crumbs genes play a role in the differentiation of the vertebrate cornea. Corneal defects associated with crumbs gene mutations are found in zebrafish mutants and human carriers of CRB1 gene mutations.
Abstract
Purpose.
To evaluate the role of crumbs genes and related epithelial polarity loci in the vertebrate cornea.
Methods.
The authors used histologic analysis and electron microscopy to evaluate the corneas of zebrafish mutant for a crumbs locus oko meduzy (ome) and in mutants of four other loci, nagie oko (nok), heart and soul (has), mosaic eyes (moe), and ncad (formerly glass onion), that function in the same or related genetic pathways. In parallel, they performed an evaluation of corneas in human carriers of a crumbs gene, CRB1, and mutations using topography and biomicroscopy. The expression of the CRB1 gene in the normal human cornea was examined by polymerase chain reaction (PCR) and immunohistochemical staining.
Results.
The corneas of zebrafish mutants display severe abnormalities of the epithelial and stromal layers. The epithelial cells do not properly adhere to each other, and fluid-filled spaces form between them. In addition, the layering of the corneal stroma is poorly formed or absent. The corneas of human carriers of CRB1 mutations display shape deviations compared with what has been observed in normal individuals. A PCR product of the correct size was obtained from normal human corneal samples. Sequence analyses confirmed its identity to be the human CRB1 gene. Immunohistochemical staining using anti-CRB1 yielded positive brown deposits in the human cornea.
Conclusions.
crumbs genes play a role in the differentiation of the vertebrate cornea. Corneal defects associated with crumbs gene mutations are very severe in the zebrafish model and, in comparison, appear clinically less pronounced in the human eye.
Optimal vision relies on the clarity and optical characteristics of the cornea. Its smooth optical surface and transparent nature allow the cornea to act as the eye's primary refractive surface. Any breakdown in this function presents a risk for a loss of visual acuity. Given the many variations of corneal abnormalities and the estimated number of affected patients, corneal diseases continue to be a valuable area of research.1–3 Corneal pathology such as trachoma, ocular trauma, and corneal ulceration make up a large portion of severe corneal morbidity, yet corneal dystrophies and degenerations also contribute a noteworthy portion to overall corneal pathologies. For example, in keratoconus—a type of corneal degeneration—prevalence has been estimated as 0.05% by one group of investigators, whereas another study reported the frequency of definite keratoconus cases of 0.12%.4,5 Although the exact prevalence of corneal dystrophies and degenerations is not always known, their clinical relevance is evident. It is worth noting that a portion of corneal dystrophies and degenerations have been suspected as genetically based, but in other cases the role of genetic factors remains unknown.2,6–9
The cornea of the vertebrate eye is a stratified structure. Each of the five primary layers that form the cornea must maintain its proper function to sustain optimal vision. The most superficial layer is the corneal epithelium, which, in the adult human eye, consists of three major components: the squamous cell layer, wing cell layer, and basal cell layer.10 Cells of the corneal epithelium are continuously added by mitotic divisions in the basal layer of the cornea and shed at the corneal surface.11 Postmitotic basal cells continuously move anteriorly, becoming wing cells and, with time, superficial epithelial cells. All corneal epithelial cells adhere to one another through desmosomes and gap junctions. In addition, the superficial squamous cells also adhere to each other through tight junctions.12,13 Beneath the corneal epithelium lies Bowman's layer, followed by a thick layer known as the stroma; both layers are rich in collagen fibrils. Bowman's layer consists of a thin, randomly structured meshwork, whereas the corneal stroma contains many lamellae of collagen fibrils, which provide thickness and strength to the cornea. In the fully developed human eye, the stroma contributes approximately 90% of the corneal thickness.13 Layers (lamellae) of the corneal stroma maintain an organized structure; collagen fiber bundles run parallel to each other within each layer, and each layer of bundles lies at an angle to adjacent layers.11,13–15 Collagen fibrils are maintained by keratocytes, which lie between stromal lamellae.13 Beneath the corneal stroma sits Descemet's layer and finally the most posterior corneal cell layer, the endothelium. Descemet's layer is a basal lamina secreted by the endothelium; with age, it increases in thickness.13 The endothelium performs an important function by acting as a pump to maintain corneal transparency and as a barrier to prevent the ingress of aqueous fluid. It removes fluid from the cornea, thus maintaining proper hydration and lamellar organization that preserve the clarity of the cornea.10 Endothelial cell membranes interdigitate with one another and are tightly bound by junctional complexes.12 The overall structure of the cornea is similar in different vertebrate species, including higher vertebrates and teleost fish.16 This provides the basis for the use of animal models in the studies of human corneal differentiation and function.
Both corneal epithelium and endothelium display apicobasal polarity. The most obvious manifestation of this polarity is the subdivision of their cell surface into two distinct domains by belts of cell junctions.13,16–20 These junctions confer mechanical strength and provide a barrier that limits the exchange of molecules between the outer environment and the extracellular spaces inside tissues. Given these characteristics, we hypothesized that mutations that cause a breakdown of epithelial polarity would also cause corneal defects. A number of genes have been shown to play essential roles in the formation of epithelial polarity in vertebrate and invertebrate genetic model organisms.21,22 One class of the major regulators of epithelial polarity is crumbs genes. Originally discovered in Drosophila,23 mutations in crumbs genes have been shown to produce apicobasal polarity defects in fly embryonic epithelia, the zebrafish retinal neuroepithelium, and in cultured mammalian epithelial sheets.24–28 To test their possible role in the cornea, we inspected zebrafish mutants at a crumbs locus, oko meduzy (ome), and found obvious abnormalities in the architecture of the corneal epithelium. Similar defects of the cornea were present in mutants of other loci that function in the ome pathway: nagie oko (nok), heart and soul (has), and mosaic eyes (moe), as well as in a cadherin mutant ncadm117.
Based on studies conducted in animal models, human carriers of crumbs gene mutations are also likely to display defects of epithelial structures. In fact, defects in one of the three human crumbs genes, CRB1, have been shown to cause several forms of photoreceptor degeneration in the human population.29–32 As photoreceptor cells display several epithelial characteristics,33 this phenotype is consistent with crumbs function in epithelial polarity. To investigate the possibility that crumbs genes function in the human cornea, we examined the expression of the CRB1 gene in the cornea and examined carriers of CRB1 mutations for corneal abnormalities. Although we found that these individuals do not display striking aberrations in the appearance of their corneas, compared with their genotypically normal siblings, their corneas do display a tendency to develop aberrant curvature.
Methods
Electron microscopy was performed as described previously.16 To quantify defects in the cornea of mutant zebrafish, we counted epithelial desmosomes at 3 days post fertilization (dpf). For each mutation, two mutant individuals and one wild-type sibling were used for measurements. Desmosomes were counted in five separate adjacent cells in the central cornea for each mutant and wild-type individual and the average number of desmosomes per cell was calculated. The length of the interface between two cell layers of the central corneal epithelium and the length of gaps that separate them were also measured and compared between mutant and wild-type siblings. All studies were performed in accordance with the ARVO Statement for Use of Animals in Ophthalmic and Vision Research and were approved by the animal welfare committee at each institution involved in this study.
Informed consent was obtained from all participants in this study according to the tenets of the Declaration of Helsinki. Clinical evaluations were conducted on a subset of patients from families carrying CRB1 mutations.32 Affected persons and their genetically normal siblings were examined. Corneal topographies were performed with a keratograph (CTK 922; Haag-Strait, Koeniz, Switzerland). Biomicroscopy was performed using standard clinical slit lamp approaches. Statistical analysis of data was performed using Wilcoxon, Mann-Whitney U, and Student's t-tests.
Polymerase chain reaction (PCR) and immunohistochemical staining were carried out to determine whether the CRB1 gene is expressed in the human cornea. Institutional review board approval was obtained for use of human eye tissues. Total RNA was extracted from normal human corneal and retinal tissues (RNeasy Mini Kit; Qiagen, Valencia, CA) according to the manufacturer's protocol. Tissues were dissected from eye bank eyes (Illinois Eye Bank, Chicago, IL) from donors 49 and 61 years of age. At least 1 μg RNA was reverse transcribed into cDNA using random hexamers (First-Strand cDNA Synthesis Kit; Fermentas, Glen Burnie, MD). PCR was performed using 500 ng corneal or retinal (positive control),34 cDNA template, and human CRB1-specific primer sets (forward, 5′-AACAACACCAGGTGCCTCTC-3′; reverse, 5′-GGCATGTAGCCTCGTTCTTG-3′). Water was used as a negative control. PCR conditions were 94°C denaturation for 1 minute, followed by 11 cycles of touchdown (94°C, 30 seconds; 65°C-55°C, decrease 1°C per cycle, 30 seconds; 72°C, 1 minute); 30 cycles of 94°C, 30 seconds; 55°C, 30 seconds, 72°C, 1 minute; and one cycle of 72°C, 7 minutes. The expected PCR product measures 516 bp. PCR products, along with DNA molecular weight marker (Marker VI; Roche Laboratories, Nutley, NJ) or 100-bp ladder markers (GeneRuler; Fermentas), were resolved on 1% agarose gels. They were subsequently column purified (QIAquick PCR Purification kit; Qiagen). Sequencing analyses were conducted to verify the identity of PCR products. The experiments were repeated three times.
For immunohistochemistry, corneas from eye bank donors 24, 25, 49, 61, 61, and 61 years of age were fixed in formalin and embedded in paraffin. Sections (5 μm) were deparaffinized and treated with boiling citrate buffer, pH 6.0, for antigen retrieval. Tissue sections were then blocked in blocking buffer (3% bovine serum albumin in phosphate-buffered saline) and incubated with goat anti–CRB1 antibody (1:100; Santa Cruz Biotechnology, Santa Cruz, CA) overnight at 4°C. The primary antibody was omitted in negative controls. The slides were further incubated with biotin-conjugated rabbit anti–goat secondary antibody (1:500; Jackson ImmunoResearch Laboratories, West Grove, PA), and the immunoreactive products were visualized (Vectastain ABC kit; Vector Laboratories, Burlingame, CA).
Zebrafish mutant alleles that were used in this analysis harbor the following defects. omem289 contains a termination codon at position 764, which eliminates approximately half the extracellular domain and the entire cytoplasmic region.26,35 The nokm227allele contains a substitution of a conserved amino acid in the PDZ domain at position 329.35,36 The strength of this allele is indistinguishable from nokm520, which involves a truncation of 150 C-terminal amino acids. The glom117 features a substitution of a tryptophane at position 2.35,37 This amino acid is very well conserved in evolution and is deemed essential for cadherin function. The hasm567 allele causes a C-terminal truncation of approximately 70 amino acids.38,39 Finally, moeb781 contains a premature termination codon within the first 20% of the polypeptide. All these alleles are predicted to produce either null or near-null phenotypes.
Results
The Role of crumbs and Related Genes in the Zebrafish Cornea
We previously showed that zebrafish and human corneas share many structural similarities, such as five major layers, keratocytes in the stroma, and a multilayered organization of the stromal matrix.16 To investigate the genetic bases of corneal differentiation, we took advantage of a large collection of zebrafish eye mutants available for analysis.40 Regulators of apicobasal polarity are one group of loci potentially important for corneal morphogenesis and maintenance. At least five such genes were found in past mutagenesis screens: oko meduzy (ome), nagie oko (nok), heart and soul (has), mosaic eyes (moe), and ncad (previously glass onion/parachute).35,41 They encode, respectively, a transmembrane protein related to the fly crumbs gene, a MAGUK scaffolding factor, an atypical protein kinase C, a FERM protein, and an adhesion molecule.26,36–39,42,43 Mutations in these loci affect the polarity of retinal neuroepithelium. Given that the cornea contains two cell layers that display apicobasal polarity, we hypothesized that these regulators are likely to play a role in corneal differentiation.
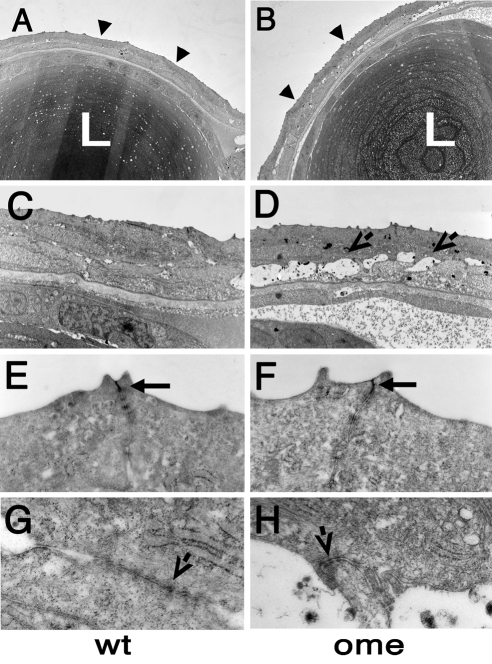
In the wild-type zebrafish cornea at 3 dpf, the two layers of epithelial cells adhere tightly to one another and are connected by cell junction complexes, most likely desmosomes16 (Figs. 1C; 2, top). By contrast, the omem289 mutation produces extensive extracellular spaces between the inner and the outer layer of the corneal epithelium (Fig. 1D). These spaces indicate a defect in adhesive properties of corneal epithelial cells. Given that ome mutations produce a defect of apical cell junctions in both the zebrafish retinal neuroepithelium and in fly embryonic epithelia,44,45 it was unexpected that the apical junctions of the corneal epithelium would be grossly intact (Fig. 1F; compare to wild-type in Fig. 1E). Some junctions also persist at the interface of the two epithelial cell layers of mutant corneas (Fig. 1H).
Figure 1.
Electron micrographs of transverse sections through eyes of wild-type and oko meduzy (ome) mutant zebrafish at 3 dpf. (A, B) Low-magnification view of the lens and the overlying cornea. Note that the surface of the mutant cornea features more protrusions than the wild-type cornea. Additionally, fluid-filled spaces are found between epithelial cells in the mutant. Arrowheads: corneal surface. (C, D) Higher-magnification images of the cornea. (D, arrows) Fluid-filled spaces between epithelial cells. (E, F) Higher magnifications of the corneal surface. Wild-type and mutant apical junctions (arrows) do not display any obvious differences. (G, H) Junctions are present on the interface between two layers of corneal epithelial cells. At least some of them persist in the mutant (arrows). L, lens.
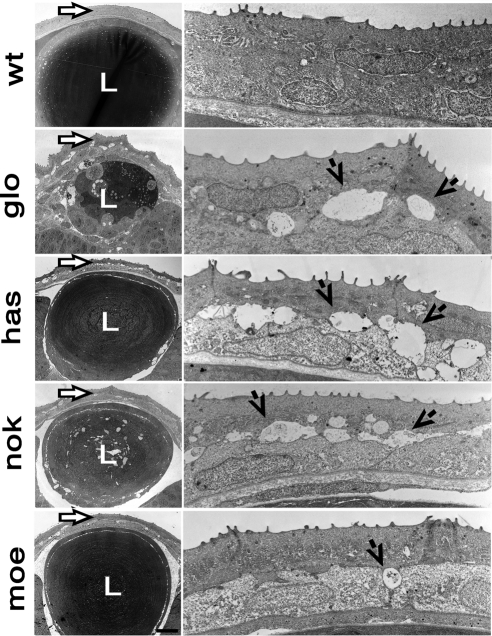
Figure 2.
Electron micrographs of transverse sections through eyes of wild-type and mutant zebrafish at 3 dpf. Left: lens and overlying cornea (arrows). Right: higher magnifications of the corneal epithelium and stromal layer. Each row of images corresponds to a different genotype. Top to bottom: corneas of the wild-type and the following mutant strains: glo, has, nok, and moe. Right, arrows: fluid-filled spaces between epithelial cells of mutant corneas. L, lens.
To extend this analysis, we investigated the corneas of mutants in four loci related to ome: nagie oko (nok), heart and soul (has), mosaic eyes (moe), and ncad. All mutants display defects similar to these seen in ome, although the magnitude of epithelial abnormalities varies (Fig. 2). To quantitatively analyze these defects, we measured the portion of the interface between the two epithelial layers occupied by intercellular spaces and recorded the number of desmosomes (Table 1). Mutations in the ncad (glom117) and has (hasm567) genes produce the strongest loss of adhesion between the outer and the inner epithelial layer. In these mutants, approximately 75% of adhesion interface is occupied by extracellular spaces, and only approximately 25% of the desmosomes are present, compared with their wild-type siblings (Table 1). Corneal epithelial defects in nok (nokm227) are similar to those in ome (omem289): approximately 30% of adhesion interface is affected, and about half the desmosomes are lost. Finally, the moe (moeb781) epithelial defect is the weakest. Approximately 20% of adhesive interface is defective, primarily at the borders between adjacent cells of the posterior layer of the corneal epithelium (Fig. 2). Similar to omem289, the apical junctions are intact in all four mutants.
Table 1.
Quantitative Evaluation of Corneal Defects in Zebrafish Epithelial Polarity Mutants
| Fish | Desmosomes (n) |
% of Cell Surface in Direct Contact with Other Cells |
|||
|---|---|---|---|---|---|
| Wt | Mt | Mt/Wt (%) | Wt | Mt | |
| glom117 | 20.8 | 5.1 | 24.6 | 97 | 24 |
| hasm567 | 21.0 | 5.3 | 25.0 | 96.2 | 20.2 |
| nokm227 | 21.5 | 10.9 | 50.7 | 97.8 | 66.9 |
| omem289 | 20.3 | 10.7 | 52.7 | 95.7 | 66.9 |
| moeb781 | 21.0 | 14.1 | 67.2 | 93.6 | 75.1 |
Wt, phenotypically wild-type siblings of each strain; Mt, phenotypically mutant siblings of each strain.
In omem289 and all other mutants, the stroma appears to be less organized. In wild-type fish, the stroma contains 8 to 10 layers of fibrils, arranged at angles to each other.16 In contrast, this regularity of layering of the stromal fibrils is compromised in all epithelial polarity mutants (data not shown).
In addition to the cornea, mutations in epithelial polarity loci also affect the lens. The glo defect is again the most severe. glom117 lens is much smaller in the mutant compared with that in its wild-type siblings. There is no defined layer of the lens epithelium; instead, the lens appears to be composed of largely undifferentiated cells (Fig. 2). In the nokm227 mutants, fluid-filled spaces are present in the central region of the lens (Fig. 2). Lens differentiation appears to be delayed in this mutant, as evidenced by the presence of cell nuclei in the central region of the lens (data not shown). Similar defects are also seen in some of the more severely affected omem289 mutant individuals (not shown). The lens in hasm567 and moeb781 mutants is largely normal, though intercellular spaces are present under the lens epithelium (Fig. 2). These are absent in their wild-type siblings.
Abnormalities in Human crumbs Mutant Corneas
Mutations in one of the human crumbs genes, CRB1, are known to cause several forms of retinal abnormalities that lead to blindness, including Leber congenital amaurosis, and several forms of retinitis pigmentosa.29,30,32,46,47 These human CRB1-associated diseases are autosomal recessive in inheritance. Studies of these disorders identified numerous human carriers of defects in the CRB1 gene48 (example in Fig. 3). Given the presence of corneal abnormalities in zebrafish crumbs mutants, we began to search for corneal defects in human carriers of crumbs mutations. To accomplish that, we performed corneal topography and biomicroscopy measurements in individuals homozygous for CRB1 genetic defects (example shown in Fig. 4).
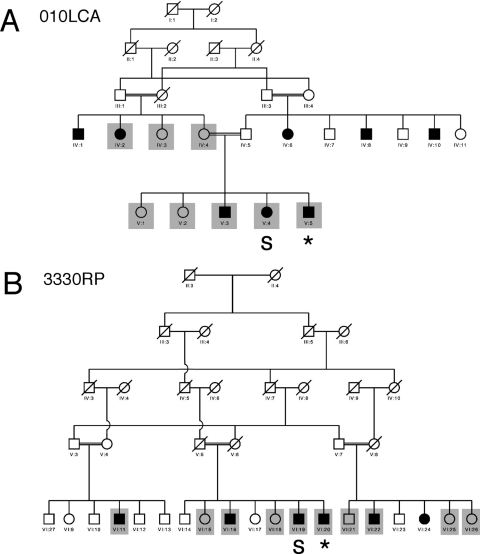
Figure 3.
Pedigrees of two Pakistani families that carry defects in the CRB1 gene based on previously published data.32 Shaded boxes: family members who were tested for corneal defects. Asterisks: patients who show signs of corneal abnormalities based on KSS analysis. S, severe corneal scarring.
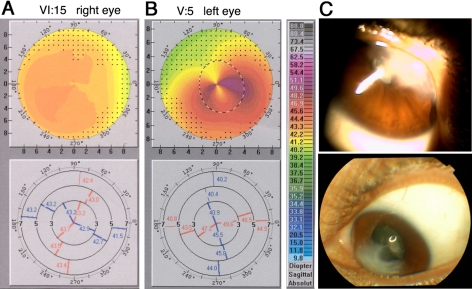
Figure 4.
Normal and CRB1 mutant corneas. (A, B) Corneal topography data from a 3330RP family control (A) and from a 010LCA family homozygous carrier of a CRB1 mutation (B). (A, B) Top: graphic representations of corneal curvature. Steeper areas are represented by warmer colors, and flatter regions are represented by cooler colors, as color-coded on the scale to the right. Bottom: raw numerical data of corneal curvature from the same two patients. The cornea of a crumbs mutation carrier is characterized by steeper curvatures and irregular astigmatism, most pronounced in the inferior temporal region. The control features much more homogeneous corneal curvatures. (C) Cornea of patient V-4 (010LCA pedigree; Fig. 3A). Severe corneal scarring, possibly a result of corneal ectasia, prevented topography measurement in this patient.
Corneal evaluations were performed on the members of two Pakistani families who were previously shown to carry CRB1 mutations32 (Fig. 3). In the 3330RP pedigree, we inspected corneal phenotypes in five homozygous carriers of the CRB1 mutation and in five control members either heterozygous or wild type at the CRB1 locus. In the 010LCA family, we inspected four homozygous carriers and four control members. We were unable to collect topography data from two family members because of severe corneal defects (labeled “S” in Fig. 3). On slit lamp examination, both persons displayed severe corneal scarring, combined in at least one case with Munson's sign (Fig. 4C). Such defects were not seen in relatives of these two persons; thus, they may represent severe examples of the CRB1 mutant phenotype. We also removed several topography measurements from the data pool because of patients' inability to focus on the target during data collection. All the instances of poor fixation were found among topography measurements of homozygous carriers of CRB1 mutations. This was not unexpected because they had poor vision and, in the most extreme cases, were blind.31,49
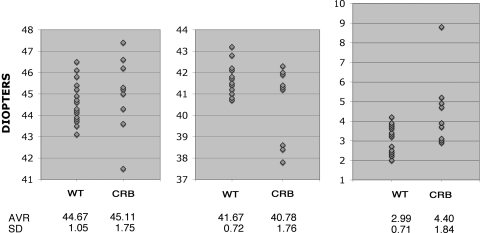
To quantitate differences between clinically normal persons and homozygous carriers of CRB1 mutations, we examined three parameters based on topographic measurements: the steepest point on the cornea, the flattest point on the cornea, and total corneal toricity. The steepest point on the cornea was steeper on average in carriers of CRB1 mutations compared with the steepest point in noncarriers, but this difference was not statistically significant (Fig. 5; 44.67 vs. 45.11 D in normal and affected persons, respectively). Similarly, the flattest point on the cornea was flatter on average in carriers of crumbs mutations. However, the difference again was not statistically significant given our sample size (41.67 vs. 40.78 D in normal and affected persons). Consistent with these findings, strong differences were present in the total corneal toricity, measured as the difference between the steepest and the flattest point (2.99 vs. 4.40 D). This difference was significant based on the Student's t-test and the Mann-Whitney U test (P < 0.05). Additionally, the first two measurements displayed dramatically higher variability in carriers of CRB1 mutations (Fig. 5).
Figure 5.
Measurements of corneal curvature in controls (WT) and in homozygous carriers of CRB1 mutations (CRB). Measurements of the steepest point on the cornea (left), the flattest point (middle), and the difference between the two (right) are provided. The vertical axis shows dioptric values, and the horizontal axis shows genotypes. Averages and standard deviations are provided below each graph.
In addition, to evaluate differences between persons homozygous for the CRB1 mutation and their normal relatives, we performed a modified version of the keratoconus severity score (KSS) analysis.1 This assessment revealed that two homozygous carriers of CRB1 mutations feature defective corneas (Fig. 3, asterisks). None of the control persons displayed this defect. Finally, specular microscopy did not reveal any obvious abnormalities in the morphology of endothelial cells (data not shown). Taken together, these observations reveal deviations in the shape of CRB1 homozygous mutant corneas compared with the corneas of control persons, either wild-type homozygous or heterozygous carriers of the CRB1 mutation. Our results suggest that crumbs gene defects create a predisposition to corneal abnormalities.
Expression of Human CRB1 Gene in Normal Human Cornea
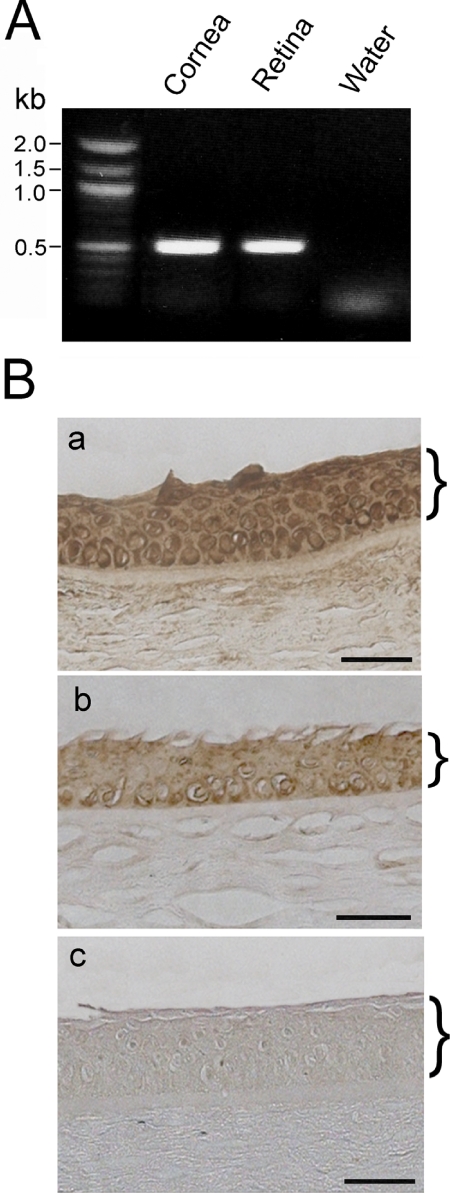
To investigate whether CRB1 is expressed in the cornea, we performed two experiments. First, a 516-bp product was obtained from the normal human corneal cDNA samples by PCR (Fig. 6A). Sequencing analyses confirmed the identity of this PCR product to be the human CRB1 gene. An identical PCR product was also detected in human retinal samples that served as the positive control but not from the negative control (Fig. 6A). In agreement with these results, immunohistochemical staining using anti-CRB1 yielded positive brown deposits in the human cornea (Figs. 6Ba, 6Bb). The most prominent staining was seen in the epithelial layer. Staining of CRB1 appeared enriched in nuclei.
Figure 6.
Expression of CRB1 gene in the human cornea. (A) PCR amplification of the human CRB1 gene. A 516-bp CRB1 PCR product was amplified from normal human corneal and retinal cDNA templates but not from the water control. (B) Immunohistochemical staining for CRB1 in the normal human cornea (a, b). Immunoreactive products (brown) were noted in the epithelial layer. Negative control (c) in which primary antibody was omitted showed minimal staining. Brackets: the corneal epithelium.
Discussion
Analysis of the zebrafish crumbs (oko meduzy) mutant cornea revealed a loss of integrity in the corneal epithelial layer, as evidenced by the appearance of fluid-filled spaces between epithelial cells. Additionally, the layering of the corneal stroma was poorly defined or absent. Such structural defects are not obviously reminiscent of those seen in human corneal dystrophies. In a keratoconic human cornea, epithelial cells display irregular or elongated shape, yet the desmosomes and interfaces between epithelial cells appear to be normal.50–52 In contrast to our observations in the zebrafish, fluid-filled spaces were reported inside, but not in between, corneal epithelial cells in keratoconus patients.50,52 Additionally, in affected humans, stromal lamellae decrease in number and are frequently irregular or wavy.14,52 These abnormalities differ from defects observed in zebrafish ome mutants. Similarly, a number of other dystrophies affect corneal structure, but their characteristics differ considerably from what we found in zebrafish ome mutants. For example, though Reis-Buckler dystrophy affects the epithelium, Bowman's layer and the superficial stroma, and epithelial basement membrane dystrophy primarily affects the epithelial basement membrane, neither produces the magnitude of stromal defects we observed in zebrafish mutants.53 On the other hand, many stromal dystrophies are characterized by the presence of stromal deposits or opacities, which frequently are visible as linear or spotted forms in the cornea.53 We did not observe these in human carriers of crumbs mutations or in the zebrafish model. Additionally, such dystrophies as posterior polymorphous dystrophy, and iridocorneal endothelial syndrome (ICE) feature corneal abnormalities originating primarily in the posterior aspect of the cornea.54 Again, their characteristics differ from those seen in zebrafish mutants. Thus, we do not see a clear similarity between zebrafish ome defects and those in well-known human corneal dystrophies.54
Given that crumbs genes function in the formation of adherens junctions,26,44 abnormal adhesion between epithelial cells in ome mutants is not surprising. It is important to note, however, that obvious differences exist between the phenotype of the corneal epithelium and those of other epithelia that were studied in crumbs mutants. Although the apical surface is disrupted in fly embryonic epithelia and in the retinal epithelium of the zebrafish ome mutant,26,44,45 the integrity of the apical surface in the corneal epithelium of crb2a (ome) mutants is intact. Our observations also show that the same is true for the corneas of mutants that carry defects in several other epithelial polarity loci related to ome. In particular, apically located cell junctions appear grossly intact in this group of mutants (Figs. 1, 2). This suggests that crumbs genes function differently in the corneal epithelium than in other epithelia. Perhaps their function is limited to regulating some aspects of protein trafficking and is not relevant to the formation of junctional structures. Mistargeting of apical polypeptides to the basolateral surface could change adhesive properties of epithelial cells and result in the appearance of fluid-filled spaces between them, as observed in ome mutants. The origins of stromal defects in zebrafish crumbs mutants are also unclear. In one scenario, they may be secondary to epithelial abnormalities, as discussed previously by others.55–58 Alternatively, a keratocyte defect could be responsible for changes in stromal architecture. This does not appear to be the case, however, because corneal keratocytes are not found in the zebrafish cornea until after 2 weeks of age.16 Finally, we cannot exclude the possibility that stromal defects are, at least in part, secondary to endothelial abnormalities.
As in zebrafish corneas, human corneas display defects in homozygous carriers of crumbs mutations. The human defects appear, however, considerably milder. Differences between animal models and human models are not without a precedent. The mouse mutants of some Usher syndrome genes, for example, do not display photoreceptor degeneration.59 Alternatively, the differences between human and zebrafish phenotypes may be caused by different strengths of mutant alleles. The zebrafish omem289 mutant allele used in this study truncates most of the protein sequence and is most likely null,26 whereas CRB1 mutations in the human pedigrees we studied involve single amino acid substitutions and thus may result in a partial loss of gene function.32 Another likely explanation for differences between zebrafish and human phenotypes could lie in the fact that the zebrafish ome locus encodes a homolog of the human CRB2 gene, whereas the affected family members we studied carry CRB1 defects. It is thus possible that human carriers of CRB2 mutations display more severe corneal defects. To our knowledge, however, human abnormalities associated with CRB2 mutations have not been reported thus far.60 Two of our patients (Fig. 4C and data not shown) displayed severe corneal scarring. These two patients may represent a more severe form of crumbs-associated corneal defects that eventually produced scarring, possibly as a result of recurrent erosion. If this is the case, their corneas may, in fact, display abnormalities reminiscent of those seen in the zebrafish model. Ultrastructural analysis of tissue in nonscarred areas of the corneas of such patients will be necessary to determine whether this is the case. We also note that in a related effort, McMahon et al.,61 while investigating a cohort of patients with Leber congenital amaurosis, determined that carriers homozygous for the CRB1 gene, and possibly the CRX gene, seem to have higher propensity of concurrent signs of keratoconus compared with other genotypes studied (AIPL1, RetGC, RPE65).61
Our analysis has identified a tendency toward corneal defects in human carriers of CRB1 gene mutations. This observation is supported by our studies that detected both CRB1 transcript and protein in the human cornea, indicating that the CRB1 gene is expressed therein. CRB1 mutations are likely to be overlooked in most patients because they display severe retinal degeneration. Our findings suggest that CRB1 patients should also be routinely examined for corneal defects. Although human carriers of homozygous CRB1 mutations have vision loss primarily caused by photoreceptor degeneration, benefit derived from the diagnosis and treatment of corneal defects may enhance their overall functional vision and may improve their quality of life.
Footnotes
Supported by National Eye Institute awards RO1 EY011882 and EY016859 (JJM), EY017571 (TTM), EY018728 (XCZ), EY03890 (BYJTY), EY01792 (Core Grant UIC), and EY10608; Research to Prevent Blindness (Department of Ophthalmology and Visual Science, The University of Texas Health Science Center at Houston); Hermann Eye Fund Awards (XCZ, RY); and Wellcome Trust Grant 063406/Z/2000/Z.
Disclosure: J. Beyer, None; X.C. Zhao, None; R. Yee, None; S. Khaliq, None; T.T. McMahon, None; H. Ying, None; B.Y.J.T. Yue, None; J.J. Malicki, None
References
- 1.McMahon TT, Szczotka-Flynn L, Barr JT, et al. A new method for grading the severity of keratoconus: the Keratoconus Severity Score (KSS). Cornea. 2006;25:794–800 [DOI] [PubMed] [Google Scholar]
- 2.Poulaki V, Colby K. Genetics of anterior and stromal corneal dystrophies. Semin Ophthalmol. 2008;23:9–17 [DOI] [PubMed] [Google Scholar]
- 3.Javadi MA, Rafee'i AB, Kamalian N, Karimian F, Ja'farinasab MR, Yazdani S. Concomitant keratoconus and macular corneal dystrophy. Cornea. 2004;23:508–512 [DOI] [PubMed] [Google Scholar]
- 4.Kennedy RH, Bourne WM, Dyer JA. A 48-year clinical and epidemiologic study of keratoconus. Am J Ophthalmol. 1986;101:267–273 [DOI] [PubMed] [Google Scholar]
- 5.Hofstetter HW. A keratoscopic survey of 13,395 eyes. Am J Optom Arch Am Acad Optom. 1959;36:3–11 [DOI] [PubMed] [Google Scholar]
- 6.Rabinowitz YS. The genetics of keratoconus. Ophthalmol Clin North Am. 2003;16:607–620, vii [DOI] [PubMed] [Google Scholar]
- 7.Edwards M, McGhee CN, Dean S. The genetics of keratoconus. Clin Exp Ophthalmol. 2001;29:345–351 [DOI] [PubMed] [Google Scholar]
- 8.Bechara SJ, Waring GO, 3rd, Insler MS. Keratoconus in two pairs of identical twins. Cornea. 1996;15:90–93 [PubMed] [Google Scholar]
- 9.Rabinowitz YS, Maumenee IH, Lundergan MK, et al. Molecular genetic analysis in autosomal dominant keratoconus. Cornea. 1992;11:302–308 [DOI] [PubMed] [Google Scholar]
- 10.Kaufman P, Alm A. Adler's Physiology of the Eye. St. Louis: Mosby; 2003 [Google Scholar]
- 11.Hanna C, Bicknell DS, O'Brien JE. Cell turnover in the adult human eye. Arch Ophthalmol. 1961;65:695–698 [DOI] [PubMed] [Google Scholar]
- 12.McLaughlin BJ, Caldwell RB, Sasaki Y, Wood TO. Freeze-fracture quantitative comparison of rabbit corneal epithelial and endothelial membranes. Curr Eye Res. 1985;4:951–961 [DOI] [PubMed] [Google Scholar]
- 13.Kaufman H, Barron B, McDonald MB. The Cornea. Boston: Butterworth-Heinemann; 1998 [Google Scholar]
- 14.Polack FM. Morphology of the cornea, I: study with silver stains. Am J Ophthalmol. 1961;51:1051–1056 [DOI] [PubMed] [Google Scholar]
- 15.McCally RL, Farrell RA. Structural implications of small-angle light scattering from cornea. Exp Eye Res. 1982;34:99–113 [DOI] [PubMed] [Google Scholar]
- 16.Zhao XC, Yee RW, Norcom E, et al. The zebrafish cornea: structure and development. Invest Ophthalmol Vis Sci. 2006;47:4341–4348 [DOI] [PubMed] [Google Scholar]
- 17.Anderson SC, Stone C, Tkach L, SundarRaj N. Rho and Rho-kinase (ROCK) signaling in adherens and gap junction assembly in corneal epithelium. Invest Ophthalmol Vis Sci. 2002;43:978–986 [PubMed] [Google Scholar]
- 18.Barry PA, Petroll WM, Andrews PM, Cavanagh HD, Jester JV. The spatial organization of corneal endothelial cytoskeletal proteins and their relationship to the apical junctional complex. Invest Ophthalmol Vis Sci. 1995;36:1115–1124 [PubMed] [Google Scholar]
- 19.Mandell KJ, Holley GP, Parkos CA, Edelhauser HF. Antibody blockade of junctional adhesion molecule-A in rabbit corneal endothelial tight junctions produces corneal swelling. Invest Ophthalmol Vis Sci. 2006;47:2408–2416 [DOI] [PubMed] [Google Scholar]
- 20.Mandell KJ, Berglin L, Severson EA, Edelhauser HF, Parkos CA. Expression of JAM-A in the human corneal endothelium and retinal pigment epithelium: localization and evidence for role in barrier function. Invest Ophthalmol Vis Sci. 2007;48:3928–3936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Knust E, Bossinger O. Composition and formation of intercellular junctions in epithelial cells. Science. 2002;298:1955–1959 [DOI] [PubMed] [Google Scholar]
- 22.Shin K, Fogg VC, Margolis B. Tight junctions and cell polarity. Annu Rev Cell Dev Biol. 2006;22:207–235 [DOI] [PubMed] [Google Scholar]
- 23.Jurgens G, Wieschaus E, Nusslein-Volhard C, Kluding C. Mutations affecting the pattern of the larval cuticle in Drosophila melanogaster. Roux Archiv of Dev Bio. 1984;193:283–295 [DOI] [PubMed] [Google Scholar]
- 24.Roh MH, Fan S, Liu CJ, Margolis B. The Crumbs3-Pals1 complex participates in the establishment of polarity in mammalian epithelial cells. J Cell Sci. 2003;116:2895–2906 [DOI] [PubMed] [Google Scholar]
- 25.Fogg VC, Liu CJ, Margolis B. Multiple regions of Crumbs3 are required for tight junction formation in MCF10A cells. J Cell Sci. 2005;118:2859–2869 [DOI] [PubMed] [Google Scholar]
- 26.Omori Y, Malicki J. oko meduzy and Related crumbs genes are determinants of apical cell features in the vertebrate embryo. Curr Biol. 2006;16:945–957 [DOI] [PubMed] [Google Scholar]
- 27.Tepass U, Theres C, Knust E. crumbs encodes an EGF-like protein expressed on apical membranes of Drosophila epithelial cells and required for organization of epithelia. Cell. 1990;61:787–799 [DOI] [PubMed] [Google Scholar]
- 28.Campbell K, Knust E, Skaer H. Crumbs stabilises epithelial polarity during tissue remodelling. J Cell Sci. 2009;122:2604–2612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.den Hollander AI, ten Brink JB, de Kok YJ, et al. Mutations in a human homologue of Drosophila crumbs cause retinitis pigmentosa (RP12). Nat Genet. 1999;23:217–221 [DOI] [PubMed] [Google Scholar]
- 30.den Hollander AI, Heckenlively JR, van den Born LI, et al. Leber congenital amaurosis and retinitis pigmentosa with Coats-like exudative vasculopathy are associated with mutations in the crumbs homologue 1 (CRB1) gene. Am J Hum Genet. 2001;69:198–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.den Hollander AI, Davis J, van der Velde-Visser SD, et al. CRB1 mutation spectrum in inherited retinal dystrophies. Hum Mutat. 2004;24:355–369 [DOI] [PubMed] [Google Scholar]
- 32.Khaliq S, Abid A, Hameed A, et al. Mutation screening of Pakistani families with congenital eye disorders. Exp Eye Res. 2003;76:343–348 [DOI] [PubMed] [Google Scholar]
- 33.Kennedy B, Malicki J. What drives cell morphogenesis: a look inside the vertebrate photoreceptor. Dev Dyn. 2009;238:2115–2138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gosens I, den Hollander AI, Cremers FP, Roepman R. Composition and function of the Crumbs protein complex in the mammalian retina. Exp Eye Res. 2008;86:713–726 [DOI] [PubMed] [Google Scholar]
- 35.Malicki J, Neuhauss SC, Schier AF, et al. Mutations affecting development of the zebrafish retina. Development. 1996;123:263–273 [DOI] [PubMed] [Google Scholar]
- 36.Wei X, Malicki J. nagie oko, encoding a MAGUK-family protein, is essential for cellular patterning of the retina. Nat Genet. 2002;31:150–157 [DOI] [PubMed] [Google Scholar]
- 37.Malicki J, Jo H, Pujic Z. Zebrafish N-cadherin, encoded by the glass onion locus, plays an essential role in retinal patterning. Dev Biol. 2003;259:95–108 [DOI] [PubMed] [Google Scholar]
- 38.Horne-Badovinac S, Lin D, Waldron S, et al. Positional cloning of heart and soul reveals multiple roles for PKC lambda in zebrafish organogenesis. Curr Biol. 2001;11:1492–1502 [DOI] [PubMed] [Google Scholar]
- 39.Peterson RT, Mably JD, Chen JN, Fishman MC. Convergence of distinct pathways to heart patterning revealed by the small molecule concentramide and the mutation heart-and-soul. Curr Biol. 2001;11:1481–1491 [DOI] [PubMed] [Google Scholar]
- 40.Avanesov A, Malicki J. Approaches to study neurogenesis in the zebrafish retina. Methods Cell Biol. 2004;76:333–384 [DOI] [PubMed] [Google Scholar]
- 41.Jensen AM, Walker C, Westerfield M. mosaic eyes: a zebrafish gene required in pigmented epithelium for apical localization of retinal cell division and lamination. Development. 2001;128:95–105 [DOI] [PubMed] [Google Scholar]
- 42.Jensen AM, Westerfield M. Zebrafish mosaic eyes is a novel FERM protein required for retinal lamination and retinal pigmented epithelial tight junction formation. Curr Biol. 2004;14:711–717 [DOI] [PubMed] [Google Scholar]
- 43.Masai I, Lele Z, Yamaguchi M, et al. N-cadherin mediates retinal lamination, maintenance of forebrain compartments and patterning of retinal neurites. Development. 2003;130:2479–2494 [DOI] [PubMed] [Google Scholar]
- 44.Grawe F, Wodarz A, Lee B, Knust E, Skaer H. The Drosophila genes crumbs and stardust are involved in the biogenesis of adherens junctions. Development. 1996;122:951–959 [DOI] [PubMed] [Google Scholar]
- 45.Malicki J, Driever W. oko meduzy mutations affect neuronal patterning in the zebrafish retina and reveal cell-cell interactions of the retinal neuroepithelial sheet. Development. 1999;126:1235–1246 [DOI] [PubMed] [Google Scholar]
- 46.den Hollander AI, Ghiani M, de Kok YJ, et al. Isolation of Crb1, a mouse homologue of Drosophila crumbs, and analysis of its expression pattern in eye and brain. Mech Dev. 2002;110:203–207 [DOI] [PubMed] [Google Scholar]
- 47.Lotery AJ, Jacobson SG, Fishman GA, et al. Mutations in the CRB1 gene cause Leber congenital amaurosis. Arch Ophthalmol. 2001;119:415–420 [DOI] [PubMed] [Google Scholar]
- 48.Easter SS, Jr, Malicki JJ. The zebrafish eye: developmental and genetic analysis. Results Probl Cell Differ. 2002:346–370 [DOI] [PubMed] [Google Scholar]
- 49.Richard M, Roepman R, Aartsen WM, et al. Towards understanding CRUMBS function in retinal dystrophies. Hum Mol Genet. 2006;15:R235–R243 [DOI] [PubMed] [Google Scholar]
- 50.Aktekin M, Sargon MF, Cakar P, Celik HH, Firat E. Ultrastructure of the cornea epithelium in keratoconus. Okajimas Folia Anat Jpn. 1998;75:45–53 [DOI] [PubMed] [Google Scholar]
- 51.Lohman LE, Rao GN, Aquavella JV. In vivo microscopic observations of human corneal epithelial abnormalities. Am J Ophthalmol. 1982;93:210–217 [DOI] [PubMed] [Google Scholar]
- 52.Wong S, Rodrigues MM, Blackman HJ, Guimaraes R, Lemp MA. Color specular microscopy of disorders involving the corneal epithelium. Ophthalmology. 1984;91:1176–1183 [DOI] [PubMed] [Google Scholar]
- 53.Miller CA, Krachmer JH. Epithelial and stromal dystrophies. In: Kaufman H, Barron B, McDonald MB. eds. The Cornea. Boston: Butterworth-Heinemann; 1998:411–452 [Google Scholar]
- 54.Miller CA, Krachmer JH. Endothelial dystrophies. In: Kaufman H, Barron B, McDonald MB. eds. The Cornea. Boston: Butterworth-Heinemann; 1998:453–475 [Google Scholar]
- 55.Wilson SE, He YG, Weng J, et al. Epithelial injury induces keratocyte apoptosis: hypothesized role for the interleukin-1 system in the modulation of corneal tissue organization and wound healing. Exp Eye Res. 1996;62:325–327 [DOI] [PubMed] [Google Scholar]
- 56.Kim WJ, Rabinowitz YS, Meisler DM, Wilson SE. Keratocyte apoptosis associated with keratoconus. Exp Eye Res. 1999;69:475–481 [DOI] [PubMed] [Google Scholar]
- 57.Sawaguchi S, Fukuchi T, Abe H, Kaiya T, Sugar J, Yue BY. Three-dimensional scanning electron microscopic study of keratoconus corneas. Arch Ophthalmol. 1998;116:62–68 [DOI] [PubMed] [Google Scholar]
- 58.Teng CC. Electron microscope study of the pathology of keratoconus, I. Am J Ophthalmol. 1963;55:18–47 [DOI] [PubMed] [Google Scholar]
- 59.Saihan Z, Webster AR, Luxon L, Bitner-Glindzicz M. Update on Usher syndrome. Curr Opin Neurol. 2009;22:19–27 [DOI] [PubMed] [Google Scholar]
- 60.van den Hurk JA, Rashbass P, Roepman R, et al. Characterization of the Crumbs homolog 2 (CRB2) gene and analysis of its role in retinitis pigmentosa and Leber congenital amaurosis. Mol Vis. 2005;11:263–273 [PubMed] [Google Scholar]
- 61.McMahon TT, Kim LS, Fishman GA, et al. CRB1 gene mutations are associated with keratoconus in patients with Leber congenital amaurosis. Invest Ophthalmol Vis Sci. 2009;50:3185–3187 [DOI] [PubMed] [Google Scholar]