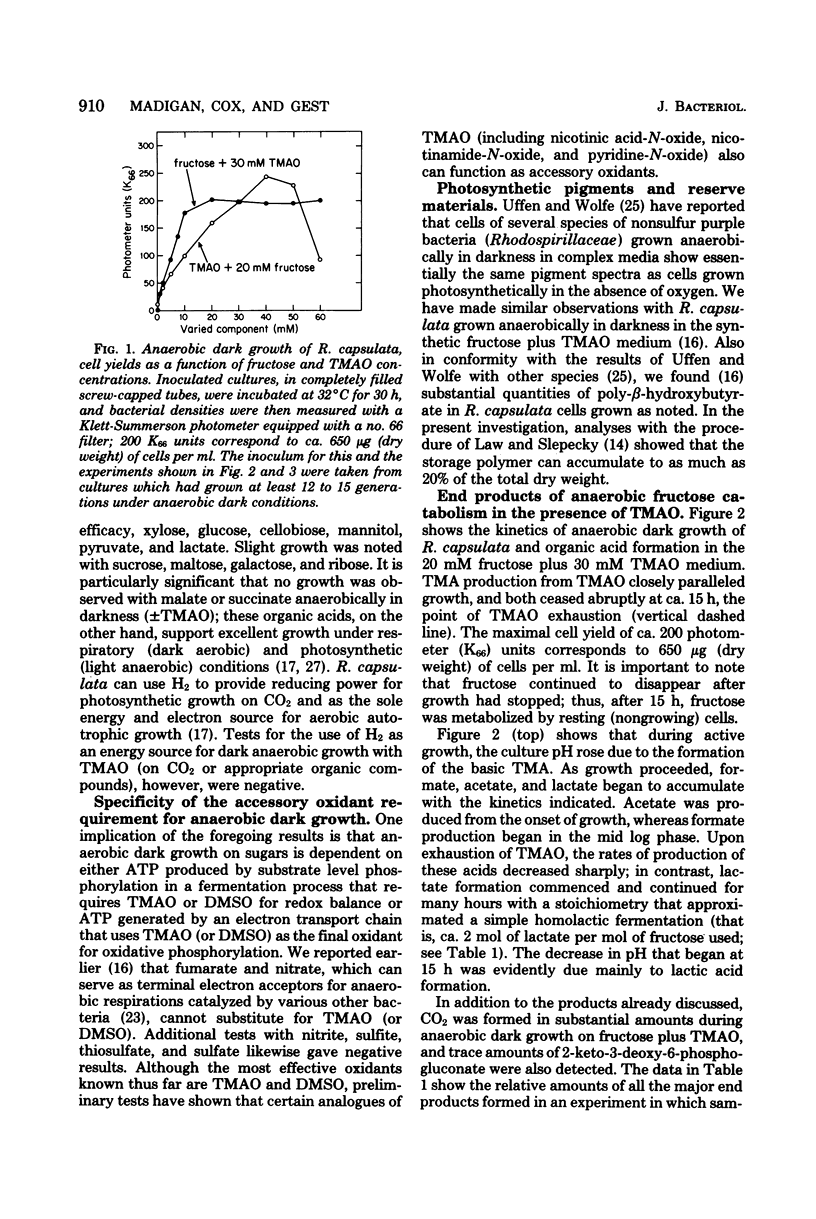
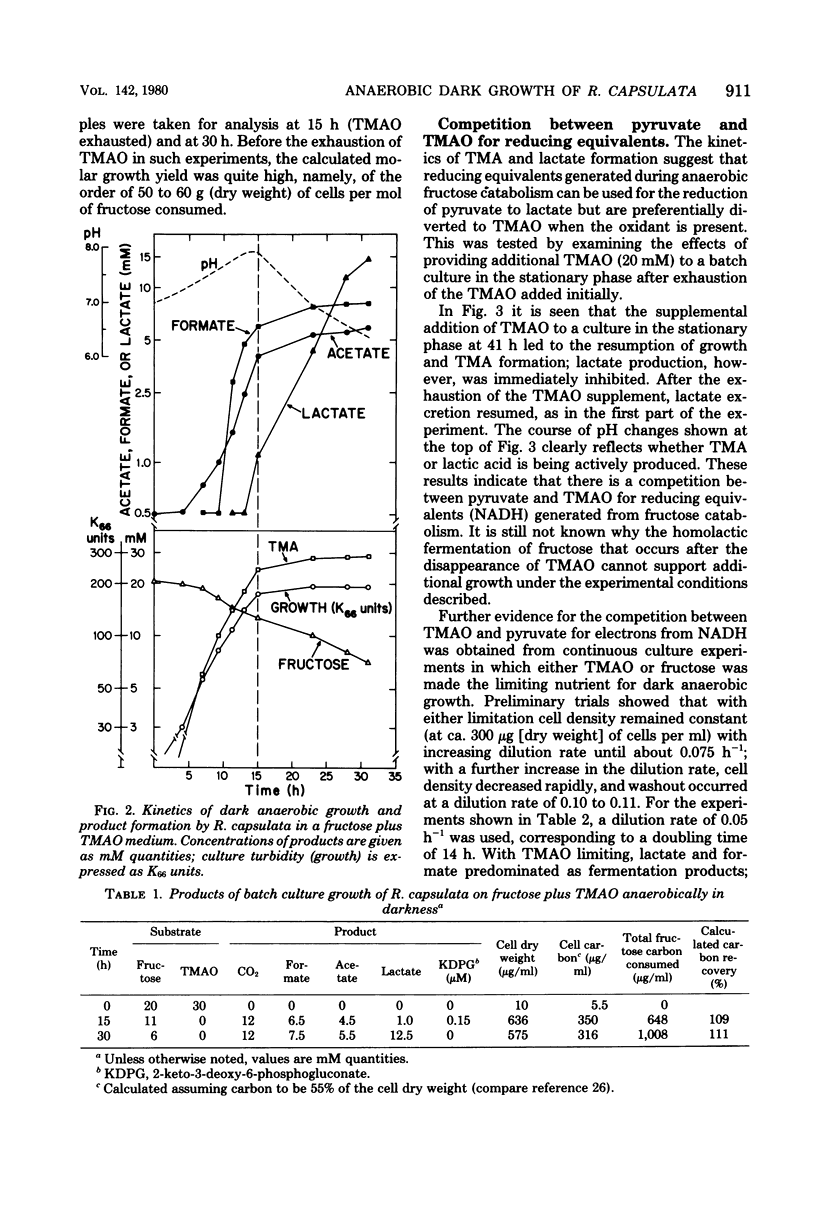
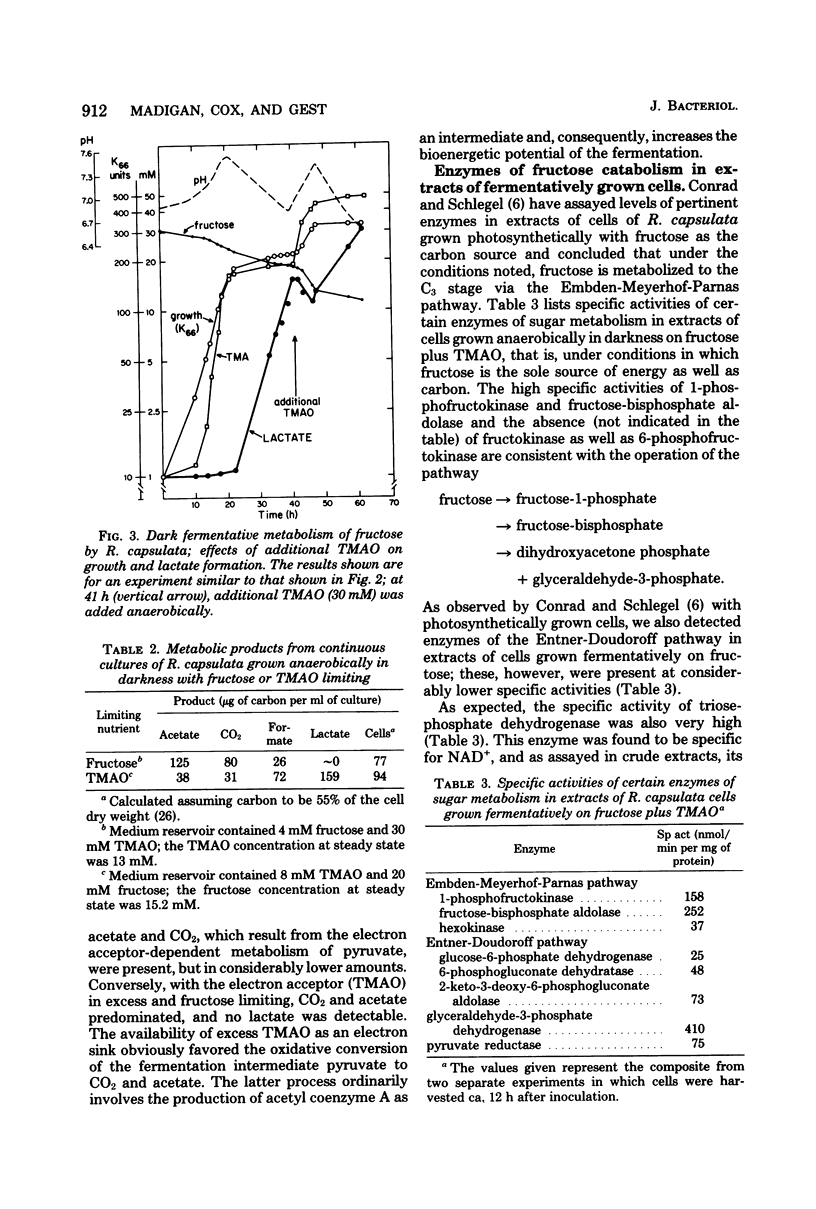
Abstract
The photosynthetic bacterium Rhodopseudomonas capsulata can grow under anaerobic conditions with light as the energy source or, alternatively, in darkness with D-fructose or certain other sugars as the sole source of carbon and energy. Growth in the latter mode requires an "accessory oxidant" such as trimethylamine-N-oxide, and the resulting cells contain the photosynthetic pigments characteristic of R. capsulata (associated with intracytoplasmic membranes) and substantial deposits of poly-beta-hydroxybutyrate. In dark anaerobic batch cultures in fructose plus trimethylamine-N-oxide medium, trimethylamine formation parallels growth, and typical fermentation products accumulate, namely, CO2 and formic, acetic, and lactic acids. These products are also found in dark anaerobic continuous cultures of R. capsulata; acetic acid and CO2 predominate when fructose is limiting, whereas formic and lactic acids are observed at elevated concentrations when trimethylamine-N-oxide is the limiting nutrient. Evidence is presented to support the conclusions that ATP generation during anaerobic dark growth of R. capsulata on fructose plus trimethylamine-N-oxide occurs by substrate level phosphorylations associated with classical glycolysis and pyruvate dissimilation, and that the required accessory oxidant functions as an electron sink to permit the management of fermentative redox balance, rather than as a terminal electron acceptor necessary for electron transport-driven phosphorylation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiking H., Sojka G. Response of Rhodopseudomonas capsulata to illumination and growth rate in a light-limited continuous culture. J Bacteriol. 1979 Aug;139(2):530–536. doi: 10.1128/jb.139.2.530-536.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad R., Schlegel H. G. Different degradation pathways for glucose and fructose in Rhodopseudomonas capsulata. Arch Microbiol. 1977 Feb 4;112(1):39–48. doi: 10.1007/BF00446652. [DOI] [PubMed] [Google Scholar]
- Crow V. L., Wittenberger C. L. Separation and properties of NAD+- and NADP+-dependent glyceraldehyde-3-phosphate dehydrogenases from Streptococcus mutans. J Biol Chem. 1979 Feb 25;254(4):1134–1142. [PubMed] [Google Scholar]
- GARDNER J. F., LASCELLES J. The requirement for acetate of a streptomycin-resistant strain of Staphylococcus aureus. J Gen Microbiol. 1962 Sep;29:157–164. doi: 10.1099/00221287-29-1-157. [DOI] [PubMed] [Google Scholar]
- Gorrell T. E., Uffen R. L. Fermentative metabolism of pyruvate by Rhodospirillum rubrum after anaerobic growth in darkness. J Bacteriol. 1977 Aug;131(2):533–543. doi: 10.1128/jb.131.2.533-543.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LANNING M. C., COHEN S. S. The detection and estimation of 2-ketohexonic acids. J Biol Chem. 1951 Mar;189(1):109–114. [PubMed] [Google Scholar]
- LAW J. H., SLEPECKY R. A. Assay of poly-beta-hydroxybutyric acid. J Bacteriol. 1961 Jul;82:33–36. doi: 10.1128/jb.82.1.33-36.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungdahl L. G. Total synthesis of acetate from CO2 by heterotrophic bacteria. Annu Rev Microbiol. 1969;23:515–538. doi: 10.1146/annurev.mi.23.100169.002503. [DOI] [PubMed] [Google Scholar]
- Madigan M. T., Gest H. Growth of a photosynthetic bacterium anaerobically in darkness, supported by "oxidant-dependent" sugar fermentation. Arch Microbiol. 1978 May 30;117(2):119–122. doi: 10.1007/BF00402298. [DOI] [PubMed] [Google Scholar]
- Madigan M. T., Gest H. Growth of the photosynthetic bacterium Rhodopseudomonas capsulata chemoautotrophically in darkness with H2 as the energy source. J Bacteriol. 1979 Jan;137(1):524–530. doi: 10.1128/jb.137.1.524-530.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madigan M. T., Wall J. D., Gest H. Dark anaerobic dinitrogen fixation by a photosynthetic microorganism. Science. 1979 Jun 29;204(4400):1429–1430. doi: 10.1126/science.204.4400.1429. [DOI] [PubMed] [Google Scholar]
- Morris D. L. Quantitative Determination of Carbohydrates With Dreywood's Anthrone Reagent. Science. 1948 Mar 5;107(2775):254–255. doi: 10.1126/science.107.2775.254. [DOI] [PubMed] [Google Scholar]
- Pfennig N., Biebl H. Desulfuromonas acetoxidans gen. nov. and sp. nov., a new anaerobic, sulfur-reducing, acetate-oxidizing bacterium. Arch Microbiol. 1976 Oct 11;110(1):3–12. doi: 10.1007/BF00416962. [DOI] [PubMed] [Google Scholar]
- Satoh T., Hoshino Y., Kitamura H. Rhodopseudomonas sphaeroides forma sp. denitrificans, a denitrifying strain as a subspecies of Rhodopseudomonas sphaeroides. Arch Microbiol. 1976 Jul;108(3):265–269. doi: 10.1007/BF00454851. [DOI] [PubMed] [Google Scholar]
- Strøm A. R., Olafsen J. A., Larsen H. Trimethylamine oxide: a terminal electron acceptor in anaerobic respiration of bacteria. J Gen Microbiol. 1979 Jun;112(2):315–320. doi: 10.1099/00221287-112-2-315. [DOI] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uffen R. L. Anaerobic growth of a Rhodopseudomonas species in the dark with carbon monoxide as sole carbon and energy substrate. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3298–3302. doi: 10.1073/pnas.73.9.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uffen R. L., Wolfe R. S. Anaerobic growth of purple nonsulfur bacteria under dark conditions. J Bacteriol. 1970 Oct;104(1):462–472. doi: 10.1128/jb.104.1.462-472.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall J. D., Johansson B. C., Gest H. A pleiotropic mutant of Rhodopseudomonas capsulata defective in nitrogen metabolism. Arch Microbiol. 1977 Dec 15;115(3):259–263. doi: 10.1007/BF00446450. [DOI] [PubMed] [Google Scholar]
- Weaver P. F., Wall J. D., Gest H. Characterization of Rhodopseudomonas capsulata. Arch Microbiol. 1975 Nov 7;105(3):207–216. doi: 10.1007/BF00447139. [DOI] [PubMed] [Google Scholar]
- Yamamoto I., Ishimoto M. Anaerobic growth of Escherichia coli on formate by reduction of nitrate, fumarate, and trimethylamine N-oxide. Z Allg Mikrobiol. 1977;17(3):235–242. doi: 10.1002/jobm.3630170309. [DOI] [PubMed] [Google Scholar]
- Yen H. C., Marrs B. Growth of Rhodopseudomonas capsulata under anaerobic dark conditions with dimethyl sulfoxide. Arch Biochem Biophys. 1977 Jun;181(2):411–418. doi: 10.1016/0003-9861(77)90246-6. [DOI] [PubMed] [Google Scholar]
- Zinder S. H., Brock T. D. Dimethyl sulfoxide as an electron acceptor for anaerobic growth. Arch Microbiol. 1978 Jan 23;116(1):35–40. doi: 10.1007/BF00408731. [DOI] [PubMed] [Google Scholar]
- Zinder S. H., Brock T. D. Dimethyl sulphoxide reduction by micro-organisms. J Gen Microbiol. 1978 Apr;105(2):335–342. doi: 10.1099/00221287-105-2-335. [DOI] [PubMed] [Google Scholar]
- van Niel C. B. THE CULTURE, GENERAL PHYSIOLOGY, MORPHOLOGY, AND CLASSIFICATION OF THE NON-SULFUR PURPLE AND BROWN BACTERIA. Bacteriol Rev. 1944 Mar;8(1):1–118. doi: 10.1128/br.8.1.1-118.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]


