Sequence variation in PROM1 should be considered in patients presenting with bull's eye maculopathy.
Abstract
Purpose.
To characterize in detail the phenotype of five unrelated families with autosomal dominant bull's eye maculopathy (BEM) due to the R373C mutation in the PROM1 gene.
Methods.
Forty-one individuals of five families of Caribbean (family A), British (families B, D, E), and Italian (family C) origin, segregating the R373C mutation in PROM1, were ascertained. Electrophysiological assessment, fundus autofluorescence (FAF) imaging, fundus fluorescein angiography (FFA), and optical coherence tomography (OCT) were performed in available subjects. Mutation screening of PROM1 was performed.
Results.
The R373C mutant was present heterozygously in all affected patients. The age at onset was variable and ranged between 9 and 58 years, with most of the individuals presenting with reading difficulties. Subjects commonly had a mild to moderate reduction in visual acuity except for members of family C who experienced markedly reduced central vision. The retinal phenotype was characterized by macular dystrophy, with retinal pigment epithelial mottling in younger subjects, progressing to typical BEM over time, with the development of macular atrophy in older patients. In addition, all members of family C had typical features of RP. The electrophysiological findings were variable both within and between families.
Conclusions.
Mutations in PROM1 have been described to cause a severe form of autosomal recessive RP in two families of Indian and Pakistani descent. The results of this study have demonstrated that a distinct redundant PROM1 mutation (R373C) can also produce an autosomal dominant, fully penetrant retinopathy, characterized by BEM with little inter- and intrafamilial variability, and retinal dystrophy with variable rod or rod–cone dysfunction and marked intra- and interfamilial variability, ranging from isolated maculopathy without generalized photoreceptor dysfunction to maculopathy associated with very severe rod–cone dysfunction.
Bull's eye maculopathy (BEM) is a distinctive macular phenotype characterized by annular retinal pigment epithelium (RPE) atrophy with central sparing of the fovea. BEM was initially described in association with chloroquine retinopathy in 1966 by Kearns and Hollenhorst.1 BEM has now also been associated with a heterogeneous group of inherited retinal disorders with both autosomal recessive and dominant inheritance patterns.2–5 This heterogeneity is illustrated by the electrophysiological findings in a large panel of patients with BEM. These findings were consistent with isolated macular dystrophy in 60% of cases, cone–rod or rod–cone dystrophy in 36% of cases, and isolated cone dystrophy in 4%.6 BEM has been reported in patients with various forms of retinitis pigmentosa (RP): (1) in syndromic RP such as Bardet-Biedl syndrome,7 (2) in nonsyndromic RP (e.g., RPGR-related RP),8 and (3) in RP with Stargardt-like maculopathy.9
A previous report has described the presence of disease-causing ABCA4 sequence variants in 35% of patients with BEM.10 The genetic heterogeneity of BEM was recently extended to the mutation R373C in PROM1.11 This was achieved by positional cloning after three large autosomal dominant pedigrees affected with bull's eye macular dystrophy (MCDR2),12 Stargardt-like disease (STGD4),13 and RP (Gaillard MC, et al. IOVS 2007;48:ARVO E-Abstract 3737) were all mapped to the short arm of chromosome 4.
In this study the clinical spectrum in carriers of the PROM1 p.R373C mutation is further explored in detail by revisiting the phenotypes of the original STGD4, MCDR2, and RP families (families A, B, and C, respectively) and by examining two newly ascertained families (D and E).
Methods
Patients and Clinical Assessment
The study adhered to the tenets of the Declaration of Helsinki (1983 Revision) and was approved by the local ethics committees. Informed consent was obtained from all subjects.
All available members of the five families had a complete clinical examination, including best corrected Snellen visual acuity (BCVA). In willing subjects, the assessment included Goldmann perimetry (Haag-Streit AG, Köniz, Switzerland) or standard automated perimetry (Humphrey Perimeter, Carl Zeiss Meditec, Dublin, CA); electrophysiological testing, including a full-field electroretinogram (ERG) and pattern ERG (PERG) incorporating the protocols recommended by the International Society for Clinical Electrophysiology of Vision (ISCEV)14,15; fundus fluorescein angiography (FFA); and fundus autofluorescence (FAF) imaging with a confocal scanning laser ophthalmoscope (cSLO; Heidelberg Retina Angiogram, HRA 2; Heidelberg Engineering, Heidelberg, Germany). Color vision testing was performed with Hardy-Rand-Rittler (HRR) plates (American Optical Company, New York, NY). Macular optical coherence tomography (OCT; Stratus OCT 3; Carl Zeiss Meditec Inc., Dublin, CA) was also performed in a significant proportion of patients. For analysis of retinal lamination abnormalities, raw scan data were exported from the OCT device for further analysis, and the light reflection profiles (LRPs) were calculated (IGOR Pro 6.03a; Wavemetrics Inc. Lake Oswego, OR).16–18 A 28-year-old normal subject was used as a control for the OCT data analysis.
Molecular Genetic Analysis
Genomic DNA was extracted from the peripheral blood of the family members by using a standard procedure. The p.R373C mutation had been identified in all affected subjects in family A (STGD4) and family B (MCDR2) after screening of the entire coding sequence and intron–exon junctions of the prominin-1 (PROM1) gene in the probands.11 Families D and E were selected for directed screening for the R373C PROM1 variant on the basis of clinical phenotype. In family C, a whole-genome linkage analysis was performed that identified a disease interval containing PROM1. The disease interval in family C was defined by markers D4S403 (LOD score 3.31 for θ = 0) and D4S419 (LOD score 3.51 for θ = 0). There were no other known or putative RP genes in the interval. Mutation screening of the entire coding sequence and intron–exon junctions of PROM1 was subsequently undertaken in the index case, as previously described.11 In brief, amplification in a thermal cycler (GeneAmp 9700; Applied Biosystems [ABI], Foster City, CA), was performed in a total volume of 20 μL. Each polymerase chain reaction (PCR) contained 100 ng genomic DNA, 1 μM of each primer, and 10 μL master mix 2× (Qiagen, Valencia, CA), with or without betaine. PCR-amplified products were screened for mutations by using denaturing high-performance liquid chromatography (DHPLC; WAVE system; Transgenomics, Crewe, UK). PCR fragments displaying DHPLC abnormal retention times were directly sequenced on both strands by using dye termination chemistry (BigDye Terminator ver. 3.1; ABI), in a final reaction volume of 10 μL, and electrophoresed on a genetic analyzer (Prism 3100; ABI). Sequences were aligned by computer (Chromas 2.23 software; Technelysium, Tewantin, QLD, Australia), with the reference genomic sequence provided by the Retina International Mutation Database (www.retina-international.org/ provided in the public domain by a consortium of societies for eye diseases). PROM1 was the only gene screened during this study.
Results
Molecular Genetic Analysis
Screening of PROM1 revealed a C-to-T transition at position 1117 (c.1117C>T) of the coding sequence (NM_006017.1), which encodes a p.R373C substitution. This mutation segregated heterozygously in all affected individuals from the five families and was absent in the unaffected family members. In family C, of the 20 individuals molecularly screened, 8 affected subjects had the mutation, and 3 spouses and 9 first- or second-degree unaffected relatives did not harbor the mutation. In another study, the R373C variant was not identified in 400 normal control subjects (800 alleles; unrelated healthy individuals of various ethnic backgrounds),11 and in the present study a further 100 control subjects (200 alleles; white western European origin) were screened and found not to harbor this mutation. The following four sequence variants were also identified during the screening of PROM1 in family C: c.IVS2–6 C>T, c.IVS13+47 T>C, c.IVS22+5 G>C, and c.IVS23+4 A>G. These are not believed to be disease-associated.
Clinical Features
To date, the patients reported herein have exhibited only an ocular phenotype.
Family A (STGD4).
The clinical findings in 14 affected subjects from this four-generation Caribbean family (Fig. 1A) are summarized in Table 1.
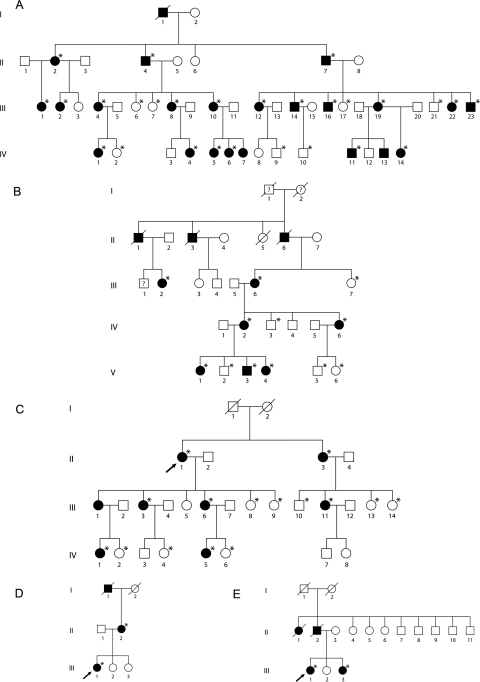
Figure 1.
Pedigrees of families A to E. (*) Subjects screened for the R373C substitution. All affected patients were found to harbor the R373C PROM1 mutation. No unaffected patients were found to have the R373C PROM1 mutation.
Table 1.
Summary of Findings
| Family/Patient | Sex | Age (y) | Symptoms | Presenting Visual Acuity OD-OS | Current Visual Acuity OD-OS | Fundus | AF Imaging | ERG | PERG | Visual Fields | Color Vision |
|---|---|---|---|---|---|---|---|---|---|---|---|
| AII:2 | F | 47 | Reduced VA (20 y) | 6/60–6/60 | NP | Beaten metal appearance, macular RPE atrophy, flecks | NP | NP | NP | NP | Moderate generalized dyschromatopsia |
| AII:4 | M | 52 | Reduced VA (17 y) | 6/120–6/120 | NP | Macular RPE atrophy and hyperpigmentation | NP | NP | NP | NP | Moderate generalized dyschromatopsia |
| AII:7 | M | 57 | Reduced VA (18 y) No driving at night (32 y) | CF-CF | NP | Extensive macular RPE atrophy and pigmentation | NP | NP | NP | NP | Moderate generalized dyschromatopsia |
| AIII:1 | F | 15 | Reduced VA (10 y) | 6/60–6/60 | NP | BEM, beaten metal appearance | NP | NP | NP | NP | Moderate generalized dyschromatopsia |
| AIII:2 | F | 30 | Asymptomatic | 6/6–6/7.5 | NP | Mild foveal pigmentary change | NP | NP | NP | NP | Moderate generalized dyschromatopsia |
| AIII:4 | F | 22 | Asymptomatic | 6/9–6/9 | NP | BEM | NP | NP | NP | NP | Moderate generalized dyschromatopsia |
| AIII:8 | F | 30 | Reduced VA (24 y) | 6/24–6/24 | NP | RPE hyperpigmentation and macular atrophy | NP | NP | NP | NP | Moderate generalized dyschromatopsia |
| AIII:10 | F | 31 | Glaucoma (10–20 y); congenital cataract OU | 6/120–6/45 | NP | BEM | NP | NP | NP | NP | Moderate generalized dyschromatopsia |
| AIII:12 | F | 35 | Reduced VA (23 y) | 6/18–6/60 | NP | BEM, macular RPE atrophy | NP | NP | NP | NP | Moderate generalized dyschromatopsia |
| AIII:14 | M | 34 | Reduced color vision (25 y) Reduced VA (29 y) | 6/48–6/48 | NP | BEM, macular RPE atrophic lesions, flecks | NP | Scotopic amplitude reduced with normal implicit time OU | NP | Central scotoma with normal peripheral field OU | Moderate generalized dyschromatopsia |
| AIII:19 | F | 32 | Reduced VA (15 y) | 6/120–6/15 | NP | BEM | NP | NP | NP | NP | NP |
| AIII:22 | F | 23 | Reduced VA (15 y) | NP | NP | BEM, flecks | NP | Mildly reduced flicker ERG amplitude with normal implicit time, normal scotopic ERG OU | NP | NP | Severe generalized dyschromatopsia |
| AIII:23 | M | 13 | Asymptomatic | 6/6–6/6 | NP | Mild RPE changes | NP | NP | NP | NP | Moderate generalized dyschromatopsia |
| AIV:1 | F | 5 | Asymptomatic | 6/6–6/6 | NP | Normal | NP | NP | NP | NP | Mild generalized dyschromatopsia |
| BIII:2 | F | 69 | Reading difficulties (24 y) Central visual field loss (24 y) |
6/4–6/4 (24 y) | 6/24–6/36 | Bilateral macular granular pigmentation (24 y) Bilateral BEM (69 y) | NP | Mildly reduced rod responses, borderline cone responses | Undetectable | Bilateral central scotomate (24 y) | Moderate generalized dyschromatopsia |
| BIII:6 | F | 67 | Reading difficulties (12 y) Photophobia (12 y) |
6/6–6/6 (12 y) | 6/18–6/9 | Bilateral BEM with a well-demarcated area of RPE atrophy at the right macula (67 y) | Bilateral “bull's eye' lesions (OS>OD): ring of decreased perifoveal AF bordered centrally > peripherally by increased AF. Area of reduced AF in OD corresponding to atrophy. | Mildly reduced rod responses | Undetectable | Bilateral central scotomata (67 y) | Mild generalized dyschromatopsia |
| BIV:2 | F | 47 | Glare at night (41 y) | 6/5–6/5 (41 y) | 6/6–6/6 | Bilateral BEM | Bilateral “bull's eye' appearance | N | Undetectable | Bilateral central scotomata (41 y) | Mild generalized dyschromatopsia |
| BIV:6 | F | 43 | Photophobia (37 y) Glare at night (37 y) | 6/5–6/5 (37 y) | 6/5–6/5 | Bilateral mild macular RPE mottling | Bilateral perifoveal ring of mildly increased AF | N | Reduced OD > OS | Normal | N |
| BV:1 Proband | F | 23 | Reading difficulties (12 y) Metamorphopsia (12 y) |
6/9–6/6 (13 y) | 6/36–6/36 | Bilateral macular RPE mottling | Bilateral perifoveal ring of increased AF | N | Reduced | Reduced central sensitivity | Moderate generalized dyschromatopsia |
| BV:3 | M | 15 | Reading difficulties (9 y) | 6/6–6/6 (9 y) | 6/9–6/9 | Bilateral prominent foveal reflex, and a red speckled appearance at the level of the RPE | Bilateral perifoveal ring of increased AF | N | NP | NP | Mild protan and deutan defect |
| BV:4 | F | 20 | Impaired color vision (14 y) | 6/5–6/5 (14 y) | 6/6–6/5 | Bilateral prominent foveal reflex, and a red speckled appearance at the level of the RPE | Bilateral perifoveal ring of mildly increased AF | N | N | NP | Mild generalized dyschromatopsia |
| CII:1 | F | 60 | Subcapsular cataract (50 y) Night blindness (58 y) |
6/6–6/6 (39 y) | 6/7.5–6/7.5 (50 y) 6/12–6/15 (58 y) |
BEM (50 y) Macular atrophy OS (58 y) OD (62 y) Peripheral pigment (55 y) Attenuated arteries (58 y) (Fig 3) |
NP | Undetectable rod and cone responses (55 y) | NP | OD 60° OS 50° (58 y) |
NP |
| CIII:1 | F | 58 | Photopsia (35 y) Reading difficulties (45 y) Night blindness (46 y) Visual field loss (58 y) |
6/6–6/6 (19 y) | HM-6/6 (46 y) <6/120–<6/120 (56 y) | BEM (46 y) Macular atrophy (56 y) Peripheral pigment (46 y) Attenuated arteries (58 y) Pale disc (58 y) |
Macular ring (Figure 3) | Moderately reduced rod responses, mildly reduced cone responses (46 y) | NP | Normal (46 y) OD Complete Loss OS 50° (58 y) |
NP |
| CIII:3 | F | 58 | Reading difficulties (36 y) Photophobia (48 y) Visual field loss (48 y) Night blindness (52 y) Photopsia (52 y) |
6/4.8–6/4.8 (34 y) | 6/4.8–6/4.8 (34 y) 6/15–6/12 (52 y) |
BEM (34 y) Mecular atrophy (52 y) Salt and pepper dystrophy (50 y) Attenuated arteries (50 y) Pale disc (56 y) |
Mottled | Undetectable rod responses, severely reduced cone responses (56 y) | NP | Normal (34 y) OU 50° (56 y) |
NP |
| CIII:6 | F | 58 | Reading difficulties (16 y) Night blindness (30 y) Subcapsular cataract (42 y) |
6/4.8–6/6 (16 y) | 6/19–6/19 (28 y) 6/60–6/30 (42 y) |
BEM (28 y) Macular atrophy (42 y) Peripheral pigment (28 y) Attenuated arteries (28 y) Pale disc (28 y) (Fig. 3) |
Mottled (Fig 3) | Moderately reduced rod responses, mildly reduced cone responses (35 y) | NP | Normal (28 y) OD 90° OS 75° (30 y) OD Complete loss OS 2–3° (58 y) |
NP |
| CIV:1 | F | 41 | Reading difficulties (24 y) | 6/6–6/15 (24 y) | 6/6–6/15 (24 y) 6/60–6/60 (39 y) |
BEM (24 y) Macular atrophy (39 y) Attenuated arteries (39 y) Pale disc (41 y) |
Macular ring | Moderately reduced rod responses, mildly reduced cone responses (39 y) | NP | Bilateral central scolomata (39 y) | NP |
| CIV:5 | F | 31 | Reading difficulties (14 y) Photophobia (29 y) Subcapsular cataract (31 y) |
6/7.5–6/4.8 (7 y) | 6/7.5–6/4.8 (7 y) 6/120–6/60 (26 y) |
BEM (7 y) Macular atrophy (26 y) Attenuated arteries (29 y) Pale disc (29 y) |
Mottled | Undetectable rod and cone responses (29 y) | NP | Normal (10 y) OU 30° (29 y) |
NP |
| DII:2 | F | 65 | Reading difficulties (40 y) | 6/60–6/60 (64 y) | 6/60–6/60 | Bilateral macular atrophy and pigmentation (64 y) (Fig. 4) | Bilateral reduced macular AF corresponding to atrophy, surrounded by high intensity AF rings (Fig. 4) | Mildly reduced rod responses and borderline cone responses | Markedly reduced | Bilateral central scotomata (64 y) | NP |
| DIII:1 Proband | F | 32 | Central scotomata (29 y) Right amblyopia |
6/18–6/9 (29 y) | 6/18–6/12 | Bilateral BEM and optic nerve head drusen (29 y) (Fig. 4) | Bilateral perifoveal rings of increased AF surrounding mildly reduced foveal AF (Fig. 4) | N | Undetectable | Bilateral central scotomata (29 y) | NP |
| EIII:1 Proband | F | 44 | Photophobia (since childhood) | 6/5–6/5 (34 y) | 6/6–6/6 | Bilateral BEM (34 y) | Bilateral “bull's eye' lesions: ring of decreased perifoveal AF bordered peripherally > centrally by annuli of increased AF | N (35 y) | Markedly reduced | Bilateral central scotomata (34 y) | Mild generalized dyschromatopsia |
| EIII:3 | F | 54 | Reading difficulties (44 y) Reduced color vision (44 y) |
6/9–6/5 (44 y) | 6/12–6/6 | Bilateral BEM and optic nerve head drusen (44 y) | Speckled appearance with areas of increased and decreased AF | N (44 y) | Markedly reduced | Bilateral central scotomata (44 y) | Mild gene ralized dyschromatopsia |
NP, not performed; N, normal.
The commonest presenting symptom was decreased visual acuity, reported by nine patients at a mean age of 19 years (range, 10–29 years). Visual acuity ranged from 6/6 to counting fingers. Sixteen subjects had evidence of macular degeneration, including macular RPE alterations (five patients), BEM associated with various degrees of RPE atrophy (seven patients), and retinal flecks (two patients). FFA was performed in five patients, revealing a dark choroid in one case and hyperfluorescent macular lesions in four other subjects. Full-field ERGs revealed evidence of generalized rod photoreceptor dysfunction in III:14.
Family B (MCDR2).
The clinical findings in seven affected subjects from this five-generation nonconsanguineous British family with autosomal dominant BEM (Fig. 1B) are summarized in Table 1.
Most patients became symptomatic in the first to third decade, with reduced central vision being the commonest complaint. The majority of patients had normal or mildly reduced visual acuity. The most severely affected subjects were BIII:2 (69 years) and BV:1 (23 years), both with visual acuity of 6/36 in at least one eye. The early macular abnormalities included an increased foveal reflex and a red-speckled macular appearance, progressing to a more classic BEM. FFA was performed in two patients (BV:1 and BIV:2) and revealed localized masking of choroidal fluorescence in the perifoveal area. A dark choroid was not seen in either patient.
Full-field ERGs were normal in five patients; the two oldest subjects exhibited mild generalized retinal dysfunction, with mildly reduced rod photoreceptor function and borderline cone responses in BIII:2 and isolated mildly reduced rod photoreceptor responses in BIII:6. Three patients (BIII:6, BIV:2, and BV:1) had repeat electrophysiological assessments 7 years after initial testing. The amplitude of the PERG had reduced further in the proband, BV:1. Full-field ERGs remained normal in BV:1 and BIV:2, but there had been a progressive reduction in rod-mediated responses in III:6, with cone responses remaining within normal limits. The electrophysiological data were consistent with isolated macular dysfunction in younger patients and suggested a very slow deterioration of generalized rod photoreceptor function, with sparing of cone responses, even in later life.
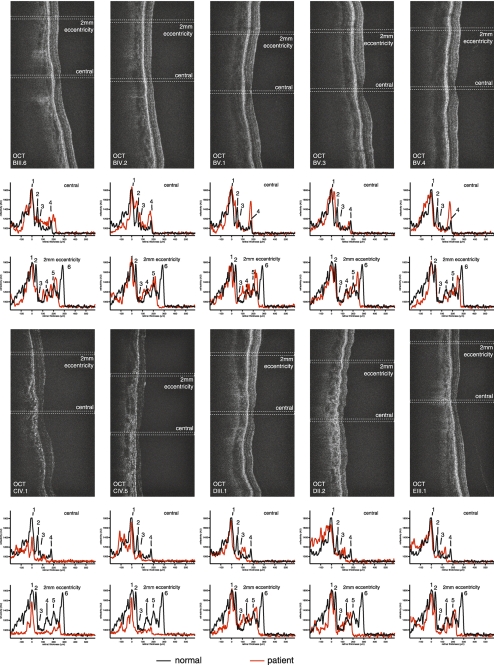
OCT was performed in five patients (BIII:6, BIV:2, BV:1, BV:3, and BV:4) and revealed a reduction in total central retinal thickness (Fig. 2). Shortening of photoreceptor outer segments, reduction of the outer nuclear layer, and a reduction in the reflectivity of the photoreceptor cell layer were present in all scans, but were more pronounced centrally (Fig. 2). The inner retinal layers were less affected than the outer retinal layers, with significant thinning of the inner plexiform and inner nuclear layer, whereas the thickness of the ganglion cell layer was only mildly reduced. Patients BV:3 and BV:4 showed outer retinal LRPs very similar to those in control subjects, whereas the inner retinal LRPs seem to be more altered. This observation was made in these two individuals only.
Figure 2.
OCT cross sections and calculated LRPs from the foveolar region (central) and the peripheral macular tissue (2 mm eccentricity) are shown below the OCT cross sections for members of families B, C, D, and E. 1, retinal pigment epithelium; 2, ellipsoid of the photoreceptors; 3, external limiting membrane; 4, outer plexiform layer; 5, inner plexiform layer; and 6, nerve fiber layer. Whereas in normal control subjects (black line) the reflectivity peaks for the ellipsoid region of the photoreceptors (2) and the external limiting membrane (3) could easily be detected, this signal was attenuated in all patients. Furthermore, in all individuals, both the foveal region and peripheral macular tissue were affected, with the outer retina (outer nuclear layer, ellipsoid) showing more pronounced signs of degeneration than the inner retinal tissue. In the peripheral macular tissue (2 mm from the center of the fovea) the damage was less pronounced than at the fovea with a preservation of lamination and well-preserved signals from the inner retina. There was some variation regarding the more peripheral changes among the different individuals, but still, the pattern of changes was very similar.
Family C.
The clinical findings in six affected subjects from this four-generation nonconsanguineous Italian family with autosomal dominant retinitis pigmentosa (adRP) (Fig. 1C) are summarized in Table 1.
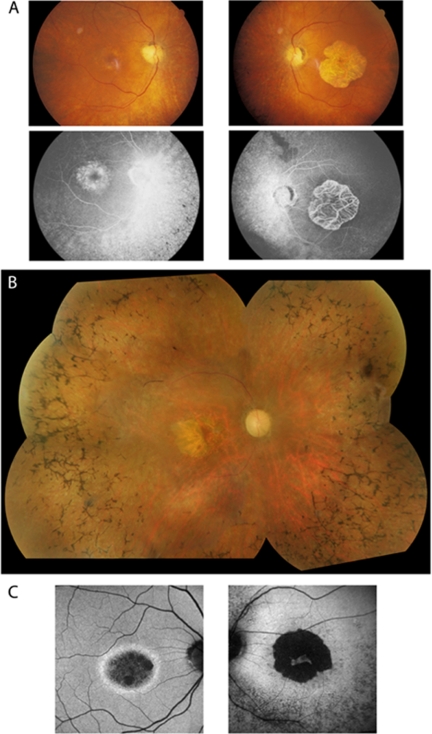
The age at onset was variable, ranging from 14 to 58 years. Reduced central vision was the most common presenting symptom, with night blindness being increasingly noticed over time. BCVA ranged from 6/4.8 to hand motion. Fundus examination revealed BEM in all patients at a mean age of 34 years (range, 7–54), which progressed over time to macular atrophy (Fig. 3). A variable combination of the triad of typical retinal features associated with RP was observed (Table 1, Fig. 3). FFA was performed in all six patients and failed to reveal any evidence of a dark choroid.
Figure 3.
(A) Fundus and fluorescein angiography showing asymmetrical macular changes in this 60-year-old patient (CII:1) with bull's eye maculopathy in the right eye and geographic atrophy in the left eye. (B) Fundus photograph showing bone spicule pigmentation throughout the peripheral retina and retinal vessel attenuation in patient CIII:6 (58 years old). (C) AF imaging revealing two distinct phenotypes: (left) a ring of high-density AF, with normal background AF of the retina inside the vascular arcade (patient CIII:1; 58 years old) and (right) a hypo- and hyperfluorescent pattern at the macula and up to the arcades (patient CIII:6; 58 years old).
Full-field ERG recordings were consistent with a rod–cone dystrophy in four patients, and rod- and cone-driven responses were undetectable in the remaining two patients.
OCT imaging showed a significant overall decrease in retinal thickness and marked changes in the outer retina, similar to those in family B (Fig. 2). Analysis of retinal lamination and calculation of LRP was performed in the two youngest patients (CIV:1 and CIV:5). In individual CIV:1, with nonconfluent macular atrophy, central and peripheral macular (2 mm eccentricity) retinal thicknesses were reduced to 106 ± 8 and 254 ± 12 μm, respectively (Fig. 2). Loss of retinal thickness was mainly caused by a loss of photoreceptor outer segments and thinning of the outer nuclear layer (Fig. 2), whereas the structure of the inner retina, especially the nerve fiber layer, showed fewer signs of atrophy. In CIV:5, with marked macular atrophy, there was evidence of more pronounced changes: central retinal thickness was reduced to 76 ± 6 μm and peripheral macular thickness to 170 ± 14 μm. This thinning was again mainly due to loss of outer retinal structures, although in this subject there was also evidence of significant loss of inner retinal layers (Fig. 2), including the nerve fiber layer.
Family D.
The clinical findings in two affected subjects from this three-generation nonconsanguineous British family (Fig. 1D) with autosomal dominant BEM are summarized in Table 1.
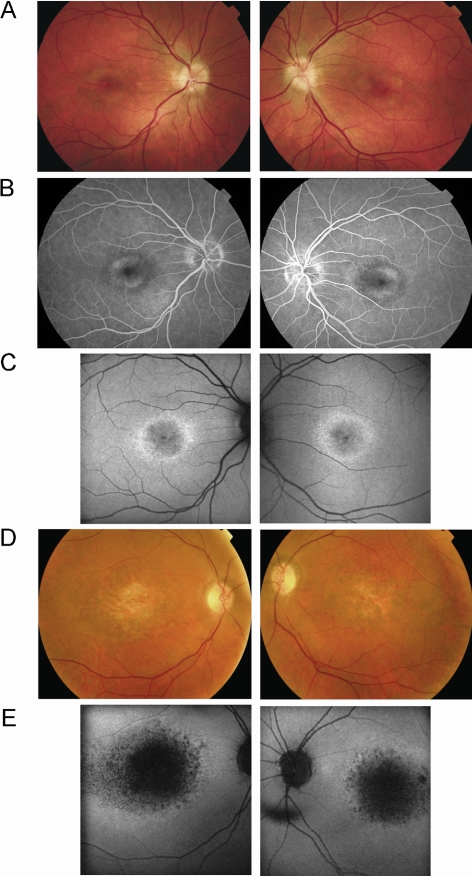
The proband (DIII:1) noted central scotomata in the third decade, and her mother (DII:2) was aware of reading difficulties in the fourth decade. The proband was mildly affected with visual acuity of 6/18 in the right eye (amblyopia) and 6/12 in the left, with her mother being severely affected with 6/60 vision in both eyes and describing a very slow deterioration over a period of two decades. The proband had bilateral BEM and optic nerve head drusen, whereas the mother had bilateral macular atrophy and areas of hyperpigmentation (Fig. 4). The proband underwent FFA, which revealed window defects at the macula consistent with BEM (Fig. 4). There was no evidence of a dark choroid.
Figure 4.
(A) Patient DIII:1 (32 years old): fundus photographs showing bilateral bull's-eye maculopathy and optic nerve head drusen. (B) Patient DIII:1: fundus fluorescein angiography showing bilateral perifoveal window defects corresponding to the retinal pigment epithelial changes seen ophthalmoscopically. (C) Patient DIII:1: fundus AF imaging revealing bilateral rings of increased perifoveal AF surrounding mildly reduced foveal AF. (D) Patient DII:2 (65 years old): fundus photographs showing bilateral macular atrophy and pigmentation. (E) Patient DII:2: fundus AF imaging showing bilateral markedly reduced macular AF corresponding to atrophy seen clinically, surrounded by high-intensity AF rings.
Full-field ERGs were normal in the proband, with mildly reduced rod photoreceptor function and borderline cone responses in her mother, suggesting gradual deterioration in generalized retinal function over time.
OCT imaging of patient DII:2 showed a generalized atrophy of the neuroretinal tissue and the underlying RPE, with more pronounced changes in the central macula (Fig. 2). There seemed to be less evidence of retinal degeneration on OCT imaging of the proband (DIII.1). However, detailed analysis revealed a significant loss of reflectivity in the central macula area with respect to outer retinal structures. This loss was most evident when looking at the thinned retina and at the loss of the signal of the photoreceptor ellipsoid region, associated with photoreceptor loss (peak 2, Fig. 2). The more peripheral macular tissue appeared to be less affected, with the retinal layering being relatively well preserved, although a reduction in the outer nuclear layer thickness and loss of signal in the ellipsoid region were noted (Fig. 2). The inner retina was far less affected than the outer retinal tissue, in keeping with all the other tested families.
Family E.
The clinical findings in two affected subjects from this three-generation nonconsanguineous British family (Fig. 1E) with autosomal dominant BEM are summarized in Table 1.
The proband reported having photophobia since childhood, and her sister became aware of reading difficulties and reduced color vision in the fifth decade. The proband was mildly affected with visual acuity of 6/6 in both eyes, but her sister was more severely affected, with 6/12 in her right eye and 6/6 in her left. Both sisters had bilateral BEM on ophthalmoscopy, with the proband's sister also having optic nerve head drusen.
Full-field ERGs were normal in the sister (EIII:3). Full-field ERGs were normal in the proband (EIII:1), when tested at age 35 years, but on repeat testing at age 42 years there was evidence of mildly reduced rod and cone photoreceptor function, suggesting gradual mild generalized retinal dysfunction over time, in keeping with the other two British families (B and D).
OCT was performed in patient EIII:1 and revealed findings very similar to those obtained in subject DIII:1 (Fig. 2). Reduced foveolar retinal thickness was observed, with loss of the highly reflective signal originating from the outer retina (Fig. 2), consistent with outer retinal disease. The more peripheral macular region appeared to be better preserved than was the central macula, although there was also evidence of a reduction of peripheral macular thickness, mainly due to changes in the outer retina (reduced outer nuclear layer thickness and outer segment shortening), in keeping with the changes observed in subject DIII:1 (Fig. 2). However, in contrast to patient DIII:1, patient EIII:1 also had more pronounced inner retinal disease, with a notable reduction of the nerve fiber layer (Fig. 2).
Discussion
In this study, we investigated in detail the PROM1 p.R373C-related phenotype in five unrelated families originating from the Caribbean, United Kingdom, and Italy. The mode of inheritance was found to be autosomal dominant with complete penetrance. Variable expressivity was observed both between and within families, with marked interocular asymmetry in members of family C (RP phenotype). The patients, predominantly females (sex ratio, 0.41), first reported reading difficulties between the first and third decades of life. The rate of progression in most of the subjects was slow, with good visual acuity being usually maintained until the sixth decade. However, in the family with RP (family C), a rapid and severe loss of central vision and the development of night blindness and marked visual field constriction were typically seen.
Affected individuals were most consistently found to have BEM. The spectrum of macular phenotypes varied from mild RPE changes to geographic atrophy, as the incipient and end-stage manifestations of the disease respectively. In family C, the characteristic macular phenotype was associated with features of typical RP: bone spicule pigmentary deposits, vascular narrowing, and pallor of the optic nerve head. In this family, full-field ERGs confirmed the rod–cone nature of the dystrophy. In the other four families, the ERG was either normal (n = 8) or displayed evidence of mild rod photoreceptor dysfunction, especially in the older patients (n = 6), whereas cone responses were normal (n = 3), borderline (n = 2), or mildly reduced (n = 1). These electrophysiological findings suggest that rod photoreceptors may be more susceptible to the deleterious effects of the PROM1 p.R373C mutation than are peripheral cone cells. Interestingly, the patients with isolated generalized rod dysfunction did not have any ophthalmoscopic features of RP. Electrophysiological findings were variable both between and within families, ranging from isolated macular dysfunction to a generalized reduction in rod and cone responses. Environmental or other genetic modifying factors, including sequence variation in PRPH2 and ROM1, may determine the extent of retinal dysfunction and phenotypic heterogeneity observed in this study.
There was both an inter- and intrafamilial variability with respect to the OCT findings. Retinal degeneration was mildest in family B (MCDR2), compared with the other three families, with all members of family B having evidence of outer retinal damage that was more pronounced in the central macula. The inner retinal layers seemed to be less affected in this family, showing a greater change in the central macula than in the peripheral macular tissue. In the members of family C, degenerative changes were again present in the outer retina, albeit to a greater degree than in family B. Although the extent of degeneration, with additional significant involvement of inner retinal tissue, was much greater than in family B, the pattern was very similar. Findings in family D were in keeping with those recorded in family C. Overall, the pattern of degeneration was similar in families B, C, and D, with more pronounced atrophy in the central macula and a greater involvement of the outer retina. In contrast, in family E, there was evidence that inner retinal atrophy (including the nerve fiber layer) was more pronounced than outer retinal degeneration; however, only one family member was available for OCT imaging. Although, it is of note that electrophysiological testing did not reveal evidence of inner retinal dysfunction in family E.
AF imaging identified two macular phenotypes with respect to the presence (11/15) or absence (4/15) of a macular ring of increased AF. The high-density ring of AF is due to an accumulation of lipofuscin, which probably reflects the inability of the RPE to process photoreceptor outer segments debris or alternatively may represent increased outer segment turnover. It has been demonstrated histologically that the number of photoreceptor cells is reduced in the presence of increased quantities of lipofuscin in the RPE, leading to the proposal that autofluorescent material may accumulate before cell death. The ring of increased AF has been proposed to represent the border between functional and dysfunctional retina and is well documented in RP patients with preserved central vision and preserved macular AF.19,20
Remarkably, the patients reported to date exhibited only an ocular phenotype, despite the ubiquitous prominin-1 expression in plasma membrane protrusions (Table 2). A possible explanation of this restricted phenotype is an absence of prominin-2 expression in the eye.21,22 By functional redundancy, this closely related protein may compensate for the absence of prominin-1 in other tissues.22 In photoreceptors, prominin-1 is concentrated in the plasma evaginations at the base of the outer segment.22,23 The essential structural role of prominin-1 in outer segment morphogenesis and photoreceptor disc genesis has been demonstrated in mice with a targeted disruption of prominin-1 (prom1−/−).22 In this animal model, both cone and rod outer segments are highly disorganized as early as P12.22
Table 2.
Prominin 1 Mutations Reported to Date
| Nucleotide Change | Amino Acid Change | Inheritance | Disease | OMIM | Reference |
|---|---|---|---|---|---|
| c.1117C>T | p.R373C | Dominant | STGD4 | 603786 | Family A11,13 |
| MCDR2 | 608051 | Family B11,12 | |||
| RP | Family C (Gaillard MC, et al. IOVS 2007;48:ARVO E-Abstract 3737) | ||||
| c.1726C>T | p.Q576X | Recessive | RP41 | 612095 | 24 |
| c.1841delG | p.G614fsX626 | Recessive | RP41 | 612095 | 23 |
| c.1349insT | p.Y452fsX12 | Recessive | CORD | 25 |
The mutation nomenclature uses the first nucleotide of the ATG codon in the RefSeq cDNA NM_006017.1 as +1. (MCDR2, macular dystrophy retinal 2; i.e., autosomal dominant bull's eye macular dystrophy; STGD4, Stargardt-like disease 4, i.e., autosomal dominant bull's eye macular dystrophy; RP41, retinitis pigmentosa 41; i.e., severe retinitis pigmentosa with macular degeneration; CORD, cone-rod dystrophy.)
The three previously reported prominin-1 nonsense mutations are recessively inherited, whereas the p.R373C missense mutation acts in a dominant manner (Table 2). It is likely that the p.G614fsX626, p.Q576X, and p.Y452fsX12 mutations would be subject to nonsense-mediated mRNA decay and that the recessively inherited retinal degenerations are due to the complete absence of prominin-1.23–25 The p.R373C missense mutation introduces an additional cysteine (Cys) residue in the predicted first extracellular loop of prominin-1. It is of note that prominin-1 contains multiple evolutionarily conserved Cys residues that are also conserved in prominin-2.21 It is tempting to speculate that the introduction of an additional Cys residue disrupts a network of disulfide bridges and, in turn, impairs homophilic protein interactions, causing a dominant phenotype. In Drosophila compound eyes, prominin-1 deficiency converts the rhabdoms from an open to a closed configuration, indicating evolutionarily conserved structural roles of prominin-1 in photoreceptor formation and maintenance.26 A comparable phenotype is observed in Drosophila compound eyes deficient in the agrin-perlecan related proteoglycan Eys (Eyes shut)/spam (spacemaker).26,27 Recently, patients affected by autosomal recessive RP carrying mutations in EYS have been described.28 Whether differential expression levels of EYS modify prominin-1-associated retinal dystrophies remains to be determined.
In conclusion, we have identified a range of disease phenotypes in families with the p.R373C mutation in PROM1, that include isolated macular dysfunction, pure rod, and rod–cone dystrophy. The phenotypic hallmark to date of the p.R373C mutation is BEM. This consistent finding suggests that sequence variation in PROM1 should be considered in patients presenting with BEM, particularly when accompanied by a dominant family history.
Footnotes
Supported by Grant 32-111948/1 from the Swiss National Science Foundation (FLM, DFS), and by grants from Moorfields Special Trustees, the British Retinitis Pigmentosa Society, the Guide Dogs for the Blind Association, the National Institute for Health Research UK to the Biomedical Research Centre for Ophthalmology based at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology, the European Commission (EVI-Genoret), and Fight for Sight (USA) (MM, ARW, DMH, ATM). KZ is supported by grants from NEI/NIH, a VA Merit Award, Foundation Fighting Blindness, the Macula Vision Research Foundation, the Ruth and Milton Steinbach Fund, Research to Prevent Blindness, and a BWF Clinical Scientist Award in Translational Research.
Disclosure: M. Michaelides, None; M.-C. Gaillard, None; P. Escher, None; L. Tiab, None; M. Bedell, None; F.-X. Borruat, None; D. Barthelmes, None; R. Carmona, None; K. Zhang, None; E. White, None; M. McClements, None; A.G. Robson, None; G.E. Holder, None; K. Bradshaw, None; D.M. Hunt, None; A.R. Webster, None; A.T. Moore, None; D.F. Schorderet, None; F.L. Munier, None
References
- 1.Kearns TP, Hollenhorst RW. Chloroquine retinopathy: evaluation by fluorescein fundus angiography. Arch Ophthalmol. 1966;76:378–384 [DOI] [PubMed] [Google Scholar]
- 2.Deutman AF. Benign concentric annular macular dystrophy. Am J Ophthalmol. 1974;78:384–396 [DOI] [PubMed] [Google Scholar]
- 3.Fishman GA, Fishman M, Maggiano J. Macular lesions associated with retinitis pigmentosa. Arch Ophthalmol. 1977;95:798–803 [DOI] [PubMed] [Google Scholar]
- 4.Michaelides M, Hardcastle AJ, Hunt DM, Moore AT. Progressive cone and cone-rod dystrophies: phenotypes and underlying molecular genetic basis. Surv Ophthalmol. 2006;51:232–258 [DOI] [PubMed] [Google Scholar]
- 5.O'Donnell FE, Jr, Welch RB. Fenestrated sheen macular dystrophy: a new autosomal dominant maculopathy. Arch Ophthalmol. 1979;97:1292–1296 [DOI] [PubMed] [Google Scholar]
- 6.Kurz-Levin MM, Halfyard AS, Bunce C, et al. Clinical variations in assessment of bull's-eye maculopathy. Arch Ophthalmol. 2002;120:567–575 [DOI] [PubMed] [Google Scholar]
- 7.Campo RV, Aaberg TM. Ocular and systemic manifestations of the Bardet-Biedl syndrome. Am J Ophthalmol. 1982;94:750–756 [DOI] [PubMed] [Google Scholar]
- 8.Koenekoop RK, Loyer M, Hand CK, et al. Novel RPGR mutations with distinct retinitis pigmentosa phenotypes in French-Canadian families. Am J Ophthalmol. 2003;136:678–687 [DOI] [PubMed] [Google Scholar]
- 9.Suzuki R, Hirose T. Bull's-eye macular dystrophy associated with peripheral involvement. Ophthalmologica. 1998;212:260–267 [DOI] [PubMed] [Google Scholar]
- 10.Michaelides M, Chen LL, Brantley MA, Jr, et al. ABCA4 mutations and discordant ABCA4 alleles in patients and siblings with bull's-eye maculopathy. Br J Ophthalmol. 2007;91:1650–1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Z, Chen Y, Lillo C, et al. Mutant prominin 1 found in patients with macular degeneration disrupts photoreceptor disk morphogenesis in mice. J Clin Invest. 2008;118:2908–2916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michaelides M, Johnson S, Poulson A, et al. An autosomal dominant bull's-eye macular dystrophy (MCDR2) that maps to the short arm of chromosome 4. Invest Ophthalmol Vis Sci. 2003;44:1657–1662 [DOI] [PubMed] [Google Scholar]
- 13.Kniazeva M, Chiang MF, Morgan B, et al. A new locus for autosomal dominant Stargardt-like disease maps to chromosome 4. Am J Hum Genet. 1999;64:1394–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holder GE, Brigell MG, Hawlina M, et al. ISCEV standard for clinical pattern electroretinography: 2007 update. Doc Ophthalmol. 2007;114:111–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marmor MF, Fulton AB, Holder GE, et al. Standard for clinical electroretinography (2008 update). Doc Ophthalmol. 2009;118:69–77 [DOI] [PubMed] [Google Scholar]
- 16.Barthelmes D, Sutter FK, Kurz-Levin MM, et al. Quantitative analysis of OCT characteristics in patients with achromatopsia and blue-cone monochromatism. Invest Ophthalmol Vis Sci. 2006;47:1161–1166 [DOI] [PubMed] [Google Scholar]
- 17.Barthelmes D, Gillies MC, Sutter FK. Quantitative OCT analysis of idiopathic perifoveal telangiectasia. Invest Ophthalmol Vis Sci. 2008;49:2156–2162 [DOI] [PubMed] [Google Scholar]
- 18.Fischer MD, Fleischhauer JC, Gillies MC, et al. A new method to monitor visual field defects caused by photoreceptor degeneration by quantitative optical coherence tomography. Invest Ophthalmol Vis Sci. 2008;49:3617–3621 [DOI] [PubMed] [Google Scholar]
- 19.Popovic P, Jarc-Vidmar M, Hawlina M. Abnormal fundus autofluorescence in relation to retinal function in patients with retinitis pigmentosa. Graefes Arch Clin Exp Ophthalmol. 2005;243:1018–1027 [DOI] [PubMed] [Google Scholar]
- 20.Robson AG, Saihan Z, Jenkins SA, et al. Functional characterisation and serial imaging of abnormal fundus autofluorescence in patients with retinitis pigmentosa and normal visual acuity. Br J Ophthalmol. 2006;90:472–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fargeas CA, Florek M, Huttner WB, Corbeil D. Characterization of prominin-2, a new member of the prominin family of pentaspan membrane glycoproteins. J Biol Chem. 2003;278:8586–8596 [DOI] [PubMed] [Google Scholar]
- 22.Zacchigna S, Oh H, Wilsch-Brauninger M, et al. Loss of the cholesterol-binding protein prominin-1/CD133 causes disk dysmorphogenesis and photoreceptor degeneration. J Neurosci. 2009;29:2297–2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maw MA, Corbeil D, Koch J, et al. A frameshift mutation in prominin (mouse)-like 1 causes human retinal degeneration. Hum Mol Genet. 2000;9:27–34 [DOI] [PubMed] [Google Scholar]
- 24.Zhang Q, Zulfiqar F, Xiao X, et al. Severe retinitis pigmentosa mapped to 4p15 and associated with a novel mutation in the PROM1 gene. Hum Genet. 2007;122:293–299 [DOI] [PubMed] [Google Scholar]
- 25.Pras E, Abu A, Rotenstreich Y, et al. Cone-rod dystrophy and a frameshift mutation in the PROM1 gene. Mol Vis. 2009;15:1709–1716 [PMC free article] [PubMed] [Google Scholar]
- 26.Zelhof AC, Hardy RW, Becker A, Zuker CS. Transforming the architecture of compound eyes. Nature. 2006;443:696–699 [DOI] [PubMed] [Google Scholar]
- 27.Husain N, Pellikka M, Hong H, et al. The agrin/perlecan-related protein eyes shut is essential for epithelial lumen formation in the Drosophila retina. Dev Cell. 2006;11:483–493 [DOI] [PubMed] [Google Scholar]
- 28.Abd El-Aziz MM, Barragan I, O'Driscoll CA, et al. EYS, encoding an ortholog of Drosophila spacemaker, is mutated in autosomal recessive retinitis pigmentosa. Nat Genet. 2008;40:1285–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]