google Scholar]
- 91.Evangelista LS, Sarna L, Brecht ML, Padilla G, Chen J. Health perceptions and risk behaviors of lung cancer survivors. Heart & Lung. 2003;32(2):131–39. doi: 10.1067/mhl.2003.12. [DOI] [PubMed] [Google Scholar]
- 92.Sarna L, Padilla G, Holmes C, Tashkin D, Brecht ML, Evangelista L. Quality of Life of Long-Term Survivors of Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2002;20(13):2920–29. doi: 10.1200/JCO.2002.09.045. [DOI] [PubMed] [Google Scholar]
- 93.Yang P, Sugimura H, Sloan J, Williams B, Cassivi S, Garces Y, Sun Z, Worra J, Midthun D, Jatoi A. Longitudinal evaluation of quality of life in long-term lung cancer survivors. [Abstract of the 11th World Conference on Lung Cancer, Presidential Symposium, July 3 - 6, 2005, Barcelona, Spain] Lung Cancer. 2005;49:S4. [Google Scholar]
- 94.Kaasa S, Mastekaasa A, Lund E. Prognostic factors for patients with inoperable non-small cell lung cancer, limited disease. Radiother. Oncol. 1989;15:235–42. doi: 10.1016/0167-8140(89)90091-1. [DOI] [PubMed] [Google Scholar]
- 95.Aziz NM. Cancer Survivorship Research: Challenge and Opportunity. J. Nutr. 2002;132:3494S–503S. doi: 10.1093/jn/132.11.3494S. [DOI] [PubMed] [Google Scholar]
- 96.Wilson IB, Cleary PD. Linking clinical variables with health-related quality of life: A conceptual model of patient outcomes. JAMA. 1995;273:59–65. [PubMed] [Google Scholar]