Abstract
Background
Growing numbers of critically ill patients receive prolonged mechanical ventilation. Little is known about their patterns of care as they transition from the acute hospital to post-acute care facilities or the associated resource utilization.
Objectives
To describe one-year trajectories of care and resource utilization for prolonged mechanical ventilation patients.
Design
One-year prospective cohort study.
Setting
5 ICUs at Duke University Medical Center.
Participants
126 prolonged mechanical ventilation patients as well as their 126 surrogates and 54 ICU physicians were enrolled consecutively during one year. Prolonged mechanical ventilation was defined as ventilation for ≥4 days with tracheostomy placement or ventilation for ≥21 days without tracheostomy.
Measurements
Patients and surrogates were interviewed in hospital, as well as 3 and 12 months later to determine patient survival, functional status, and facility type and duration of post-discharge care. Physicians were interviewed in-hospital to elicit prognoses. Institutional billing records were used to assign costs for acute care, outpatient care, and inter-facility transportation. We used Medicare claims data to assign costs for post-acute care.
Results
103 (82%) hospital survivors experienced 457 separate transitions in post-discharge care location (median 4 [interquartile range 3, 5]), including 68 (67%) patients who were readmitted at least once. Patients spent an average of 74% (CI, 68% to 80%) of all days alive in a hospital, post-acute care facility, or receiving home health care. At one year, 11 (9%) patients had a good outcome (alive with no functional dependency), 33 (26%) had a fair outcome (alive with moderate dependency), and 82 (65%) had a poor outcome (either alive with complete functional dependency (n=4, 21%) or dead (n=56, 44%). Patients experiencing a poor outcome were older, had more comorbidities, and were more frequently discharged to a post-acute care facility than patients with either fair or good outcomes (all p <0.05). Costs per patient were $306,135 (SD $285,467) and total cohort costs totaled $38.1 million, for an estimated $3.5 million per one-year independently functioning survivor.
Limitations
The results of this single center study may not be applicable to other centers.
Conclusions
Prolonged mechanical ventilation patients experience multiple transitions of care, resulting in extraordinary health care costs and persistent, profound disability. The optimism of surrogate decision makers should be balanced by discussions of these outcomes when considering a course of prolonged life support.
INTRODUCTION
The 300,000 patients per year who receive life support in intensive care units (ICUs) for much longer than average have been labeled as receiving “prolonged mechanical ventilation” (1, 2). These patients utilize a disproportionately large amount of health care resources and experience relatively poor long-term outcomes (2–4). Patients report a diminished quality of life, demonstrate important functional and cognitive limitations, require prolonged informal caregiving assistance, and suffer high one-year mortality (5–7). Despite these outcomes, the number of annual prolonged mechanical ventilation recipients will likely exceed 600,000 within a decade, with associated hospital costs of $50–60 billion (2). Decision makers’ hope for patient survival coupled with an incomplete understanding of the specific implications of prolonged mechanical ventilation provision may contribute to the increasing incidence (8).
Prolonged mechanical ventilation patients’ disproportionately high acute care costs and frequent utilization of post-acute care facilities compared to other patient groups makes them of special interest to health care payors like the Centers for Medicare and Medicaid Services as well as to the post-acute care industry (3). Due in part to their substantial annual costs, the high reimbursement rates for their facility-based care, and the high margins reported by post-acute care facilities, the 2005 Deficit Reduction Act directed the Centers for Medicare and Medicaid Services to reform the current system of post-acute care payment (9).
For these reasons, a clearer description of patients’ post-discharge paths through different care facilities, the associated burden of functional limitations, and patient-level factors associated with high resource utilization and poor outcomes can assist in clinical decision making, institutional planning, payment reform, and the design of future interventions targeted to these unique patients. Therefore, we aimed to describe the summative trajectories of care location and costs for a consecutively enrolled cohort of prolonged mechanical ventilation recipients.
METHODS
Study design and participants
We conducted a one-year, prospective cohort study in the adult general surgical and trauma, neurological, cardiothoracic surgery, cardiac, and medical ICUs at Duke University. Participants, including 126 patients, patients’ 126 surrogates, and patients’ 54 primary ICU physicians, were enrolled beginning in April 2006 through daily screening of ICUs by study staff with follow up completed in April 2008. Details about enrollment, exclusion and refusal rates, as well as characteristics of the surrogates and physicians have been described elsewhere (5).
Patients were eligible for the study if they were ≥18 years of age and met either of two common definitions of prolonged mechanical ventilation: (a) mechanical ventilation for ≥21 days with <48 hours of unassisted breathing or (b) ≥4 days of ventilation and placement of a tracheostomy for an expected prolonged requirement for ventilatory support (10). Exclusion criteria were (1) lack of an identifiable surrogate, (2) English fluency poor enough to require a translator, (3) tracheostomy placement for either emergency indications or for an ear, nose, or throat-related diagnosis, or (4) pre-admission receipt of a tracheostomy. We defined the surrogate as the person most involved in the decision to place a tracheostomy and the one most likely to provide the majority of post-discharge care. Physicians enrolled were those self-identified as the primary ICU physician for each patient.
Data collection and variables
We collected data from medical records, administrative billing records, and participant interviews. Study staff abstracted clinical data from patients’ charts and hospital electronic records to record admitting diagnoses and operative procedures, sociodemographics, Charlson comorbidity scores (11), acute physiology scores representing illness severity on the day of tracheostomy placement (12), mechanical ventilation course, and hospital and ICU lengths of stay.
Patients, surrogates, and physicians were interviewed in person within 48 hours of meeting study eligibility criteria. Follow-up interviews were performed with patients and surrogates either by telephone or in person 3 and 12 months later, with 3-month interviews primarily informing vital status and resource utilization calculations. We completed 100% of interviews with surrogates and patients, excluding patients who had died (36 [29%] at 3 months and 56 [44%] at 12 months) or who demonstrated clinically significant cognitive impairment (36 [29%] at 3 months and 31 [25%] at 12 months) as defined by a Folstein mini-mental status questionnaire score <20 (13). The six-item activities of daily living instrument quantified dependencies in basic functioning including bathing, dressing, feeding, transferring from bed to chair, bladder and bowel control, and use of the toilet (14). Quality of life was assessed with the EuroQOL-5D, an instrument with evidence of validity both among survivors of critical illness and as a surrogate-completed proxy measure (15, 16). We adjusted survival for life quality by multiplying each patient’s 3- and 12-month EuroQOL-5D index scores (range, 1=excellent quality of life to −0.1=worse than death) by the total days alive in the two periods (0 to 3 months and 4 to 12 months) preceding each measurement. We defined “poor quality of life” as an index score ≤0.44, a value two standard deviations below the population average for persons aged 55–65 (17). “Good quality of life” was defined as a score >0.80, the US population average for similar age groups, with “fair quality of life” assigned to intermediate scores (18). Because nearly a third of patients were too disabled to complete interviews during follow up, we used surrogate assessments of patients’ quality of life and functional status in analyses. EuroQol-5D scores were highly correlated (r=0.94, p <0.0001) between surrogates and cognitively intact patients. Surrogates and physicians also reported if they expected patients to survive and to have complete functional independence at one year, with responses of “strongly agree” or “agree” considered as “high expectations” and responses of “don’t know,” “disagree,” “strongly disagree” recorded as “low expectations” for either item.
Data on resource utilization were obtained by review of medical records, administrative billing records, and participant interviews (Appendix). Costs for the primary hospitalization were determined using itemized charges from each patient’s administrative billing record and converted to costs using department-specific cost-to-charge ratios obtained from the Centers for Medicare and Medicaid hospital cost reports (19). We used participant interviews to record any hospital readmission, post-acute care facility admission, or use of home health service. Duration of care for each episode, including length of ICU care during readmissions, was verified by review of medical records whenever possible. Costs for post-discharge care episodes were estimated using 2006 region-specific mean daily ICU, hospital, and post-acute care costs obtained from Medicare claims. Physician costs were estimated at 17% of hospital costs as used in previous analyses (20). Clinic visits and ambulatory surgical procedures were recorded from medical charts and assigned costs based on relevant current procedural terminology codes. Air and ground transportation costs were assigned for interfacility transfers based on administrative billing records.
Statistical analyses
The primary outcomes of the study were one-year survival, functional status, and healthcare-associated resource utilization. We also described health outcomes by combining one-year survival and functional status into three simple categories designated to be equally interpretable by patients, surrogates and physicians: “good outcome” (alive with no dependencies in activities of daily living), “fair outcome” (alive but with 1 – 5 dependencies in activities of daily living), or “poor outcome” (either dead or alive but with dependencies in all 6 activities of daily living).
We present categorical data using number (percentage) and continuous data as means (standard deviations) or medians (interquartile ranges [IQR]). We examined factors associated with grouped health outcomes (good, fair, poor) as appropriate for data distribution using Pearson’s chi-square and Fisher’s exact tests for categorical variables and analysis of variance tests or Kruskal-Wallis tests for continuous variables. Because cost data were skewed, we used log-transformed values in analyses. Additional ventilator outcomes data are shown in the Appendix. We used Stata software, version 11 (College Station, TX) for all analyses and considered a p <0.05 to be significant. The Duke University Institutional Review Board approved all study procedures. This study was not supported by external funding.
RESULTS
Baseline characteristics and hospital course
Patients were middle aged, insured, well educated, and had few pre-morbid functional limitations and medical comorbidities on average (Table 1). Admission diagnoses were nearly equivalent in proportion between trauma, non-trauma surgical, neurological, and medical etiologies. Patients had a median of 27 ventilator days (IQR 18, 27) and a median hospital length of stay of 39 days. Eighty-six (68%) patients were ultimately liberated from ventilation (Appendix). The 23 (18%) patients who died in the hospital had a greater length of stay (53 days [IQR 33, 82] vs. 28 days [IQR 27, 52]) and received more ventilator days than survivors (46 days [IQR 32, 81] vs. 25 days [IQR 20, 34], p=0.0001). All who died during the initial hospitalization were receiving ventilation via tracheostomy at the time of death. The majority (74%) of survivors were discharged to a post-acute care facility.
Table 1.
Baseline characteristics and hospital outcomes
| Characteristic | Value |
|---|---|
| Age | |
| Mean | 55 (16) |
| Range | 19 – 85 |
| Female | 50 (40%) |
| Race & ethnicity | |
| White | 67 (53%) |
| African-American | 48 (38%) |
| Native American | 7 (5%) |
| Asian | 2 (2%) |
| Hispanic | 2 (2%) |
| Place of residency before admission | |
| Home | 124 (98%) |
| Nursing facility | 2 (2%) |
| Employment status | |
| Employed full- or part-time | 41 (32%) |
| Not currently employed, retired, disabled | 85 (68%) |
| Education level, <high school, n=108* | |
| Patients | 15 (12%) |
| Surrogates | 9 (8%) |
| Insurance status | |
| Private | 72 (57%) |
| Government (Medicare or Medicaid) | 34 (27%) |
| Self-pay | 20 (16%) |
| Comorbidities | 2 (0, 4) |
| Dependencies in activities of daily living | 0 (0, 0) |
| Primary ICU admission diagnosis† | |
| Respiratory failure | 29 (23%) |
| Neurological | 29 (23%) |
| Trauma | 27 (21%) |
| Post-operative | 26 (21%) |
| Septic shock | 10 (8%) |
| Cardiac | 5 (4%) |
| Acute physiology score‡ | |
| ICU day 1 | 19 (15, 24) |
| Day of tracheostomy | 15 (12, 19) |
| Ventilator days before tracheostomy, n=125§ | 11 (8, 17) |
| Ventilator days, total | 27 (18, 24) |
| Length of stay | |
| ICU | 26 (22, 42) |
| Hospital | 39 (28, 57) |
| Hospital discharge disposition, n=102 | |
| Home without paid home health care | 6 (5%) |
| Home with paid home health care | 14 (11%) |
| Long-term acute care facility | 36 (29%) |
| Skilled nursing facility | 17 (13%) |
| Rehabilitation facility | 23 (18%) |
| Other hospital | 3 (2%) |
| Still in acute care hospital at one year | 1 (1%) |
| Inpatient hospice facility | 3 (2%) |
| Dead | 23 (18%) |
n=126 unless noted. Results as number (%) or median (interquartile range).
Refused to answer (18 [14%]).
Categories include: respiratory (pneumonia, aspiration, and pulmonary embolus); neurological (ischemic stroke, subarachnoid hemorrhage, Guillain-Barre, and status epilepticus); trauma; non-trauma surgical (immediate post-operative general and cardiothoracic); and cardiac (myocardial infarction and out of hospital cardiac arrest).
From APACHE II classification (12).
One patient did not undergo tracheostomy.
One-year outcomes and trajectories of care
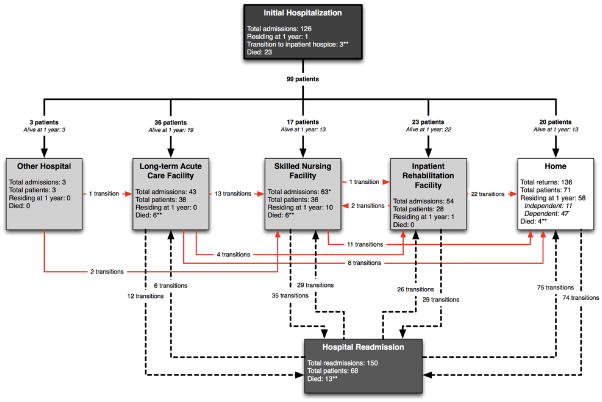
At one year, 70 (56%) were alive, although only 11 (9%) were independently functioning and just 19 (27%) had a good quality of life (Table 2). Sixty-eight of these survivors were ultimately decannulated, all within one month of ventilator liberation. Those who died during follow up lived a median of only 79 days (IQR 46, 125). Patients experienced 457 transitions in care location (median 4, [IQR 3, 5]) during follow up (Figure 1), receiving a total of 14,552 days of inpatient hospital and facility care. There were 150 readmissions among 68 (67%) of the 103 hospital survivors. Most readmissions (n=96, 65%) occurred within 3 months; nearly half were related to sepsis. The average patient spent 74% (95% CI, 68% to 80%) of all days alive in a hospital, post-acute care facility, or receiving home health care; 61% (95% CI 54% to 68%) of study days were facility-based. Only 3 (2%) patients were both initially discharged to home and remained there, while just 3 of 54 (6%) previously employed patients ever returned to work. Nineteen (27%) surrogates reported a good quality of life for one-year survivors.
Table 2.
One-year patient and process of care outcomes
| Value | |
|---|---|
| Survival | 70 (56%) |
| Quality of life at 1-year among survivors, n=70* | |
| Good | 19 (27%) |
| Fair | 17 (24%) |
| Poor | 34 (49%) |
| Quality-adjusted life-days during 1 year, total† | 66 (16, 223) |
| Disposition at one year | |
| Home without paid home care | 11 (9%) |
| Home with paid home care | 47 (37%) |
| Skilled nursing facility | 10 (8%) |
| Inpatient rehabilitation facility | 1 (1%) |
| Residing in a hospital, never discharged | 1 (1%) |
| Dead | 56 (44%) |
| Location of death over one year, n=56 | |
| Withdrawal of mechanical ventilation in hospital | 22 (39%) |
| Receiving full support on ventilator in hospital or facility | 24 (43%) |
| Hospice | 7 (13%) |
| Home | 3 (5%) |
| Mechanical ventilation outcomes | |
| Liberated from ventilator | 86 (68%) |
| During initial hospitalization | 69 |
| At long-term acute care facility | 13 |
| At skilled nursing facility | 4 |
| At another hospital | 1 |
| Not liberated from ventilator | 40 (32%) |
| Alive, still ventilated at one year | 3 |
| Died, ventilator-dependent | 37 |
| Duration of ventilator support | |
| Ventilator days if liberated from ventilator, n=86 | 21 (16, 37) |
| Ventilator days if never liberated from ventilator, n=40 | 42 (33, 74) |
| Transitions among care locations over 1 year, n=457 | |
| To a lower or equal level of care, n=88 patients‡ | 305 (65%) |
| To a higher level of care, n=69 patients‡ | 152 (35%) |
| Readmissions§ | 150 |
| Inpatient rehabilitation to skilled nursing facility | 2 |
| Hospital readmissions by diagnostic category, n=150|| | |
| Sepsis | 56 (45%) |
| Respiratory failure | 26 (21%) |
| Surgical complications | 22 (17%) |
| Neurological complications | 7 (6%) |
| Other medical | 39 (31%) |
| Post-acute care utilization, days | |
| Long-term acute care facility, 43 admissions among 38 patients | 29 (21, 70) |
| Skilled nursing facility, n=63 admissions among 36 patients | 42 (27, 228) |
| Rehabilitation facility, n=54 admissions among 28 patients | 28 (21, 45) |
| Home health services, n=44 episodes among 36 patients | 84 (34, 250) |
n=126 unless noted. Values displayed as n (%) or median (interquartile range).
By surrogate assessment using EuroQol-5D index score; includes poor (≤0.44), fair (0.45–0.79), and good (≥0.80).
Calculated by adjusting all days alive during one year with corresponding EuroQol-5D index scores obtained at 3 and 12 months follow up.
Total of patients who experienced either lower or higher level of care transitions include 58 patients who experienced both transitions to higher and to lower levels of care.
Among 68 of the 102 patients discharged from the index hospitalization to a destination other than hospice.
Readmission categories include: sepsis (urinary tract infection, pneumonia, catheter infections, other); respiratory failure (congestive heart failure, pneumonia, pulmonary embolism); surgical complications or repeat surgery related to primary admission; neurological complications (seizure, intracerebral hemorrhage, subdural hematoma); other medical (dehydration, mental status change, gastrointestinal hemorrhage, admission for chemotherapy, fall, renal failure, pancreatitis, gastrostomy complication).
Figure 1. Trajectories of care for prolonged mechanical ventilation cohort members over the first year post-discharge.
126 patients are depicted entering the hospital, with 99 (79%) discharges (23 died and one patient remained in the hospital) then experiencing 457 transitions in care location during follow up. Arrows between care locations depict both the direction of patient transitions as well as the total number of patients transferred over one year between locations. Bold lines represent initial transitions between the hospital and other locations. Dashed lines represent subsequent hospital readmissions and discharges involving post-discharge care locations. Red lines represent transitions among post-discharge care locations, including home. Within each box representing a location of care, a summary is provided of the total numbers of both readmissions and patients admitted, as well as how many remained or had died in each at one year. *One skilled nursing facility to skilled nursing facility transition not shown. **Seven transitions to inpatient hospice (and death) not shown graphically (three from the acute hospitalization and one each from home, long-term acute care facility, skilled nursing facility, hospital readmission).
At one year, there were 11 (9%) patients with a good health outcome, 33 (26%) with a fair outcome, and 82 (65%) with a poor outcome (Table 2). Only 23 (18%) patients either improved in outcome category or sustained a good outcome between 3 and 12 months (Figure 2). All one year survivors were residing at home in the fair or good outcome groups, except one nursing facility resident with a fair outcome. Compared to patients with poor outcomes, those with fair outcomes had more transitions (5 [IQR 4, 7] vs. 2 [IQR 0, 5]) and were more likely both to be readmitted (53% vs. 27%, p=0.02) and to receive facility-based post-acute care (81% vs. 61%, p=0.03).
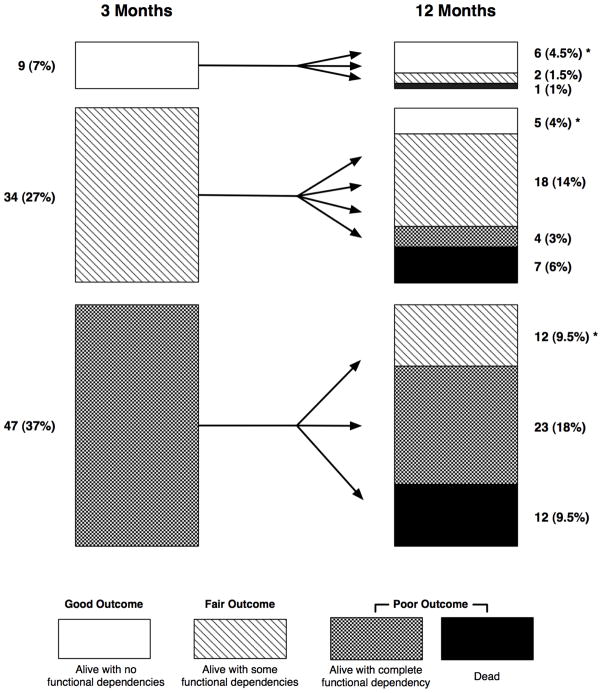
Figure 2. One-year patient trajectories by health outcomes groupings.
Each bar chart shows patients at 3- and 12-month intervals grouped by survival and number of functional limitations in basic activities of daily living in three categories (good outcome: alive with no activities of daily living dependencies [white boxes]; fair outcome: alive but with 1–5 dependencies in activities of daily living [boxes with wavy lines]; and poor outcome: either alive and completely dependent or dead [boxes for those alive with cross-hatches and black boxes for those dead]). The arrows depict group members’ subsequent longitudinal transitions to other health outcomes. For example, between 3 and 12 months, 34 patients with a fair 3-month outcome improved to a good outcome (n=5), remained as fair (n=18), or worsened to a poor outcome (2 patients with a fair outcome and 1 with a poor outcome). Percentages in both 3-and 12-month outcomes categories are calculated by including 36 (29%) patients (not shown) who were dead at 3 months. * Designates the 23 (18%) patients who improved or remained in the good outcome grouping between 3 and 12 months.
Patients with a poor outcome were older, had more comorbidities, and were more frequently discharged still receiving mechanical ventilation than those with better outcomes (Table 3). Those with fair outcomes were most frequently uninsured and less severely ill on the day of tracheostomy placement. Fair and poor group members were discharged in equal proportion to long-term acute care and skilled nursing facilities (42 [51%] vs. 16 [48%]). Patients with good outcomes tended to be admitted with trauma and were more frequently discharged home than others. The proportion of patients with moderate or greater severity of illness (acute physiology scores >15) on the day of tracheostomy was the same in both poor (64%) and good (63%) outcome groups. The proportion of surrogates with high expectations for either survival or functional independence did not differ based on subsequent health outcome categorization (all p>0.05). Physicians were somewhat less optimistic about patients’ survival, though reported high expectations for functional independence for 36 (44%) patients who later experienced poor outcomes, 17 (52%) with fair outcomes, and 11 (100%) with good outcomes (p=0.002).
Table 3.
Baseline and hospital characteristics associated with 1 year health outcomes groupings
| Characteristic | Health Outcome Grouping* | p† | ||
|---|---|---|---|---|
| Poor | Fair | Good | ||
| n=82 | n=33 | n=11 | ||
| Age | 59 (14) | 47 (17) | 51 (22) | 0.001 |
| ≥1 dependency in activities of daily living | 13 (16%) | 6 (18%) | 1 (9%) | 0.8 |
| Comorbidities | 2 (1, 4) | 1 (0, 3) | 0 (0, 2) | 0.001 |
| Insurance status | 0.04 | |||
| Private | 43 (53%) | 19 (58%) | 10 (91%) | |
| Government (Medicare or Medicaid) | 29 (35%) | 4 (12%) | 1 (9%) | |
| Self-pay | 10 (12%) | 10 (30%) | 0 (0%) | |
| Primary ICU admission diagnostic category‡ | 0.02 | |||
| Trauma | 9 (11%) | 13 (39%) | 5 (45%) | |
| Non-trauma surgical | 28 (34%) | 5 (15%) | 3 (27%) | |
| Medicine | 45 (55%) | 15 (45%) | 3 (27%) | |
| Acute physiology score >15 on day of tracheostomy | 52 (63%) | 12 (36%) | 7 (64%) | 0.03 |
| Physicians with high expectations for: | ||||
| Survival | 36 (44%) | 24 (73%) | 11 (100%) | <0.0001 |
| Functional independence | 36 (44%) | 17 (52%) | 11 (100%) | 0.002 |
| Surrogates with high expectations for: | ||||
| Survival | 74 (90%) | 32 (97%) | 11 (100%) | 0.3 |
| Functional independence | 54 (66%) | 25 (76%) | 11 (100%) | 0.05 |
| Mechanical ventilation at hospital discharge | 42 (52%) | 5 (15%) | 1 (9%) | <0.0001 |
| Discharge disposition | <0.0001 | |||
| Home | 8 (10%) | 6 (18%) | 6 (55%) | |
| Long-term acute care, skilled nursing facility, other hospital | 42 (51%) | 16 (48%) | 2 (18%) | |
| Inpatient rehabilitation facility | 9 (11%) | 11 (33%) | 3 (27%) | |
| Dead | 23 (28%) | 0 (0%) | 0 (0%) | |
| Transitions of care location | 2 (0, 5) | 5 (4, 7) | 2 (2, 5) | 0.001 |
n=126. Values shown as number (%), mean (standard deviation), or median (interquartile range).
Health outcomes categories defined as: good outcome (alive with no activities of daily living dependencies), fair outcome (alive but with 1–5 dependencies in activities of daily living), and poor outcome (either alive and completely dependent or dead).
p based on Pearson’s chi-square, Fisher’s exact, one-way analysis of variance, or Kruskal-Wallis tests.
Categories include: trauma, non-trauma surgical (immediate post-operative general and cardiothoracic), and medicine (pulmonary, infectious disease, neurological, and cardiac diagnoses).
Resource utilization
Mean total one-year costs of health care for cohort members were $306,135 (SD $285,467), while costs for the entire cohort exceeded $38.5 million (Table 3). The majority of these costs ($28.1 million, or 73%) were incurred by the initial hospitalization. The highest mean cost for post-acute care was for individuals receiving long-term acute care ($91,277), followed by care in a skilled nursing facility ($31,892), in an inpatient rehabilitation facility ($21,244), and home health service care ($6,669). Outpatient costs averaged $551, though transportation costs exceeded $10,000 per patient. There were no differences in one year costs by health outcomes grouping (p=0.4).
DISCUSSION
Our study offers new insights into a growing population of relatively understudied critically ill patients. Over one year, a cohort of 126 patients who received prolonged mechanical ventilation experienced a median of four transitions of care location each, while spending nearly 75% of all days alive either in hospitals, post-acute care facilities, or receiving paid home care. One year survivors were left with a serious burden of pervasive, persistent disability despite aggressive care that cost a total of $38 million, or approximately $3.5 million for each one-year survivor without serious functional dependencies.
These findings are important for patients, families, clinicians, and policymakers alike. First, the impact of prolonged mechanical ventilation patients on the US health care system has likely been substantially underestimated (2, 3, 21). Past estimates of these patients’ resource utilization have not focused on cumulative acute and post-discharge costs (22, 23). We found that while the initial hospitalization accounted for the majority of costs, post-acute care facilities and readmissions contributed substantially to resource utilization. The pattern of patients’ deaths may have accentuated this distribution of costs, as patients who died during hospitalization had an average length of stay over two weeks greater than those who survived. Because the risk of dying remained high throughout follow up, the opportunity to utilize post-discharge resources was attenuated. Still, the high cost of acute critical care is clearly a major factor, as cohort members’ hospital costs alone were 15 times greater than an average Medicare patient with critical illness (24). Also, the readmission rate we observed was nearly 50% higher than that reported among Medicare beneficiaries who survive a hospitalization that includes mechanical ventilation (25). It is therefore concerning that the number of prolonged mechanical ventilation patients is expected to increase substantially during the coming decade (2).
These data also are relevant to post-acute care payment reform efforts initiated with the 2005 Deficit Reduction Act (26). Currently, there are widely disparate payments made for prolonged mechanical ventilation care, with acute care hospitals often receiving far less than post-acute care facilities (27). Some have proposed basing payment for the treatment of the chronically critically ill on the quality of longitudinal care, seeking to reward lower-cost providers who can reduce costly transitions and readmissions (28, 29). However, the singularly high readmission rate from post-acute care may be associated with patient characteristics impossible to modify such as age and comorbidities, and should be investigated further before a benchmark rate is considered as a quality modifier of payment (30). The complexity of patients’ trajectories of care highlights the need to define quality indicators for this population that are transportable across institution type with the goals of improving patients’ overall care and the efficiency with which care is delivered (26).
Several studies have documented the extensive impact of critical illness on patients’ and families’ physical, mental, and financial well being (7, 31–33). However, both the magnitude of disability and the infrequency of post-discharge recovery are noteworthy. Our findings that those with poor outcomes were more likely to be elderly, have comorbid conditions, and be receiving ventilation at discharge are generally similar to prior studies, as is our observation that illness severity scores at the time tracheostomy do not accurately discriminate between patients with good or poor outcomes (34, 35). Similarly, we observed that the majority of those with a good functional recovery were admitted because of trauma (36). However, patients with intermediate outcomes, alive but with moderate functional dependency, may be the most challenging to manage because of the perceived uncertainty associated with their prognosis. These previously high-functioning patients were less severely ill than other patients. Despite their decision makers’ initial optimism, however, they rarely improved over time, instead cycling frequently among post-acute care facilities and hospitals.
Our study confirms that prolonged mechanical ventilation is a highly resource-intense condition with a generally poor outcome. However, the circumstances under which prolonged mechanical ventilation decision making occur are not ideal, likely favoring the pursuit of aggressive care (8). First, the content of physician-surrogate communication is inadequate for fully shared decision making (37). Nelson et al. reported that 80% and 93% of surrogates of patients with prolonged mechanical ventilation received no information about patients’ possible functional dependency or expected one year survival, respectively (8). Second, both clinicians and surrogates substantially overestimate patients’ prospects for recovery and do not anticipate the amount and intensity of caregiving that will be required (5). A new prognostic model has shown promise for this population, though requires further validation (34). Third, previous research has demonstrated that most internists are uncomfortable discussing uncertain prognoses, as may be the case for a patient who survives an acute critical illness but still requires life support (38). However, surrogates acknowledge the inevitable uncertainty in critical illness outcomes, and still desire prognostic estimates in the setting of end of life decisions (39). Finally, it can simply be challenging for providers to explain the complexities of critical illness in terms that surrogates understand and value. The simple health outcomes groupings we have reported may help in this regard, and may also lend themselves to incorporation in future decision support tools for this population. El-Jawahri et al. have shown that decision tools that use simple categorizations of choices and outcomes are more effective than verbal descriptions alone in end of life considerations (40).
We enrolled critically ill patients near the time of tracheotomy. This is a period when the physician determines that timely ventilator liberation is unlikely and the surrogate decision maker acknowledges that the patient would desire prolonged life support. Although tracheotomy is being performed increasingly earlier in the course of ventilation, there is little persuasive evidence that either its early (less than a week) or late (greater than two to three weeks) timing confers important clinical benefit (41–43). This uncertainty has likely contributed to the substantial variation in practice seen across physicians, hospitals, and regions (44).
Our study has several limitations. We used participants’ self-reports to quantify the duration of post-discharge care. Although this strategy may result in inaccuracies, data suggest that costs would be underestimated, rather than inflated (45, 46). Similarly, we were unable to quantify the notable financial strain of critical illness on patients and their caregivers, also reducing its true economic impact. Additionally, although we enrolled participants consecutively and with few (20%) refusals, our findings may not represent prolonged mechanical ventilation recipients at other institutions or who have different sociocultural or linguistic backgrounds. The Durham, NC area has a relatively high long-term acute care facility penetration compared to other regions of the US, which may lead to a greater number of care transitions. Further study in larger datasets may allow a more robust characterization of potentially modifiable risk factors for resource utilization.
The incidence of prolonged mechanical ventilation is likely to increase in the coming years, in the process consuming substantial health care resources. Given the disproportionately high costs and associated disability of prolonged mechanical ventilation, clinicians need to reconsider their approach to its provision. Currently, the prolonged mechanical ventilation decision making process is marked by unrealistic expectations and poor communication. It seems prudent that in the context of prolonged mechanical ventilation physicians not only discuss long-term outcomes with surrogates in terms they can easily understand, but also explicitly convey the likely demands of treatment and future functional dependence patients will likely experience.
Table 4.
One Year Resource Utilization
| Individual | Cohort Total | |
|---|---|---|
| Total one year costs | $306,135 ($285,467) | $38,577,935 |
| Initial hospitalization | $223,406 ($278,165) | $28,149,128 |
| Post-acute care | ||
| Total | $57,730 ($77,735) | $5,504,902 |
| Long-term acute care facility | $91,277 ($103,017) | $3,468,519 |
| Skilled nursing facility | $31,892 ($34,727) | $1,148,122 |
| Rehabilitation facility | $21,244 ($15,718) | $594,839 |
| Home health services | $6,669 ($4,754) | $293,422 |
| Hospital readmissions | $54,818 ($87,204) | $3,727,631 |
| Transportation costs* | $10,906 ($6,951) | $1,155,998 |
| Outpatient costs† | $551 ($585) | $40,276 |
n=126. Values displayed as total or mean (standard deviation).
Includes air and ground transport between acute and post-acute care facilities.
Includes emergency department visits, clinic visits, and outpatient surgeries.
Acknowledgments
Grant support: Career development awards from National Institutes of Health grants K23 HL081048 (CEC), K23 HL082650 (JMK), and K23 HL067068 (SSC) provided salary support.
Appendix: Cost calculations and supplementary tables
Cost calculations
All costs are adjusted for inflation by the medical component of the consumer price index for the US Southeastern region, urban, to 2007 US dollars (A1).
Initial hospitalization
Costs for the primary hospitalization (including physician fees) during which patients were enrolled in the study were determined using itemized charges from each patient’s administrative billing record, and converted to costs using department-specific cost-to-charge ratios obtained from the Centers for Medicare and Medicaid Services Healthcare Cost Information System (A2).
Hospital readmissions
Costs for subsequent hospital admissions were obtained by combining reported intensive care unit and hospital lengths of stay with estimated average daily ICU and hospital costs using the Centers for Medicare and Medicaid Services Medicare Provider Analysis Review. First we identified all 2006 Medicare Provider Analysis Review discharges within the Dartmouth Atlas-defined Durham, NC Hospital Referral Region involving intensive care. Next we estimated total costs for each hospitalization by multiplying departmental charges with departmental cost-to-charge ratios from the Medicare cost reports. We then used observed ICU and hospital length stay to estimate average daily costs, weighting initial, second and subsequent ICU days using a previously validated approach (A3, A4). After adjustment by the medical component of the consumer price index, daily hospital ward costs were $1,303. For those requiring ICU care, costs were estimated at $8,545 for day 1, $4,126 for day 2, and $2,231 for subsequent days of care. Professional fees were estimated by adding 17% of hospital costs (A5).
Long-term acute care facilities
Utilizing the Medicare Provider Analysis Review database, we evaluated all patients transferred to a long-term acute care facility from an acute care hospital in the Durham, NC hospital referral region in 2006. Total daily costs were estimated as $1,657 from the sum of department-specific charges multiplied by department-specific cost-to-charge ratios obtained from 2006 Medicare costs reports (A3, A4).
Skilled nursing facilities
Using the Medicare Provider Analysis Review database, we first identified all 2006 skilled nursing facility admissions in the three digit ZIP code 277xx, which encompasses Durham, NC and the surrounding area. Because this included only 19 skilled nursing facilities, we also included North Carolina skilled nursing facilities that admitted patients who lived in a 277xx ZIP code, for a total of 39 facilities. Next, we obtained average daily costs ($260) by multiplying total charges for the 8,112 Medicare admissions to these facilities by the skilled nursing facility-specific cost to charge ratio taken from the Medicare Healthcare Cost Report Information System database, dividing by the length of stay.
Rehabilitation facilities
We followed a similar procedure to that described above under “skilled nursing facilities” to determine daily rehabilitation facility costs ($458).
Home health care costs
We calculated home health care costs per Medicare guidelines described in the 2007 Federal Register (A6). In general, a base payment ($2,337 in 2007) is made for the first 60 days of care that is itself adjusted for clinical status, functional status, and recent service utilization, as well as geographical differences in wage. This overall case-mix assessment, done using the Outcome and Assessment Information Set instrument, is a composite measure of clinical status, functional status, and recent service utilization. In this model, we assumed patients fit the case-mix category of C1F2S2, representing low-moderate disability. Therefore we multiplied the 2007 base payment ($2,337) by the case-mix adjustment factor corresponding to C1F2S2 (0.9393) to obtain the standard 60-day rate ($2,195). Next, we multiplied the case-mix adjusted rate by the labor factor (0.77082) and adjusted this to the wage index of Durham county, NC (0.9816) to calculate the adjusted labor component of the total cost ($1,661). The non-labor component was calculated by multiplying the non-labor proportion (0.22918) by the case-mix adjusted rate ($2,195). Finally, the total home health care costs were then calculated to be $2,164 by summing the labor and non-labor components. For persons receiving ≤4 days of home health care, a per diem rate of $48 was applied, assuming the care of a home health care aide (rather than nurse). Home ventilation was assigned a cost of $950 per month (A7).
Inter-facility transportation
Hospital data were reviewed to account for all episodes of air (helicopter and fixed-wing plane) travel to and from the study hospital during the initial hospitalization. Ground ambulance transport was assumed to occur during all transitions between hospitals and post-acute care facilities. Operational costs (labor, supplies, vehicle) were obtained from institutional billing sources to estimate transportation episode costs per episode ($2,983 for ground and $9,270 for air).
Outpatient clinic and ambulatory surgery costs
Patient charts were abstracted to quantify episodes of care in the emergency department, outpatient clinics, and ambulatory surgeries during follow up. Costs were based on average North Carolina payment for services based on current procedural terminology codes listed in the American Medical Association database (A8). We assigned costs for emergency department visits based on code 99284 and code 99213 (Level 3 established patient return visit) for clinic visits. Ambulatory surgical procedure costs also were estimated based on procedures documented in the medical record using relevant current procedural terminology codes. These data were incomplete for 8 (6%) patients and missing for 4 (3%) patients.
Appendix Table 1.
Mechanical ventilation and one-year outcomes by ventilator liberation status
| Characteristic | All patients | Liberated from ventilation | Not liberated from ventilation |
|---|---|---|---|
| n=126 | n=86 | n=40 | |
| Mechanical ventilation duration, days | 27 (18, 24) | 21 (16, 37) | 43 (33, 74) |
| Duration of mechanical ventilation | |||
| <7 days | 3 (2%) | 3 (3%) | 0 |
| 7–13 days | 13 (10%) | 13 (15%) | 0 |
| 14–20 days | 24 (19%) | 22 (26%) | 2 (5%) |
| ≥21 days | 86 (68%) | 48 (56%) | 38 (95%) |
| Duration of ventilation before tracheostomy, days* | 11 (8, 17) range 3 – 47 |
10 (7, 16) range 3 – 47 |
14 (9, 17) range 3 – 30 |
| Tracheostomy decannulated | 82 (65%) | 82 (95%) | 0 |
| Hospital length of stay, days | 39 (28, 57) | 38 (28, 52) | 43 (31, 77) |
| Hospital discharge disposition | |||
| Home without paid home health care | 6 (5%) | 6 (7%) | 0 |
| Home with paid home health care | 14 (11%) | 12 (14%) | 2 (5%) |
| Long-term acute care facility | 36 (29%) | 24 (28%) | 12 (30%) |
| Skilled nursing facility | 17 (13%) | 15 (17%) | 2 (5%) |
| Rehabilitation facility | 23 (18%) | 23 (27%) | 0 |
| Other hospital | 3 (2%) | 3 (3.5%) | 0 |
| Still in acute care hospital at one year | 1 (1%) | 0 | 1 (3%) |
| Inpatient hospice facility | 3 (2%) | 3 (3.5%) | 0 |
| Dead | 23 (18%) | 0 | 23 (57%) |
| Transitions in care location, n† | 4 (2, 6) | 5 (3, 7) | 2 (1, 3) |
| Percent of all days alive receiving facility-based care or home health care‡ | 74% (CI 68%, 80%) | 62% (CI 54%, 70%) | 100% (CI 100%, 100%) |
| One-year survival | 70 (56%) | 67 (78%) | 3 (8%) |
| One-year health outcomes grouping§ | |||
| Good | 9 (7%) | 9 (11%) | 0 |
| Fair | 34 (27%) | 33 (38%) | 1 (3%) |
| Poor | 83 (66%) | 44 (51%) | 39 (97%) |
n=126. Values shown as number (%), median (interquartile range), or percent (95% confidence interval [CI]).
Does not include 1 patient who never underwent tracheostomy.
Transitions of care location over 1 year for 103 hospital survivors overall, 86 hospital survivors among those liberated, and 17 hospital survivors among those never liberated.
Includes acute hospitalization, post-acute care facilities, and home health care received during the entire study period (1 year).
Health outcomes categories measured at 1 year and defined as: good outcome (alive with no activities of daily living dependencies), fair outcome (alive but with 1–5 dependencies in activities of daily living), and poor outcome (either alive and completely dependent or dead).
Appendix Table 2.
Three-month patient and process of care outcomes
| Value | |
|---|---|
| Survival at 3 months | 90 (71%) |
| Disposition at 3 months | |
| Home without paid home care | 13 (10%) |
| Home with paid home care | 19 (15%) |
| Long-term acute care facility | 10 (8%) |
| Skilled nursing facility | 14 (11%) |
| Inpatient rehabilitation facility | 12 (10%) |
| Other hospital (transfer or readmission) | 13 (10%) |
| Residing in acute care hospital, never discharged | 6 (5%) |
| Dead | 36 (29%) |
| Liberated from ventilator and alive at 3 months | 78 (87%) |
| Patients readmitted within 3 months, among 103 hospital survivors* | 62 (60%) |
| Quality of life at 3 months among 3-month survivors, n=90† | |
| Good | 12 (13%) |
| Fair | 24 (27%) |
| Poor | 54 (60%) |
| Quality-adjusted life-days during first 3 months, total‡ | 21 (14, 35) |
| Health outcomes groupings at 3 months§ | |
| Good | 9 (7%) |
| Fair | 47 (37%) |
| Poor | 70 (56%) |
| Total 3-month costs|| | $278,733 ($279,855) |
n=126 unless noted. Values displayed as n (%), mean (standard deviation), or median (interquartile range).
These 62 patients were readmitted a total of 96 separate episodes.
By assessment of 3 month survivors’ surrogates using the EuroQol-5D index score; includes poor (≤0.44), fair (0.45–0.79), and good (≥0.80).
Calculated by adjusting all days alive during 3 months with corresponding EuroQol-5D index scores obtained at 3 month follow up.
Health outcomes categories measured at 3 months and defined as: good outcome (alive with no activities of daily living dependencies), fair outcome (alive but with 1–5 dependencies in activities of daily living), and poor outcome (either alive and completely dependent or dead).
Includes acute and post-acute care facilities.
Appendix Table 3.
One-year trajectories of care and resource utilization by ventilator and health outcomes
| Characteristic | Transitions of Care | p* | Read-missions | p* | Post-acute care days | p* | Total costs | p* |
|---|---|---|---|---|---|---|---|---|
| Mechanical ventilation characteristics & outcomes | ||||||||
| Mechanical ventilation <21 days, n=40 | 4 (2, 6) | 0.10 | 1 (0, 2) | 0.35 | 86 (25, 285) | 0.08 | $226,114 ($167,101) | 0.03 |
| Mechanical ventilation ≥21 days, n=86 | 3 (0, 6) | 1 (0, 2) | 51 (0, 213) | $343,819 ($320,161) | ||||
| Mechanical ventilation <28 days, n=65 | 4 (2, 6) | 0.02 | 1 (0, 2) | 0.10 | 80 (23, 304) | 0.007 | $235,229 ($141,057) | 0.004 |
| Mechanical ventilation ≥28 days, n=61 | 2 (0, 6) | 0 (0, 2) | 43 (0, 116) | $381,690 ($370,540) | ||||
| <14 ventilator days before tracheostomy, n=72 | 3 (1, 5) | 0.99 | 1 (0, 2) | 0.59 | 51 (17, 250) | 0.50 | $305,741 ($356,426) | 0.99 |
| ≥14 ventilator days before tracheostomy, n=54 | 3 (1, 6) | 1 (0, 2) | 62 (0, 237) | $306,660 ($148,362) | ||||
| Liberated from ventilation, n=86† | 5 (3, 7) | 0.0001 | 2 (0, 2) | 0.0001 | 82 (32, 290) | 0.0001 | $274,823 ($157,475) | 0.08 |
| Not liberated from ventilation, n=40 | 0 (0, 2) | 0 (0, 0) | 0 (0, 41) | $373,456 ($447,585) | ||||
| Health outcomes groupings‡ | ||||||||
| Good, n=9 | 2 (2, 5) | 0.0001 | 0 (0, 2) | 0.005 | 22 (14, 45) | 0.02 | $316,476 ($326,988) | 0.40 |
| Fair, n=34 | 5 (4, 7) | 2 (1, 3) | 80 (49, 258) | $321,757 ($185,483) | ||||
| Poor, n=83 | 2 (0, 5) | 0 (0, 2) | 44 (0, 264) | $151,751 ($80,534) | ||||
| Quality of life groupings§ | ||||||||
| Good, n=19 | 4 (2, 5) | 0.05 | 1 (0, 2) | 0.04 | 55 (21, 112) | 0.04 | $230,835 ($119,741) | 0.50 |
| Fair, n=17 | 6 (4, 7) | 2 (2, 3) | 288 (38, 327) | $320,884 ($179,454) | ||||
| Poor, n=34 | 5 (2, 7) | 2 (1, 3) | 145 (56, 310) | $346,374 ($469,166) | ||||
n=126. Values shown as median (interquartile range) or mean (standard deviation).
p based on one-way analysis of variance or Kruskal-Wallis tests.
Liberated during the study period of 1 year follow up.
Health outcomes categories measured at 1 year follow up and defined as: good outcome (alive with no activities of daily living dependencies), fair outcome (alive but with 1–5 dependencies in activities of daily living), and poor outcome (either alive and completely dependent or dead).
By assessment of one-year survivors’ surrogates using the EuroQol-5D index score; includes poor (≤0.44), fair (0.45–0.79), and good (≥0.80).
Footnotes
Reproducible Research Statement
Protocol: Available from Dr. Cox (christopher.cox@duke.edu).
Statistical code: Not available.
Data set: Available to other investigators or approved individuals through written agreements with Dr. Cox (christopher.cox@duke.edu).
Contributor Information
Mark Unroe, Email: mark.unroe@duke.edu.
Jeremy M. Kahn, Email: jmkahn@mail.med.upenn.edu.
Shannon S. Carson, Email: scarson@med.unc.edu.
Joseph A. Govert, Email: gover001@mc.duke.edu.
Tereza Martinu, Email: tereza.martinu@duke.edu.
Shailaja J. Sathy, Email: sathy@uw.edu.
Alison S. Clay, Email: alison.clay@duke.edu.
Jessica Chia, Email: chia0002@mc.duke.edu.
Alice Gray, Email: alice.gray@duke.edu.
James A. Tulsky, Email: jtulsky@duke.edu.
Christopher E. Cox, Email: christopher.cox@duke.edu.
References
- 1.Carson SS, Bach PB. The epidemiology and costs of chronic critical illness. Crit Care Clin. 2002;18:461–76. doi: 10.1016/s0749-0704(02)00015-5. [DOI] [PubMed] [Google Scholar]
- 2.Zilberberg MD, Shorr AF. Prolonged acute mechanical ventilation and hospital bed utilization in 2020 in the United States: implications for budgets, plant and personnel planning. BMC Health Serv Res. 2008;8:242. doi: 10.1186/1472-6963-8-242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cox CE, Carson SS, Govert JA, Chelluri L, Sanders GD. An economic evaluation of prolonged mechanical ventilation. Crit Care Med. 2007;35:1918–27. doi: 10.1097/01.CCM.0000275391.35834.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson JE, Meier DE, Litke A, Natale DA, Siegel RE, Morrison RS. The symptom burden of chronic critical illness. Crit Care Med. 2004;32:1527–34. doi: 10.1097/01.ccm.0000129485.08835.5a. [DOI] [PubMed] [Google Scholar]
- 5.Cox CE, Martinu T, Sathy SJ, Clay AS, Chia J, Gray AL, et al. Expectations and outcomes of prolonged mechanical ventilation. Crit Care Med. 2009;37:2888–2894. doi: 10.1097/CCM.0b013e3181ab86ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nelson JE, Tandon N, Mercado AF, Camhi SL, Ely EW, Morrison RS. Brain dysfunction: another burden for the chronically critically ill. Arch Intern Med. 2006;166:1993–9. doi: 10.1001/archinte.166.18.1993. [DOI] [PubMed] [Google Scholar]
- 7.Van Pelt DC, Milbrandt EB, Qin L, Weissfeld LA, Rotondi AJ, Schulz R, et al. Informal caregiver burden among survivors of prolonged mechanical ventilation. Am J Respir Crit Care Med. 2007;175:167–73. doi: 10.1164/rccm.200604-493OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nelson JE, Mercado AF, Camhi SL, Tandon N, Wallenstein S, August GI, et al. Communication about chronic critical illness. Arch Intern Med. 2007;167:2509–15. doi: 10.1001/archinte.167.22.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deficit Reduction Act of 2005 [serial on-line] Government USF. [6 August 2009];PUBLIC LAW 109-171. 2006 S:1932. Accessed at www.gpo.gov:80/fdsys/pkg/PLAW-109publ171/pdf/PLAW-109publ171.pdf.
- 10.MacIntyre NR, Epstein SK, Carson S, Scheinhorn D, Christopher K, Muldoon S. Management of patients requiring prolonged mechanical ventilation: report of a NAMDRC consensus conference. Chest. 2005;128:3937–54. doi: 10.1378/chest.128.6.3937. [DOI] [PubMed] [Google Scholar]
- 11.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 12.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–29. [PubMed] [Google Scholar]
- 13.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 14.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. The index of ADL: a standardized measure of biological and physiological function. JAMA. 1963;185:914–19. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 15.The EuroQol Group. EuroQol—a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. doi: 10.1016/0168-8510(90)90421-9. [DOI] [PubMed] [Google Scholar]
- 16.Angus DC, Carlet J. Surviving Intensive Care: a report from the 2002 Brussels Roundtable. Intensive Care Med. 2003;29:368–77. doi: 10.1007/s00134-002-1624-8. [DOI] [PubMed] [Google Scholar]
- 17.Kind P, Hardman G, Macran S. [18 April 2008];UK population norms for the EQ-5D [serial on-line] Accessed at www.york.ac.uk/inst/che/pdf/DP172.pdf.
- 18.Luo N, Johnson JA, Shaw JW, Feeny D, Coons SJ. Self-reported health status of the general adult U.S. population as assessed by the EQ-5D and Health Utilities Index. Med Care. 2005;43:1078–86. doi: 10.1097/01.mlr.0000182493.57090.c1. [DOI] [PubMed] [Google Scholar]
- 19.Taira DA, Seto TB, Siegrist R, Cosgrove R, Berezin R, Cohen DJ. Comparison of analytic approaches for the economic evaluation of new technologies alongside multicenter clinical trials. Am Heart J. 2003;145:452–8. doi: 10.1067/mhj.2003.3. [DOI] [PubMed] [Google Scholar]
- 20.Ellis SG, Miller DP, Brown KJ, Omoigui N, Howell GL, Kutner M, et al. In-hospital cost of percutaneous coronary revascularization. Circulation. 1995;92:741–7. doi: 10.1161/01.cir.92.4.741. [DOI] [PubMed] [Google Scholar]
- 21.Heyland DK, Konopad E, Noseworthy TW, Johnston R, Gafni A. Is it ‘worthwhile’ to continue treating patients with a prolonged stay (>14 days) in the ICU? Chest. 1998;114:192–8. doi: 10.1378/chest.114.1.192. [DOI] [PubMed] [Google Scholar]
- 22.Cox CE, Carson SS, Hoff-Linquist JA, Olsen MA, Govert JA, Chelluri L. Differences in one-year health outcomes and resource utilization by definition of prolonged mechanical ventilation. Crit Care. 2007;11:R9. doi: 10.1186/cc5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dewar DM, Kurek CJ, Lambrinos J, Cohen IL, Zhong Y. Patterns in costs and outcomes for patients with prolonged mechanical ventilation undergoing tracheostomy. Crit Care Med. 1999;27:2640–7. doi: 10.1097/00003246-199912000-00006. [DOI] [PubMed] [Google Scholar]
- 24.Cooper LM, Linde-Zwirble WT. Medicare intensive care unit use: analysis of incidence, cost, and payment. Crit Care Med. 2004;32:2247–53. doi: 10.1097/01.ccm.0000146301.47334.bd. [DOI] [PubMed] [Google Scholar]
- 25.Wunsch H, Guerra C, Barnato AE, Angus DC, Li G, Linde-Zwirble WT. Three-year outcomes for Medicare beneficiaries who survive intensive care. JAMA. 2010;303:849–56. doi: 10.1001/jama.2010.216. [DOI] [PubMed] [Google Scholar]
- 26.RTI International. Post-acute care payment reform demonstration [serial on-line] [12 November 2009]; Accessed at www.pacdemo.rti.org.
- 27.Centers for Medicare and Medicaid Services. Overview of the prospective payment system [serial on-line] [15 November 2009]; Accessed at www.cms.hhs.gov/ProspMedicareFeeSvcPmtGen.
- 28.Davis K. Paying for care episodes and care coordination. N Engl J Med. 2007;356:1166–8. doi: 10.1056/NEJMe078007. [DOI] [PubMed] [Google Scholar]
- 29.Bodenheimer T. Coordinating care—a perilous journey through the health care system. N Engl J Med. 2008;358:1064–71. doi: 10.1056/NEJMhpr0706165. [DOI] [PubMed] [Google Scholar]
- 30.Nasraway SA, Button GJ, Rand WM, Hudson-Jinks T, Gustafson M. Survivors of catastrophic illness: outcome after direct transfer from intensive care to extended care facilities. Crit Care Med. 2000;28:19–25. doi: 10.1097/00003246-200001000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Angus DC, Musthafa AA, Clermont G, Griffin MF, Linde-Zwirble WT, Dremsizov TT, et al. Quality-adjusted survival in the first year after the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2001;163:1389–94. doi: 10.1164/ajrccm.163.6.2005123. [DOI] [PubMed] [Google Scholar]
- 32.Covinsky KE, Goldman L, Cook EF, Oye R, Desbiens N, Reding D, et al. The impact of serious illness on patients’ families. JAMA. 1994;272:1839–44. doi: 10.1001/jama.272.23.1839. [DOI] [PubMed] [Google Scholar]
- 33.Herridge MS, Cheung AM, Tansey CM, Matte-Martyn A, Diaz-Granados N, Al-Saidi F, et al. One-year outcomes in survivors of the acute respiratory distress syndrome. N Engl J Med. 2003;348:683–93. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- 34.Carson SS, Garrett J, Hanson LC, Lanier J, Govert J, Brake MC, et al. A prognostic model for one-year mortality in patients requiring prolonged mechanical ventilation. Crit Care Med. 2008;36:2061–9. doi: 10.1097/CCM.0b013e31817b8925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carson SS, Bach PB. Predicting mortality in patients suffering from prolonged critical illness: an assessment of four severity-of-illness measures. Chest. 2001;120:928–33. doi: 10.1378/chest.120.3.928. [DOI] [PubMed] [Google Scholar]
- 36.Engoren M, Arslanian-Engoren C, Fenn-Buderer N. Hospital and long-term outcome after tracheostomy for respiratory failure. Chest. 2004;125:220–7. doi: 10.1378/chest.125.1.220. [DOI] [PubMed] [Google Scholar]
- 37.White DB, Braddock CH, Bereknyei S, Curtis JR. Toward shared decision making at the end of life in intensive care units: opportunities for improvement. Arch Intern Med. 2007;167:461–7. doi: 10.1001/archinte.167.5.461. [DOI] [PubMed] [Google Scholar]
- 38.Christakis NA, Iwashyna TJ. Attitude and self-reported practice regarding prognostication in a national sample of internists. Arch Intern Med. 1998;158:2389–95. doi: 10.1001/archinte.158.21.2389. [DOI] [PubMed] [Google Scholar]
- 39.Evans LR, Boyd EA, Malvar G, Apatira L, Luce JM, Lo B, et al. Surrogate decision-makers’ perspectives on discussing prognosis in the face of uncertainty. Am J Respir Crit Care Med. 2009;179:48–53. doi: 10.1164/rccm.200806-969OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.El-Jawahri A, Podgurski LM, Eichler AF, Plotkin SR, Temel JS, Mitchell SL, et al. Use of video to facilitate end-of-life discussions with patients with cancer: a randomized controlled trial. J Clin Oncol. 2010;28:305–10. doi: 10.1200/JCO.2009.24.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cox CE, Carson SS, Holmes GM, Howard A, Carey TS. Increase in tracheostomy for prolonged mechanical ventilation in North Carolina, 1993–2002. Crit Care Med. 2004;32:2219–26. doi: 10.1097/01.ccm.0000145232.46143.40. [DOI] [PubMed] [Google Scholar]
- 42.Terragni PP, Antonelli M, Fumagalli R, Faggiano C, Berardino M, Pallavicini FB, et al. Early vs late tracheotomy for prevention of pneumonia in mechanically ventilated adult ICU patients: a randomized controlled trial. JAMA. 2010;303:1483–9. doi: 10.1001/jama.2010.447. [DOI] [PubMed] [Google Scholar]
- 43.Griffiths J, Barber VS, Morgan L, Young JD. Systematic review and meta-analysis of studies of the timing of tracheostomy in adult patients undergoing artificial ventilation. BMJ. 2005;330:1243. doi: 10.1136/bmj.38467.485671.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nathens AB, Rivara FP, Mack CD, Rubenfeld GD, Wang J, Jurkovich GJ, et al. Variations in rates of tracheostomy in the critically ill trauma patient. Crit Care Med. 2006;34:2919–24. doi: 10.1097/01.CCM.0000243800.28251.AE. [DOI] [PubMed] [Google Scholar]
- 45.Kahn JM, Rubenfeld GD, Rohrbach J, Fuchs BD. Cost savings attributable to reductions in intensive care unit length of stay for mechanically ventilated patients. Med Care. 2008;46:1226–33. doi: 10.1097/MLR.0b013e31817d9342. [DOI] [PubMed] [Google Scholar]
- 46.Rapoport J, Teres D, Zhao Y, Lemeshow S. Length of stay data as a guide to hospital economic performance for ICU patients. Med Care. 2003;41:386–97. doi: 10.1097/01.MLR.0000053021.93198.96. [DOI] [PubMed] [Google Scholar]
References for Appendix
- A1.Bureau of Labor Statistics. [9 August 2009];Consumer price index [serial on-line] Accessed at www.bls.gov/cpi/home.htm.
- A2.Taira DA, Seto TB, Siegrist R, Cosgrove R, Berezin R, Cohen DJ. Comparison of analytic approaches for the economic evaluation of new technologies alongside multicenter clinical trials. Am Heart J. 2003;145:452–8. doi: 10.1067/mhj.2003.3. [DOI] [PubMed] [Google Scholar]
- A3.Kahn JM, Rubenfeld GD, Rohrbach J, Fuchs BD. Cost savings attributable to reductions in intensive care unit length of stay for mechanically ventilated patients. Med Care. 2008;46:1226–33. doi: 10.1097/MLR.0b013e31817d9342. [DOI] [PubMed] [Google Scholar]
- A4.Rapoport J, Teres D, Zhao Y, Lemeshow S. Length of stay data as a guide to hospital economic performance for ICU patients. Med Care. 2003;41:386–97. doi: 10.1097/01.MLR.0000053021.93198.96. [DOI] [PubMed] [Google Scholar]
- A5.Ellis SG, Miller DP, Brown KJ, Omoigui N, Howell GL, Kutner M, et al. In-hospital cost of percutaneous coronary revascularization. Critical determinants and implications. Circulation. 1995;92:741–7. doi: 10.1161/01.cir.92.4.741. [DOI] [PubMed] [Google Scholar]
- A6.Department of Health and Human Services. Washington, D.C.: 2007. [9 August 2009]. Medicare Program; Home Health Prospective Payment System Refinement and Rate Update for Calendar Year 2008; Final Rule [serial on-line] pp. 49762–49945. Accessed at edocket.access.gpo.gov/2007/pdf/07-4184.pdf. [Google Scholar]
- A7.Lewarski JS, Gay PC. Current issues in home mechanical ventilation. Chest. 2007;132:671–6. doi: 10.1378/chest.07-0558. [DOI] [PubMed] [Google Scholar]
- A8.American Medical Association. [12 February 2010];Current Procedual Terminology Code Online Search. Accessed at: catalog.ama-assn.org/Catalog/cpt/cpt_search.jsp.