Innervation to monkey and human lateral rectus muscles is segregated into well-defined superior and inferior zones, so that the lateral rectus may function as two parallel muscles under separate control. Differential activation of the two lateral rectus zones could impart previously unrecognized torsional and vertical actions to this nominally “horizontal” rectus muscle, potentially resolving an important paradox in ocular kinematics.
Abstract
Purpose.
Skeletal and craniofacial muscles are frequently composed of multiple neuromuscular compartments that serve different physiological functions. Evidence of possible regional selectivity in LR intramuscular innervation was sought in a study of the anatomic potential of lateral rectus (LR) muscle compartmentalization.
Methods.
Whole orbits of two humans and five macaque monkeys were serially sectioned at 10-μm thickness and stained with Masson trichrome. The abducens nerve (CN6) was traced anteriorly from the deep orbit as it branched to enter the LR and arborized among extraocular muscle (EOM) fibers. Three-dimensional reconstruction was performed in human and monkey orbits.
Results.
Findings were in concordance in the monkey and human orbits. External to the LR global surface, CN6 bifurcated into approximately equal-sized trunks before entering the global layer. Subsequent arborization showed a systematic topography, entering a well-defined inferior zone 0.4 to 2.5 mm more posteriorly than branches entering the largely nonoverlapping superior zone. Zonal innervation remained segregated anteriorly and laterally within the LR.
Conclusions.
Consistent segregation of intramuscular CN6 arborization in humans and monkeys suggests functionally distinct superior and inferior zones for the LR. Since the LR is shaped as a broad vertical strap, segregated control of the two zones could activate them separately, potentially mediating previously unappreciated but substantial torsional and vertical oculorotary LR actions.
Muscles need not be controlled as if they were monolithic structures. Many human skeletal muscles consist of multiple neuromuscular compartments in which different muscular regions are controlled by different populations of motor neurons.1–3 For example, the human cricothyroid muscle has three distinct bellies controlled by separate motor nerve branches apparently serving different motor tasks.4 Numerous skeletal muscles are compartmentally organized and controlled by somatotopically organized motor neuron pools.5 Compartmentalization adds diversity to the functional repertoire of a muscle.
Extraocular muscle (EOM) fibers and motor neurons are known to exist in superabundance relative to the requirements of the conventionally recognized mechanisms of eye movements.6,7 It is theoretically plausible that individual EOMs would have more than one function. The active pulley hypothesis (APH) proposes that the orbital layers (OLs) of the EOMs shift connective tissue structures to influence EOM pulling directions, whereas the global layers (GLs) directly exert oculorotary torque. Aside from the APH, there is little knowledge or speculation about possible compartmentalization in EOMs.
The lateral rectus (LR) is the most obvious candidate rectus EOM for compartmentalization. Clues are already available that the LR may be a compartmentalized EOM. The LR has traditionally been recognized to have a dual-headed origin described as either dividing the superior orbital fissure into inferior and superior compartments8 or arising as two distinct slips at the common tendinous ring.9 Classic embryology maintained that the LR arises from what were originally considered two different myotomes.10,11 Modern embryology recognizes that the avian LR muscle arises from somitomeres 4 and 512,13 and that the abducens nerve (CN6) arises from both rhombomeres 5 and 6.12 More recently, longitudinal splitting of the human LR has been observed by magnetic resonance imaging (MRI) in congenital cranial dysinnervation disorders, including congenital fibrosis of the extraocular muscles type 1 (CFEOM1),14 Duane syndrome,15–17 congenital trochlear palsy,18 and congenital oculomotor palsy.18
In domestic mammals, CN6 innervates the LR after giving off branches to the retractor bulbi (RB) muscle.19 The accessory lateral rectus muscle (ALR), an EOM seen consistently in monkeys but normally absent in humans,20 is believed to be the vestigial homologue of the RB21 and has been reported once in a human with congenital third nerve palsy.22 The ALR shares an origin with the LR.23 In the cat, 18% of abducens nucleus neurons innervating the ALR also innervate the LR.19 These observations suggest that the ontogenic role of CN6 is to innervate multiple functional EOM units.
Innervation of the LR may often be grossly bifid in humans. Autopsy evidence indicates an 8% to 15% rate of unilateral or bilateral duplication of CN6 in humans,24–27 with splitting sometimes as proximal as the pons itself.27 Split innervation from CN6 to the RB and LR in nonhuman mammals, combined with the dual origin of the LR and frequent duplication of CN6 in humans, supports the idea that CN6 may contain topographically distinct branches that may innervate separate LR functional compartments.
We therefore hypothesized that that the primate LR may contain two functional zones, controllable by topographically segregated innervation that may confer some degree of independence. We sought evidence to support this proposition by re-examining peripheral and intramuscular LR innervation in human and nonhuman primates.
Materials and Methods
Specimen Preparation
In conformity with legal requirements, orbits were harvested from 4-year-old (H6) and 17-month-old (H7) male human cadavers that had been donated to a tissue bank (IIAM, Scranton, PA). The heads were frozen to −78°C within 24 hours of death, and then slowly thawed in 10% neutral buffered formalin for 1 week before orbit extraction. The orbits were removed en bloc with the periorbita intact and fixed in 10% neutral phosphate-buffered formalin. After a 24-hour decalcification in 0.003 M EDTA and 1.35 N HCl, the orbits were dehydrated in alcohol and xylenes, placed in a vacuum chamber for paraffin embedding, serially sectioned in a coronal plane at 10-μm thickness, and mounted on 50 × 75-mm gelatin-coated glass slides. Sections were prepared by using Masson's trichrome stain.28
After surgical implantation of scleral magnetic search coils, five macaque monkeys participated in behavioral studies of eye movements and strabismus at Washington University. All experiments were performed in compliance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Washington University Animal Care and Use Committee. At the conclusion of behavioral studies at Washington University, the monkeys were terminally anesthetized and perfused via the left ventricle with a solution containing 2.6% paraformaldehyde, 0.1 M lysine-HCl, 0.8% NaIO4, and 0.8% iodoacetic acid (pH 7.4; modified PLP-fixative). The brains were removed for anatomic analysis, and the heads were immersed in 10% neutral phosphate-buffered formalin. The magnetic search coils were removed by minimal dissection. The orbits were removed, fixed, and stained by using the same procedure as for the human orbits.
Histologic Processing
The orbits were prepared intact for histologic examination. They were then removed in continuity with the eyelids and orbital bones, carefully thinned under magnification by using a high-speed drill and rongeurs, decalcified for 24 hours at room temperature in 0.003 M EDTA and 1.35 N HCl, embedded in paraffin, and processed for sectioning in the quasicoronal plane (perpendicular to the long axis of the orbit) at 10 μm thickness, as described for the human orbits. Serial sections were mounted on 50 × 75-mm glass slides and examined microscopically after Masson's trichrome (MT) staining.28 MT stains EOM fibers red and distinctly stains nerves purple.29,30
Microscopy
Digital photographs were taken with a light microscope (Eclipse E800; Nikon, Tokyo, Japan) fitted with a digital camera (D1X; Nikon) with ×0.5 to ×40 objectives. The images were then processed (Photoshop CS4; Adobe Systems, San Jose, CA; and Canvas X; ACD Systems, Miami, FL).
Reconstruction
In the deep orbits of human H6 and monkey M3, primary bifurcations of CN6 were identified and highlighted in the layers (Photoshop; Adobe Systems). Working anteriorly from this location, we highlighted these secondary nerve trunks and all their daughter branches by using corresponding colors as far anteriorly as identifiable nerve bundles were present. The nerve branches corresponding to each primary bifurcation of CN6 were reconstructed with ImageJ (developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html). Three-dimensional reconstruction with interpolation and multidirectional perspectives was computationally intensive and was performed with an eight-processor computer (Mac Pro; Apple Computer, Cupertino, CA). Nerve cross sections were also determined with ImageJ.
Results
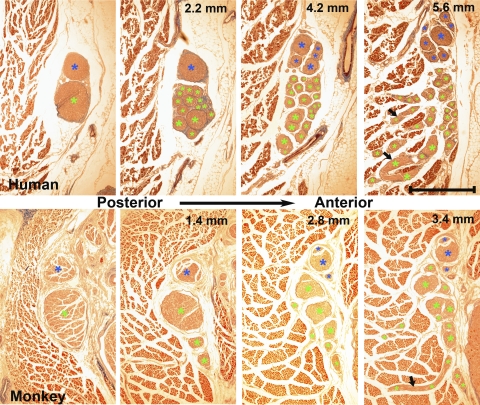
Findings were similar in all human and monkey orbits. In the orbital apex, CN6 began as a single circular trunk, gradually elongating and dividing as it approached the global surface of the LR or was bifurcated into two parallel trunks. This primary bifurcation sometimes was already evident in the most apical sections examined in most orbits, but was always proximal to the CN6 entry into the LR. Before entering the LR from the global surface, CN6 exhibited two major levels of bifurcation in addition to several minor divisions as it progressed toward and entered the GL. The cross-sectional areas of the inferior and superior major CN6 trunks were computed before any further bifurcations. In human orbits H5 and H6, the ratio of inferior trunk cross section to the sum of the inferior and superior cross sections was 0.26 and 0.66, respectively. In the five monkey orbits, this ratio averaged 0.52 ± 0.08 SEM, not significantly different from a value of 0.5 corresponding to an equal average distribution into the two major trunks. The anterior distance from the initial CN6 bifurcation to the point of GL entry ranged 2.2 to 5.0 mm. This pattern is displayed in representative images from human (H6) and monkey (M3) specimens (Fig. 1).
Figure 1.
Sequential coronal sections showed two major nerve bifurcations of CN6 before entry into the LR GL in human H6 (top) and monkey M3 (bottom). Distances are anterior to initial panel bifurcation at left. Arrows: nerve branches traveling laterally into the adjacent LR. (*) Bifurcations of the two major bifurcations: blue for the superior and green for the inferior branches. Masson trichrome. Scale bar, 1 mm.
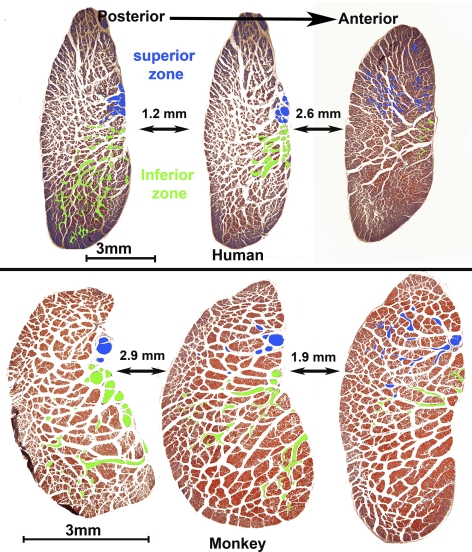
Intramuscular features of CN6 were consistent in human and monkey specimens. After the two major external bifurcations, the branches of CN6 further divided into arborizations within distinct, nonoverlapping inferior and superior zones of EOM fibers. At the LR surface, the inferior nerve branches entered the LR more posteriorly, whereas the superior branches had not yet further divided. Proceeding 0.4 to 3.8 mm anteriorly, the superior CN6 branches entered and bifurcated within the LR. Segregation of innervation to the superior and inferior zones was maintained throughout the LR. Progressing anteriorly, CN6 branches coursed laterally between EOM fibers in a uniform fashion, traveling along a boundary between the inferior and superior zones. This course may be seen in cross sections marked in representative sections in Figure 2, where intramuscular nerve arborizations to the two zones are distinguished by colored overlays. Although fine terminal motor nerve arborizations were observed in the orbital layer (OL), zonal separation was indeterminable based on nerve tracings. It was not possible to distinguish separate innervation to the OL and GL.
Figure 2.
Human and monkey LR cross sections, stained with Masson trichrome. Blue: CN6 superior zone branches that enter the LR more anteriorly. Green: inferior zone innervation that enters the LR more posteriorly, with little or no overlap between innervation zones.
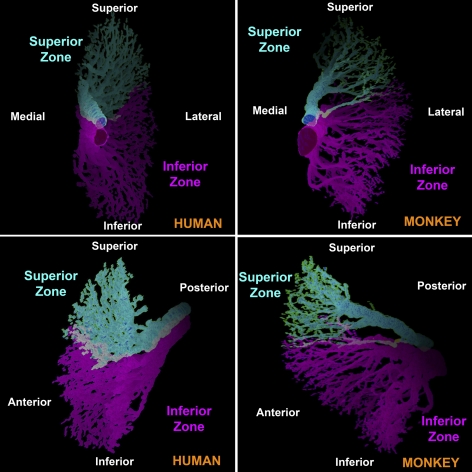
Three-dimensional reconstructions of intramuscular branches of motor nerves in human (H6) and monkey (M3) rectus EOMs were performed by outlining corresponding intramuscular nerves and tracing, beginning in the deep orbit and continuing anteriorly as far as possible, the distributions of the two major branches of CN6. These reconstructions clearly delineated, in both monkey and human, two distinct intramuscular nerve distributions that innervate the superior and inferior zones of the LR (Fig. 3). There was little or no overlap between zones, confirming the anatomic impression obtained from individual sections of all specimens.
Figure 3.
Three-dimensional reconstructions, in two perspectives each, of intramuscular CN6 arborizations in human H6 and monkey M3, showing segregation of arborizations to the superior and inferior zones.
Discussion
Peripheral LR innervation by CN6 exhibited consistent features in the human and monkey specimens. There is a primary bifurcation of CN6 external to the LR on its global surface in the posterior orbit, or even more proximally. Branches of CN6 innervate a distinct inferior zone more posteriorly than they innervate a corresponding superior zone. The boundary between the inferior and superior zones is respected by intramuscular innervation throughout the LR and even most peripherally and anteriorly as the CN6 branches traverse the GL toward the OL. Although there was no connective tissue specialization such as a fascial plane separating the LR inferior and superior zones, the segregated intramuscular innervation pattern suggests the potential for selective compartmental activation of the LR. In a structural neuromuscular sense, the peripheral LR may be controllable as though it consisted of two separate EOM compartments. Innervation to the superior and inferior zones is segregated at least central to the primary bifurcation of the main CN6 trunk external to the LR and probably more centrally.
Structural separation of distinct superior and inferior LR zones is occasionally visible in high-resolution MRI scans of living humans. This split is often quite obvious in high-resolution MRI scans of humans with CFEOM1,14 CFEOM3,31 Duane syndrome,15,16 and congenital oculomotor18 and trochlear18 palsies and is also observed in phenotypically normal humans who harbor the R262C or D417N mutations in the neuron-specific β-tubulin isotype III (TUBB3),31 which may cause CFEOM3.32 Although it is plausible that such a structural separation of anatomic superior and inferior regions in the LR could also correspond with the distinct innervational zones, this possibility remains to be demonstrated.
Although the LR, like all other rectus EOMs, has the shape of a broad, thin strap, it has traditionally been assumed to behave homogeneously so that force acting at a single point could summarize its entire oculorotary action. The present finding of consistent segregation of intramuscular CN6 arborizations in humans and monkeys suggests that the inferior and superior zones may comprise two functional subdivisions for LR oculorotary action. These two zones may be anterior continuations of the classic dual-headed LR origin; however, it was not possible to define the LR origin in the current specimens.
Compartmental LR organization seemingly permits a degree of differential contraction of its two distinct zones. It is not clear how this segregation may relate to postulated separated actions of the OL and GL, since innervation to the OL crossed the GL in both zones, but was too fine to be traced within the OL by the present method. The APH proposes that OL and GL fibers are under at least partially differential central neural control and have distinct mechanical actions29,33–35 despite the presence of some motor neurons innervating both the OL and GL.36 The current suggestion of compartmental organization of the LR therefore neither challenges nor supports the APH, but adds another layer of complexity to the peripheral oculomotor apparatus.
Compartmental LR function may have important kinematic implications. Ocular torsion around the line of sight is a unique function of horizontal and vertical eye position.37 With the head upright and stationary and for distant targets, Listing's law (LL) specifies that eye torsion in any gaze direction is that reached by a single rotation from primary eye position about an axis lying in a plane.38 For empiric purposes, LL is satisfied if the ocular rotational velocity axis shifts by half of the shift in ocular orientation.39
It was formerly believed that all ocular torsion was explicitly computed by the brain, which was supposed to command the cyclovertical extraocular muscles (the obliques and vertical rectus EOMs) to twist the eyes appropriately to enforce LL.40–44 However, motor neurons activating the cyclovertical EOMs of behaving monkeys do not encode the signals corresponding to LL torsion during smooth pursuit.45 Furthermore, electrical stimulation to the CN6 of behaving monkeys evokes saccadelike movements that conform to LL, an outcome that would be impossible if the required torsion were neurally computed.46 The APH alternatively explains the absence of such neural signals for torsion by proposing that systematic changes in rectus EOM pulling directions generate the torsion required by LL.34,35,47,48 According to the APH, pulleys, composed of connective tissue rings through which the rectus EOMs pass, are shifted by the OLs of the rectus EOMs themselves.29,34,35,47,49 Although the anteroposterior locations of the pulleys are under neural control for positioning the pulleys appropriately,49 pulley positioning is postulated not to have a simple relationship to eye torsion, because the dependence of torsion on eye position is mediated by the geometry of rectus EOM paths. The kinematic default behavior of the oculomotor apparatus therefore appears to be LL.
Nonetheless, a serious kinematic paradox has emerged. The vestibulo-ocular reflex (VOR) does not conform to LL. When the head rotates, velocity axes of the resulting ocular counterrotations change by one fourth of eye position, not one half, as specified by LL.50 Ghasia and Angelaki45 found that motor neurons driving cyclovertical EOMs not only did not command half-angle LL torsion, but also did not command the VOR's quarter-angle torsion.45 These striking negative findings indicate that neural commands for the eye torsion necessary to override the half-angle mechanical behavior dictated by the APH are not executed by the cyclovertical EOMs. Where could this torsional behavior arise? An early suggestion that quarter-angle behavior could be implemented by differential activation of the OLs and GLs of rectus EOMs to retract their pulleys29 has been shown to be unrealistic in many conditions42 and was long ago abandoned.51,52 Furthermore, mechanical pulley retraction would be inconsistent with observed zero-latency transition between quarter-angle VOR, and half-angle saccade behavior.50
Resolving the foregoing paradox therefore requires that neurally commanded quarter-angle torsional violations of LL be executed by one or both horizontal rectus EOMs. Surgical shifting of rectus EOM insertions circumferential to the corneoscleral limbus is recognized to impart torsional actions to the transposed EOMs.53,54 Because the human LR tendon is a thin strap 10 to 11 mm wide at the scleral insertion,51 the effective vertical location of the LR force centroid could be shifted by more than ±2.5 mm by differential activation of the two LR zones. Simulation using a computational biomechanical model (Orbit 1.8; Eidactics, San Francisco, CA) suggests that such a shift would impart vertical action ±13% to 15% of total LR tendon force and torsional action ±16% to 22% of total LR tendon force.55 Differential compartmental LR activation would, at no additional latency, alter the effective transverse location of a rectus EOM's functional origin without a shift in location of the pulley ring.34 Violations of LL could be implemented by differential activation of the superior versus inferior LR zones by innervation patterns limited to activity within CN6 itself. Such compartmental activation may be demonstrated experimentally by simultaneous recording of superior and inferior zone innervation or electromyographic activity, but would not be evident from separate recording of activity in either zone.
Anatomically, compartmental innervation in CN6 appears to be sufficiently segregated external to the LR to permit selective lesion, tracer, and stimulation experiments to test putative selective functions physiologically and to determine possible specificity of central brain stem connections. Duplication of CN6 is a relatively frequent anatomic variation in humans24–27 and may correspond to segregated projections to the LR's superior and inferior zones. It is presently unknown whether any other rectus EOMs have corresponding zonal segregation of intramuscular innervation. The feline inferior oblique EOM is innervated by distinct medial and lateral branches, which, when electrically stimulated, generate different contractile responses; however, motor neurons in the oculomotor nucleus are not topographically segregated.56 Topographic segregation of motor neurons in the brain stem would not be necessary for functional specificity.
Despite the present anatomic evidence of compartmental LR innervation, the physiological implications remain to be confirmed. The plausibility of putative selective oculorotary actions of LR compartments could be tested by biomechanical, neuroanatomic, and neurophysiologic data. For example, selective zonal oculorotary behavior could occur only if there is incomplete interzonal mechanical force coupling among parallel EOM fibers, a likely proposition that could be tested biomechanically. Selective zonal control of the LR would require some degree of selective neural control at level of nuclear and probably prenuclear inputs to the LR, a proposition that could be tested neuroanatomically and neurophysiologically. Zonal LR innervation need not be absolutely selective to be physiologically important. For example, the existence of some overlapping neural projections to both zones, analogous to projections of a minority of feline CN6 motor neurons to both the LR and ALR, would not prevent differential control of the LR by the remaining motor neurons that project to single LR zones.
Recent findings regarding the selective subnuclear organization of each of the ocular motor nuclei for control of multiply innervated fibers suggest that much complexity in the peripheral ocular motor system remains to be elucidated.57–59 Conventional concepts of the organization of the peripheral ocular motor system no longer suffice to explain its physiological repertoire. The possibility of compartmental LR activation deserves consideration.
Footnotes
Supported by U.S. Public Health Service, National Eye Institute Grants EY08313 and EY00331, and Research to Prevent Blindness. JLD is the Leonard Apt Professor of Ophthalmology.
Disclosure: M. Peng, None; V. Poukens, None; R.M. da Silva Costa, None; L. Yoo, None; L. Tychsen, None; J.L. Demer, None
References
- 1.English AW, Wolf SL, Segal RL. Compartmentalization of muscles and their motor nuclei: the partitioning hypothesis. Phys Ther. 1993;73:857–867 [DOI] [PubMed] [Google Scholar]
- 2.Holtermann A, Roeleveld K, Mork PJ, et al. Selective activation of neuromuscular compartments within the human trapezius muscle. J Electromyogr Kinesiol. 2009;29:896–902 [DOI] [PubMed] [Google Scholar]
- 3.Urquhart DM, Hodges PW. Differential activity of regions of transversus abdominis during trunk rotation. Eur Spine. 2005;14:393–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mu L, Sanders I. The human cricothyroid muscle: three muscle bellies and their innervation patterns. J Voice. 2007;23:21–28 [DOI] [PubMed] [Google Scholar]
- 5.Marshall CD, Hsu RH, Herring SW. Somatotopic organization of perioral musculature innervation within the pig facial motor nucleus. Brain Behav Evol. 2005;66:22–34 [DOI] [PubMed] [Google Scholar]
- 6.Goldberg SJ, Wilson KE, Shall MS. Summation of extraocular motor unit tensions in the lateral rectus muscle of the cat. Muscle Nerve. 1997;20:1229–1235 [DOI] [PubMed] [Google Scholar]
- 7.Goldberg SJ, Meredith MA, Shall MS. Extraocular motor unit and whole-muscle responses in the lateral rectus muscle of the squirrel monkey. J Neurosci. 1998;18:10629–10639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Govsa F, Kayalioglu G, Erturk M, Ozgur T. The superior orbital fissure and its contents. J Surg Radiol Anat. 1999;21:181–185 [DOI] [PubMed] [Google Scholar]
- 9.Spencer RF, Porter JD. Biological organization of the extraocular muscles. Prog Brain Res. 2006;151:43–80 [DOI] [PubMed] [Google Scholar]
- 10.Gilbert PW. The origin and development of the extrinsic ocular muscles in the domestic cat. Contrib Embryol. 1957;36:61–78 [PubMed] [Google Scholar]
- 11.Neal HV. Ths history of the eye muscles. J Morphol. 1918;30:433–453 [Google Scholar]
- 12.Wahl CM, Noden DM, Baker R. Developmental relations between sixth nerve motor neurons and their targets in the chick embryo. Dev Dynam. 1994;20:191–202 [DOI] [PubMed] [Google Scholar]
- 13.Evans DJ, Noden DM. Spatial relations between avian craniofacial neural crest and paraxial mesoderm cells. Dev Dynam. 2006;235:1310–1325 [DOI] [PubMed] [Google Scholar]
- 14.Demer JL, Clark RA, Engle EC. Magnetic resonance imaging evidence for widespread orbital dysinnervation in congenital fibrosis of extraocular muscles due to mutations in KIF21A. Invest Ophthalmol Vis Sci. 2005;46:530–539 [DOI] [PubMed] [Google Scholar]
- 15.Demer JL, Clark RA, Lim KH, Engle EC. Magnetic resonance imaging evidence for widespread orbital dysinnervation in dominant Duane's retraction syndrome linked to the DURS2 locus. Invest Ophthalmol Vis Sci. 2007;48:194–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demer JL, Clark RA, Lim K-H, Engle EC. Magnetic resonance imaging of innervational and extraocular muscle abnormalities in Duane-radial ray syndrome. Invest Ophthalmol Vis Sci. 2007;48:5505–5511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okanobu H, Kono R, Ohtsuki H, Miyake K. Magnetic resonance imaging findings in Duane's retraction syndrome type III (in Japanese). Rinsho Ganka (Jpn Clin Ophthalmol). 2008;62:65–69 [Google Scholar]
- 18.Okanobu H, Kono R, Miyake K, Ohtsuki H. Splitting of the extraocular horizontal rectus muscle in congenital cranial dysinnervation disorders. Am J Ophthalmol. 2009;147:550–556 [DOI] [PubMed] [Google Scholar]
- 19.Crandall WF, Goldberg SJ, Wilson JS, McClung JR. Muscle units divided among rectractor bulbi muscle slips and between the lateral rectus and retractor bulbi muscles in cat. Exp Brain Res. 1980;71:251–260 [DOI] [PubMed] [Google Scholar]
- 20.Narasimhan A, Tychsen LT, Poukens V, Demer JL. Horizontal rectus muscle anatomy in naturally and artificially strabismic monkeys. Invest Ophthalmol Vis Sci. 2007;48:2576–2588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schnyder H. The innervation of the monkey accessory lateral rectus muscle. Brain Res. 1984;296:139–144 [DOI] [PubMed] [Google Scholar]
- 22.Park CY, Oh SY. Accessory lateral rectus muscle in a patient with congenital third-nerve palsy. Am J Ophthalmol. 2003;136:355–356 [DOI] [PubMed] [Google Scholar]
- 23.Boothe RG, Quick MW, Joosse MV, Abbas MA, Anderson DC. Accessory lateral rectus orbital geometry in normal and naturally strabismic monkeys. Invest Ophthalmol Vis Sci. 1990;31:1168–1174 [PubMed] [Google Scholar]
- 24.Nathan H, Ouaknine G, Kosary IZ. The abducens nerve: anatomical variations in its course. J Neurosurg. 1974;42:561–566 [DOI] [PubMed] [Google Scholar]
- 25.Jain KK. Aberrant roots of the abducens nerve. J Neurosurg. 1964;21:349–351 [DOI] [PubMed] [Google Scholar]
- 26.Iaconetta G, Tessitore E, Samii M. Duplicated abducent nerve and its course: microanatomical study and surgery-related considerations. J Neurosurg. 2001;95:853–858 [DOI] [PubMed] [Google Scholar]
- 27.Ozeren MF, Sam B, Akdemir I, LAlkan A, Tekdemir I, Deda H. Duplication of the abducens nerve at the petroclival region: an anatomic study. Neurosurgery. 2003;52:645–651 [DOI] [PubMed] [Google Scholar]
- 28.Sheehan DC, Hrapchak BB. Theory and Practice of Histotechnology. St. Louis: Mosby; 1973 [Google Scholar]
- 29.Demer JL, Oh SY, Poukens V. Evidence for active control of rectus extraocular muscle pulleys. Invest Ophthalmol Vis Sci. 2000;41:1280–1290 [PubMed] [Google Scholar]
- 30.Oh SY, Poukens V, Demer JL. Quantitative analysis of rectus extraocular muscle layers in monkey and humans. Invest Ophthalmol Vis Sci. 2001;42:10–16 [PubMed] [Google Scholar]
- 31.Demer JL, Clark RA, Tischfield MA, Engle EC. Evidence of an asymmetrical endophenotype in congenital fibrosis of extraocular muscles type 3 resulting from TUBB3 mutations. Invest Ophthalmol Vis Sci. 2010;51:4600–4611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tischfield MA, Baris HN, Gupta ML, et al. Human TUBB3 mutations perturb microtubule dynamics, kinesin interactions, and neuronal circuitry Cell. 2010;140:74–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller JM. Understanding and misunderstanding extraocular muscle pulleys. J Vision. 2007;7:1–15 [DOI] [PubMed] [Google Scholar]
- 34.Demer JL. Current concepts of mechanical and neural factors in ocular motility. Cur Opin Neurol. 2006;19:4–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Demer JL. Mechanics of the orbita. Dev Ophthalmol. 2007;40:132–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shall MS, Goldberg SJ. Lateral rectus EMG and contractile responses elicited by cat abducens motoneurons. Musc Nerve. 1995;18:948–955 [DOI] [PubMed] [Google Scholar]
- 37.Tweed D, Vilis T. Implications of rotational kinematics for the oculomotor system in three dimensions. J Neurophysiol. 1987;58:832–849 [DOI] [PubMed] [Google Scholar]
- 38.Ruete CGT. Ocular physiology. Strabismus. 1999;7:43–60 [DOI] [PubMed] [Google Scholar]
- 39.Tweed D, Vilis T. Geometric relations of eye position and velocity vectors during saccades. Vision Res. 1990;30:111–127 [DOI] [PubMed] [Google Scholar]
- 40.Crawford JD, Vilis T. Symmetry of oculomotor burst neuron coordinates about Listing's plane. J Neurophysiol. 1992;68:432–448 [DOI] [PubMed] [Google Scholar]
- 41.Tweed D. Visual-motor optimization in binocular control. Vis Res. 1997;37:1939–1951 [DOI] [PubMed] [Google Scholar]
- 42.Misslisch H, Tweed D. Neural and mechanical factors in eye control. J Neurophysiol. 2001;86:1877–1883 [DOI] [PubMed] [Google Scholar]
- 43.Angelaki DE, Hess BJ. Control of eye orientation: where does the brain's role end and the muscle's begin? Eur J Neurosci. 2004;19:1–10 [DOI] [PubMed] [Google Scholar]
- 44.Angelaki DE. Three-dimensional ocular kinematics during eccentric rotations: evidence for functional rather than mechanical constraints. J Neurophysiol. 2003;89:2685–2696 [DOI] [PubMed] [Google Scholar]
- 45.Ghasia FF, Angelaki DE. Do motoneurons encode the noncommutativity of ocular rotations? Neuron. 2005;47:281–293 [DOI] [PubMed] [Google Scholar]
- 46.Klier EM, Meng H, Angelaki DE. Three-dimensional kinematics at the level of the oculomotor plant. J Neurosci. 2006;26:2732–2737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Demer JL. Pivotal role of orbital connective tissues in binocular alignment and strabismus. The Friedenwald lecture. Invest Ophthalmol Vis Sci. 2004;45:729–738 [DOI] [PubMed] [Google Scholar]
- 48.Demer JL. The orbital pulley system: a revolution in concepts of orbital anatomy. Ann NY Acad Sci. 2002;956:17–32 [DOI] [PubMed] [Google Scholar]
- 49.Kono R, Clark RA, Demer JL. Active pulleys: magnetic resonance imaging of rectus muscle paths in tertiary gazes. Invest Ophthalmol Vis Sci. 2002;43:2179–2188 [PubMed] [Google Scholar]
- 50.Crane BT, Tian J, Demer JL. Kinematics of vertical saccades during the yaw vestibulo-ocular reflex in humans. Invest Ophthalmol Vis Sci. 2005;46:2800–2809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Demer JL. Anatomy of strabismus. In: Taylor D, Hoyt C. eds. Pediatric Ophthalmology and Strabismus. 3rd ed.London: Elsevier; 2005:849–861 [Google Scholar]
- 52.Demer JL, Kono R, Wright W. Magnetic resonance imaging of human extraocular muscles in convergence. J Neurophysiol. 2003;89:2072–2085 [DOI] [PubMed] [Google Scholar]
- 53.Kono R, Ohtsuki H, Okanobu H, Kingugasa K. Displacement of rectus muscle pulleys by torsional muscle surgery for treatment of full macular translocation-induced incyclotropia. Am J Ophthalmol. 2005;140:144–146 [DOI] [PubMed] [Google Scholar]
- 54.Iwata EA, Sato M, Ukai K, Terasaki H. Magnetic resonance imaging of the extraocular muscle path before and after strabismus surgery for a large degree of cyclotorsion induced by macular translocation surgery. Jpn J Ophthalmol. 2008;53:131–137 [DOI] [PubMed] [Google Scholar]
- 55.Miller JM, Pavlovski DS, Shaemeva I. Orbit 1.8 Gaze Mechanics Simulation. San Francisco: Eidactics; 1999 [Google Scholar]
- 56.Shall MS, Sorg PJ, McClung JR, Gilliam EE, Goldberg SJ. Relationship of the mechanical properties of the cat inferior oblique muscle to the anatomy of its motoneurons and nerve branches. Acta Anat (Basel). 1995;153:151–160 [DOI] [PubMed] [Google Scholar]
- 57.Buttner-Ennever JA, Eberhorn A, Horn AKE. Motor and sensory innervation of extraocular eye muscles. Ann N Y Acad Sci. 2003;1004:40–49 [DOI] [PubMed] [Google Scholar]
- 58.Buttner-Ennever JA, Horn AK. The neuroanatomical basis of oculomotor disorders: the dual motor control of extraocular muscles and its possible role in proprioception. Cur Opin Neurol. 2002;15:35–43 [DOI] [PubMed] [Google Scholar]
- 59.Buttner-Ennever JA, Konacki KZ, Blumer R. Sensory control of extraocular muscles. Prog Brain Res. 2006;151:81–93 [DOI] [PubMed] [Google Scholar]